MTL-CEBPA Combined with Immunotherapy or RFA Enhances Immunological Anti-Tumor Response in Preclinical Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Tumor Cell Lines
2.3. BNL Model Experimental Groups and Treatment Schedule
- 1.
- Control
- 2.
- RFA
- 3.
- Anti-PD1
- 4.
- MTL-CEBPA
- 5.
- Anti-PD1+ MTL-CEBPA
- 6.
- RFA + anti-PD1
- 7.
- RFA+ MTL-CEBPA
- 8.
- RFA+ Anti-PD1+ MTL-CEBPA
2.4. Radiofrequency Ablation (RFA) Treatment
2.5. TILs Isolation
2.6. Flow Cytometry
2.7. CT26 Model Experimental Group and Treatment Schedule
- 1.
- Control group
- 2.
- MTL-CEBPA
- 3.
- Anti-PD1
- 4.
- Anti-PD1+ MTL-CEBPA
2.8. Nanostring Analysis of CT26 Frozen Tumor Samples
2.9. Statistics Analysis
3. Results
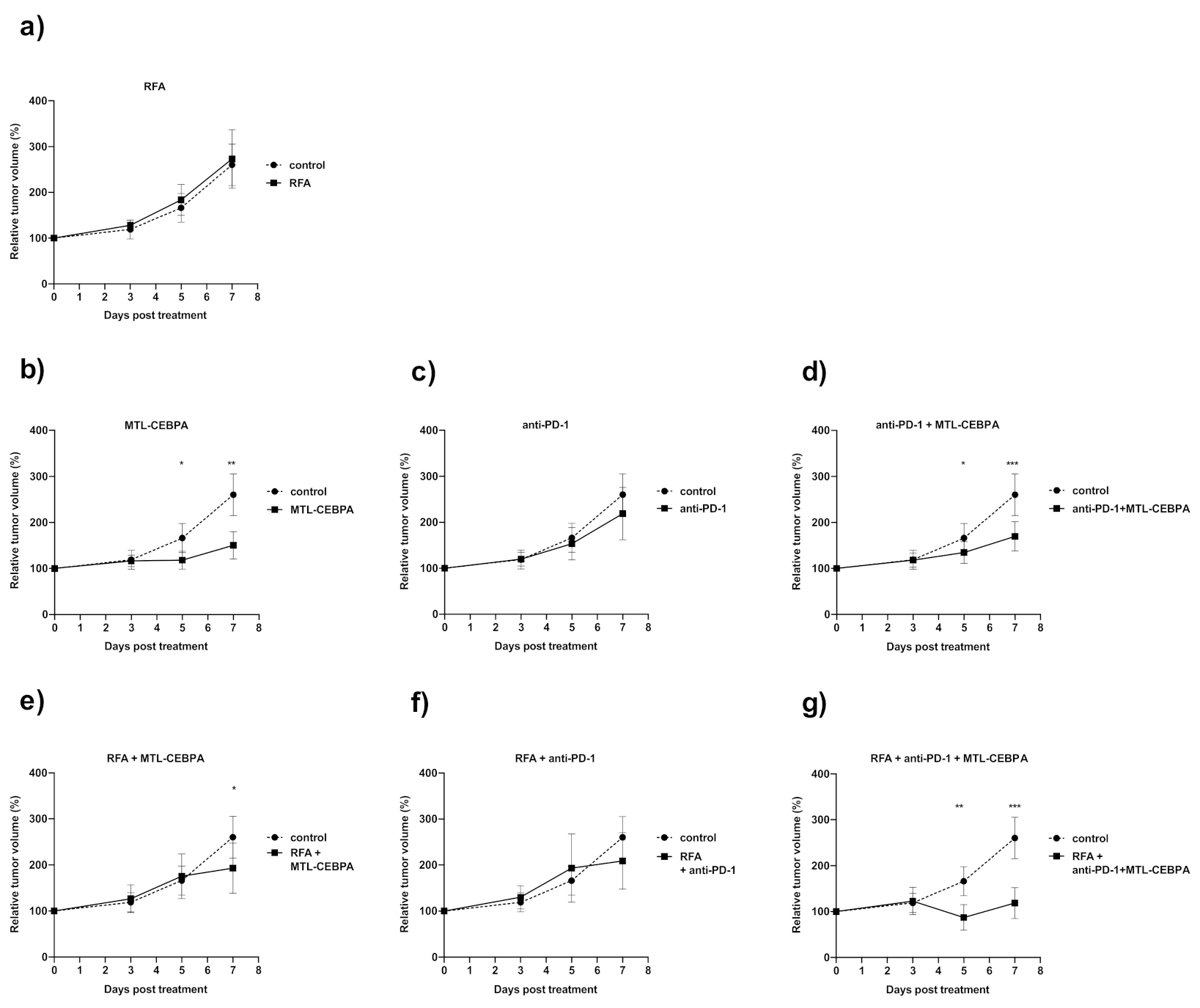
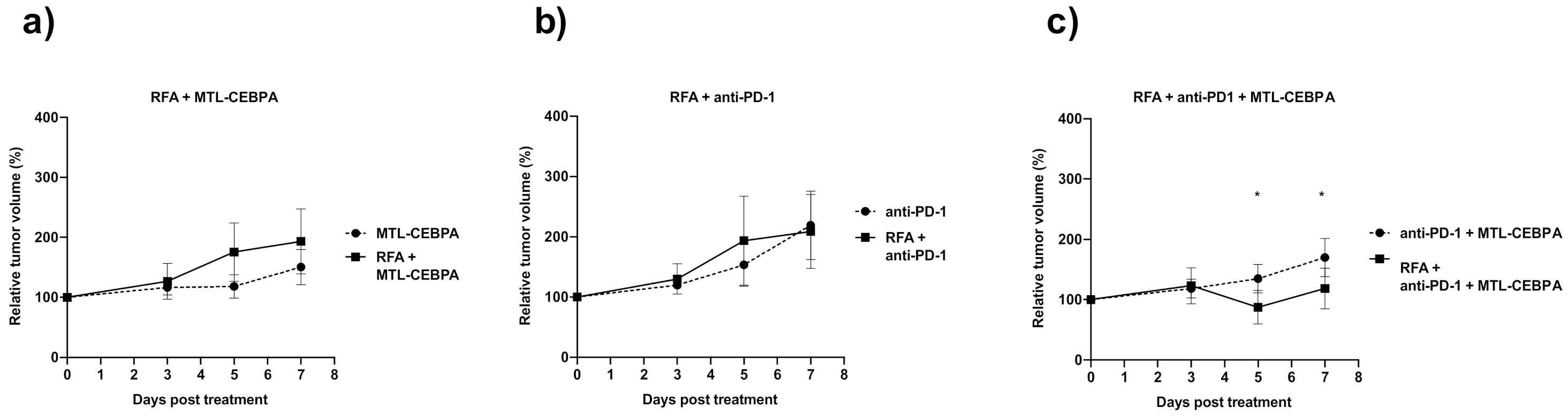
3.1. Combination of RFA, Anti-PD-1 and MTL-CEBPA Synergistically Reduce BNL Tumor Volume
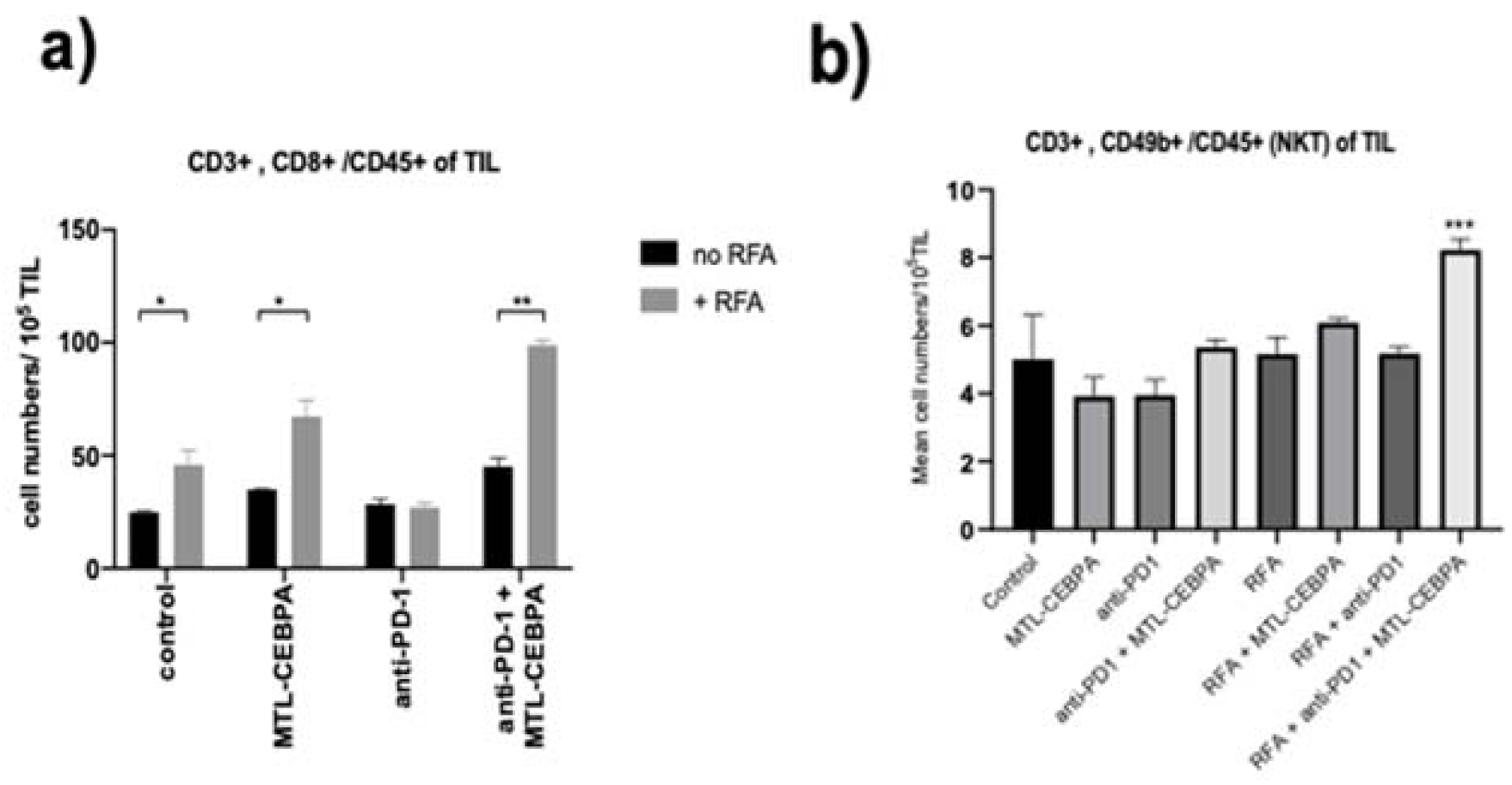
3.2. MTL-CEBPA Enhances CD8+ and NKT Cells Infiltrating in BNL Tumor
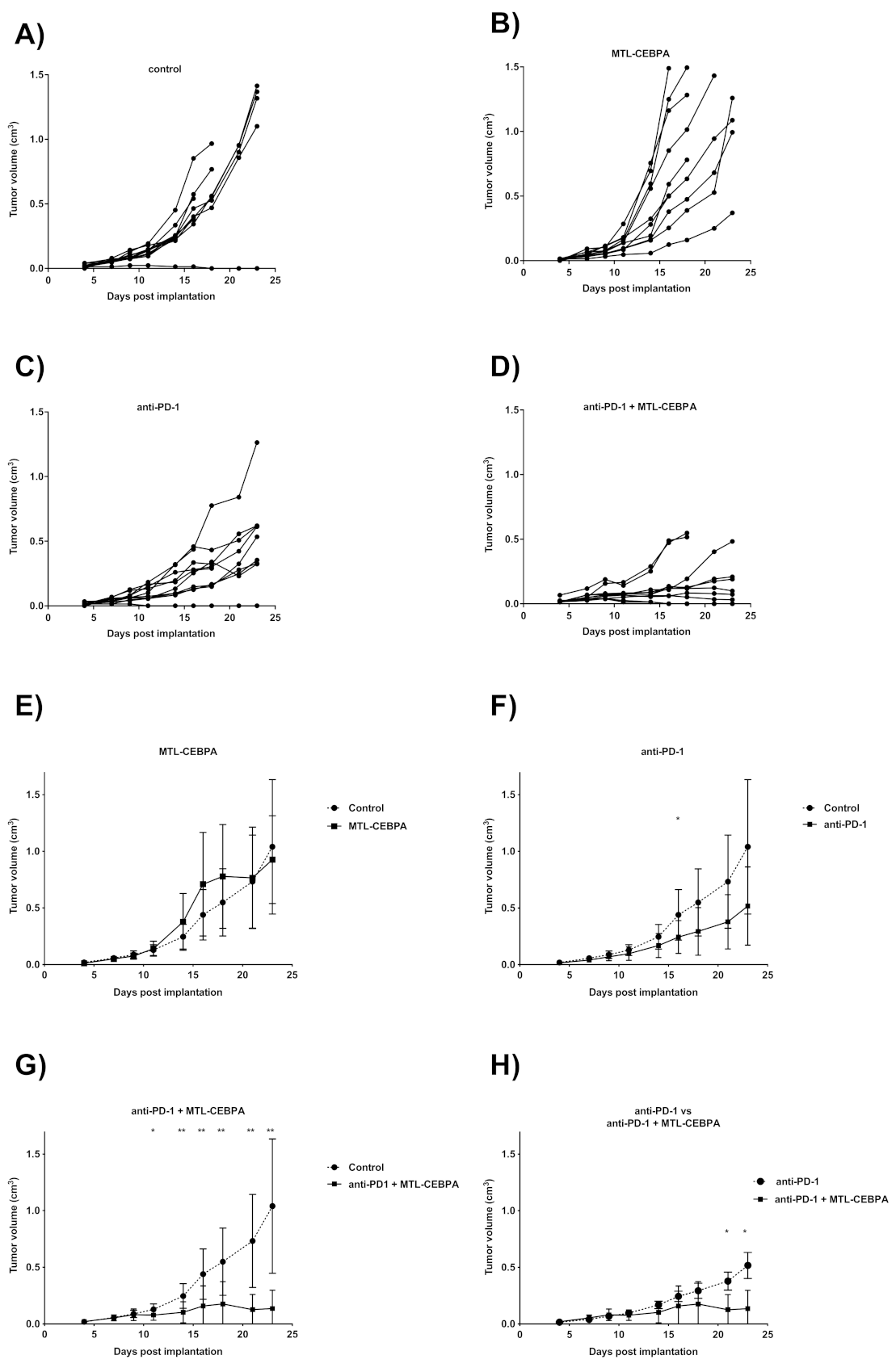
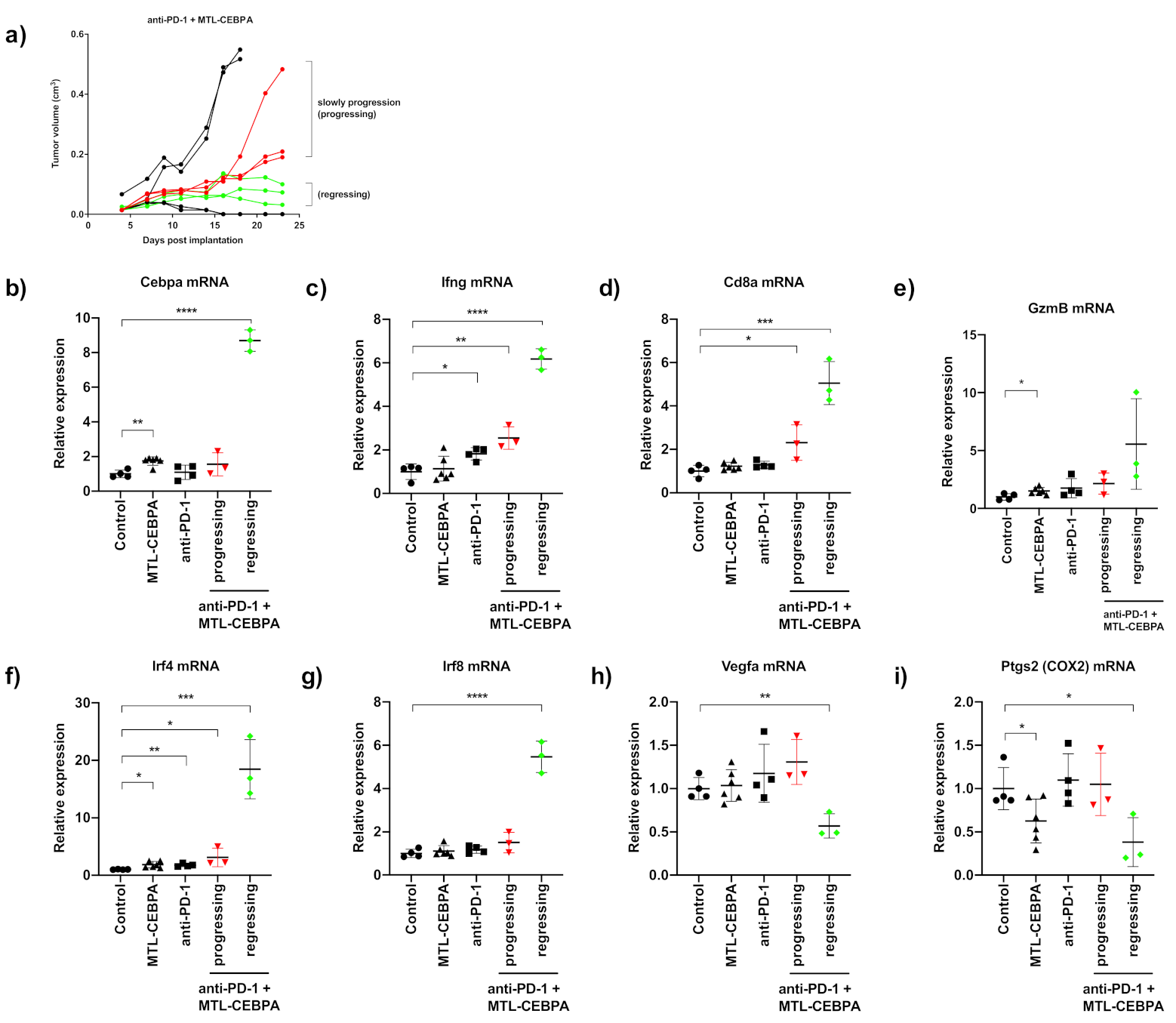
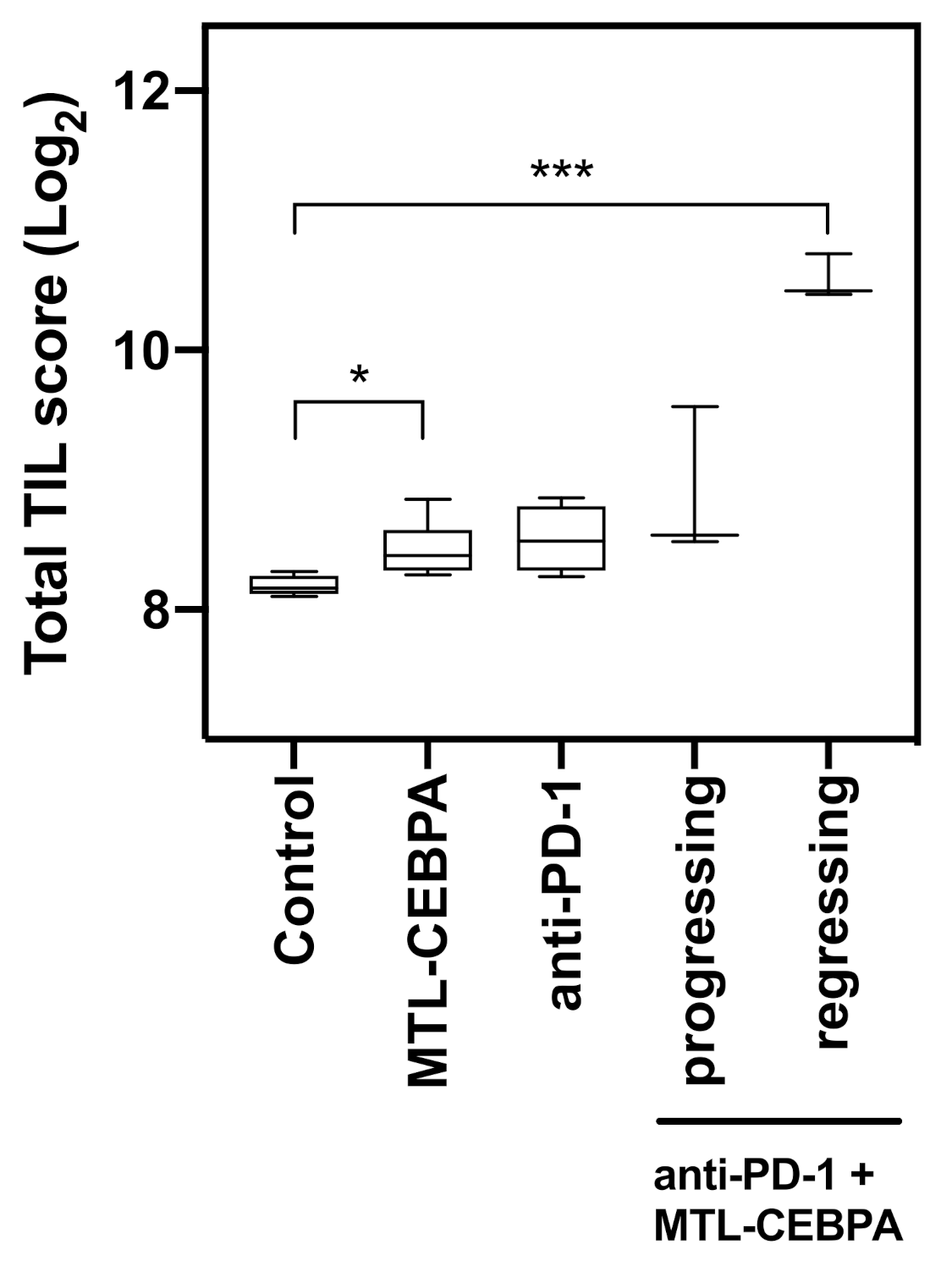
3.3. Combination of Anti-PD-1 and MTL-CEBPA Have Synergistic Efficacy in CT26 Syngeneic Mouse Model
3.4. Gene Expression Analysis of CT26 Tumor Reveals That Anti-PD-1+MTL-CEBPA Treated Group Have Anti-Tumor Transcriptional Profile
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Avellino, R.; Delwel, R. Expression and regulation of C/EBPalpha in normal myelopoiesis and in malignant transformation. Blood 2017, 129, 2083–2091. [Google Scholar] [CrossRef]
- Avellino, R.; Havermans, M.; Erpelinck, C.; Sanders, M.A.; Hoogenboezem, R.; van de Werken, H.J.; Rombouts, E.; van Lom, K.; van Strien, P.M.; Gebhard, C.; et al. An autonomous CEBPA enhancer specific for myeloid-lineage priming and neutrophilic differentiation. Blood 2016, 127, 2991–3003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackert, J.R.; Qu, P.; Min, Y.; Johnson, P.F.; Yang, L.; Lin, P.C. Dual negative roles of C/EBPalpha in the expansion and pro-tumor functions of MDSCs. Sci. Rep. 2017, 7, 14048. [Google Scholar] [CrossRef]
- Torroella-Kouri, M.; Ma, X.; Perry, G.; Ivanova, M.; Cejas, P.J.; Owen, J.L.; Iragavarapu-Charyulu, V.; Lopez, D.M. Diminished expression of transcription factors nuclear factor kappaB and CCAAT/enhancer binding protein underlies a novel tumor evasion mechanism affecting macrophages of mammary tumor-bearing mice. Cancer Res. 2005, 65, 10578–10584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setten, R.L.; Lightfoot, H.L.; Habib, N.A.; Rossi, J.J. Development of MTL-CEBPA: Small Activating RNA Drug for Hepatocellular Carcinoma. Curr. Pharm. Biotechnol. 2018, 19, 611–621. [Google Scholar] [CrossRef]
- Voutila, J.; Reebye, V.; Roberts, T.C.; Protopapa, P.; Andrikakou, P.; Blakey, D.C.; Habib, R.; Huber, H.; Saetrom, P.; Rossi, J.J.; et al. Development and Mechanism of Small Activating RNA Targeting CEBPA, a Novel Therapeutic in Clinical Trials for Liver Cancer. Mol. Ther.J. Am. Soc. Gene Ther. 2017, 25, 2705–2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reebye, V.; Saetrom, P.; Mintz, P.J.; Huang, K.W.; Swiderski, P.; Peng, L.; Liu, C.; Liu, X.; Lindkaer-Jensen, S.; Zacharoulis, D.; et al. Novel RNA oligonucleotide improves liver function and inhibits liver carcinogenesis in vivo. Hepatology 2014, 59, 216–227. [Google Scholar] [CrossRef] [Green Version]
- Reebye, V.; Huang, K.W.; Lin, V.; Jarvis, S.; Cutilas, P.; Dorman, S.; Ciriello, S.; Andrikakou, P.; Voutila, J.; Saetrom, P.; et al. Gene activation of CEBPA using saRNA: Preclinical studies of the first in human saRNA drug candidate for liver cancer. Oncogene 2018, 37, 3216–3228. [Google Scholar] [CrossRef]
- Huan, H.; Wen, X.; Chen, X.; Wu, L.; Liu, W.; Habib, N.A.; Bie, P.; Xia, F. C/EBPalpha Short-Activating RNA Suppresses Metastasis of Hepatocellular Carcinoma through Inhibiting EGFR/beta-Catenin Signaling Mediated EMT. PLoS ONE 2016, 11, e0153117. [Google Scholar] [CrossRef]
- Zhao, X.; Voutila, J.; Ghobrial, S.; Habib, N.A.; Reebye, V. Treatment of Liver Cancer by C/EBPA saRNA. Adv. Exp. Med. Biol. 2017, 983, 189–194. [Google Scholar] [CrossRef]
- Sarker, D.; Plummer, E.R.; Basu, B.; Meyer, T.; Huang, K.-W.; Evans, T.R.J.; Spalding, D.; Ma, Y.T.; Palmer, D.H.; Chee, C.E.; et al. First-in-human, first-in-class phase I study of MTL-CEBPA, a small activating RNA (saRNA) targeting the transcription factor C/EBP-α in patients with advanced liver cancer. J. Clin. Oncol. 2017, 35, TPS2612. [Google Scholar] [CrossRef]
- Sarker, D.; Plummer, E.R.; Basu, B.; Meyer, T.; Huang, K.-W.; Evans, T.R.J.; Spalding, D.; Ma, Y.T.; Palmer, D.H.; Chee, C.E.; et al. Preliminary results of a first-in-human, first-in-class phase I study of MTL-CEBPA, a small activating RNA (saRNA) targeting the transcription factor C/EBP-α in patients with advanced liver cancer. J. Clin. Oncol. 2018, 36, abstr 2509. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Qi, X.; Zhao, Y.; Li, H.; Guo, X.; Han, G. Management of hepatocellular carcinoma: An overview of major findings from meta-analyses. Oncotarget 2016, 7, 34703–34751. [Google Scholar] [CrossRef]
- Evrard, S.; Menetrier-Caux, C.; Biota, C.; Neaud, V.; Mathoulin-Pelissier, S.; Blay, J.Y.; Rosenbaum, J. Cytokines pattern after surgical radiofrequency ablation of liver colorectal metastases. Gastroenterol. Clin. Biol. 2007, 31, 141–145. [Google Scholar] [CrossRef]
- Haen, S.P.; Pereira, P.L.; Salih, H.R.; Rammensee, H.G.; Gouttefangeas, C. More than just tumor destruction: Immunomodulation by thermal ablation of cancer. Clin. Dev. Immunol. 2011, 2011, 160250. [Google Scholar] [CrossRef]
- den Brok, M.H.; Sutmuller, R.P.; Nierkens, S.; Bennink, E.J.; Frielink, C.; Toonen, L.W.; Boerman, O.C.; Figdor, C.G.; Ruers, T.J.; Adema, G.J. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br. J. Cancer 2006, 95, 896–905. [Google Scholar] [CrossRef] [Green Version]
- Dromi, S.A.; Walsh, M.P.; Herby, S.; Traughber, B.; Xie, J.; Sharma, K.V.; Sekhar, K.P.; Luk, A.; Liewehr, D.J.; Dreher, M.R.; et al. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology 2009, 251, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Zerbini, A.; Pilli, M.; Penna, A.; Pelosi, G.; Schianchi, C.; Molinari, A.; Schivazappa, S.; Zibera, C.; Fagnoni, F.F.; Ferrari, C.; et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006, 66, 1139–1146. [Google Scholar] [CrossRef] [Green Version]
- Napoletano, C.; Taurino, F.; Biffoni, M.; De Majo, A.; Coscarella, G.; Bellati, F.; Rahimi, H.; Pauselli, S.; Pellicciotta, I.; Burchell, J.M.; et al. RFA strongly modulates the immune system and anti-tumor immune responses in metastatic liver patients. Int. J. Oncol. 2008, 32, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Waight, J.D.; Netherby, C.; Hensen, M.L.; Miller, A.; Hu, Q.; Liu, S.; Bogner, P.N.; Farren, M.R.; Lee, K.P.; Liu, K.; et al. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J. Clin. Investig. 2013, 123, 4464–4478. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Kang, K.; Cha, J.S.; Kim, J.W.; Lee, H.G.; Kim, Y.; Yang, Y.; Lee, M.S.; Lim, J.S. Interferon regulatory factor 4 (IRF4) controls myeloid-derived suppressor cell (MDSC) differentiation and function. J. Leukoc. Biol. 2016, 100, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Obermajer, N.; Muthuswamy, R.; Lesnock, J.; Edwards, R.P.; Kalinski, P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood 2011, 118, 5498–5505. [Google Scholar] [CrossRef] [PubMed]
- Schueller, G.; Kettenbach, J.; Sedivy, R.; Bergmeister, H.; Stift, A.; Fried, J.; Gnant, M.; Lammer, J. Expression of heat shock proteins in human hepatocellular carcinoma after radiofrequency ablation in an animal model. Oncol. Rep. 2004, 12, 495–499. [Google Scholar] [CrossRef]
- Ito, F.; Ku, A.W.; Bucsek, M.J.; Muhitch, J.B.; Vardam-Kaur, T.; Kim, M.; Fisher, D.T.; Camoriano, M.; Khoury, T.; Skitzki, J.J.; et al. Immune Adjuvant Activity of Pre-Resectional Radiofrequency Ablation Protects against Local and Systemic Recurrence in Aggressive Murine Colorectal Cancer. PLoS ONE 2015, 10, e0143370. [Google Scholar] [CrossRef] [Green Version]
- Schneider, T.; Hoffmann, H.; Dienemann, H.; Herpel, E.; Heussel, C.P.; Enk, A.H.; Ring, S.; Mahnke, K. Immune Response After Radiofrequency Ablation and Surgical Resection in Nonsmall Cell Lung Cancer. Semin. Thorac. Surg. 2016, 28, 585–592. [Google Scholar] [CrossRef]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Chen, L.; Wu, C.; Zhu, Y.; Xu, B.; Zheng, X.; Sun, M.; Wen, W.; Dai, X.; Yang, M.; et al. PD-1 Blockade Boosts Radiofrequency Ablation-Elicited Adaptive Immune Responses against Tumor. Clin. Cancer Res. 2016, 22, 1173–1184. [Google Scholar] [CrossRef] [Green Version]
| Group | CR | PR | SD | PD |
|---|---|---|---|---|
| control | 0 | 0 | 0 | 8 (100%) |
| RFA | 0 | 0 | 0 | 8 (100%) |
| anti-PD1 | 0 | 0 | 3 (33.3%) | 6 (66.7%) |
| MTL-CEBPA | 0 | 3 (33.3%) | 3 (33.3%) | 3 (33.3%) |
| anti-PD1 + MTL-CEBPA | 0 | 2 (22.2%) | 4 (44.4%) | 3 (33.3%) |
| RFA + anti-PD1 | 0 | 0 | 5 (50%) | 5 (50%) |
| RFA + MTL-CEBPA | 0 | 4 (50%) | 2 (25%) | 2 (25%) |
| RFA + anti-PD1 + MTL-CEBPA | 2 (25%) | 5 (62.5%) | 1 (12.5%) | 0 |
| Group | Mean Volume Change over Starting Size (%) +/− SD | p vs. Control |
|---|---|---|
| control | 260.4 ± 45.4 | - |
| MTL-CEBPA | 150.5 ± 29.4 | 0.001 |
| anti-PD1 | 219.1 ± 57.1 | n.s |
| anti-PD1 + MTL-CEBPA | 169.8 ± 31.6 | 0.0005 |
| RFA | 273.1 ± 63.8 | n.s |
| RFA + MTL-CEBPA | 193.2 ± 54.2 | 0.029 |
| RFA + anti-PD1 | 209.0 ± 61.1 | n.s |
| RFA + anti-PD1 + MTL-CEBPA | 118.6 ± 33.6 | 0.0006 |
| Group | Mean +/− SD of Tumor Infiltrating Cells (Cell Numbers/105 TIL) | |||
|---|---|---|---|---|
| CD4 | CD8 | NK | NKT | |
| control | 93.4 ± 1.59 | 24.8 ± 5.9 | 7.83 ± 3.23 | 5.01 ± 1.30 |
| MTL-CEBPA | 104.1 ± 5.42 | 34.9 ± 2.9 | 10.9 ± 1.98 | 3.92 ± 0.56 |
| anti-PD1 | 93.4 ± 1.59 | 28.5 ± 2.18 | 9.33 ± 1.82 | 3.95 ± 0.45 |
| anti-PD1 + MTL-CEBPA | 89.5 ± 6.03 | 45.2 ± 3.45 | 17.6 ± 1.50 * | 5.36 ± 0.23 |
| RFA | 128.3 ± 21.2 | 45.8 ± 6.57 * | 13.5 ± 2.93 | 5.16 ± 0.50 |
| RFA + MTL-CEBPA | 150.2 ± 20.9 | 67.2 ± 7.19 * | 16.0 ± 2.34 | 6.09 ± 0.13 |
| RFA + anti-PD1 | 95.3 ± 12.3 | 26.9 ± 1.96 | 12.7 ± 0.02 | 5.18 ± 0.20 |
| RFA + anti-PD1 + MTL-CEBPA | 116.2 ± 5.9 | 98.9 ± 2.23 * | 21.2 ± 2.58 * | 8.23 ± 0.32 *** |
| Group | Mean Tumor Volume (cm3) | p vs. Control |
|---|---|---|
| control | 1.040 ± 0.593 | - |
| MTL-CEBPA | 0.927 ± 0.388 | n.s |
| anti-PD1 | 0.517 ± 0.345 | n.s |
| anti-PD1 + MTL-CEBPA | 0.136 ± 0.161 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.-W.; Tan, C.P.; Reebye, V.; Chee, C.E.; Zacharoulis, D.; Habib, R.; Blakey, D.C.; Rossi, J.J.; Habib, N.; Sodergren, M.H. MTL-CEBPA Combined with Immunotherapy or RFA Enhances Immunological Anti-Tumor Response in Preclinical Models. Int. J. Mol. Sci. 2021, 22, 9168. https://doi.org/10.3390/ijms22179168
Huang K-W, Tan CP, Reebye V, Chee CE, Zacharoulis D, Habib R, Blakey DC, Rossi JJ, Habib N, Sodergren MH. MTL-CEBPA Combined with Immunotherapy or RFA Enhances Immunological Anti-Tumor Response in Preclinical Models. International Journal of Molecular Sciences. 2021; 22(17):9168. https://doi.org/10.3390/ijms22179168
Chicago/Turabian StyleHuang, Kai-Wen, Choon Ping Tan, Vikash Reebye, Cheng Ean Chee, Dimitris Zacharoulis, Robert Habib, David C. Blakey, John J. Rossi, Nagy Habib, and Mikael H. Sodergren. 2021. "MTL-CEBPA Combined with Immunotherapy or RFA Enhances Immunological Anti-Tumor Response in Preclinical Models" International Journal of Molecular Sciences 22, no. 17: 9168. https://doi.org/10.3390/ijms22179168
APA StyleHuang, K.-W., Tan, C. P., Reebye, V., Chee, C. E., Zacharoulis, D., Habib, R., Blakey, D. C., Rossi, J. J., Habib, N., & Sodergren, M. H. (2021). MTL-CEBPA Combined with Immunotherapy or RFA Enhances Immunological Anti-Tumor Response in Preclinical Models. International Journal of Molecular Sciences, 22(17), 9168. https://doi.org/10.3390/ijms22179168






