Combination of Drugs and Cell Transplantation: More Beneficial Stem Cell-Based Regenerative Therapies Targeting Neurological Disorders
Abstract
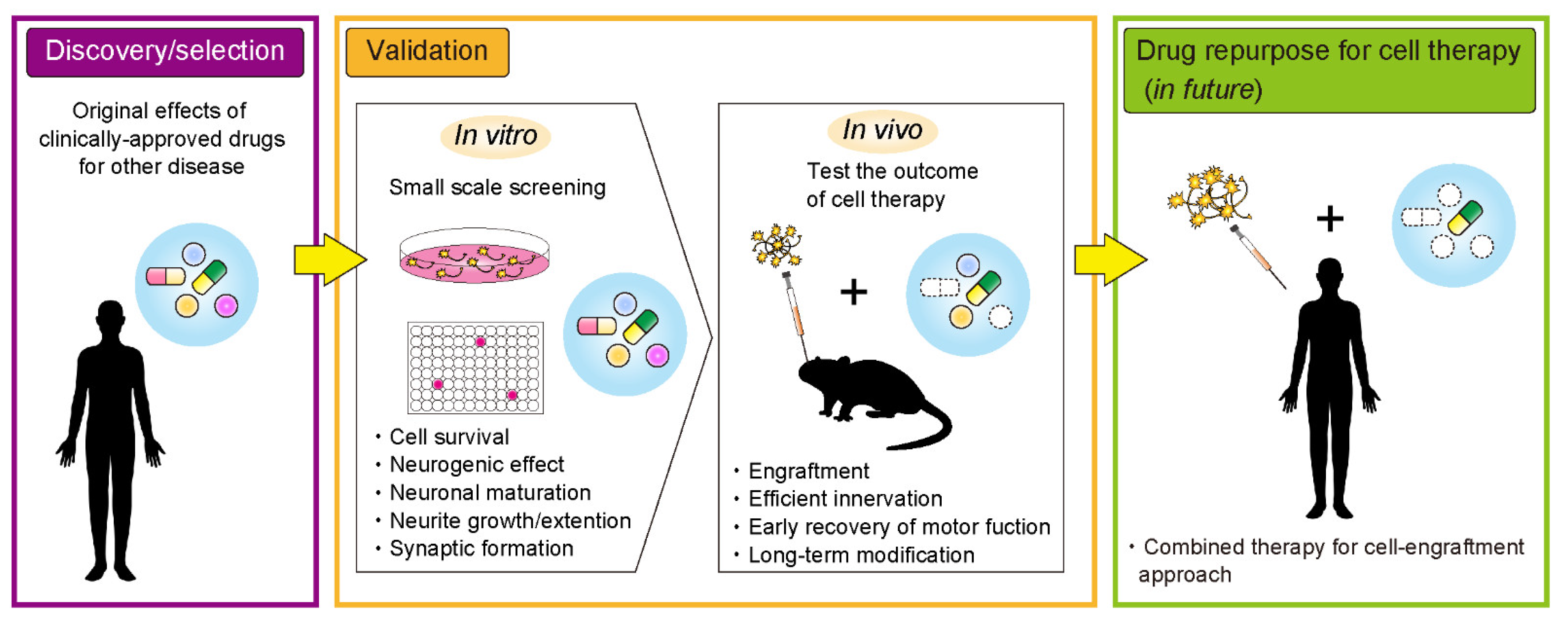
1. Introduction
2. Translational Approach of Endogenous Growth Factors for Effective Cell Transplantation Therapy
3. Drug Repurposing Approach toward Effective Cell Transplantation Therapy
3.1. Immunosuppressants
3.2. Rho-Kinase Inhibitors
3.3. Chondroitinase ABC (ChABC)
3.4. Valproic Acid (VPA)
3.5. Zonisamide
3.6. 17β-Estradiol (E2)
4. Future Prospects
5. Conclusions
Funding
Conflicts of Interest
References
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous induced stem-cell–derived retinal cells for macular degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Morizane, A.; Kikuchi, T.; Hayashi, T.; Mizuma, H.; Takara, S.; Doi, H.; Mawatari, A.; Glasser, M.F.; Shiina, T.; Ishigaki, H.; et al. MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat. Commun. 2017, 8, 385. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Cha, Y.; Ko, S.; Jeon, J.; Lee, N.; Seo, H.; Park, K.; Lee, I.; Lopes, C.; Feitosa, M.; et al. Human autologous iPSC–derived dopaminergic progenitors restore motor function in Parkinson’s disease models. J. Clin. Investig. 2020, 130, 904–920. [Google Scholar] [CrossRef] [PubMed]
- Osborn, T.M.; Hallett, P.J.; Schumacher, J.M.; Isacson, O. Advantages and recent developments of autologous cell therapy for Parkinson’s disease patients. Front. Cell. Neurosci. 2020, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Kamao, H.; Mandai, M.; Okamoto, S.; Sakai, N.; Suga, A.; Sugita, S.; Kiryu, J.; Takahashi, M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2014, 2, 205–218. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Okada, Y.; Itakura, G.; Iwai, H.; Nishimura, S.; Yasuda, A.; Nori, S.; Hikishima, K.; Konomi, T.; Fujiyoshi, K.; et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS ONE 2012, 7, e52787. [Google Scholar] [CrossRef]
- Kikuchi, T.; Morizane, A.; Doi, D.; Magotani, H.; Onoe, H.; Hayashi, T.; Mizuma, H.; Takara, S.; Takahashi, R.; Inoue, H.; et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 2017, 548, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; Zabierowski, S.; Dubose, B.N.; Hill, E.J.; Navare, M.; Claros, N.; Rosen, S.; Ramnarine, K.; Horn, C.; Fredrickson, C.; et al. Preclinical efficacy and safety of a human embryonic stem cell-derived midbrain dopamine progenitor product, MSK-DA01. Cell Stem Cell 2021, 28, 217–229. [Google Scholar] [CrossRef]
- Nicoleau, C.; Viegas, P.; Peschanski, M.; Perrier, A.L. Human pluripotent stem cell therapy for Huntington’s disease: Technical, immunological, and safety challenges. Neurotherapeutics 2011, 8, 562–576. [Google Scholar] [CrossRef]
- Iwai, H.; Shimada, H.; Nishimura, S.; Kobayashi, Y.; Itakura, G.; Hori, K.; Hikishima, K.; Ebise, H.; Negishi, N.; Shibata, S.; et al. Allogeneic neural stem/progenitor cells derived from embryonic stem cells promote functional recovery after transplantation into injured spinal cord of nonhuman primates. Stem Cells Transl. Med. 2015, 4, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Takahashi, J. Drug discovery toward successful cell transplantation therapy for Parkinson’s disease using human pluripotent stem cells. Adv. Regen. Biol. 2016, 3, 31772. [Google Scholar] [CrossRef][Green Version]
- Katsukawa, M.; Nakajima, Y.; Fukumoto, A.; Doi, D.; Takahashi, J. Fail-safe therapy by gamma-ray irradiation against tumor formation by human-induced pluripotent stem cell-derived neural progenitors. Stem Cells Dev. 2016, 25, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, Y.; Yamasaki, T.; Nagoshi, N.; Nishiyama, Y.; Nori, S.; Nishimura, S.; Iida, T.; Ozaki, M.; Tsuji, O.; Ji, B.; et al. In vivo monitoring of remnant undifferentiated neural cells following human induced pluripotent stem cell-derived neural stem/progenitor cells transplantation. Stem Cells Transl. Med. 2020, 9, 465–477. [Google Scholar] [CrossRef]
- Lan, M.L.; Acharya, M.M.; Tran, K.K.; Bahari-Kashani, J.; Patel, N.H.; Strnadel, J.; Giedzinski, E.; Limoli, C.L. Characterizing the radioresponse of pluripotent and multipotent human stem cells. PLoS ONE 2012, 7, e50048. [Google Scholar] [CrossRef]
- Zhang, H.; Song, F.; Xu, C.; Liu, H.; Wang, Z.; Li, J.; Wu, S.; Shen, Y.; Chen, Y.; Zhu, Y.; et al. Spatiotemporal PET imaging of dynamic metabolic changes after therapeutic approaches of induced pluripotent stem cells, neuronal stem cells, and a Chinese patent medicine in stroke. J. Nucl. Med. 2015, 56, 1774–1779. [Google Scholar] [CrossRef]
- Nishimura, K.; Murayama, S.; Takahashi, J. Identification of neurexophilin 3 as a novel supportive factor for survival of induced pluripotent stem cell-derived dopaminergic progenitors. Stem Cells Transl. Med. 2015, 4, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Ohira, K.; Furuta, T.; Hioki, H.; Nakamura, K.C.; Kuramoto, E.; Tanaka, Y.; Funatsu, N.; Shimizu, K.; Oishi, T.; Hayashi, M.; et al. Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat. Neurosci. 2010, 13, 173–179. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Rizk, P.; Muriel, M.P.; Duyckaerts, C.; Oertel, W.H.; Caille, I.; Hirsch, E.C. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004, 7, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef]
- Pöyhönen, S.; Er, S.; Domanskyi, A.; Airavaara, M. Effects of neurotrophic factors in glial cells in the central nervous system: Expression and properties in neurodegeneration and injury. Front. Physiol. 2019, 10, 1–20. [Google Scholar] [CrossRef]
- Apostolides, C.; Sanford, E.; Hong, M.; Mendez, I. Glial cell line-derived neurotrophic factor improves intrastriatal graft survival of stored dopaminergic cells. Neuroscience 1998, 83, 363–372. [Google Scholar] [CrossRef]
- Thompson, L.H.; Grealish, S.; Kirik, D.; Björklund, A. Reconstruction of the nigrostriatal dopamine pathway in the adult mouse brain. Eur. J. Neurosci. 2009, 30, 625–638. [Google Scholar] [CrossRef]
- Kirik, D.; Cederfjäll, E.; Halliday, G.; Petersén, Å. Gene therapy for Parkinson’s disease: Disease modification by GDNF family of ligands. Neurobiol. Dis. 2017, 97, 179–188. [Google Scholar] [CrossRef]
- Tatarewicz, S.M.; Wei, X.; Gupta, S.; Masterman, D.; Swanson, S.J.; Moxness, M.S. Development of a maturing T-cell-mediated immune response in patients with idiopathic Parkinson’s disease receiving r-metHuGDNF via continuous intraputaminal infusion. J. Clin. Immunol. 2007, 27, 620–627. [Google Scholar] [CrossRef]
- Sidorova, Y.A.; Bespalov, M.M.; Wong, A.W.; Kambur, O.; Jokinen, V.; Lilius, T.O.; Suleymanova, I.; Karelson, G.; Rauhala, P.V.; Karelson, M.; et al. A novel small molecule GDNF receptor RET agonist, BT13, promotes neurite growth from sensory neurons in vitro and attenuates experimental neuropathy in the rat. Front. Pharmacol. 2017, 8, 365. [Google Scholar] [CrossRef]
- Mahato, A.K.; Kopra, J.; Renko, J.M.; Visnapuu, T.; Korhonen, I.; Pulkkinen, N.; Bespalov, M.M.; Domanskyi, A.; Ronken, E.; Piepponen, T.P.; et al. Glial cell line–derived neurotrophic factor receptor rearranged during transfection agonist supports dopamine neurons in vitro and enhances dopamine release in vivo. Mov. Disord. 2020, 35, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Ueno, M.; Kamiya, D.; Nishiyama, A.; Matsumura, M.; Wataya, T.; Takahashi, J.B.; Nishikawa, S.; Nishikawa, S.I.; Muguruma, K.; et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007, 25, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Ross, H.H.; Ambrosio, F.; Trumbower, R.D.; Reier, P.J.; Behrman, A.L.; Wolf, S.L. Neural stem cell therapy and rehabilitation in the central nervous system: Emerging partnerships. Phys. Ther. 2016, 96, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Shimogawa, T.; Sakaguchi, H.; Kikuchi, T.; Tsuchimochi, R.; Sano, N.; Torikoshi, S.; Ito, A.; Aoyama, T.; Iihara, K.; Takahashi, J. Therapeutic effects of combined cell transplantation and locomotor training in rats with brain injury. NPJ Regen. Med. 2019, 4, 1–8. [Google Scholar] [CrossRef]
- Gantner, C.W.; de Luzy, I.R.; Kauhausen, J.A.; Moriarty, N.; Niclis, J.C.; Bye, C.R.; Penna, V.; Hunt, C.P.J.; Ermine, C.M.; Pouton, C.W.; et al. Viral delivery of GDNF promotes functional integration of human stem cell grafts in Parkinson’s disease. Cell Stem Cell 2020, 26, 511–526. [Google Scholar] [CrossRef]
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.-Y.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q.; et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N. Engl. J. Med. 2020, 382, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.A.; Parmar, M.; Studer, L.; Takahashi, J. Human trials of stem cell-derived dopamine neurons for Parkinson’s disease: Dawn of a new era. Cell Stem Cell 2017, 21, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Clipstone, N.A.; Crabtree, G.R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 1992, 357, 695–697. [Google Scholar] [CrossRef]
- Strömberg, I.; Bygdeman, M.; Goldstein, M.; Seiger, Å.; Olson, L. Human fetal substantia nigra grafted to the dopamine-denervated striatum of immunosuppressed rats: Evidence for functional reinnervation. Neurosci. Lett. 1986, 71, 271–276. [Google Scholar] [CrossRef]
- Brundin, P.; Strecker, R.E.; Widner, H.; Clarke, D.J.; Nilsson, O.G.; Åstedt, B.; Lindvall, O.; Björklund, A. Human fetal dopamine neurons grafted in a rat model of Parkinson’s disease: Immunological aspects, spontaneous and drug-induced behaviour, and dopamine release. Exp. Brain Res. 1988, 70, 192–208. [Google Scholar] [CrossRef]
- Howard, M.A.; Dacey, R.G.; Winn, H.R. Brain xenografts: The effect of cyclosporin A on graft survival. J. Neurosurg. 1988, 69, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Rehncrona, S.; Brundin, P.; Gustavii, B.; Åstedt, B.; Widner, H.; Lindholm, T.; Bjorklund, A.; Leenders, K.L.; Rothwell, J.C.; et al. Human fetal dopamine neurons grafted into the striatum in two patients with severe parkinson’s disease: A detailed account of methodology and a 6-month follow-up. Arch. Neurol. 1989, 46, 615–631. [Google Scholar] [CrossRef]
- Freed, C.R.; Breeze, R.E.; Rosenberg, N.L.; Schneck, S.A.; Kriek, E.; Qi, J.; Lone, T.; Zhang, Y.; Snyder, J.A.; Wells, T.H.; et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson’s disease. N. Engl. J. Med. 1992, 327, 1549–1555. [Google Scholar] [CrossRef]
- Spencer, D.D.; Robbins, R.J.; Naftolin, F.; Marek, K.L.; Vollmer, T.; Leranth, C.; Roth, R.H.; Price, L.H.; Gjedde, A.; Bunney, B.S.; et al. Unilateral transplantation of human fetal mesencephalic tissue into the caudate nucleus of patients with Parkinson’s disease. N. Engl. J. Med. 1992, 327, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, B.J.; Leenders, K.L.; Young, D.; Gerhardt, G.; Zerbe, G.O.; Bygdeman, M.; Seiger, Å.; Olson, L.; Strömberg, I.; Freedman, R. Eighteen-month course of two patients with grafts of fetal dopamine neurons for severe Parkinson’s disease. Exp. Neurol. 1992, 118, 243–252. [Google Scholar] [CrossRef]
- Kriks, S.; Shim, J.W.; Piao, J.; Ganat, Y.M.; Wakeman, D.R.; Xie, Z.; Carrillo-Reid, L.; Auyeung, G.; Antonacci, C.; Buch, A.; et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 2011, 480, 547–551. [Google Scholar] [CrossRef]
- Kirkeby, A.; Grealish, S.; Wolf, D.A.; Nelander, J.; Wood, J.; Lundblad, M.; Lindvall, O.; Parmar, M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012, 1, 703–714. [Google Scholar] [CrossRef]
- Sundberg, M.; Bogetofte, H.; Lawson, T.; Jansson, J.; Smith, G.; Astradsson, A.; Moore, M.; Osborn, T.; Cooper, O.; Spealman, R.; et al. Improved cell therapy protocols for Parkinson’s disease based on differentiation efficiency and safety of hESC-, hiPSC-, and non-human primate iPSC-derived dopaminergic neurons. Stem Cells 2013, 31, 1548–1562. [Google Scholar] [CrossRef] [PubMed]
- Samata, B.; Kikuchi, T.; Miyawaki, Y.; Morizane, A.; Mashimo, T.; Nakagawa, M.; Okita, K.; Takahashi, J. X-linked severe combined immunodeficiency (X-SCID) rats for xeno-transplantation and behavioral evaluation. J. Neurosci. Methods 2015, 243, 68–77. [Google Scholar] [CrossRef]
- Adler, A.F.; Cardoso, T.; Nolbrant, S.; Mattsson, B.; Hoban, D.B.; Jarl, U.; Wahlestedt, J.N.; Grealish, S.; Björklund, A.; Parmar, M. hESC-derived dopaminergic transplants integrate into basal ganglia circuitry in a preclinical model of Parkinson’s disease. Cell Rep. 2019, 28, 3462–3473. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Kim, H.S.; Hong, C.P.; Li, E.; Jeon, I.; Park, H.J.; Lee, N.; Pei, Z.; Song, J. Neural transplants from human induced pluripotent stem cells rescue the pathology and behavioral defects in a rodent model of Huntington’s disease. Front. Neurosci. 2020, 14, 558204. [Google Scholar] [CrossRef] [PubMed]
- Tornero, D.; Wattananit, S.; Madsen, M.G.; Koch, P.; Wood, J.; Tatarishvili, J.; Mine, Y.; Ge, R.; Monni, E.; Devaraju, K.; et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain 2013, 136, 3561–3577. [Google Scholar] [CrossRef] [PubMed]
- Shirai, H.; Mandai, M.; Matsushita, K.; Kuwahara, A.; Yonemura, S.; Nakano, T.; Assawachananont, J.; Kimura, T.; Saito, K.; Terasaki, H.; et al. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc. Natl. Acad. Sci. USA 2016, 113, E81–E90. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.M.; Peattie, D.A.; Fitzgibbon, M.J.; Thomson, J.A. Solution structure of the major binding protein for the immunosuppressant FK506. Nature 1991, 351, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.W. FK-506—How much potential? Immunol. Today 1989, 10, 6–9. [Google Scholar] [CrossRef]
- Maki, N.; Sekiguchi, F.; Nishimaki, J.; Miwa, K.; Hayano, T.; Takahashi, N.; Suzuki, M. Complementary DNA encoding the human T-cell FK506-binding protein, a peptidylprolyl cis-trans isomerase distinct from cyclophilin. Proc. Natl. Acad. Sci. USA 1990, 87, 5440–5443. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Javed, E.; Scura, D.; Hala, T.J.; Seetharam, S.; Falnikar, A.; Richard, J.P.; Chorath, A.; Maragakis, N.J.; Wright, M.C.; et al. Human iPS cell-derived astrocyte transplants preserve respiratory function after spinal cord injury. Exp. Neurol. 2015, 271, 479–492. [Google Scholar] [CrossRef]
- Kitahara, T.; Sakaguchi, H.; Morizane, A.; Kikuchi, T.; Miyamoto, S.; Takahashi, J. Axonal extensions along corticospinal tracts from transplanted human cerebral organoids. Stem Cell Rep. 2020, 15, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Meredith, J.E.; Fazeli, B.; Schwartz, M.A. The extracellular matrix as a cell survival factor. Mol. Biol. Cell 1993, 4, 953–961. [Google Scholar] [CrossRef]
- Sortwell, C.E.; Pitzer, M.R.; Collier, T.J. Time course of apoptotic cell death within mesencephalic cell suspension grafts: Implications for improving grafted dopamine neuron survival. Exp. Neurol. 2000, 165, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, M.; Takahashi, J.; Arakawa, Y.; Doi, D.; Fukuda, H.; Hayashi, H.; Narumiya, S. Nobuo Hashimoto Inhibition of the Rho/ROCK pathway reduces apoptosis during transplantation of embryonic stem cell-derived neural precursors. J. Neurosci. Res. 2007, 3253, 3244–3253. [Google Scholar] [CrossRef]
- Boomkamp, S.D.; Riehle, M.O.; Wood, J.; Olson, M.F.; Barnett, S.C. The development of a rat in vitro model of spinal cord injury demonstrating the additive effects of rho and ROCK inhibitors on neurite outgrowth and myelination. Glia 2012, 60, 441–456. [Google Scholar] [CrossRef]
- Novozhilova, E.; Englund-Johansson, U.; Kale, A.; Jiao, Y.; Olivius, P. Effects of ROCK inhibitor Y27632 and EGFR inhibitor PD168393 on human neural precursors co-cultured with rat auditory brainstem explant. Neuroscience 2015, 287, 43–54. [Google Scholar] [CrossRef]
- Ichikawa, M.; Yoshida, J.; Saito, K.; Sagawa, H.; Tokita, Y.; Watanabe, M. Differential effects of two ROCK inhibitors, Fasudil and Y-27632, on optic nerve regeneration in adult cats. Brain Res. 2008, 1201, 23–33. [Google Scholar] [CrossRef]
- Laabs, T.; Carulli, D.; Geller, H.M.; Fawcett, J.W. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr. Opin. Neurobiol. 2005, 15, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef]
- Matsuyama, Y.; Chiba, K.; Iwata, H.; Seo, T.; Toyama, Y. A multicenter, randomized, double-blind, dose-finding study of condoliase in patients with lumbar disc herniation. J. Neurosurg. Spine 2018, 28, 499–511. [Google Scholar] [CrossRef]
- Bukhari, N.; Torres, L.; Robinson, J.K.; Tsirka, S.E. Axonal regrowth after spinal cord injury via chondroitinase and the tissue plasminogen activator (tPA)/plasmin system. J. Neurosci. 2011, 31, 14931–14943. [Google Scholar] [CrossRef] [PubMed]
- Alilain, W.J.; Horn, K.P.; Hu, H.; Dick, T.E.; Silver, J. Functional regeneration of respiratory pathways after spinal cord injury. Nature 2011, 475, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Kauhausen, J.A.; Thompson, L.H.; Parish, C.L. Chondroitinase improves midbrain pathway reconstruction by transplanted dopamine progenitors in Parkinsonian mice. Mol. Cell. Neurosci. 2015, 69, 22–29. [Google Scholar] [CrossRef]
- Nori, S.; Khazaei, M.; Ahuja, C.S.; Yokota, K.; Ahlfors, J.E.; Liu, Y.; Wang, J.; Shibata, S.; Chio, J.; Hettiaratchi, M.H.; et al. Oligodendrogenic neural progenitor cells delivered with chondroitinase ABC facilitate functional repair of chronic spinal cord injury. Stem Cell Rep. 2018, 11, 1433–1448. [Google Scholar] [CrossRef]
- Führmann, T.; Anandakumaran, P.N.; Payne, S.L.; Pakulska, M.M.; Varga, B.V.; Nagy, A.; Tator, C.; Shoichet, M.S. Combined delivery of chondroitinase ABC and human induced pluripotent stem cell-derived neuroepithelial cells promote tissue repair in an animal model of spinal cord injury. Biomed. Mater. 2018, 13, 024103. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.; Nakashima, K.; Kuwabara, T.; Mejia, E.; Gage, F.H. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16659–16664. [Google Scholar] [CrossRef]
- Ehashi, T.; Suzuki, N.; Ando, S.; Sumida, K.; Saito, K. Effects of valproic acid on gene expression during human embryonic stem cell differentiation into neurons. J. Toxicol. Sci. 2014, 39, 383–390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chu, W.; Yuan, J.; Huang, L.; Xiang, X.; Zhu, H.; Chen, F.; Chen, Y.; Lin, J.; Feng, H. Valproic acid arrests proliferation but promotes neuronal differentiation of adult spinal NSPCs from SCI rats. Neurochem. Res. 2015, 40, 1472–1486. [Google Scholar] [CrossRef] [PubMed]
- Abematsu, M.; Tsujimura, K.; Yamano, M.; Saito, M.; Kohno, K.; Kohyama, J.; Namihira, M.; Komiya, S.; Nakashima, K. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J. Clin. Investig. 2010, 120, 3255–3266. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Homma, K.; Okamoto, S.; Yamada, C.; Nomori, A.; Takahashi, M. Adequate time window and environmental factors supporting retinal graft cell survival in rd mice. Cell Med. 2012, 4, 45–54. [Google Scholar] [CrossRef][Green Version]
- Yoshikawa, T.; Samata, B.; Ogura, A.; Miyamoto, S.; Takahashi, J. Systemic administration of valproic acid and zonisamide promotes differentiation of induced pluripotent stem cell–derived dopaminergic neurons. Front. Cell. Neurosci. 2013, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.E.; Moritoyo, T.; Yabe, H.; Nishikawa, N.; Nagai, M.; Kubo, M.; Matsuda, S.; Nomoto, M. Zonisamide attenuates MPTP neurotoxicity in marmosets. J. Pharmacol. Sci. 2010, 114, 298–303. [Google Scholar] [CrossRef]
- Sonsalla, P.K.; Wong, L.Y.; Winnik, B.; Buckley, B. The antiepileptic drug zonisamide inhibits MAO-B and attenuates MPTP toxicity in mice: Clinical relevance. Exp. Neurol. 2010, 221, 329–334. [Google Scholar] [CrossRef][Green Version]
- Yokoyama, H.; Yano, R.; Kuroiwa, H.; Tsukada, T.; Uchida, H.; Kato, H.; Kasahara, J.; Araki, T. Therapeutic effect of a novel anti-parkinsonian agent zonisamide against MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) neurotoxicity in mice. Metab. Brain Dis. 2010, 25, 305–313. [Google Scholar] [CrossRef]
- Costa, C.; Tozzi, A.; Luchetti, E.; Siliquini, S.; Belcastro, V.; Tantucci, M.; Picconi, B.; Ientile, R.; Calabresi, P.; Pisani, F. Electrophysiological actions of zonisamide on striatal neurons: Selective neuroprotection against complex I mitochondrial dysfunction. Exp. Neurol. 2010, 221, 217–224. [Google Scholar] [CrossRef]
- Yagi, H.; Ohkawara, B.; Nakashima, H.; Ito, K.; Tsushima, M.; Ishii, H.; Noto, K.; Ohta, K.; Masuda, A.; Imagama, S.; et al. Zonisamide enhances neurite elongation of primary motor neurons and facilitates peripheral nerve regeneration in vitro and in a mouse model. PLoS ONE 2015, 10, e0142786. [Google Scholar] [CrossRef]
- Miyawaki, Y.; Samata, B.; Kikuchi, T.; Nishimura, K.; Takahashi, J. Zonisamide promotes survival of human-induced pluripotent stem cell-derived dopaminergic neurons in the striatum of female rats. J. Neurosci. Res. 2020, 98, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H.; Ibi, M.; Kihara, T.; Honda, K.; Nakamizo, T.; Kanki, R.; Nakanishi, M.; Sakka, N.; Akaike, A.; Shimohama, S. Estradiol protects dopaminergic neurons in a MPP+ Parkinson’s disease model. Neuropharmacology 2002, 42, 1056–1064. [Google Scholar] [CrossRef]
- Bains, M.; Roberts, J.L. Estrogen protects against dopamine neuron toxicity in primary mesencephalic cultures through an indirect P13K/Akt mediated astrocyte pathway. Neurosci. Lett. 2016, 610, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Callier, S.; Morissette, M.; Grandbois, M.; Pélaprat, D.; Di Paolo, T. Neuroprotective properties of 17β-estradiol, progesterone, and raloxifene in MPTP C57Bl/6 mice. Synapse 2001, 41, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Vidal, Y.; Morales-Montor, J.; Gómez de León, C.T.; Ostoa-Saloma, P.; Díaz-Zaragoza, M.; Montes, S.; Arteaga-Silva, M.; Monroy-Noyola, A. Protection induced by estradiol benzoate in the MPP+ rat model of Parkinson’s disease is associated with the regulation of the inflammatory cytokine profile in the nigro striatum. J. Neuroimmunol. 2020, 349. [Google Scholar] [CrossRef]
- Bourque, M.; Morissette, M.; Sweidi, S. Al; Donatella Caruso; Melcangi, R.C.; Paolo, T. Di Neuroprotective Effect of progesterone in MPTP-treated male mice. Neuroendocrinology 2016, 103, 300–314. [Google Scholar] [CrossRef]
- Samantaray, S.; Das, A.; Matzelle, D.C.; Yu, S.P.; Wei, L.; Varma, A.; Ray, S.K.; Banik, N.L. Administration of low dose estrogen attenuates persistent inflammation, promotes angiogenesis, and improves locomotor function following chronic spinal cord injury in rats. J. Neurochem. 2016, 137, 604–617. [Google Scholar] [CrossRef]
- Siriphorn, A.; Chompoopong, S.; Floyd, C.L. 17β-Estradiol protects Schwann cells against H2O2-induced cytotoxicity and increases transplanted Schwann cell survival in a cervical hemicontusion spinal cord injury model. J. Neurochem. 2010, 115, 864–872. [Google Scholar] [CrossRef]
- Namjoo, Z.; Moradi, F.; Aryanpour, R.; Piryaei, A.; Joghataei, M.T.; Abbasi, Y.; Hosseini, A.; Hassanzadeh, S.; Taklimie, F.R.; Beyer, C.; et al. Combined effects of rat Schwann cells and 17β-estradiol in a spinal cord injury model. Metab. Brain Dis. 2018, 33, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Namjoo, Z.; Mortezaee, K.; Joghataei, M.T.; Moradi, F.; Piryaei, A.; Abbasi, Y.; Hosseini, A.; Majidpoor, J. Targeting axonal degeneration and demyelination using combination administration of 17β-estradiol and Schwann cells in the rat model of spinal cord injury. J. Cell. Biochem. 2018, 119, 10195–10203. [Google Scholar] [CrossRef]
- Nishimura, K.; Doi, D.; Samata, B.; Murayama, S.; Tahara, T.; Onoe, H.; Takahashi, J. Estradiol facilitates functional integration of iPSC-derived dopaminergic neurons into striatal neuronal circuits via activation of integrin α5β1. Stem Cell Rep. 2016, 6, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.A.; Zhou, L.; Wilkars, W.; Fester, L.; Lanowski, J.S.; Paysen, D.; König, A.; Rune, G.M. Roles of 17β-estradiol involve regulation of reelin expression and synaptogenesis in the dentate gyrus. Cereb. Cortex 2010, 20, 2985–2995. [Google Scholar] [CrossRef][Green Version]
- Sekine, K.; Kawauchi, T.; Kubo, K.I.; Honda, T.; Herz, J.; Hattori, M.; Kinashi, T.; Nakajima, K. Reelin controls neuronal positioning by promoting cell-matrix adhesion via inside-out activation of integrin α5β1. Neuron 2012, 76, 353–369. [Google Scholar] [CrossRef]
- Würth, R.; Thellung, S.; Bajetto, A.; Mazzanti, M.; Florio, T.; Barbieri, F. Drug-repositioning opportunities for cancer therapy: Novel molecular targets for known compounds. Drug Discov. Today 2016, 21, 190–199. [Google Scholar] [CrossRef]
- Turanli, B.; Grøtli, M.; Boren, J.; Nielsen, J.; Uhlen, M.; Arga, K.Y.; Mardinoglu, A. Drug repositioning for effective prostate cancer treatment. Front. Physiol. 2018, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Guhr, A.; Kobold, S.; Seltmann, S.; Seiler Wulczyn, A.E.M.; Kurtz, A.; Löser, P. Recent trends in research with human pluripotent stem cells: Impact of research and use of cell lines in experimental research and clinical trials. Stem Cell Rep. 2018, 11, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Pluripotent stem cell-based cell therapy—Promise and challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- Dazert, P.; Suofu, Y.; Grube, M.; Popa-Wagner, A.; Kroemer, H.K.; Jedlitschky, G.; Kessler, C. Differential regulation of transport proteins in the periinfarct region following reversible middle cerebral artery occlusion in rats. Neuroscience 2006, 142, 1071–1079. [Google Scholar] [CrossRef]
- Ginsberg, G.; Hattis, D.; Russ, A.; Sonawane, B. Pharmacokinetic and pharmacodynamic factors that can affect sensitivity to neurotoxic sequelae in elderly individuals. Environ. Health Perspect. 2005, 113, 1243–1249. [Google Scholar] [CrossRef]
- Schwartz, J.B. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin. Pharmacol. Ther. 2007, 82, 87–96. [Google Scholar] [CrossRef]
- Vanhaelen, Q.; Mamoshina, P.; Aliper, A.M.; Artemov, A.; Lezhnina, K.; Ozerov, I.; Labat, I.; Zhavoronkov, A. Design of efficient computational workflows for in silico drug repurposing. Drug Discov. Today 2017, 22, 210–222. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, K.; Takata, K. Combination of Drugs and Cell Transplantation: More Beneficial Stem Cell-Based Regenerative Therapies Targeting Neurological Disorders. Int. J. Mol. Sci. 2021, 22, 9047. https://doi.org/10.3390/ijms22169047
Nishimura K, Takata K. Combination of Drugs and Cell Transplantation: More Beneficial Stem Cell-Based Regenerative Therapies Targeting Neurological Disorders. International Journal of Molecular Sciences. 2021; 22(16):9047. https://doi.org/10.3390/ijms22169047
Chicago/Turabian StyleNishimura, Kaneyasu, and Kazuyuki Takata. 2021. "Combination of Drugs and Cell Transplantation: More Beneficial Stem Cell-Based Regenerative Therapies Targeting Neurological Disorders" International Journal of Molecular Sciences 22, no. 16: 9047. https://doi.org/10.3390/ijms22169047
APA StyleNishimura, K., & Takata, K. (2021). Combination of Drugs and Cell Transplantation: More Beneficial Stem Cell-Based Regenerative Therapies Targeting Neurological Disorders. International Journal of Molecular Sciences, 22(16), 9047. https://doi.org/10.3390/ijms22169047





