Zebrafish Model as a Screen to Prevent Cyst Inflation in Autosomal Dominant Polycystic Kidney Disease
Abstract
1. Introduction
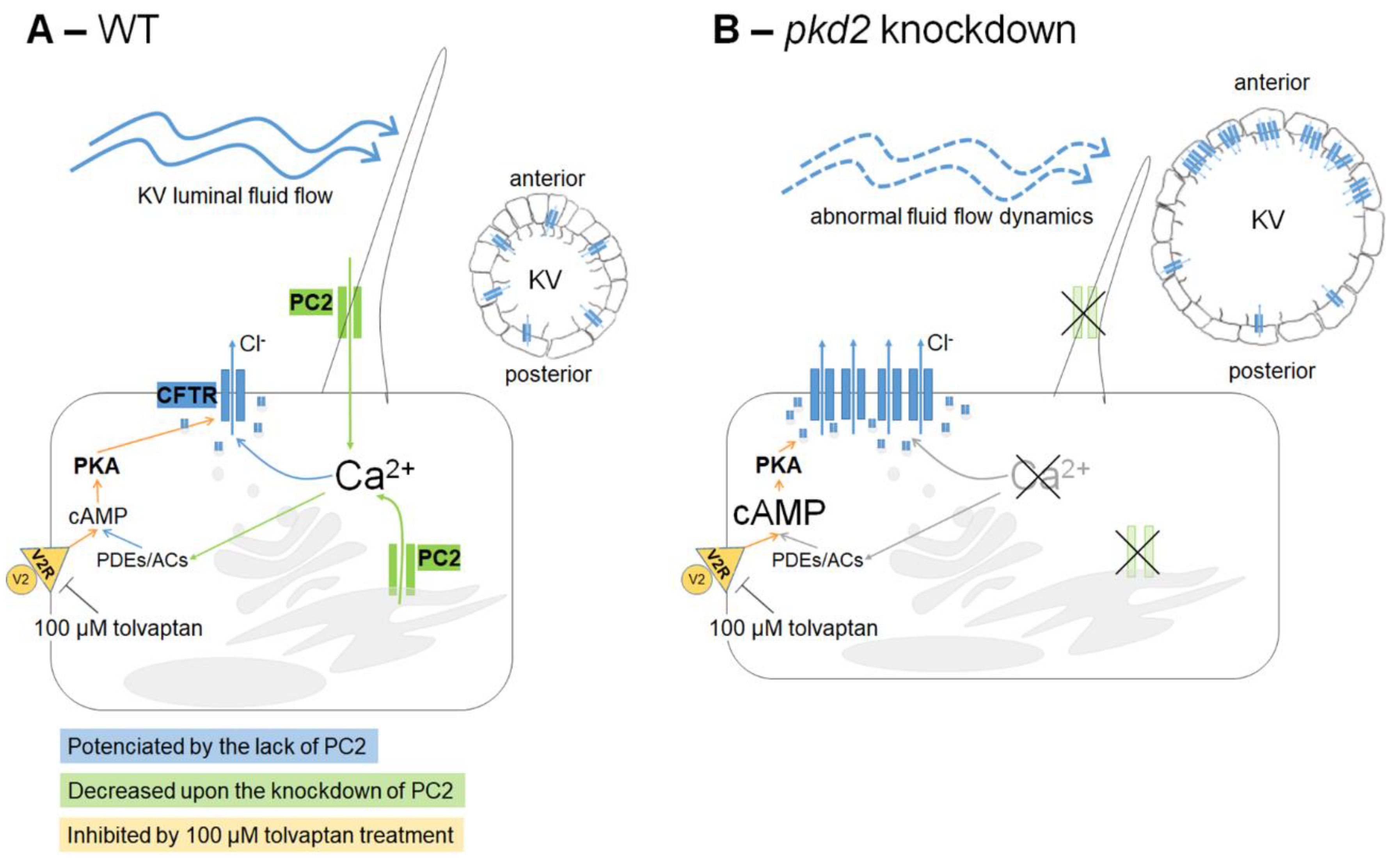
2. Results and Discussion
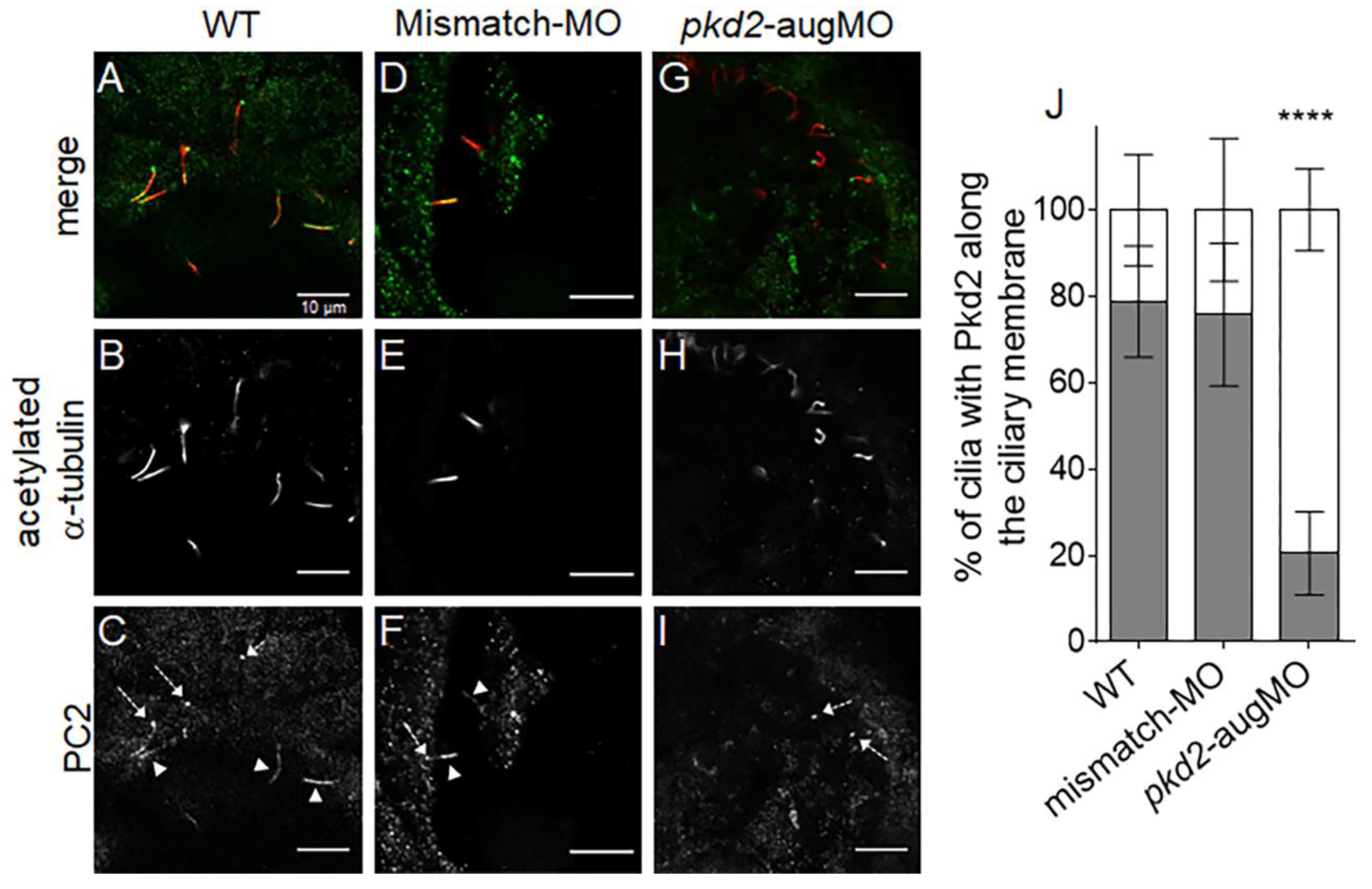
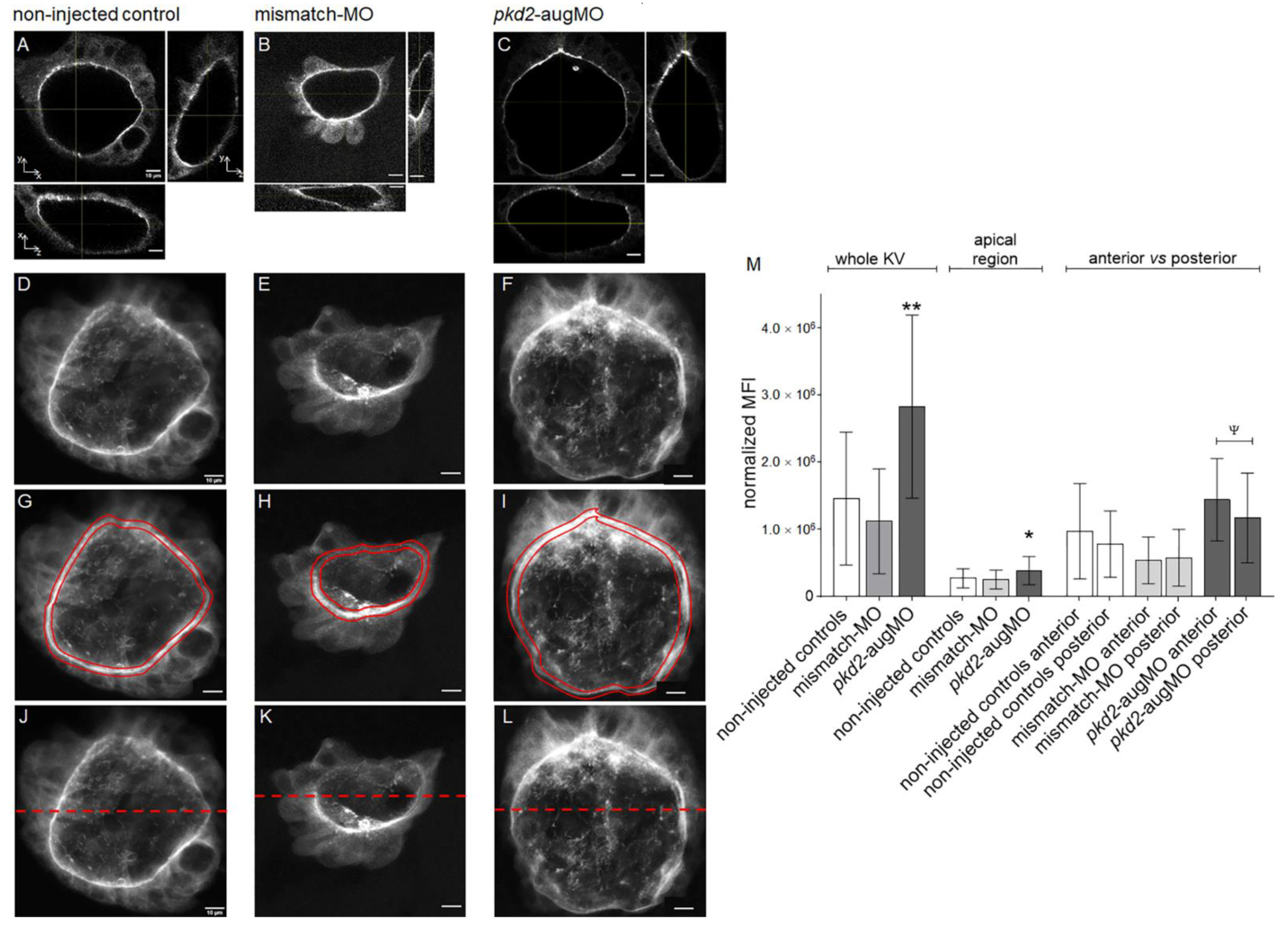
2.1. PC2 Knockdown
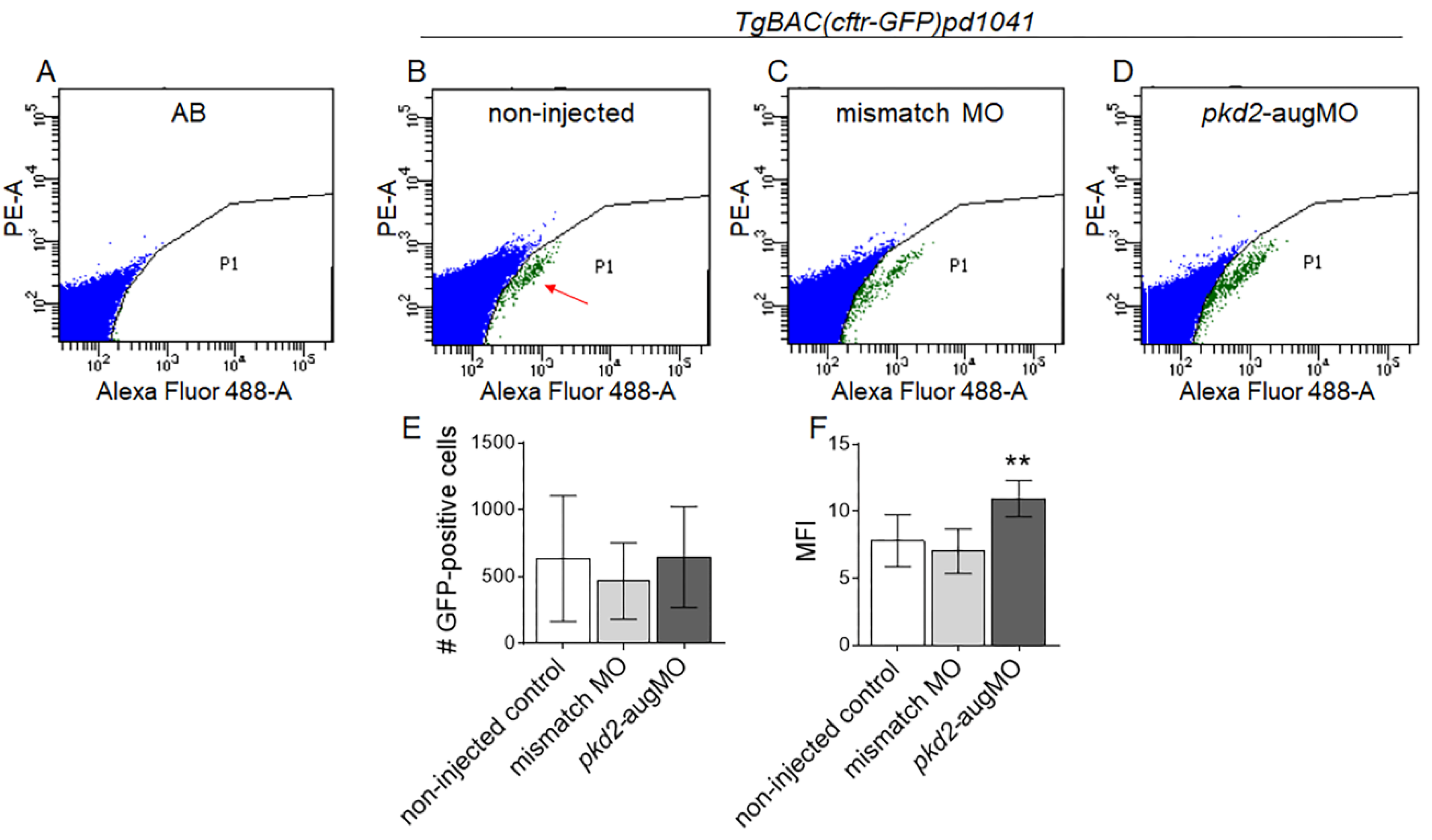
2.2. Knockdown of PC2 Leads to the Apical Accumulation of CFTR Protein
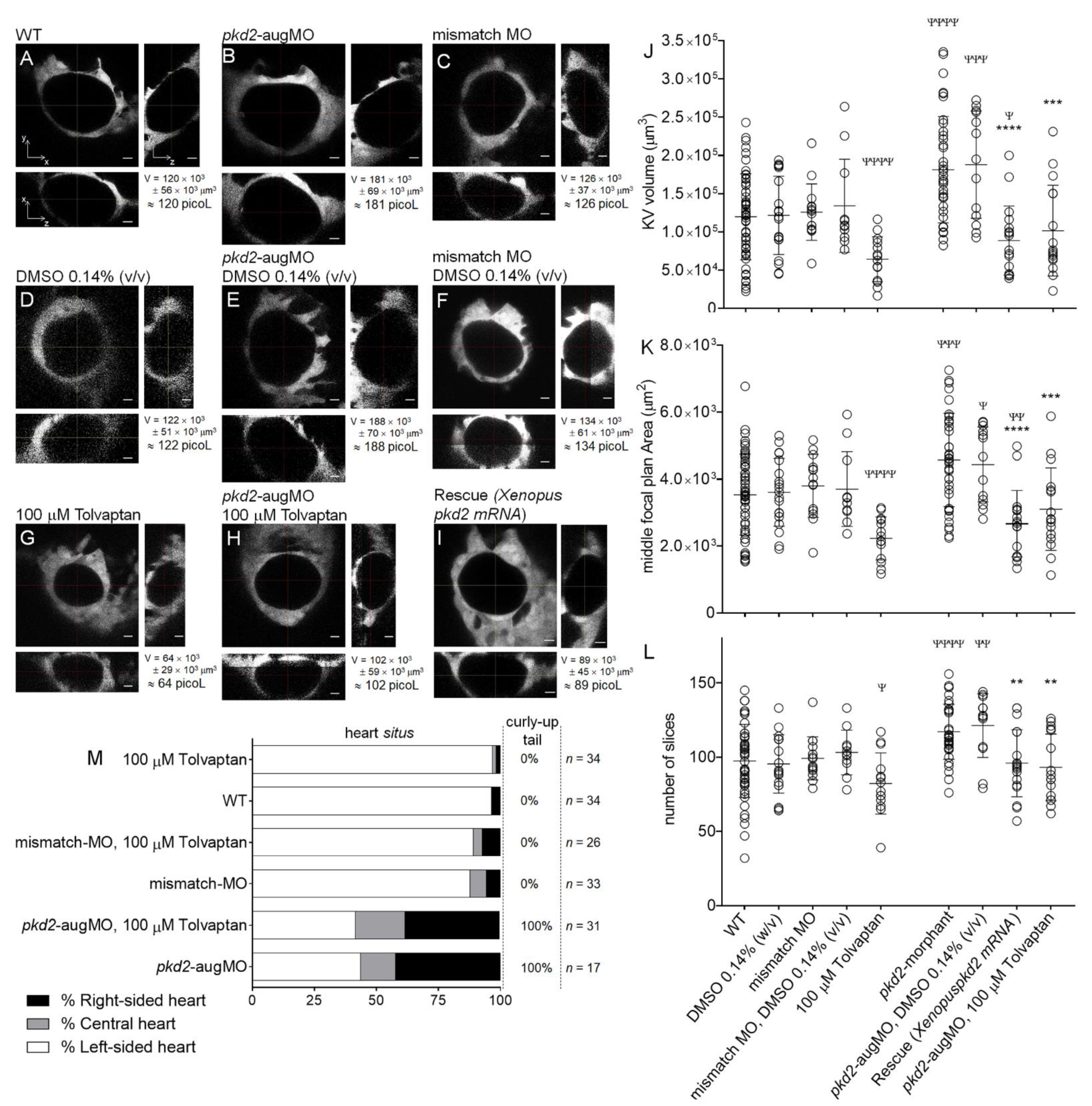
2.3. Tolvaptan Effect
3. Materials and Methods
3.1. Fish Strains
3.2. Morpholino Microinjections
3.3. Pharmacological Treatments
3.4. Heart Laterality Scoring
3.5. Immunofluorescence on Whole-Mount Embryos
3.6. Live-Imaging and KV Volume Determination
3.7. Evaluation of CFTR-GFP Mean Fluorescence Intensity
3.8. Flow Cytometry Analysis
3.9. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalgaard, O.Z. Bilateral polycystic disease of the kidneys: A follow-up of two hundred and eighty-four patients and their families. Acta Med. Scand. Suppl. 1957, 328, 1–255. [Google Scholar]
- Lanktree, M.B.; Haghighi, A.; Guiard, E.; Iliuta, I.A.; Song, X.; Harris, P.C.; Paterson, A.D.; Pei, Y. Prevalence Estimates of Polycystic Kidney and Liver Disease by Population Sequencing. J. Am. Soc. Nephrol. 2018, 29, 2593–2600. [Google Scholar] [CrossRef]
- Heyer, C.M.; Sundsbak, J.L.; Abebe, K.Z.; Chapman, A.B.; Torres, V.E.; Grantham, J.J.; Bae, K.T.; Schrier, R.W.; Perrone, R.D.; Braun, W.E.; et al. Predicted Mutation Strength of Nontruncating PKD1 Mutations Aids Genotype-Phenotype Correlations in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 2872–2884. [Google Scholar] [CrossRef] [PubMed]
- Semmo, M.; Köttgen, M.; Hofherr, A. The TRPP subfamily and polycystin-1 proteins. Handb. Exp. Pharmacol. 2014, 222, 675–711. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.C.; Torres, V.E. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J. Clin. Investig. 2014, 124, 2315–2324. [Google Scholar] [CrossRef]
- Delling, M.; Indzhykulian, A.A.; Liu, X.; Li, Y.; Xie, T.; Corey, D.P.; Clapham, D.E. Primary cilia are not calcium-responsive mechanosensors. Nature 2016, 531, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.V.; Keynton, J.L.; Grimes, D.T.; Sreekumar, V.; Williams, D.J.; Esapa, C.; Wu, D.; Knight, M.M.; Norris, D.P. Ciliary exclusion of Polycystin-2 promotes kidney cystogenesis in an autosomal dominant polycystic kidney disease model. Nat. Commun. 2019, 10, 4072. [Google Scholar] [CrossRef]
- Li, Y.; Wright, J.M.; Qian, F.; Germino, G.G.; Guggino, W.B. Polycystin 2 interacts with type I inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling. J. Biol. Chem. 2005, 280, 41298–41306. [Google Scholar] [CrossRef]
- Torres, V.E.; Harris, P.C. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J. Am. Soc. Nephrol. 2014, 25, 18–32. [Google Scholar] [CrossRef]
- Reif, G.A.; Yamaguchi, T.; Nivens, E.; Fujiki, H.; Pinto, C.S.; Wallace, D.P. Tolvaptan inhibits ERK-dependent cell proliferation, Cl⁻ secretion, and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. Am. J. Physiol. Ren. Physiol. 2011, 301, F1005–F1013. [Google Scholar] [CrossRef]
- Li, H.; Yang, W.; Mendes, F.; Amaral, M.D.; Sheppard, D.N. Impact of the cystic fibrosis mutation F508del-CFTR on renal cyst formation and growth. Am. J. Physiol. Ren. Physiol. 2012, 303, F1176–F1186. [Google Scholar] [CrossRef] [PubMed]
- Yuajit, C.; Muanprasat, C.; Gallagher, A.R.; Fedeles, S.V.; Kittayaruksakul, S.; Homvisasevongsa, S.; Somlo, S.; Chatsudthipong, V. Steviol retards renal cyst growth through reduction of CFTR expression and inhibition of epithelial cell proliferation in a mouse model of polycystic kidney disease. Biochem. Pharmacol. 2014, 88, 412–421. [Google Scholar] [CrossRef]
- Yang, B.; Sonawane, N.D.; Zhao, D.; Somlo, S.; Verkman, A.S. Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol. 2008, 19, 1300–1310. [Google Scholar] [CrossRef]
- Hanaoka, K.; Devuyst, O.; Schwiebert, E.M.; Wilson, P.D.; Guggino, W.B. A role for CFTR in human autosomal dominant polycystic kidney disease. Am. J. Physiol. 1996, 270, C389–C399. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.D.; Hutt, D.M.; Tomati, V.; Botelho, H.M.; Pedemonte, N. CFTR processing, trafficking and interactions. J. Cyst. Fibros. 2020, 19, S33–S36. [Google Scholar] [CrossRef] [PubMed]
- Mantoo, M.R.; Kabra, M.; Kabra, S.K. Cystic Fibrosis Presenting as Pseudo-Bartter Syndrome: An Important Diagnosis that is Missed! Indian J. Pediatr. 2020, 87, 726–732. [Google Scholar] [CrossRef]
- Amaral, M.D.; Farinha, C.M. Rescuing mutant CFTR: A multi-task approach to a better outcome in treating cystic fibrosis. Curr. Pharm. Des. 2013, 19, 3497–3508. [Google Scholar] [CrossRef]
- Farinha, C.M.; Matos, P.; Amaral, M.D. Control of cystic fibrosis transmembrane conductance regulator membrane trafficking: Not just from the endoplasmic reticulum to the Golgi. FEBS J. 2013, 280, 4396–4406. [Google Scholar] [CrossRef]
- Lobo, M.J.; Amaral, M.D.; Zaccolo, M.; Farinha, C.M. EPAC1 activation by cAMP stabilizes CFTR at the membrane by promoting its interaction with NHERF1. J. Cell Sci. 2016, 129, 2599–2612. [Google Scholar] [CrossRef]
- Farinha, C.M.; King-Underwood, J.; Sousa, M.; Correia, A.R.; Henriques, B.J.; Roxo-Rosa, M.; Da Paula, A.C.; Williams, J.; Hirst, S.; Gomes, C.M.; et al. Revertants, Low Temperature, and Correctors Reveal the Mechanism of F508del-CFTR Rescue by VX-809 and Suggest Multiple Agents for Full Correction. Chem. Biol. 2013, 20, 943–955. [Google Scholar] [CrossRef]
- Horie, S. Will introduction of tolvaptan change clinical practice in autosomal dominant polycystic kidney disease? Kidney Int. 2015, 88, 14–16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Devuyst, O.; Chapman, A.B.; Shoaf, S.E.; Czerwiec, F.S.; Blais, J.D. Tolerability of Aquaretic-Related Symptoms Following Tolvaptan for Autosomal Dominant Polycystic Kidney Disease: Results From TEMPO 3:4. Kidney Int. Rep. 2017, 2, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Fong, P.; Cheng, J.; Boletta, A.; Qian, F.; Zhang, X.M.; Cai, H.; Germino, G.G.; Guggino, W.B. A regulatory role of polycystin-1 on cystic fibrosis transmembrane conductance regulator plasma membrane expression. Cell Physiol. Biochem. 2006, 18, 9–20. [Google Scholar] [CrossRef]
- Asawa, R.R.; Danchik, C.; Zahkarov, A.; Chen, Y.; Voss, T.; Jadhav, A.; Wallace, D.P.; Trott, J.F.; Weiss, R.H.; Simeonov, A.; et al. A high-throughput screening platform for Polycystic Kidney Disease (PKD) drug repurposing utilizing murine and human ADPKD cells. Sci. Rep. 2020, 10, 4203. [Google Scholar] [CrossRef]
- Li, H.; Findlay, I.A.; Sheppard, D.N. The relationship between cell proliferation, Cl- secretion, and renal cyst growth: A study using CFTR inhibitors. Kidney Int. 2004, 66, 1926–1938. [Google Scholar] [CrossRef]
- Franke, M.; Baeßler, B.; Vechtel, J.; Dafinger, C.; Höhne, M.; Borgal, L.; Göbel, H.; Koerber, F.; Maintz, D.; Benzing, T.; et al. Magnetic resonance T2 mapping and diffusion-weighted imaging for early detection of cystogenesis and response to therapy in a mouse model of polycystic kidney disease. Kidney Int. 2017, 92, 1544–1554. [Google Scholar] [CrossRef]
- Roxo-Rosa, M.; Jacinto, R.; Sampaio, P.; Lopes, S.S. The zebrafish Kupffer’s vesicle as a model system for the molecular mechanisms by which the lack of Polycystin-2 leads to stimulation of CFTR. Biol. Open 2015, 4, 1356–1366. [Google Scholar] [CrossRef]
- Navis, A.; Marjoram, L.; Bagnat, M. Cftr controls lumen expansion and function of Kupffer’s vesicle in zebrafish. Development 2013, 140, 1703–1712. [Google Scholar] [CrossRef]
- Tavares, B.; Jacinto, R.; Sampaio, P.; Pestana, S.; Pinto, A.; Vaz, A.; Roxo-Rosa, M.; Gardner, R.; Lopes, T.; Schilling, B.; et al. Notch/Her12 signalling modulates, motile/immotile cilia ratio downstream of Foxj1a in zebrafish left-right organizer. Elife 2017, 6. [Google Scholar] [CrossRef]
- Sampaio, P.; Ferreira, R.R.; Guerrero, A.; Pintado, P.; Tavares, B.; Amaro, J.; Smith, A.A.; Montenegro-Johnson, T.; Smith, D.J.; Lopes, S.S. Left-right organizer flow dynamics: How much cilia activity reliably yields laterality? Dev. Cell 2014, 29, 716–728. [Google Scholar] [CrossRef]
- Pintado, P.; Sampaio, P.; Tavares, B.; Montenegro-Johnson, T.D.; Smith, D.J.; Lopes, S.S. Dynamics of cilia length in left-right development. R. Soc. Open Sci. 2017, 4, 161102. [Google Scholar] [CrossRef]
- Smith, D.; Montenegro-Johnson, T.; Lopes, S. Organized chaos in Kupffer’s vesicle: How a heterogeneous structure achieves consistent left-right patterning. Bioarchitecture 2014, 4, 119–125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, D.J.; Montenegro-Johnson, T.D.; Lopes, S.S. Symmetry-Breaking Cilia-Driven Flow in Embryogenesis. Annu. Rev. Fluid Mech. 2019, 51, 105–128. [Google Scholar] [CrossRef]
- Jacinto, R.; Sampaio, P.; Roxo-Rosa, M.; Pestana, S.; Lopes, S.S. Pkd2 Affects Cilia Length and Impacts LR Flow Dynamics and. Front. Cell Dev. Biol. 2021, 9, 624531. [Google Scholar] [CrossRef]
- Sun, Z.; Amsterdam, A.; Pazour, G.J.; Cole, D.G.; Miller, M.S.; Hopkins, N. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 2004, 131, 4085–4093. [Google Scholar] [CrossRef]
- Schottenfeld, J.; Sullivan-Brown, J.; Burdine, R.D. Zebrafish curly up encodes a Pkd2 ortholog that restricts left-side-specific expression of southpaw. Development 2007, 134, 1605–1615. [Google Scholar] [CrossRef]
- Farinha, C.M.; Miller, E.; McCarty, N. Protein and lipid interactions—Modulating CFTR trafficking and rescue. J. Cyst. Fibros. 2018, 17, S9–S13. [Google Scholar] [CrossRef]
- Gokey, J.J.; Ji, Y.; Tay, H.G.; Litts, B.; Amack, J.D. Kupffer’s vesicle size threshold for robust left-right patterning of the zebrafish embryo. Dev. Dyn. 2016, 245, 22–33. [Google Scholar] [CrossRef]
- England, S.J.; Campbell, P.C.; Banerjee, S.; Swanson, A.J.; Lewis, K.E. Identification and Expression Analysis of the Complete Family of Zebrafish pkd Genes. Front. Cell Dev. Biol. 2017, 5, 5. [Google Scholar] [CrossRef]
- Roxo-Rosa, M.; Susana Santos, L. The Zebrafish Kupffer’s Vesicle: A Special Organ in a Model Organism to Study Human Diseases. In Zebrafish in Biomedical Research; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Kamura, K.; Kobayashi, D.; Uehara, Y.; Koshida, S.; Iijima, N.; Kudo, A.; Yokoyama, T.; Takeda, H. Pkd1l1 complexes with Pkd2 on motile cilia and functions to establish the left-right axis. Development 2011, 138, 1121–1129. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Kikuchi, Y.; Kuroiwa, A.; Takeda, H.; Stainier, D.Y. The yolk syncytial layer regulates myocardial migration by influencing extracellular matrix assembly in zebrafish. Development 2006, 133, 4063–4072. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, I.; Jacinto, R.; Pestana, S.; Nolasco, F.; Calado, J.; Lopes, S.S.; Roxo-Rosa, M. Zebrafish Model as a Screen to Prevent Cyst Inflation in Autosomal Dominant Polycystic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9013. https://doi.org/10.3390/ijms22169013
Oliveira I, Jacinto R, Pestana S, Nolasco F, Calado J, Lopes SS, Roxo-Rosa M. Zebrafish Model as a Screen to Prevent Cyst Inflation in Autosomal Dominant Polycystic Kidney Disease. International Journal of Molecular Sciences. 2021; 22(16):9013. https://doi.org/10.3390/ijms22169013
Chicago/Turabian StyleOliveira, Inês, Raquel Jacinto, Sara Pestana, Fernando Nolasco, Joaquim Calado, Susana Santos Lopes, and Mónica Roxo-Rosa. 2021. "Zebrafish Model as a Screen to Prevent Cyst Inflation in Autosomal Dominant Polycystic Kidney Disease" International Journal of Molecular Sciences 22, no. 16: 9013. https://doi.org/10.3390/ijms22169013
APA StyleOliveira, I., Jacinto, R., Pestana, S., Nolasco, F., Calado, J., Lopes, S. S., & Roxo-Rosa, M. (2021). Zebrafish Model as a Screen to Prevent Cyst Inflation in Autosomal Dominant Polycystic Kidney Disease. International Journal of Molecular Sciences, 22(16), 9013. https://doi.org/10.3390/ijms22169013





