Characterization of Lipids in Saliva, Tears and Minor Salivary Glands of Sjögren’s Syndrome Patients Using an HPLC/MS-Based Approach
Abstract
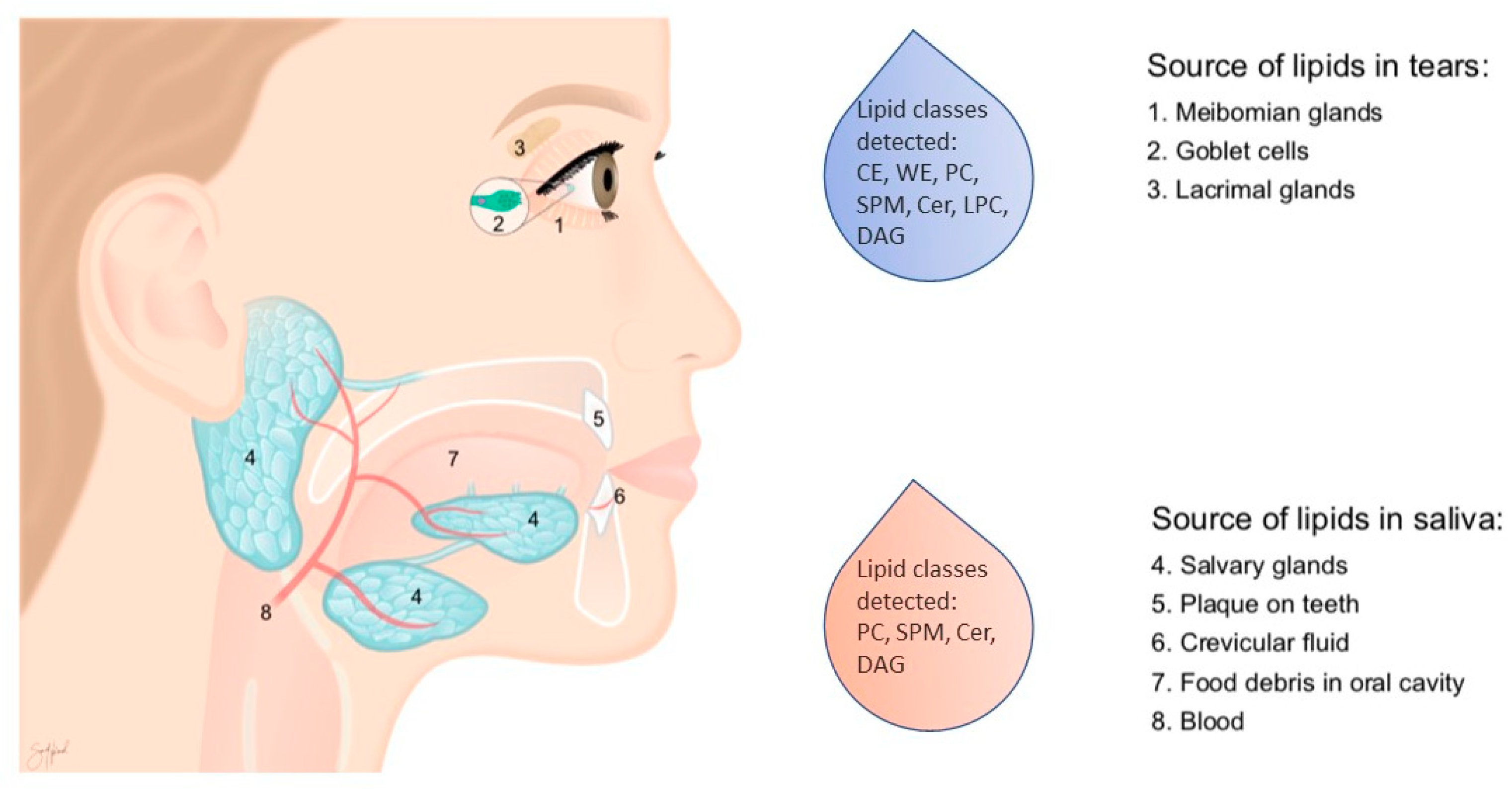
:1. Introduction
2. Results
2.1. Patient Characteristics
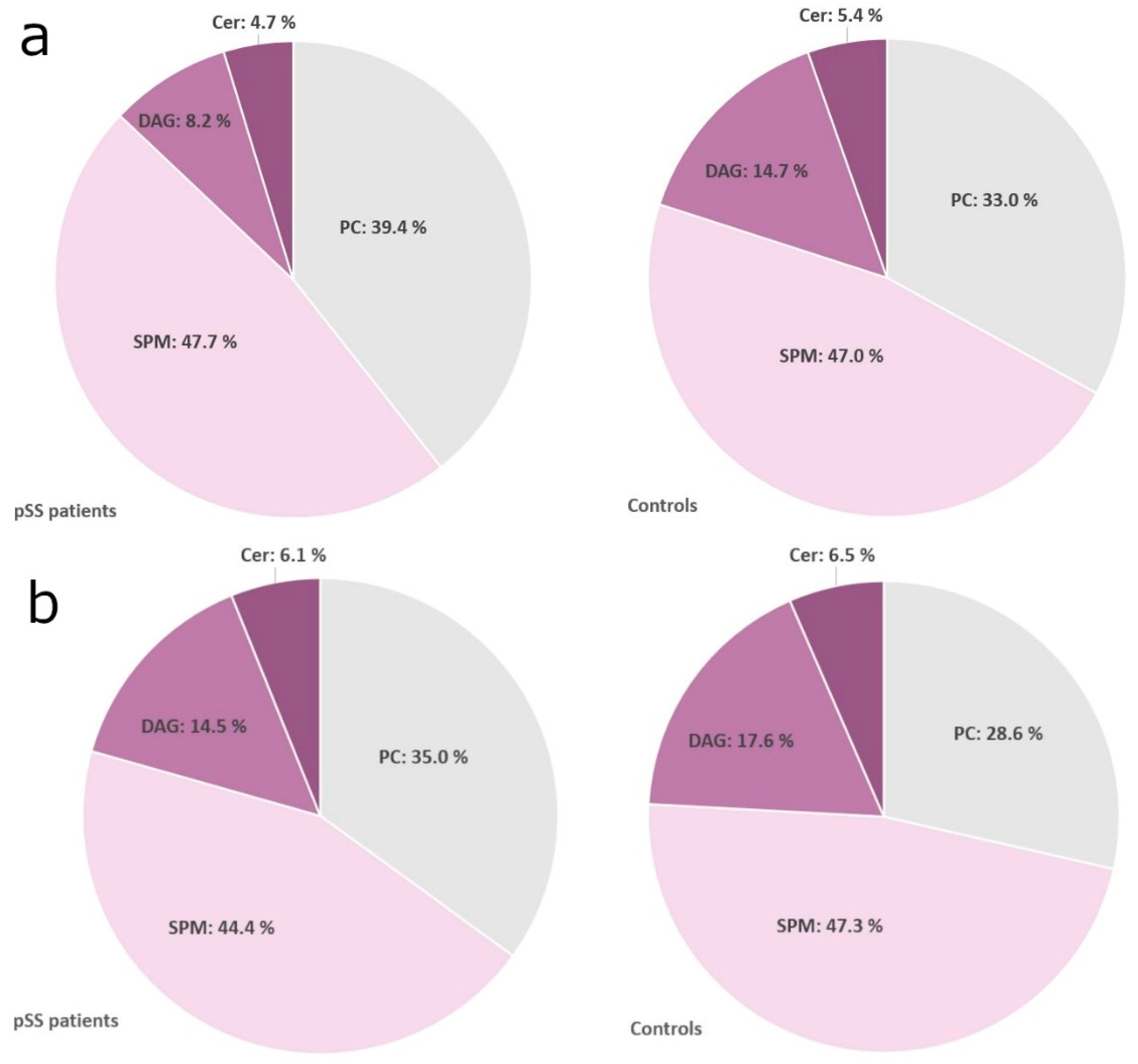
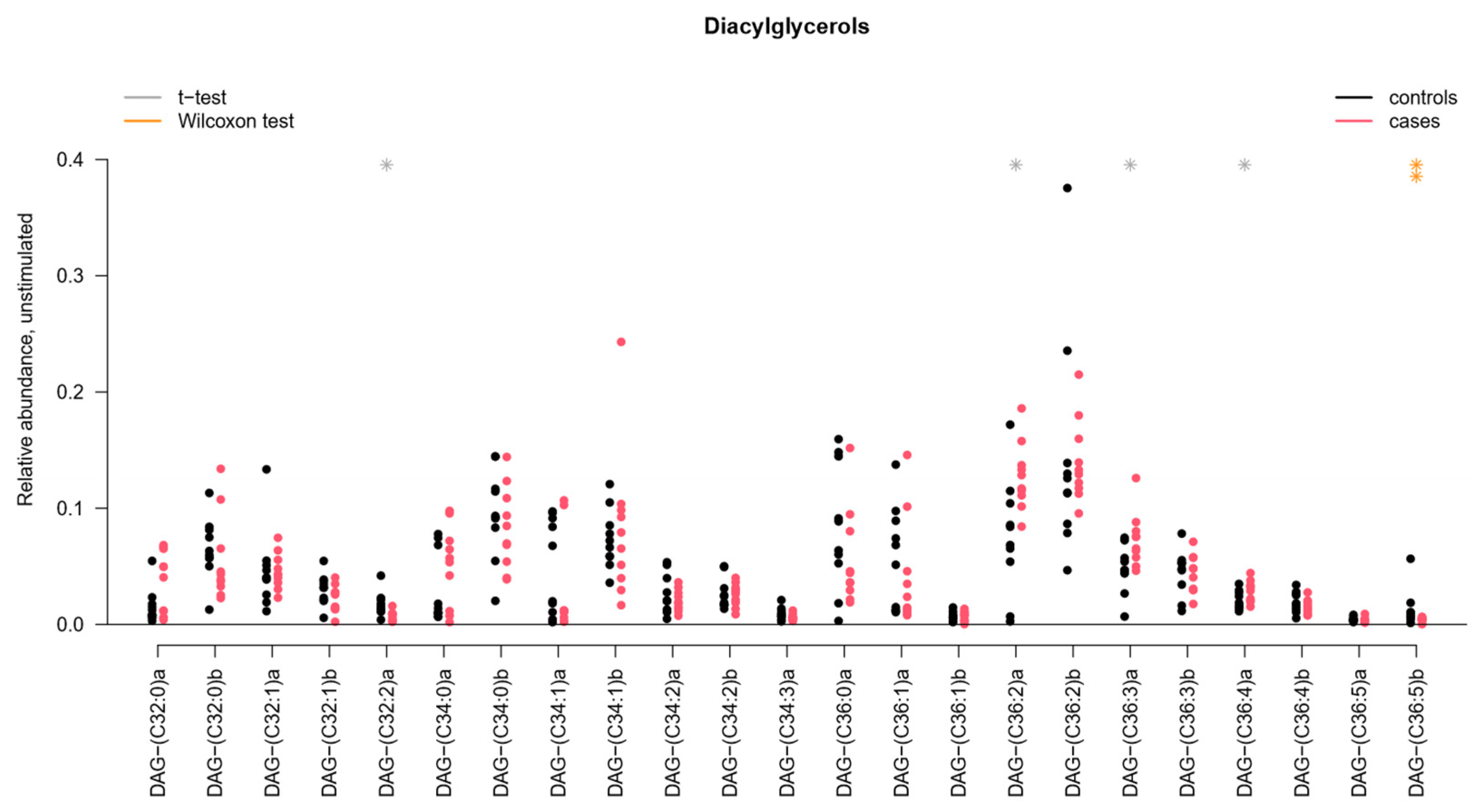
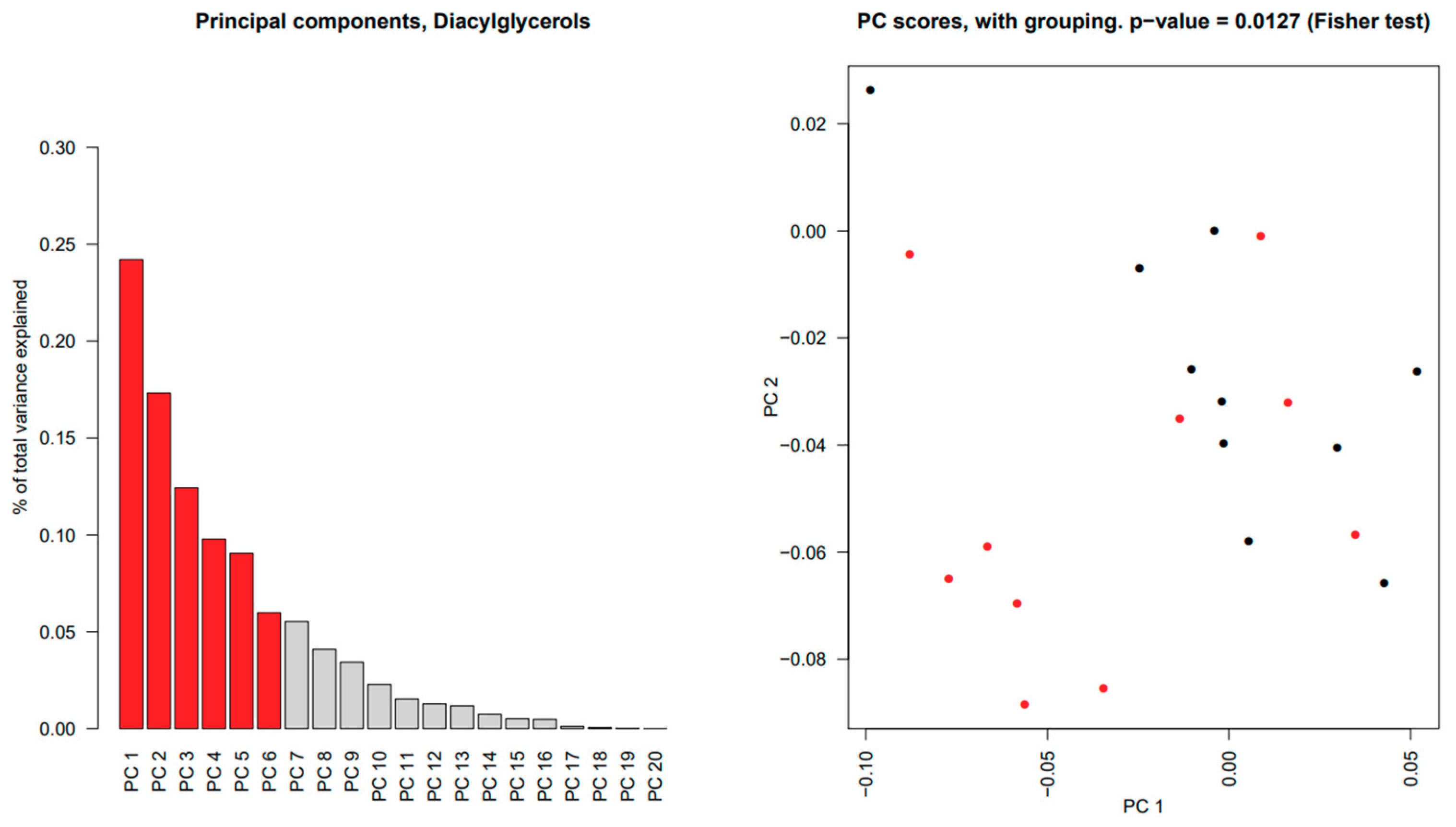
2.2. Sphingomyelins and Diacylglycerophosphocholines Are Increased While Diacylglycerols and Ceramides Are Decreased in Unstimulated Whole Saliva from pSS Patients
2.3. Diacylglycerophosphocholines Are Increased While Sphingomyelins, Diacylglycerols and Ceramides Are Decreased in Stimulated Whole Saliva from pSS Patients
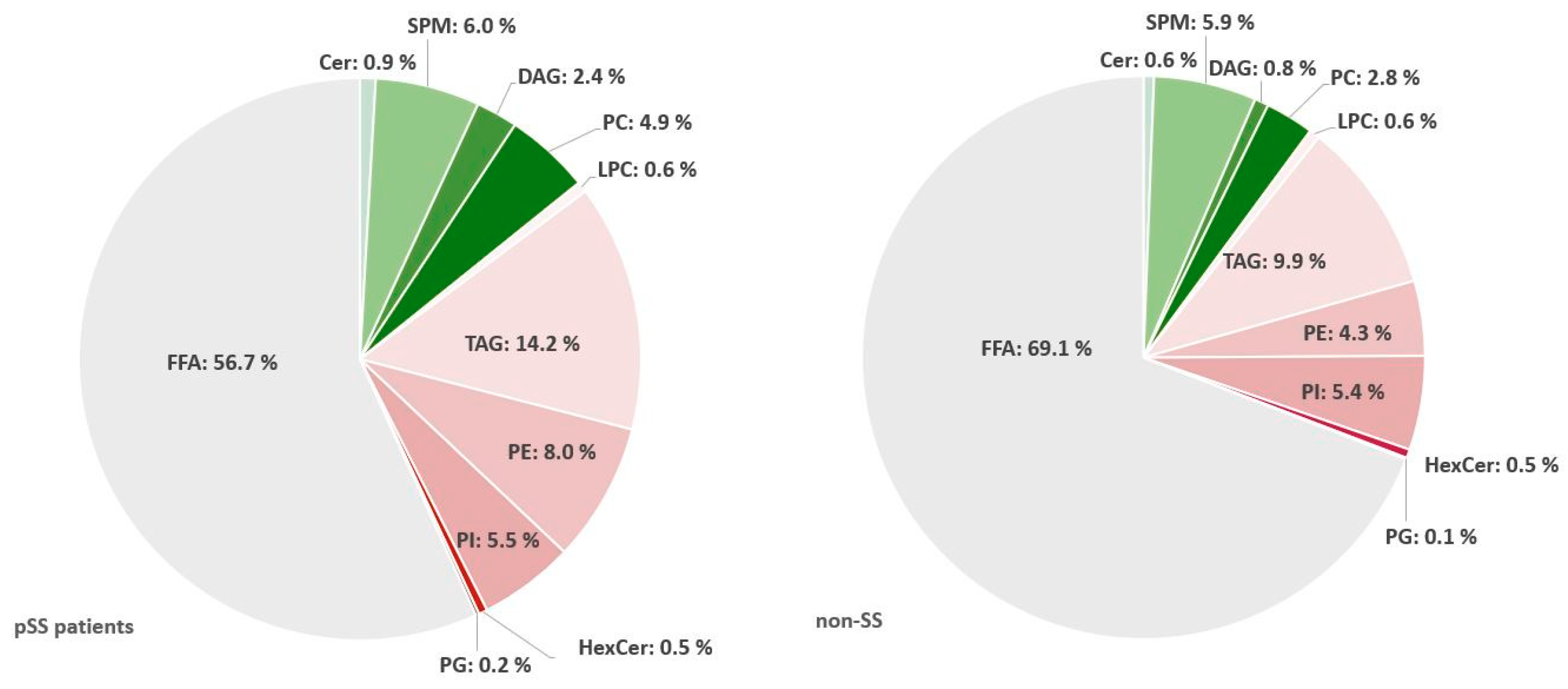
2.4. Triacylglycerols Are Increased While Diacylglycerophosphoinositol and Sphingomyelins Are Decreased in Salivary Glands from pSS Patients
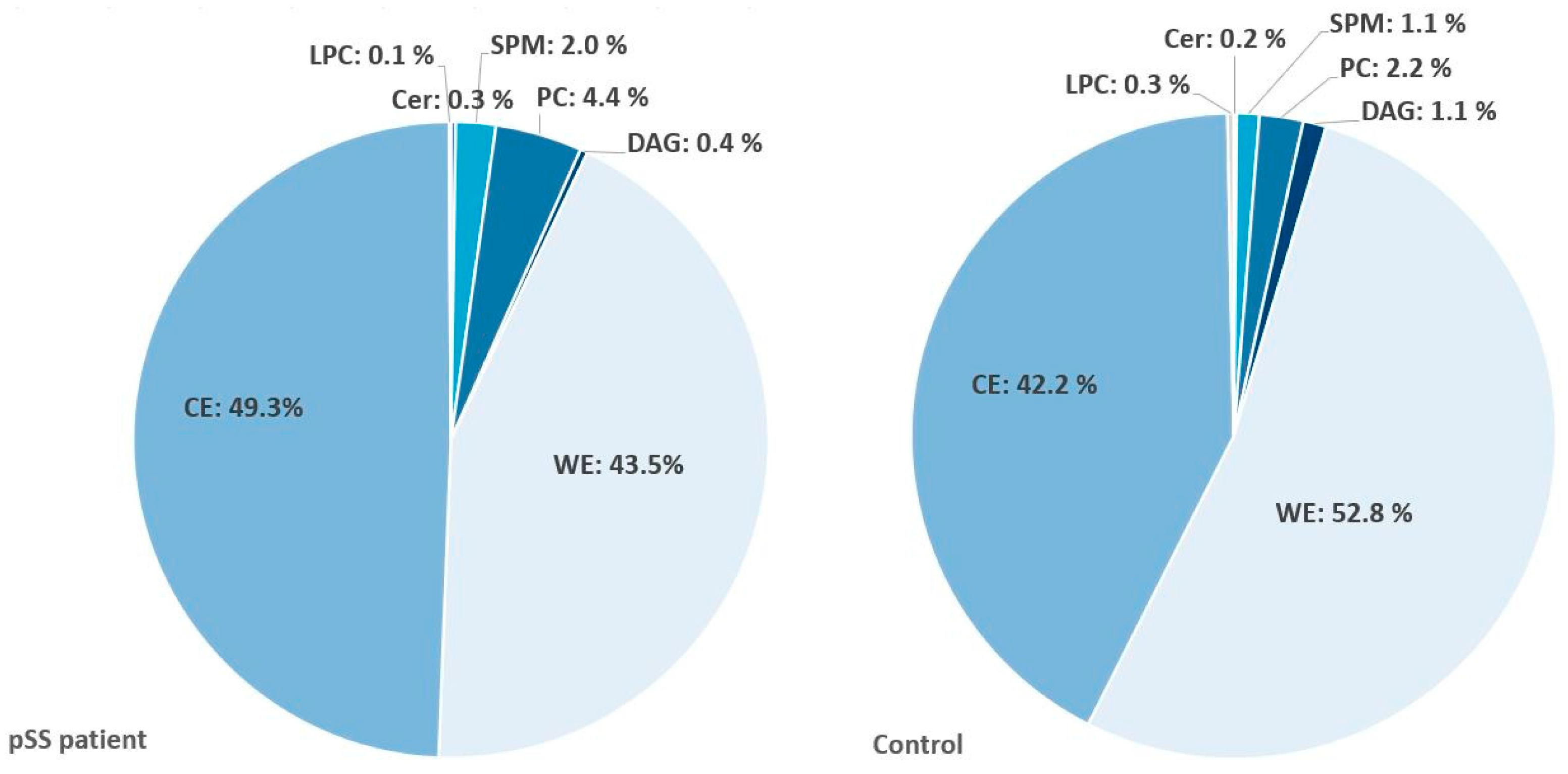
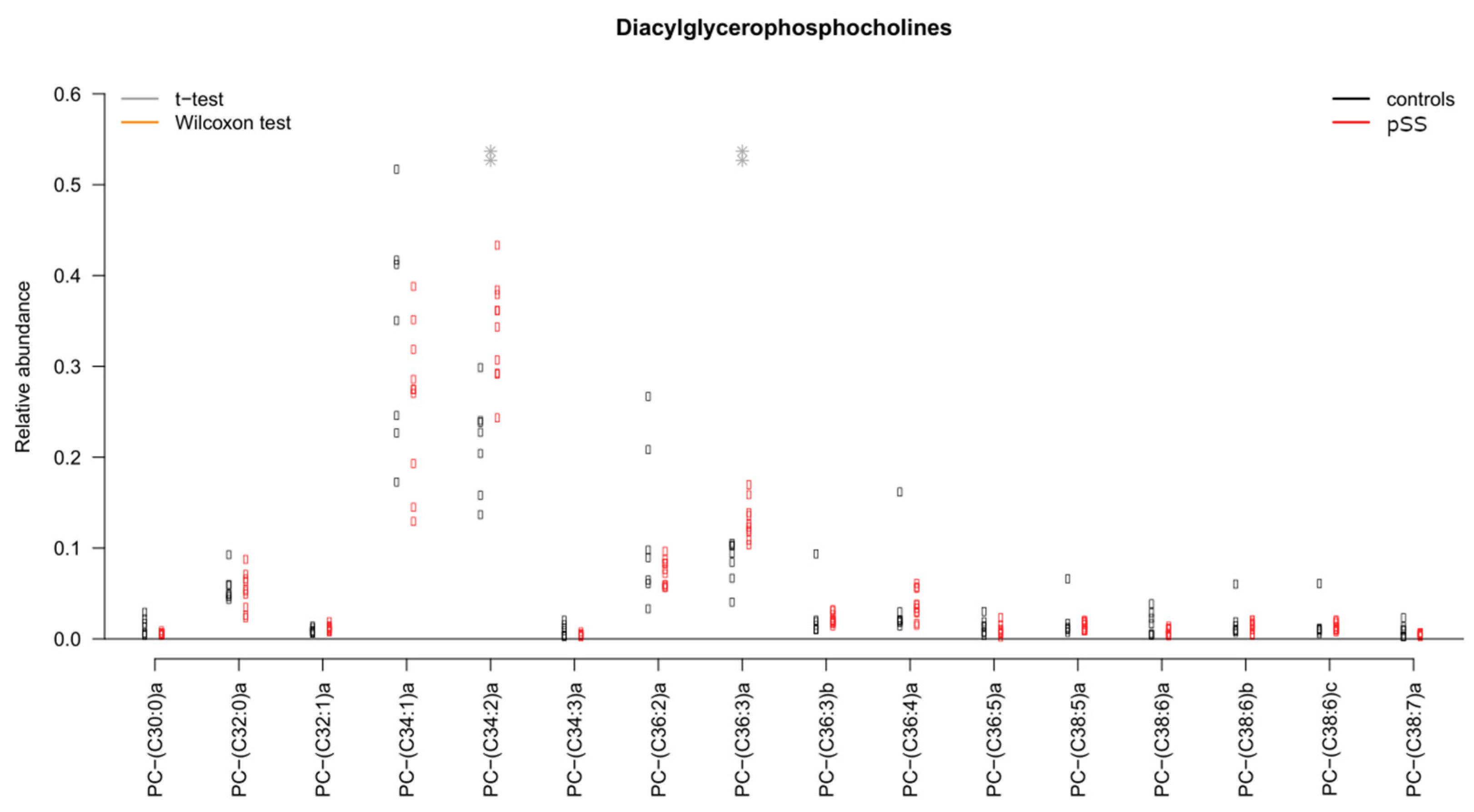
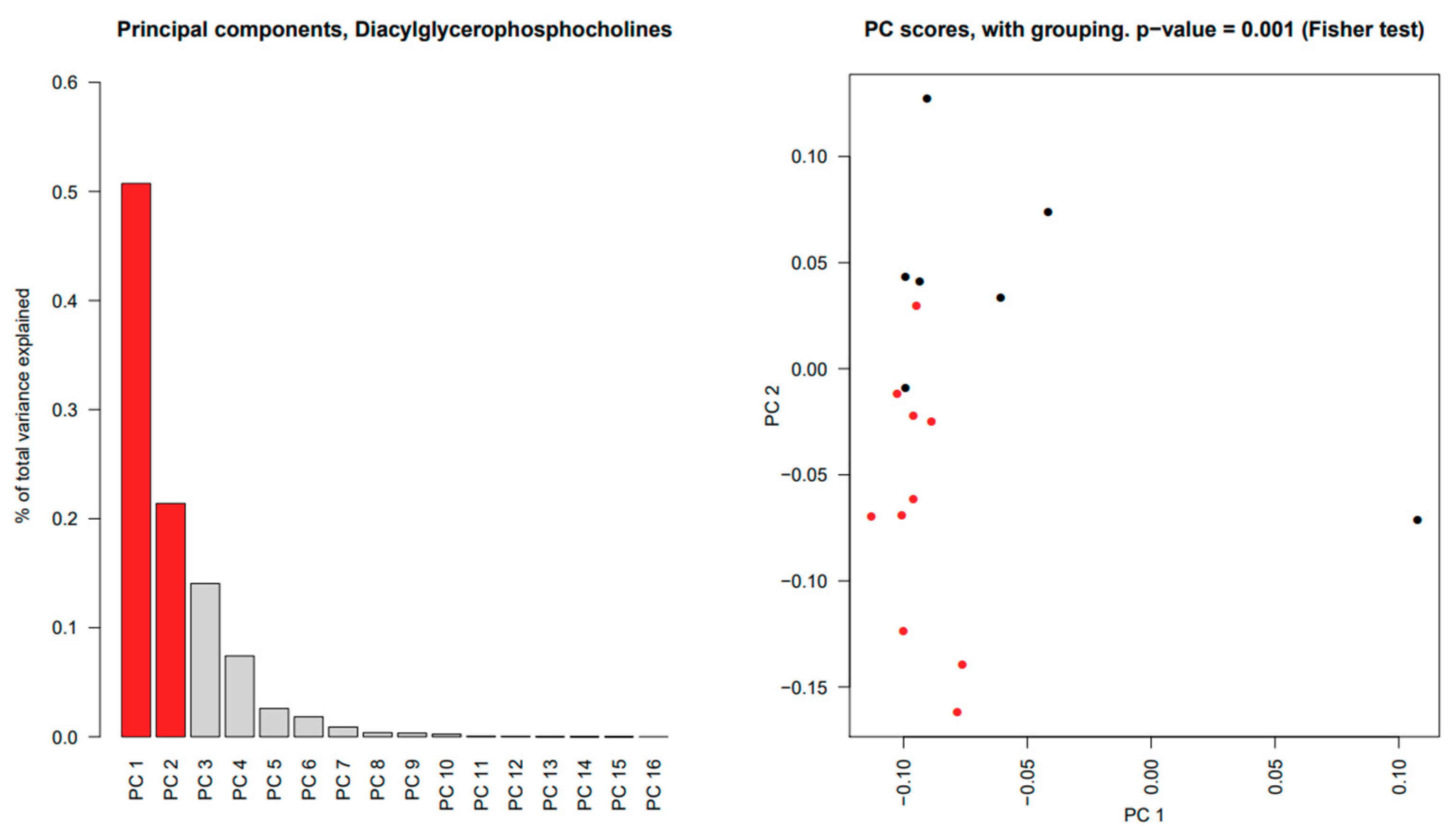
2.5. Significant Changes in Individual Species of Diacylglycerophosphocholine, Lysophosphatidylcholine, Sphingomyelins and Wax Esters in Tear Fluid from pSS Patients
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Saliva Collection
4.3. Biopsies of Labial Salivary Glands
4.4. Tear Fluid Collection
4.5. Lipid Extraction and Quantification
4.6. Untargeted Comprehensive Lipidomic Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjogren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef] [Green Version]
- Theander, E.; Jacobsson, L.T. Relationship of Sjogren’s syndrome to other connective tissue and autoimmune disorders. Rheum. Dis Clin. N. Am. 2008, 34, 935–947. [Google Scholar] [CrossRef]
- Enger, T.B.; Aure, M.H.; Jensen, J.L.; Galtung, H.K. Calcium Signaling and cell volume regulation are altered in Sjogren’s Syndrome. Acta Odontol. Scand. 2014, 72, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Barrera, M.J.; Bahamondes, V.; Sepulveda, D.; Quest, A.F.; Castro, I.; Cortes, J.; Aguilera, S.; Urzua, U.; Molina, C.; Perez, P.; et al. Sjogren’s syndrome and the epithelial target: A comprehensive review. J. Autoimmun. 2013, 42, 7–18. [Google Scholar] [CrossRef]
- Patel, R.; Shahane, A. The epidemiology of Sjogren’s syndrome. Clin. Epidemiol. 2014, 6, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Qin, B.; Wang, J.; Yang, Z.; Yang, M.; Ma, N.; Huang, F.; Zhong, R. Epidemiology of primary Sjogren’s syndrome: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 1983–1989. [Google Scholar] [CrossRef]
- Tashbayev, B.; Rusthen, S.; Young, A.; Herlofson, B.B.; Hove, L.H.; Singh, P.B.; Rykke, M.; Aqrawi, L.A.; Chen, X.; Utheim, O.A.; et al. Interdisciplinary, Comprehensive Oral and Ocular Evaluation of Patients with Primary Sjogren’s Syndrome. Sci. Rep. 2017, 7, 10761. [Google Scholar] [CrossRef] [PubMed]
- Aqrawi, L.A.; Galtung, H.K.; Vestad, B.; Ovstebo, R.; Thiede, B.; Rusthen, S.; Young, A.; Guerreiro, E.M.; Utheim, T.P.; Chen, X.; et al. Identification of potential saliva and tear biomarkers in primary Sjogren’s syndrome, utilising the extraction of extracellular vesicles and proteomics analysis. Arthritis Res. Ther. 2017, 19, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Aqrawi, L.A.; Utheim, T.P.; Tashbayev, B.; Utheim, O.A.; Reppe, S.; Hove, L.H.; Herlofson, B.B.; Singh, P.B.; Palm, O.; et al. Elevated cytokine levels in tears and saliva of patients with primary Sjogren’s syndrome correlate with clinical ocular and oral manifestations. Sci. Rep. 2019, 9, 7319. [Google Scholar] [CrossRef] [PubMed]
- Aqrawi, L.A.; Jensen, J.L.; Oijordsbakken, G.; Ruus, A.K.; Nygard, S.; Holden, M.; Jonsson, R.; Galtung, H.K.; Skarstein, K. Signalling pathways identified in salivary glands from primary Sjogren’s syndrome patients reveal enhanced adipose tissue development. Autoimmunity 2018, 51, 135–146. [Google Scholar] [CrossRef]
- Skarstein, K.; Aqrawi, L.A.; Oijordsbakken, G.; Jonsson, R.; Jensen, J.L. Adipose tissue is prominent in salivary glands of Sjogren’s syndrome patients and appears to influence the microenvironment in these organs. Autoimmunity 2016, 49, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Matczuk, J.; Zendzian-Piotrowska, M.; Maciejczyk, M.; Kurek, K. Salivary lipids: A review. Adv. Clin. Exp. Med. 2017, 26, 1021–1029. [Google Scholar] [CrossRef]
- Fabian, T.K.; Fejerdy, P.; Csermely, P. Salivary Genomics, Transcriptomics and Proteomics: The Emerging Concept of the Oral Ecosystem and their Use in the Early Diagnosis of Cancer and other Diseases. Curr. Genom. 2008, 9, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Fabian, T.K.; Hermann, P.; Beck, A.; Fejerdy, P.; Fabian, G. Salivary defense proteins: Their network and role in innate and acquired oral immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320. [Google Scholar] [CrossRef]
- Hopcraft, M.S.; Tan, C. Xerostomia: An update for clinicians. Aust. Dent. J. 2010, 55, 238–244. [Google Scholar] [CrossRef]
- Brasser, A.J.; Barwacz, C.A.; Dawson, D.V.; Brogden, K.A.; Drake, D.R.; Wertz, P.W. Presence of wax esters and squalene in human saliva. Arch. Oral Biol. 2011, 56, 588–591. [Google Scholar] [CrossRef] [Green Version]
- Tomita, Y.; Miyake, N.; Yamanaka, S. Lipids in human parotid saliva with regard to caries experience. J. Oleo Sci. 2008, 57, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Larsson, B.; Olivecrona, G.; Ericson, T. Lipids in human saliva. Arch. Oral Biol. 1996, 41, 105–110. [Google Scholar] [CrossRef]
- Slomiany, B.L.; Murty, V.L.; Slomiany, A. Salivary lipids in health and disease. Prog. Lipid Res. 1985, 24, 311–324. [Google Scholar] [CrossRef]
- Slomiany, B.L.; Zdebska, E.; Murty, V.L.; Slomiany, A.; Petropoulou, K.; Mandel, I.D. Lipid composition of human labial salivary gland secretions. Arch. Oral Biol. 1983, 28, 711–714. [Google Scholar] [CrossRef]
- Dawes, C.; Pedersen, A.M.; Villa, A.; Ekstrom, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef]
- Slomiany, B.L.; Kosmala, M.; Nadziejko, C.; Murty, V.L.; Gwozdzinski, K.; Slomiany, A.; Mandel, I.D. Lipid composition and viscosity of parotid saliva in Sjogren syndrome in man. Arch. Oral Biol. 1986, 31, 699–702. [Google Scholar] [CrossRef]
- Tishler, M.; Yaron, I.; Raz, A.; Meyer, F.A.; Yaron, M. Salivary eicosanoid concentration in patients with Sjogren’s syndrome. Ann. Rheum. Dis. 1996, 55, 202–204. [Google Scholar] [CrossRef] [Green Version]
- Willcox, M.D.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U. TFOS DEWS II tear film report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef] [Green Version]
- McCulley, J.P.; Shine, W. A compositional based model for the tear film lipid layer. Trans. Am. Ophthalmol. Soc. 1997, 95, 79–88, discussion 88–93. [Google Scholar] [PubMed]
- Butovich, I.A.; Uchiyama, E.; Di Pascuale, M.A.; McCulley, J.P. Liquid chromatography-mass spectrometric analysis of lipids present in human meibomian gland secretions. Lipids 2007, 42, 765–776. [Google Scholar] [CrossRef]
- Butovich, I.A.; Uchiyama, E.; McCulley, J.P. Lipids of human meibum: Mass-spectrometric analysis and structural elucidation. J. Lipid Res. 2007, 48, 2220–2235. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Green-Church, K.B.; Nichols, K.K. Shotgun lipidomic analysis of human meibomian gland secretions with electrospray ionization tandem mass spectrometry. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6220–6231. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Tong, L.; Yong, S.S.; Li, B.; Chaurasia, S.S.; Shui, G.; Wenk, M.R. Meibum lipid composition in Asians with dry eye disease. PLoS ONE 2011, 6, e24339. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Nichols, K.K.; Wilson, L.; Barnes, S.; Nichols, J.J. Untargeted lipidomic analysis of human tears: A new approach for quantification of O-acyl-omega hydroxy fatty acids. Ocul. Surf. 2019, 17, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.H.; Kunnen, C.M.; Duchoslav, E.; Dolla, N.K.; Kelso, M.J.; Papas, E.B.; Lazon de la Jara, P.; Willcox, M.D.; Blanksby, S.J.; Mitchell, T.W. A comparison of patient matched meibum and tear lipidomes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7417–7424. [Google Scholar] [CrossRef]
- Shine, W.E.; McCulley, J.P. Polar lipids in human meibomian gland secretions. Curr. Eye Res. 2003, 26, 89–94. [Google Scholar] [CrossRef]
- Chen, X.; Utheim, O.A.; Xiao, J.; Adil, M.Y.; Stojanovic, A.; Tashbayev, B.; Jensen, J.L.; Utheim, T.P. Meibomian gland features in a Norwegian cohort of patients with primary Sjogren’s syndrome. PLoS ONE 2017, 12, e0184284. [Google Scholar] [CrossRef]
- Zaura, E.; Brandt, B.W.; Prodan, A.; Teixeira de Mattos, M.J.; Imangaliyev, S.; Kool, J.; Buijs, M.J.; Jagers, F.L.; Hennequin-Hoenderdos, N.L.; Slot, D.E.; et al. On the ecosystemic network of saliva in healthy young adults. ISME J. 2017, 11, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.Y.W.; Chua, J.W.M.; Gill, S.; Brownlee, I.A. Analysis of the Lipolytic Activity of Whole-Saliva and Site-Specific Secretions from the Oral Cavity of Healthy Adults. Nutrients 2019, 11, 191. [Google Scholar] [CrossRef] [Green Version]
- Hamosh, M.; Burns, W.A. Lipolytic activity of human lingual glands (Ebner). Lab. Investig. 1977, 37, 603–608. [Google Scholar]
- Drotleff, B.; Roth, S.R.; Henkel, K.; Calderon, C.; Schlotterbeck, J.; Neukamm, M.A.; Lammerhofer, M. Lipidomic profiling of non-mineralized dental plaque and biofilm by untargeted UHPLC-QTOF-MS/MS and SWATH acquisition. Anal. Bioanal. Chem. 2020, 412, 2303–2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, S.M.; Tong, L.; Duan, X.; Petznick, A.; Wenk, M.R.; Shui, G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J. Lipid Res. 2014, 55, 289–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucker, A.D.; Nichols, J.J. Analysis of meibum and tear lipids. Ocul. Surf. 2012, 10, 230–250. [Google Scholar] [CrossRef]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Tang, D.; Ho, D.V. Spectroscopic evaluation of human tear lipids. Chem. Phys. Lipids 2007, 147, 87–102. [Google Scholar] [CrossRef]
- Butovich, I.A. On the lipid composition of human meibum and tears: Comparative analysis of nonpolar lipids. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3779–3789. [Google Scholar] [CrossRef] [Green Version]
- Glasgow, B.J.; Abduragimov, A.R. Interaction of ceramides and tear lipocalin. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 399–408. [Google Scholar] [CrossRef]
- Lam, S.M.; Tong, L.; Reux, B.; Duan, X.; Petznick, A.; Yong, S.S.; Khee, C.B.; Lear, M.J.; Wenk, M.R.; Shui, G. Lipidomic analysis of human tear fluid reveals structure-specific lipid alterations in dry eye syndrome. J. Lipid Res. 2014, 55, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.H.; Kunnen, C.M.; Papas, E.B.; Lazon de la Jara, P.; Willcox, M.D.; Blanksby, S.J.; Mitchell, T.W. Intersubject and Interday Variability in Human Tear and Meibum Lipidomes: A Pilot Study. Ocul. Surf. 2016, 14, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Borchman, D.; Yappert, M.C.; Foulks, G.N. Changes in human meibum lipid with meibomian gland dysfunction using principal component analysis. Exp. Eye Res. 2010, 91, 246–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, I.; Stretch, C.; Barnaby, P.; Bhatnager, K.; Rankin, K.; Fu, H.; Weljie, A.; Jha, N.; Slupsky, C. Understanding the human salivary metabolome. NMR Biomed. 2009, 22, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Norheim, F.; Bjellaas, T.; Hui, S.T.; Chella Krishnan, K.; Lee, J.; Gupta, S.; Pan, C.; Hasin-Brumshtein, Y.; Parks, B.W.; Li, D.Y.; et al. Genetic, dietary, and sex-specific regulation of hepatic ceramides and the relationship between hepatic ceramides and IR. J. Lipid Res. 2018, 59, 1164–1174. [Google Scholar] [CrossRef] [Green Version]
- Norheim, F.; Chella Krishnan, K.; Bjellaas, T.; Vergnes, L.; Pan, C.; Parks, B.W.; Meng, Y.; Lang, J.; Ward, J.A.; Reue, K.; et al. Genetic regulation of liver lipids in a mouse model of insulin resistance and hepatic steatosis. Mol. Syst. Biol. 2021, 17, e9684. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 22 March 2021).
- Johnson, R.A.; Wichern, D.W. Applied Multivariate Statistical Analysis; Prentice Hall: Upper Saddle River, NJ, USA, 2002; Volume 5. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fineide, F.; Chen, X.; Bjellaas, T.; Vitelli, V.; Utheim, T.P.; Jensen, J.L.; Galtung, H.K. Characterization of Lipids in Saliva, Tears and Minor Salivary Glands of Sjögren’s Syndrome Patients Using an HPLC/MS-Based Approach. Int. J. Mol. Sci. 2021, 22, 8997. https://doi.org/10.3390/ijms22168997
Fineide F, Chen X, Bjellaas T, Vitelli V, Utheim TP, Jensen JL, Galtung HK. Characterization of Lipids in Saliva, Tears and Minor Salivary Glands of Sjögren’s Syndrome Patients Using an HPLC/MS-Based Approach. International Journal of Molecular Sciences. 2021; 22(16):8997. https://doi.org/10.3390/ijms22168997
Chicago/Turabian StyleFineide, Fredrik, Xiangjun Chen, Thomas Bjellaas, Valeria Vitelli, Tor Paaske Utheim, Janicke Liaaen Jensen, and Hilde Kanli Galtung. 2021. "Characterization of Lipids in Saliva, Tears and Minor Salivary Glands of Sjögren’s Syndrome Patients Using an HPLC/MS-Based Approach" International Journal of Molecular Sciences 22, no. 16: 8997. https://doi.org/10.3390/ijms22168997
APA StyleFineide, F., Chen, X., Bjellaas, T., Vitelli, V., Utheim, T. P., Jensen, J. L., & Galtung, H. K. (2021). Characterization of Lipids in Saliva, Tears and Minor Salivary Glands of Sjögren’s Syndrome Patients Using an HPLC/MS-Based Approach. International Journal of Molecular Sciences, 22(16), 8997. https://doi.org/10.3390/ijms22168997





