Synthesis of New AIEE-Active Chalcones for Imaging of Mitochondria in Living Cells and Zebrafish In Vivo
Abstract
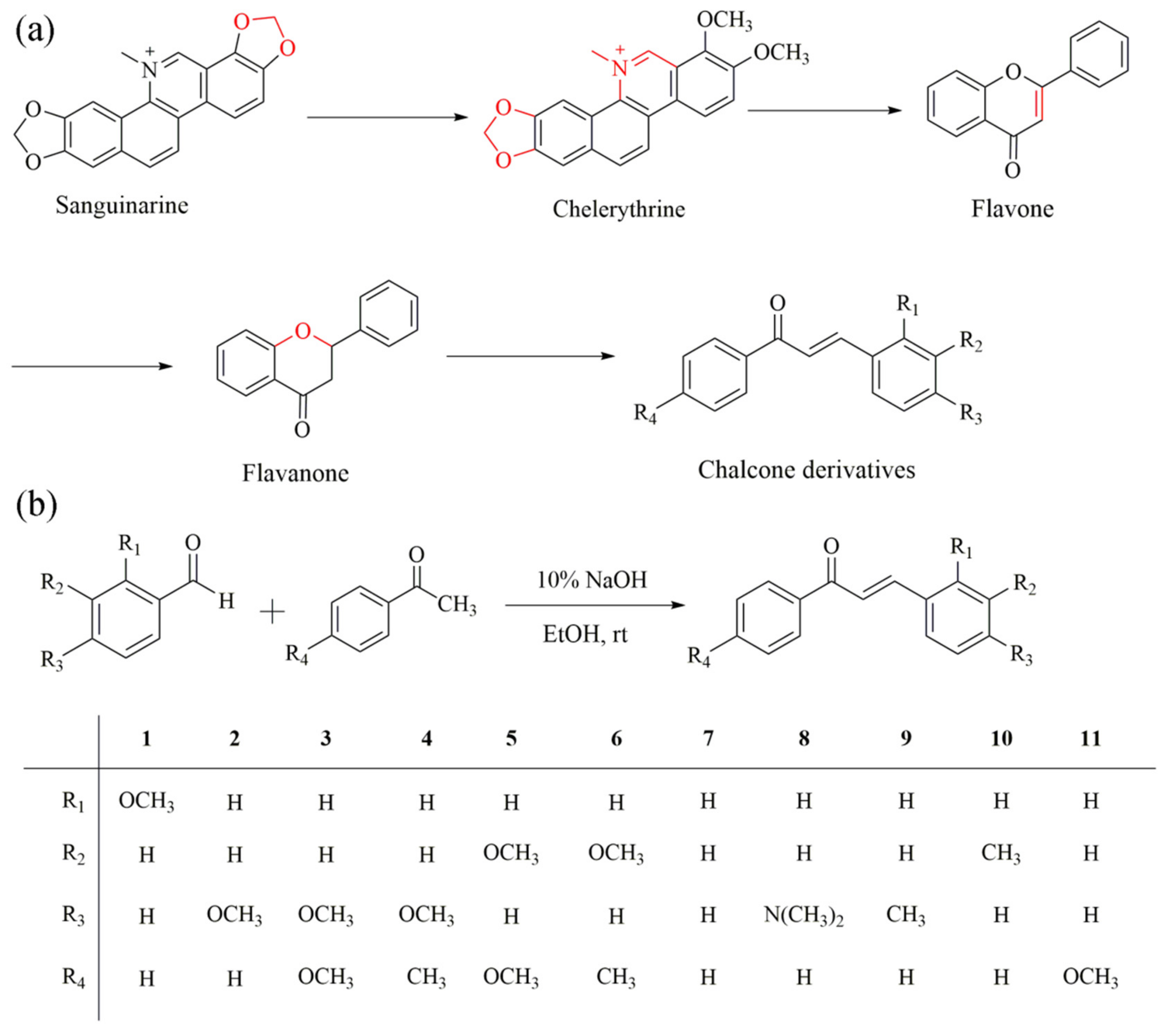
:1. Introduction
2. Results and Discussion
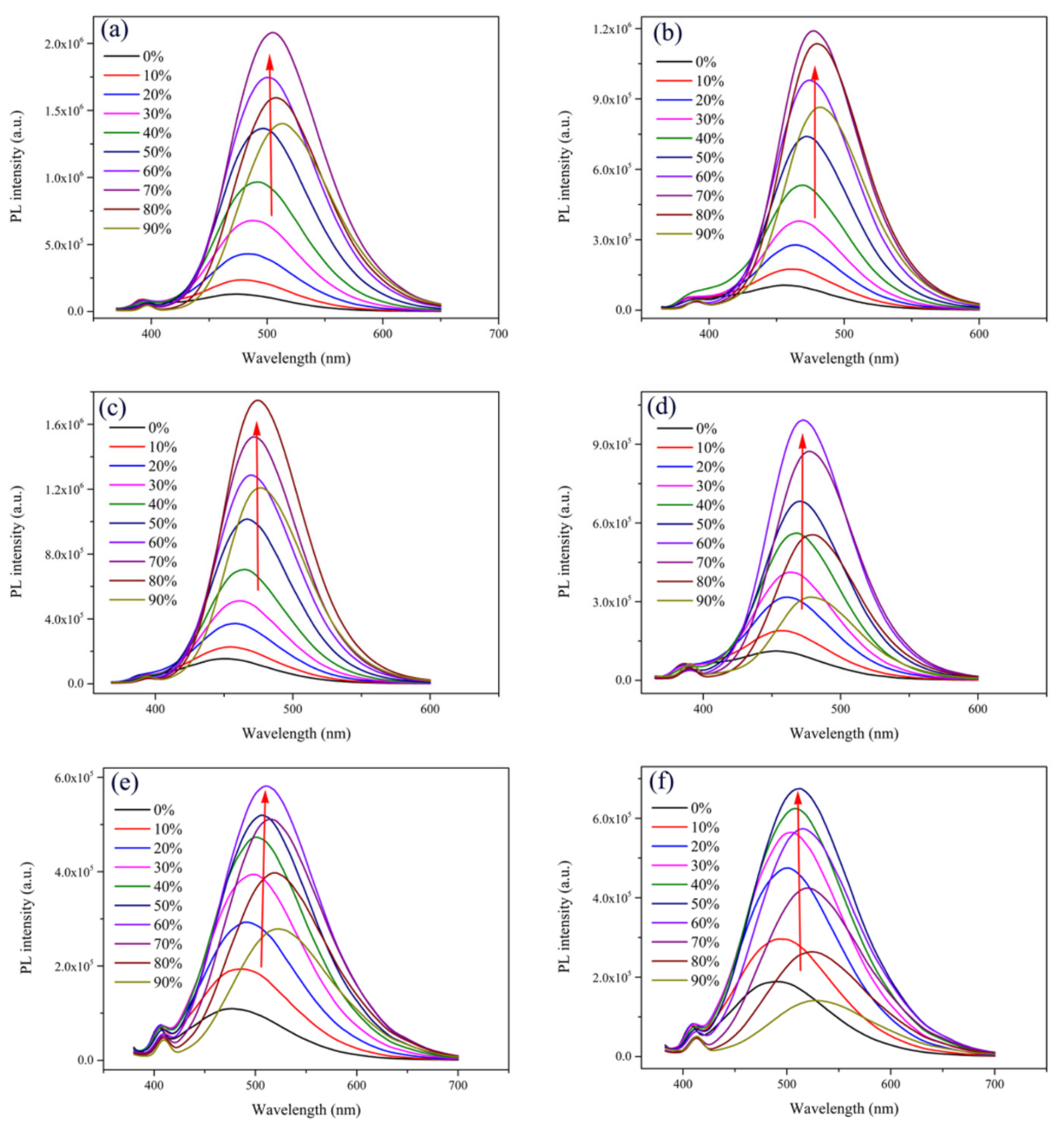
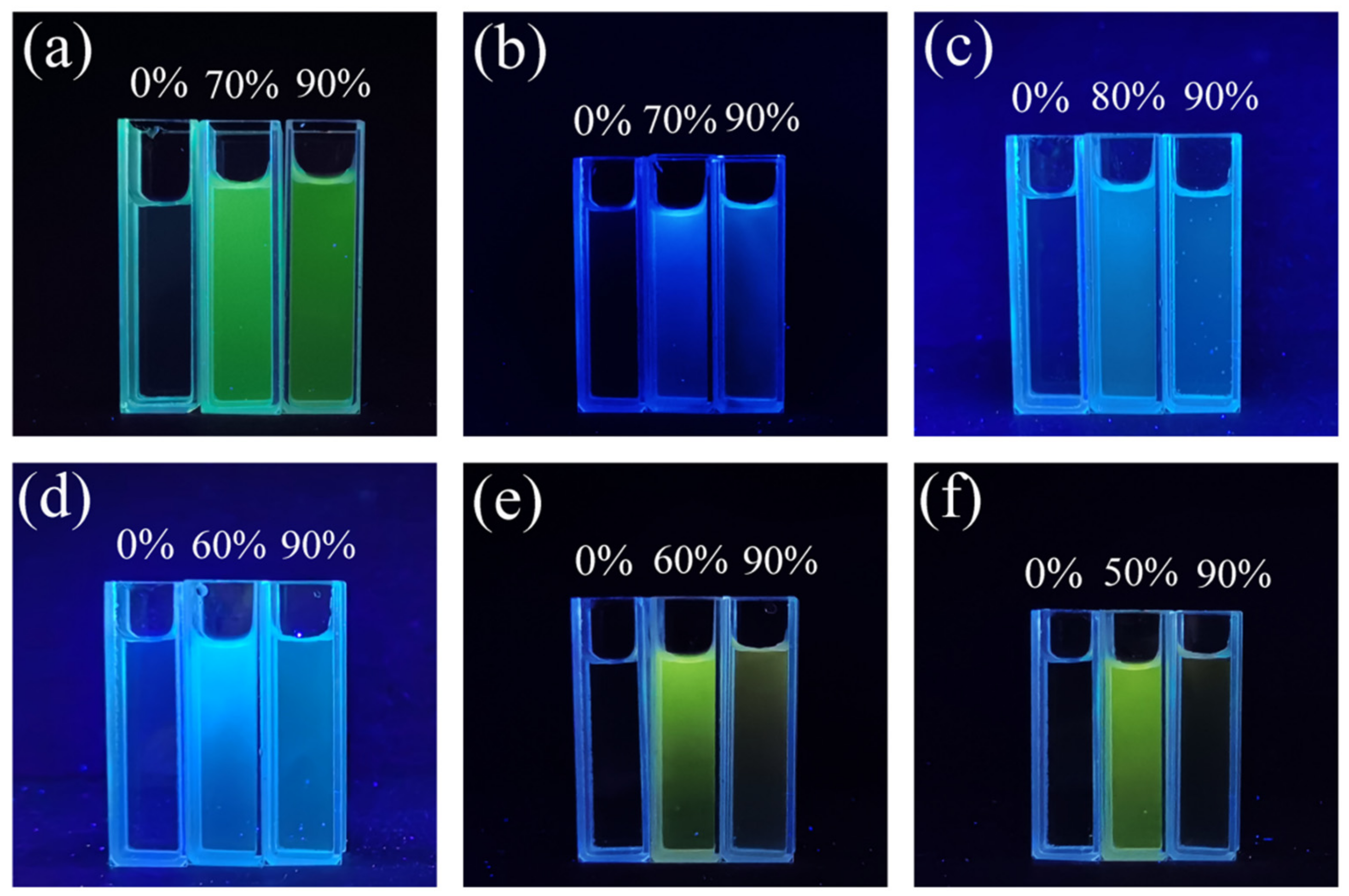
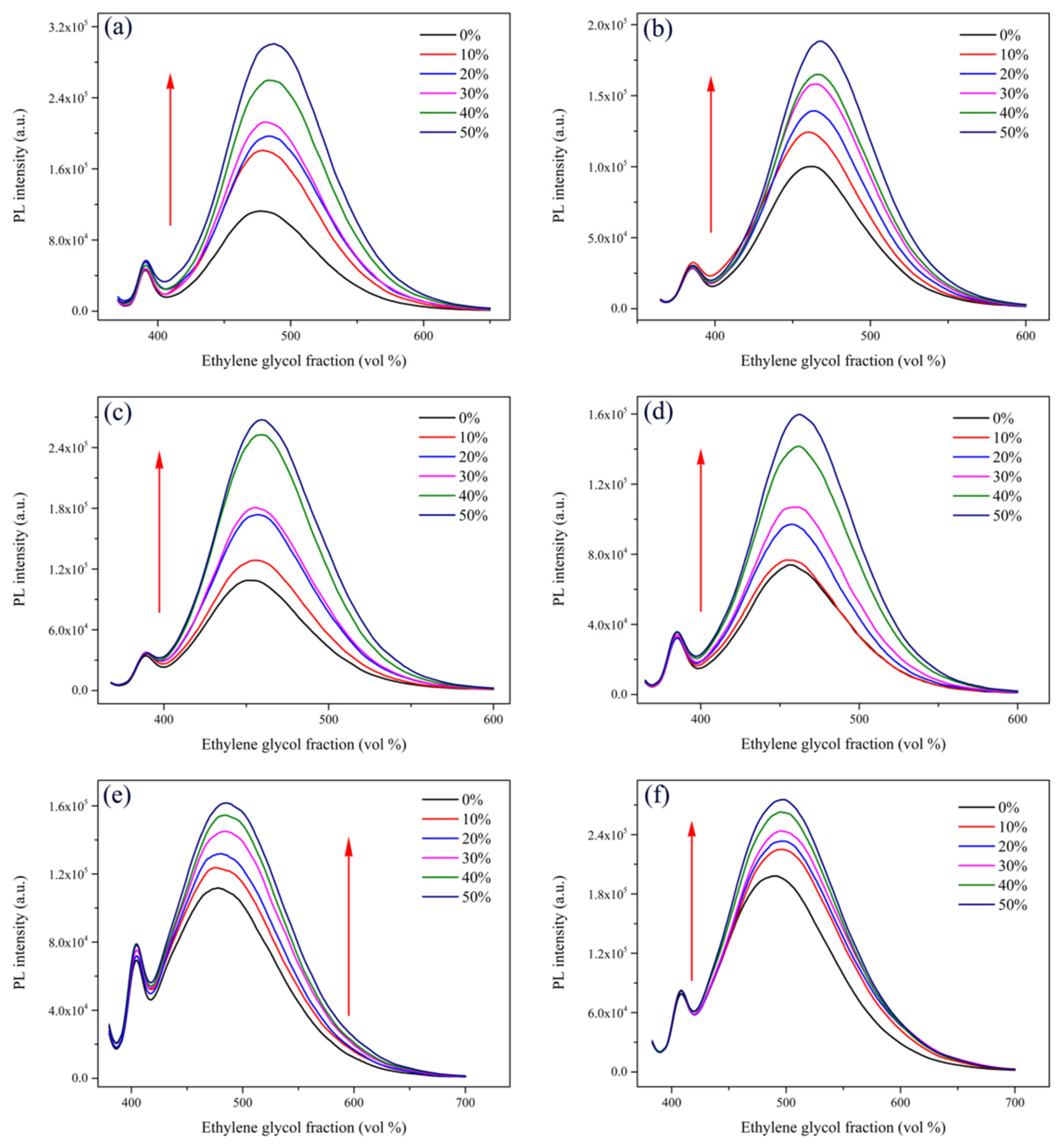
2.1. Optical Properties and Aggregation-Induced Emission Enhancement Properties
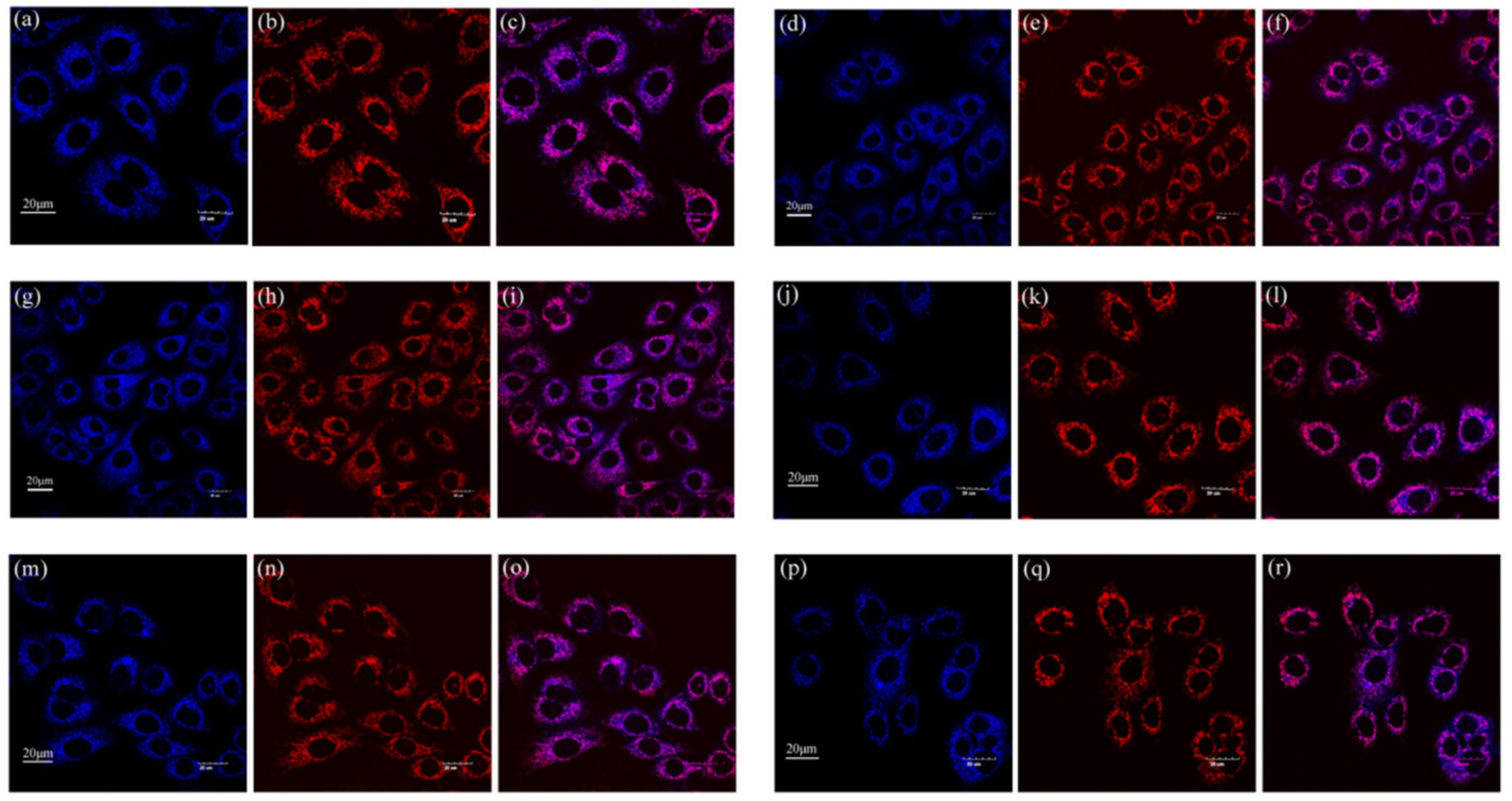
2.2. Cell Imaging
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis of Chalcones Derivatives
3.2.1. Synthesis of Compound 1
3.2.2. Synthesis of Compound 2
3.2.3. Synthesis of Compound 3
3.2.4. Synthesis of Compound 4
3.2.5. Synthesis of Compound 5
3.2.6. Synthesis of Compound 6
3.2.7. Synthesis of Compound 7
3.2.8. Synthesis of Compound 8
3.2.9. Synthesis of Compound 9
3.2.10. Synthesis of Compound 10
3.2.11. Synthesis of Compound 11
3.2.12. Characterization of Compounds 1–11
3.3. Preparatory Work before UV–Vis Spectra, PL Spectra, and SEM Measurements
3.3.1. Preparatory Work before UV–Vis Spectra and PL Spectra Measurements
3.3.2. Preparatory Work before SEM Measurements
3.4. Preparatory Work before Ethylene Glycol (EG) Measurements
3.5. Cell Culture
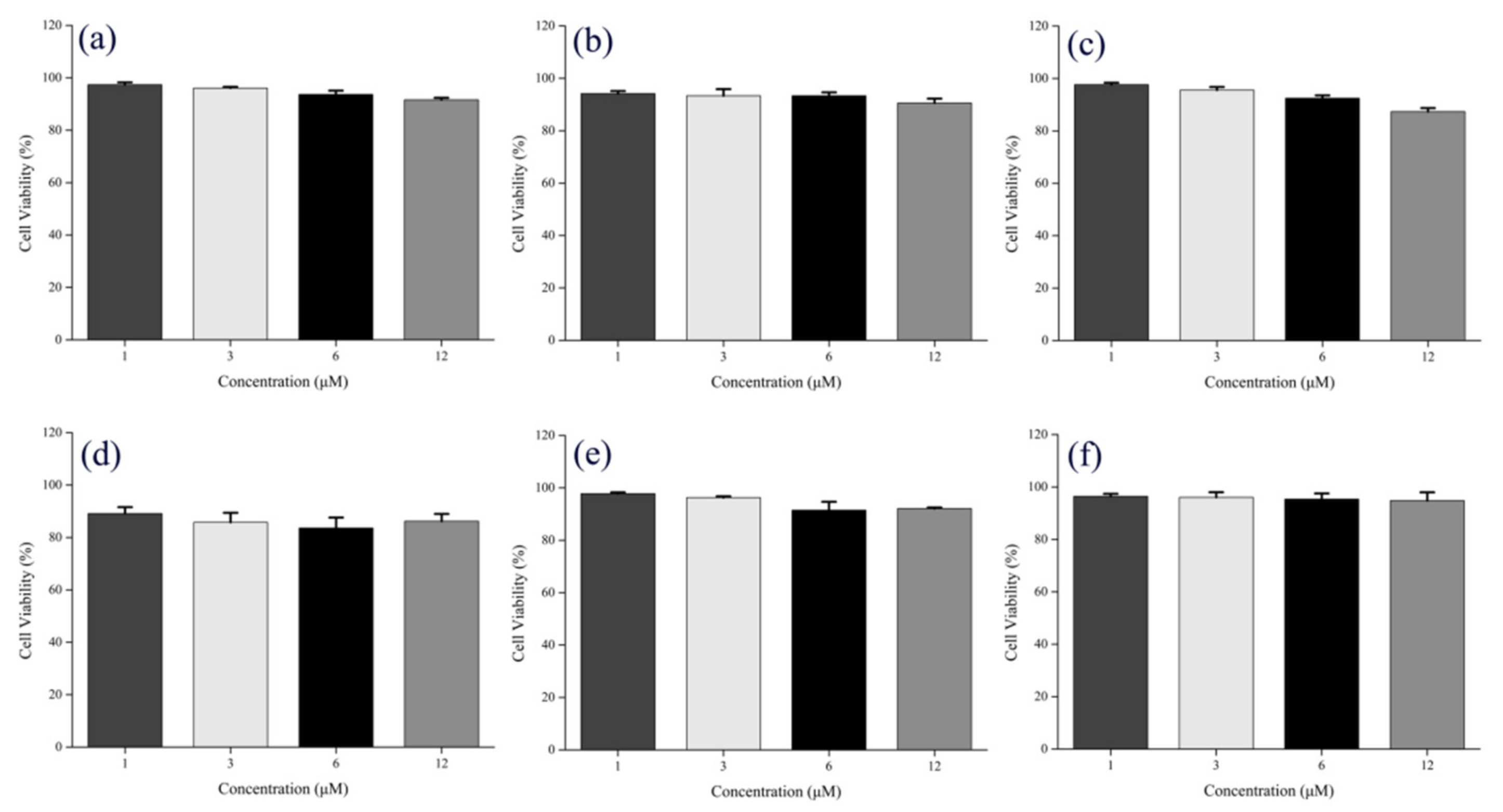
3.6. Evaluation of Cell Viability
3.7. Cell Imaging
3.8. Zebrafish Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bera, M.K.; Pal, P.; Malik, S. Solid-state emissive organic chromophores: Design, strategy and building blocks. J. Mater. Chem. C 2020, 8, 788–802. [Google Scholar] [CrossRef]
- He, X.J.; Chen, H.; Xu, C.C.; Fan, J.Y.; Xu, W.; Li, Y.H.; Deng, H.; Shen, J.L. Ratiometric and colorimetric fluorescent probe for hypochlorite monitor and application for bioimaging in living cells, bacteria and zebrafish. J. Hazard. Mater. 2020, 388, 122029. [Google Scholar] [CrossRef]
- Jing, L.; Sun, M.; Xu, P.; Yao, K.; Yang, J.; Wang, X.; Liu, H.; Sun, M.; Sun, Y.; Ni, R.; et al. Noninvasive In Vivo Imaging and Monitoring of 3D-Printed Polycaprolactone Scaffolds Labeled with an NIR Region II Fluorescent Dye. ACS Applied Bio Materials 2021, 4, 3189–3202. [Google Scholar] [CrossRef]
- Zhao, E.; Chen, S. Materials with aggregation-induced emission characteristics for applications in diagnosis, theragnosis, disease mechanism study and personalized medicine. Mat. Chem. Front. 2021, 5, 3322–3343. [Google Scholar] [CrossRef]
- Feng, G.; Liu, B. Aggregation-Induced Emission (AIE) Dots: Emerging Theranostic Nanolights. Acc. Chem. Res. 2018, 51, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Wurthner, F. Aggregation-Induced Emission (AIE): A Historical Perspective. Angew. Chem.-Int. Edit. 2020, 59, 14192–14196. [Google Scholar] [CrossRef]
- Feng, H.T.; Yuan, Y.X.; Xiong, J.B.; Zheng, Y.S.; Tang, B.Z. Macrocycles and cages based on tetraphenylethylene with aggregation-induced emission effect. Chem. Soc. Rev. 2018, 47, 7452–7476. [Google Scholar] [CrossRef] [PubMed]
- An, B.K.; Gierschner, J.; Park, S.Y. pi-Conjugated Cyanostilbene Derivatives: A Unique Self-Assembly Motif for Molecular Nanostructures with Enhanced Emission and Transport. Acc. Chem. Res. 2012, 45, 544–554. [Google Scholar] [CrossRef]
- Hong, Y.N.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [Green Version]
- Garg, K.; Ganapathi, E.; Rajakannu, P.; Ravikanth, M. Stereochemical modulation of emission behaviour in E/Z isomers of diphenyldipyrroethene from aggregation induced emission to crystallization induced emission. Phys. Chem. Chem. Phys. 2015, 17, 19465–19473. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Hong, Y.; Lam, J.W.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-induced emission: The whole is more brilliant than the parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Drummen, G.P.C.; Konishi, G.-I. Recent advances in twisted intramolecular charge transfer (TICT) fluorescence and related phenomena in materials chemistry. J. Mater. Chem. C 2016, 4, 2731–2743. [Google Scholar] [CrossRef] [Green Version]
- Yakali, G. Examination of aggregation-induced enhanced emission in a propeller-shaped chiral nonconjugated blue emitter from restricted intramolecular rotation and J-type pipi stacking interactions. Phys. Chem. Chem. Phys. 2021, 23, 11388–11399. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Zhang, H.; Xie, Z.; Xie, W.; Xu, H.; Li, B.; Shen, F.; Ye, L.; Hanif, M.; et al. Tight intermolecular packing through supramolecular interactions in crystals of cyano substituted oligo(para-phenylene vinylene): A key factor for aggregation-induced emission. Chem. Commun. 2007, 3, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, C.; Tang, L.; Qin, A.; Hu, R.; Sun, J.Z.; Tang, B.Z. Specific detection of D-glucose by a tetraphenylethene-based fluorescent sensor. J. Am. Chem. Soc. 2011, 133, 660–663. [Google Scholar] [CrossRef]
- Sun, X.; Zebibula, A.; Dong, X.; Li, G.; Zhang, G.; Zhang, D.; Qian, J.; He, S. Targeted and imaging-guided in vivo photodynamic therapy for tumors using dual-function, aggregation-induced emission nanoparticles. Nano Res. 2018, 11, 2756–2770. [Google Scholar] [CrossRef]
- Yan, L.; Xie, Y.; Li, J.; Zhu, W. A red fluorogen: AIEE characteristic, photoluminescence mechanism and its application as chemosensor for ClO−. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2020, 228, 117794. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, N.; Liu, Y.H.; Jiao, S.Y.; Zhang, W.W.; Pu, X.M.; Yu, X.Q. 1,4-Dihydropyridines: Discovery of minimal AIEEgens and their mitochondrial imaging applications. J. Mat. Chem. B 2017, 5, 464–469. [Google Scholar] [CrossRef]
- Lei, Y.; Liu, L.; Tang, X.; Yang, D.; Yang, X.; He, F. Sanguinarine and chelerythrine: Two natural products for mitochondria-imaging with aggregation-induced emission enhancement and pH-sensitive characteristics. RSC Adv. 2018, 8, 3919–3927. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, M.; Harada, Y. Direct observation of the reversible unwinding of a single DNA molecule caused by the intercalation of ethidium bromide. Nucleic Acids Res. 2007, 35, e125. [Google Scholar] [CrossRef]
- Over, B.; McCarren, P.; Artursson, P.; Foley, M.; Giordanetto, F.; Gronberg, G.; Hilgendorf, C.; Lee, M.D.t.; Matsson, P.; Muncipinto, G.; et al. Impact of stereospecific intramolecular hydrogen bonding on cell permeability and physicochemical properties. J. Med. Chem. 2014, 57, 2746–2754. [Google Scholar] [CrossRef]
- Liu, L.; Lei, Y.; Zhang, J.; Li, N.; Zhang, F.; Wang, H.; He, F. Rational Design for Multicolor Flavone-Based Fluorophores with Aggregation-Induced Emission Enhancement Characteristics and Applications in Mitochondria-Imaging. Molecules 2018, 23, 2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Liu, L.; Luo, H.; Wang, H.; Yang, D.; He, F. Flavanone-Based Fluorophores with Aggregation-Induced Emission Enhancement Characteristics for Mitochondria-Imaging and Zebrafish-Imaging. Molecules 2020, 25, 3298. [Google Scholar] [CrossRef]
- Chen, H.; Fang, S.; Wang, L.; Liu, X.; Yan, J.; Zhang, N.; Zheng, K. Tetraphenylene-chalcone hybrid derivatives: Synthesis, structural, fluorescence properties and imaging in living cells. J. Mol. Liq. 2021, 321, 114913. [Google Scholar] [CrossRef]
- Sachdeva, T.; Milton, M.D. AIEE active novel red-emitting D-pi-A phenothiazine chalcones displaying large Stokes shift, solvatochromism and “turn-on” reversible mechanofluorochromism. Dyes Pigment. 2020, 181, 108539. [Google Scholar] [CrossRef]
- Vaz, P.A.A.M.; Rocha, J.; Silva, A.M.S.; Guieu, S. Aggregation-induced emission enhancement in halochalcones. New J. Chem. 2016, 40, 8198–8201. [Google Scholar] [CrossRef]
- Maria, K.; Dimitra, H.L.; Maria, G. Synthesis and Anti-Inflammatory Activity of Chalcones and Related Mannich Bases. Med. Chem. 2008, 4, 586–596. [Google Scholar] [CrossRef]
- Machala, M.; Kubinova, R.; Horavova, P.; Suchy, V. Chemoprotective potentials of homoisoflavonoids and chalcones of Dracaena cinnabari: Modulations of drug-metabolizing enzymes and antioxidant activity. Phytother. Res. 2001, 15, 114–118. [Google Scholar] [CrossRef]
- Coskun, D.; Erkisa, M.; Ulukaya, E.; Coskun, M.F.; Ari, F. Novel 1-(7-ethoxy-1-benzofuran-2-yl) substituted chalcone derivatives: Synthesis, characterization and anticancer activity. Eur. J. Med. Chem. 2017, 136, 212–222. [Google Scholar] [CrossRef]
- Cole, A.L.; Hossain, S.; Cole, A.M.; Phanstiel, O.t. Synthesis and bioevaluation of substituted chalcones, coumaranones and other flavonoids as anti-HIV agents. Bioorg. Med. Chem. 2016, 24, 2768–2776. [Google Scholar] [CrossRef]
- Ayati, A.; Esmaeili, R.; Moghimi, S.; Oghabi Bakhshaiesh, T.; Eslami, S.Z.; Majidzadeh, A.K.; Safavi, M.; Emami, S.; Foroumadi, A. Synthesis and biological evaluation of 4-amino-5-cinnamoylthiazoles as chalcone-like anticancer agents. Eur. J. Med. Chem. 2018, 145, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, Z. A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem. 2007, 42, 125–137. [Google Scholar] [CrossRef]
- Rozmer, Z.; Perjési, P. Naturally occurring chalcones and their biological activities. Phytochem. Rev. 2014, 15, 87–120. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Chi, Z.G.; Yu, T.; Zhang, X.Q.; Chen, M.N.; Xu, B.J.; Liu, S.W.; Zhang, Y.; Xu, J.R. Triphenylethylene carbazole derivatives as a new class of AIE materials with strong blue light emission and high glass transition temperature. J. Mater. Chem. 2009, 19, 5541–5546. [Google Scholar] [CrossRef]
- Li, S.; Fei, X.; Jiao, X.; Lin, D.; Zhang, B.; Cao, L. Synthesis and characterization of the fluorescent probes for the labeling of Microthrix parvicella. Appl. Microbiol. Biotechnol. 2016, 100, 2883–2894. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Gao, Z.; Zhang, R.; Li, A.; Zhang, F.; Li, J.; Xie, J.; Wu, Y.; Wu, M.; Guo, K. A–π–D–π–A carbazole derivatives with remarkable solvatochromism and mechanoresponsive luminescence turn-on. J. Mater. Chem. C 2017, 5, 6136–6143. [Google Scholar] [CrossRef]
- Dong, J.; Solntsev, K.M.; Tolbert, L.M. Activation and Tuning of Green Fluorescent Protein Chromophore Emission by Alkyl Substituent-Mediated Crystal Packing. J. Am. Chem. Soc. 2009, 131, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Wang, H.P.; Chen, Z.Y.; Duan, W.Z. Solid-state fluorescence emission and second-order nonlinear optical properties of coumarin-based fluorophores. J. Fluoresc. 2013, 23, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yu, Z.; Yang, M.; Jin, F.; Zhang, Q.; Zhou, H.; Wu, J.; Tian, Y. Substituent group variations directing the molecular packing, electronic structure, and aggregation-induced emission property of isophorone derivatives. J. Org. Chem. 2013, 78, 3222–3234. [Google Scholar] [CrossRef]
- Li, Y.; Chen, T.; Miao, X.; Yi, X.; Wang, X.; Zhao, H.; Lee, S.M.; Zheng, Y. Zebrafish: A promising in vivo model for assessing the delivery of natural products, fluorescence dyes and drugs across the blood-brain barrier. Pharmacol. Res. 2017, 125, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Ba, M.; Zhou, H.; Cao, Y.; Tang, C.; Yang, Y.; He, R.; Liang, Y.; Zhang, X.; Li, Z.; et al. 2,4,5-Trisubstituted thiazole derivatives: A novel and potent class of non-nucleoside inhibitors of wild type and mutant HIV-1 reverse transcriptase. Eur. J. Med. Chem. 2014, 85, 27–42. [Google Scholar] [CrossRef] [PubMed]




| Compound | MW | Logp | nON |
|---|---|---|---|
| 1 | 239.11 | 3.46 | 2 |
| 2 | 239.11 | 3.46 | 2 |
| 3 | 269.12 | 3.33 | 3 |
| 4 | 253.12 | 3.95 | 2 |
| 5 | 269.12 | 3.33 | 3 |
| 6 | 253.12 | 3.95 | 2 |
| 7 | 209.10 | 3.59 | 1 |
| 8 | 252.14 | 3.87 | 2 |
| 9 | 223.11 | 4.07 | 1 |
| 10 | 223.11 | 4.07 | 1 |
| 11 | 239.11 | 3.46 | 2 |
| Compound | Solvents | Quantum Yields (ϕF) |
|---|---|---|
| 1 | CH3OH | 0.03 |
| CH3OH/H2O (3:7) | 0.59 | |
| CH3OH/H2O (1:9) | 0.34 | |
| 2 | CH3OH | 0.01 |
| CH3OH/H2O (3:7) | 0.19 | |
| CH3OH/H2O (1:9) | 0.12 | |
| 3 | CH3OH | 0.07 |
| CH3OH/H2O (2:8) | 0.21 | |
| CH3OH/H2O (1:9) | 0.15 | |
| 4 | CH3OH | 0.02 |
| CH3OH/H2O (4:6) | 0.18 | |
| CH3OH/H2O (1:9) | 0.10 | |
| 5 | CH3OH | 0.04 |
| CH3OH/H2O (4:6) | 0.08 | |
| CH3OH/H2O (1:9) | 0.05 | |
| 6 | CH3OH | 0.04 |
| CH3OH/H2O (5:5) | 0.14 | |
| CH3OH/H2O (1:9) | 0.09 |
| Compound | Corresponding R Values (Pearson Correlation Coefficient) |
|---|---|
| 1 | 0.87 |
| 2 | 0.83 |
| 3 | 0.87 |
| 4 | 0.88 |
| 5 | 0.86 |
| 6 | 0.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Li, N.; Liu, L.; Wang, H.; He, F. Synthesis of New AIEE-Active Chalcones for Imaging of Mitochondria in Living Cells and Zebrafish In Vivo. Int. J. Mol. Sci. 2021, 22, 8949. https://doi.org/10.3390/ijms22168949
Luo H, Li N, Liu L, Wang H, He F. Synthesis of New AIEE-Active Chalcones for Imaging of Mitochondria in Living Cells and Zebrafish In Vivo. International Journal of Molecular Sciences. 2021; 22(16):8949. https://doi.org/10.3390/ijms22168949
Chicago/Turabian StyleLuo, Huiqing, Na Li, Liyan Liu, Huaqiao Wang, and Feng He. 2021. "Synthesis of New AIEE-Active Chalcones for Imaging of Mitochondria in Living Cells and Zebrafish In Vivo" International Journal of Molecular Sciences 22, no. 16: 8949. https://doi.org/10.3390/ijms22168949
APA StyleLuo, H., Li, N., Liu, L., Wang, H., & He, F. (2021). Synthesis of New AIEE-Active Chalcones for Imaging of Mitochondria in Living Cells and Zebrafish In Vivo. International Journal of Molecular Sciences, 22(16), 8949. https://doi.org/10.3390/ijms22168949






