Neuroprotective and Symptomatic Effects of Cannabidiol in an Animal Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Results
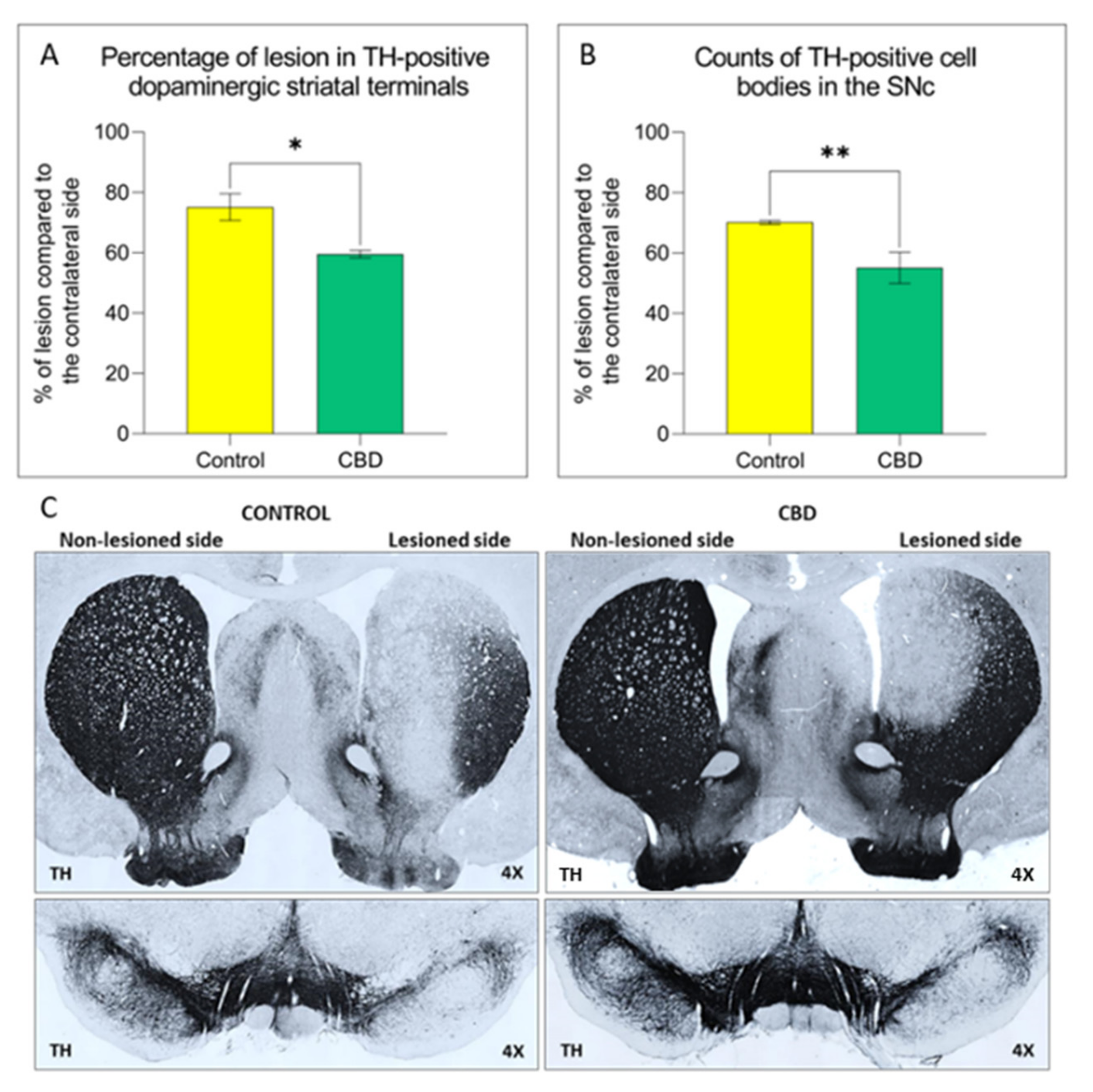
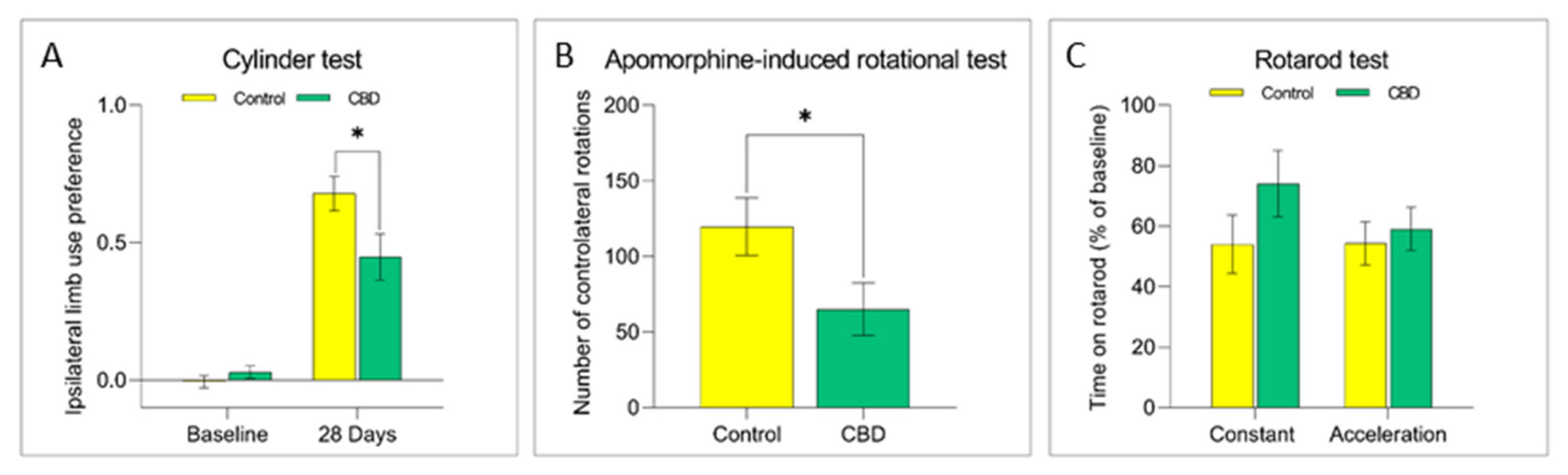
2.1. Cannabidiol Treatment Attenuates Nigrostriatal Degeneration and Improves Motor Performance
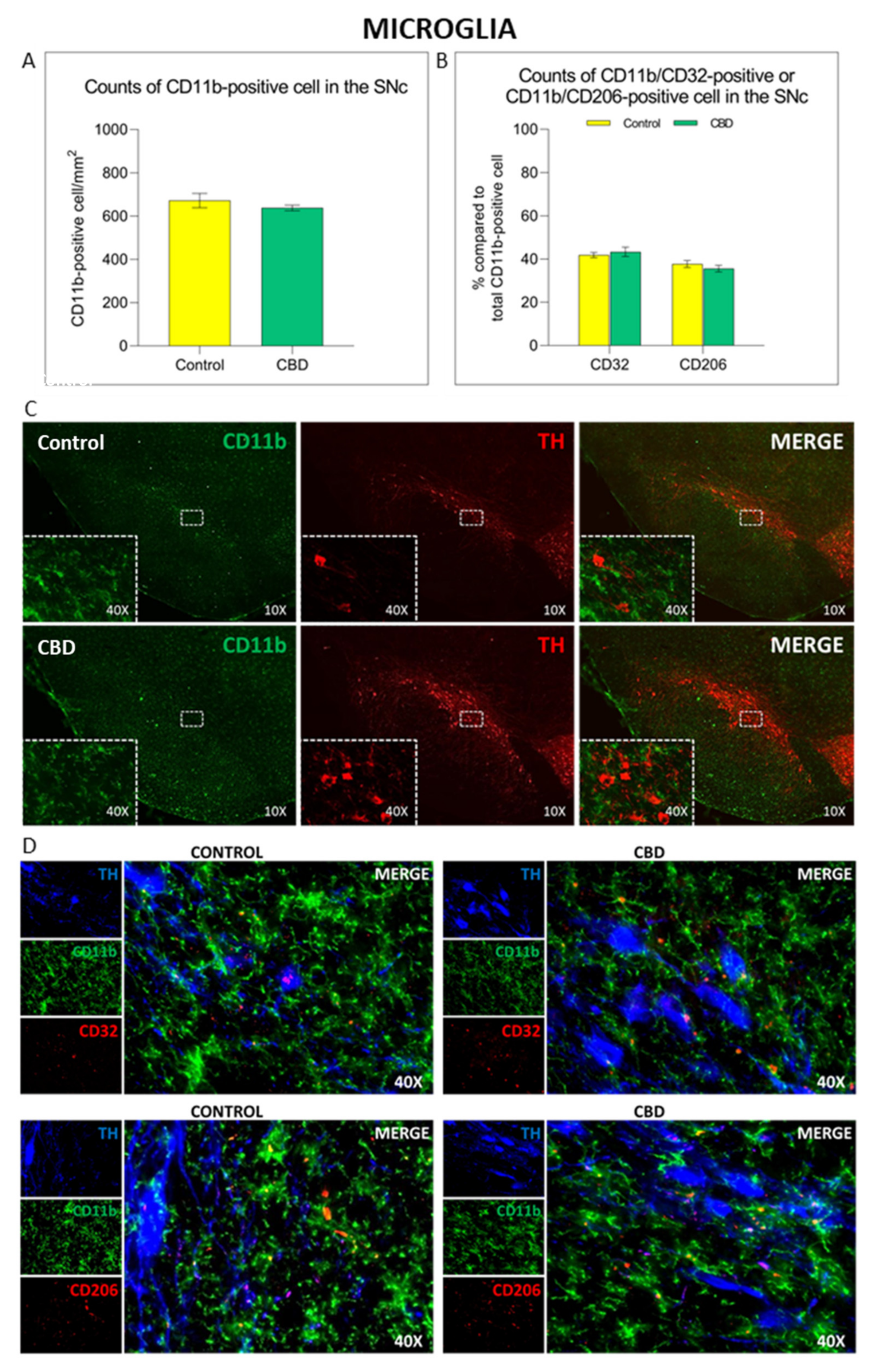
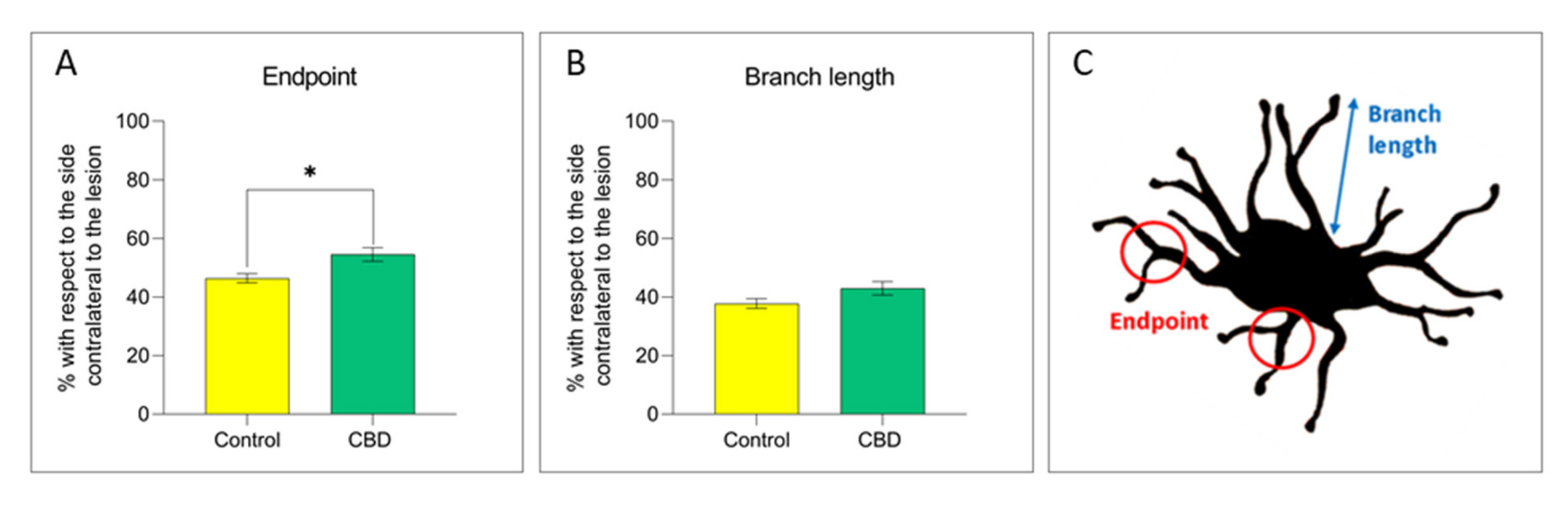
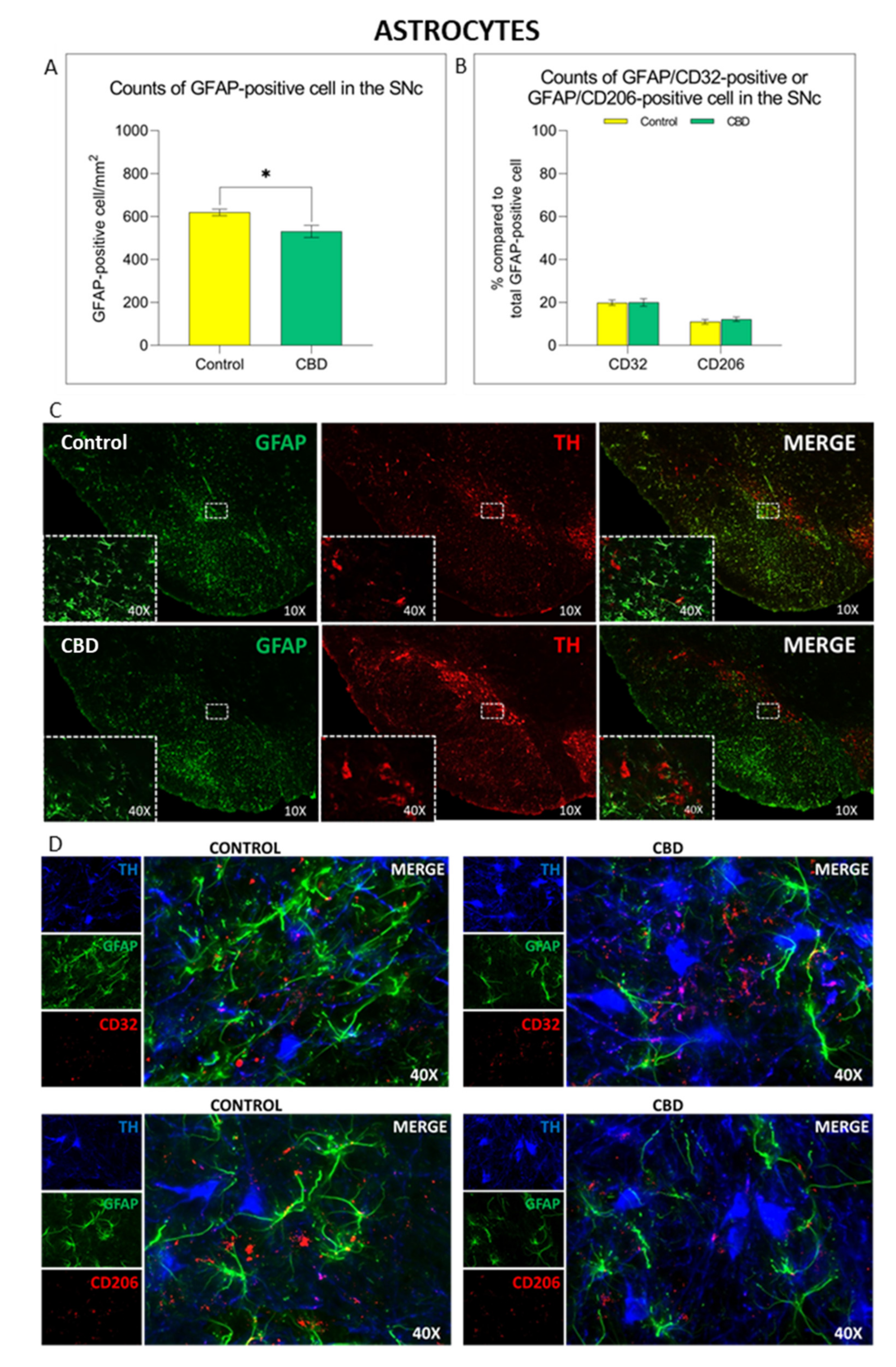
2.2. Cannabidiol Modulates the Neuroinflammatory Process in the SNc through a Preferential Action on Astrocytes
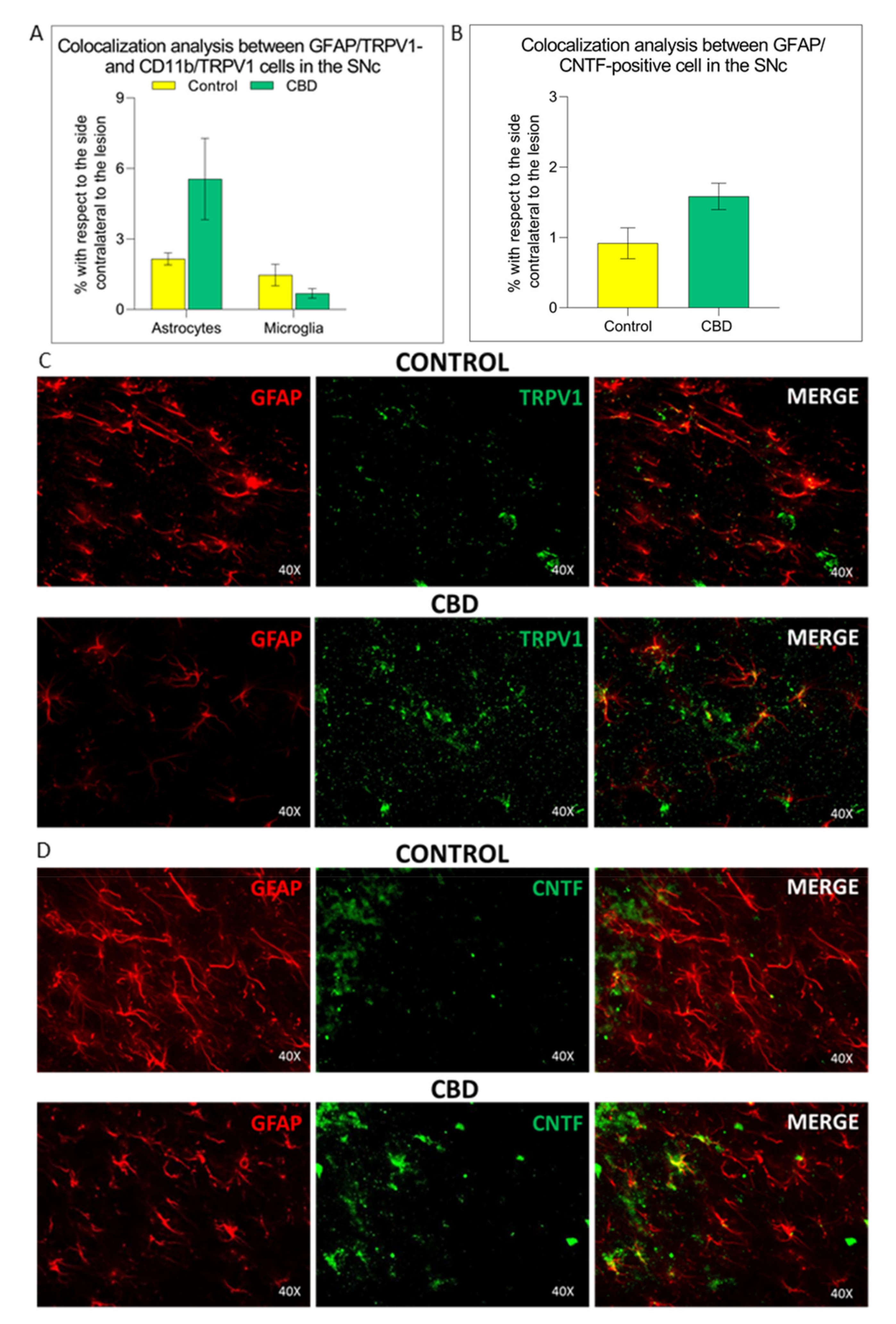
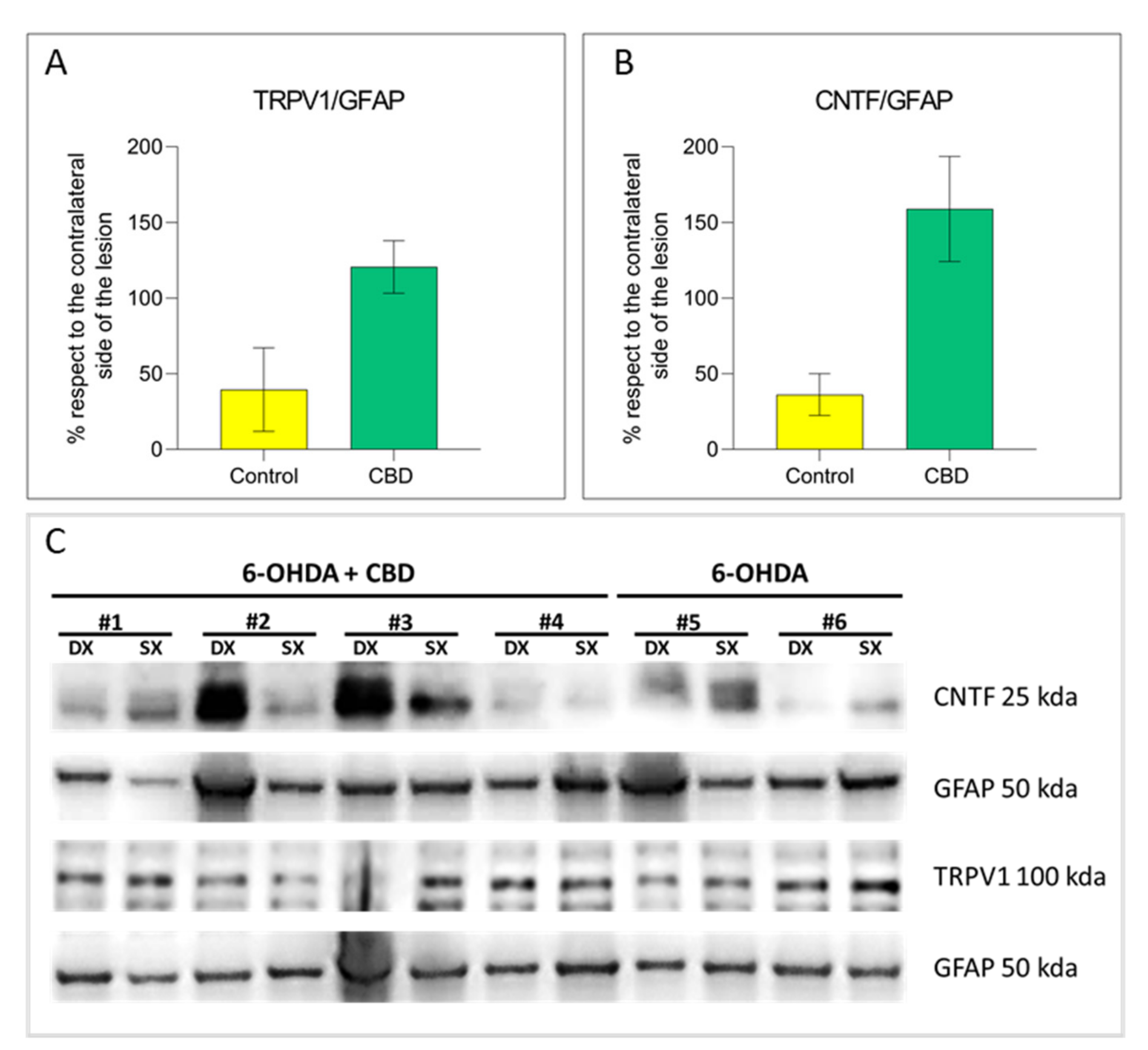
2.3. Cannabidiol Induces the Activation of TRPV1-CNTF Cascade in the Astrocytes
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drug and Study Design
4.3. Behavioral Evaluation
4.4. Immunohistochemical Staining
4.5. Image Analysis
4.6. Western Blot
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The emerging evidence of the Parkinson pandemic. J. Parkinsons Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, E.C.; Standaert, D.G. Ten unsolved questions about neuroinflammation in Parkinson’s disease. Mov. Disord. 2021, 36, 16–24. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Subramaniam, S.R.; Federoff, H.J. Targeting microglial activation states as a therapeutic avenue in Parkinson’s disease. Front. Aging Neurosci. 2017, 9, 176. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, Y.; Wang, Y.; Li, D.; Liu, J.; Zhang, F. Age-associated dopaminergic neuron loss and midbrain glia cell phenotypic polarization. Neuroscience 2019, 415, 89–96. [Google Scholar] [CrossRef]
- Han, Q.W.; Yuan, Y.H.; Chen, N.H. The therapeutic role of cannabinoid receptors and its agonists or antagonists in Parkinson’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 96, 109745. [Google Scholar] [CrossRef] [PubMed]
- Lastres-Becker, I.; Molina-Holgado, F.; Ramos, J.A.; Mechoulam, R.; Fernandez-Ruiz, J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: Relevance to Parkinson’s disease. Neurobiol. Dis. 2005, 19, 96–107. [Google Scholar] [CrossRef]
- Da Silva, V.K.; de Freitas, B.S.; Garcia, R.C.L.; Monteiro, R.T.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.S.; Schroder, N. Antiapoptotic effects of cannabidiol in an experimental model of cognitive decline induced by brain iron overload. Transl. Psychiatry 2018, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, C.; Steardo, L.; Esposito, G. Cannabidiol promotes amyloid precursor protein ubiquitination and reduction of beta amyloid expression in SHSY5YAPP+ cells through PPARgamma involvement. Phytother. Res. 2014, 28, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Libro, R.; Diomede, F.; Scionti, D.; Piattelli, A.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol modulates the expression of Alzheimer’s disease-related genes in mesenchymal stem cells. Int. J. Mol. Sci. 2016, 18, 26. [Google Scholar] [CrossRef] [Green Version]
- Kozela, E.; Juknat, A.; Kaushansky, N.; Ben-Nun, A.; Coppola, G.; Vogel, Z. Cannabidiol, a non-psychoactive cannabinoid, leads to EGR2-dependent anergy in activated encephalitogenic T cells. J. Neuroinflammation 2015, 12, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, D.M.; Singh, N.; Nagarkatti, M.; Nagarkatti, P.S. Cannabidiol attenuates experimental autoimmune encephalomyelitis model of multiple sclerosis through induction of myeloid-derived suppressor cells. Front. Immunol. 2018, 9, 1782. [Google Scholar] [CrossRef]
- Raman, C.; McAllister, S.D.; Rizvi, G.; Patel, S.G.; Moore, D.H.; Abood, M.E. Amyotrophic lateral sclerosis: Delayed disease progression in mice by treatment with a cannabinoid. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2004, 5, 33–39. [Google Scholar] [CrossRef]
- Mechoulam, R.; Panikashvili, D.; Shohami, E. Cannabinoids and brain injury: Therapeutic implications. Trends Mol. Med. 2002, 8, 58–61. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaca, M.V.; Sonego, A.B.; Guimaraes, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res. 2016, 112, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Vallee, A.; Vallee, J.N.; Lecarpentier, Y. Potential role of cannabidiol in Parkinson’s disease by targeting the WNT/beta-catenin pathway, oxidative stress and inflammation. Aging 2021, 13, 10796–10813. [Google Scholar] [CrossRef]
- Cocetta, V.; Governa, P.; Borgonetti, V.; Tinazzi, M.; Peron, G.; Catanzaro, D.; Berretta, M.; Biagi, M.; Manetti, F.; Dall’Acqua, S.; et al. Cannabidiol isolated from Cannabis sativa L. protects intestinal barrier from in vitro inflammation and oxidative stress. Front. Pharmacol. 2021, 12, 641210. [Google Scholar] [CrossRef]
- Liu, C.; Ma, H.; Slitt, A.L.; Seeram, N.P. Inhibitory effect of cannabidiol on the activation of NLRP3 inflammasome is associated with its modulation of the P2X7 receptor in human monocytes. J. Nat. Prod. 2020, 83, 2025–2029. [Google Scholar] [CrossRef]
- Bisogno, T.; Hanus, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Park, E.S.; Won, S.Y.; Lee, Y.A.; Kim, K.I.; Jeong, J.Y.; Baek, J.Y.; Cho, E.J.; Jin, M.; Chung, Y.C.; et al. TRPV1 on astrocytes rescues nigral dopamine neurons in Parkinson’s disease via CNTF. Brain 2015, 138 Pt 12, 3610–3622. [Google Scholar] [CrossRef] [Green Version]
- Baek, J.Y.; Jeong, J.Y.; Kim, K.I.; Won, S.Y.; Chung, Y.C.; Nam, J.H.; Cho, E.J.; Ahn, T.B.; Bok, E.; Shin, W.H.; et al. Inhibition of microglia-derived oxidative stress by ciliary neurotrophic factor protects dopamine neurons in vivo from MPP(+) neurotoxicity. Int. J. Mol. Sci. 2018, 19, 3543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.I.; Baek, J.Y.; Jeong, J.Y.; Nam, J.H.; Park, E.S.; Bok, E.; Shin, W.H.; Chung, Y.C.; Jin, B.K. Delayed treatment of capsaicin produces partial motor recovery by enhancing dopamine function in MPP(+)-lesioned rats via ciliary neurotrophic factor. Exp. Neurobiol. 2019, 28, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.I.; Baek, J.Y.; Chung, Y.C.; Nam, J.H.; Shin, W.H.; Jin, B.K. p70S6K on astrocytes protects dopamine neurons from 1-methyl-4-phenylpyridinium neurotoxicity. Glia 2021, 69, 2133–2145. [Google Scholar] [CrossRef]
- Blandini, F.; Armentero, M.T. Animal models of Parkinson’s disease. FEBS J. 2012, 279, 1156–1166. [Google Scholar] [CrossRef] [Green Version]
- Giuliano, C.; Siani, F.; Mus, L.; Ghezzi, C.; Cerri, S.; Pacchetti, B.; Bigogno, C.; Blandini, F. Neuroprotective effects of lignan 7-hydroxymatairesinol (HMR/lignan) in a rodent model of Parkinson’s disease. Nutrition 2020, 69, 110494. [Google Scholar] [CrossRef]
- Farooqui, T.; Farooqui, A.A. Lipid-mediated oxidative stress and inflammation in the pathogenesis of Parkinson’s disease. Parkinsons Dis. 2011, 2011, 247467. [Google Scholar] [CrossRef] [Green Version]
- Niranjan, R. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: Focus on astrocytes. Mol. Neurobiol. 2014, 49, 28–38. [Google Scholar] [CrossRef]
- Mecha, M.; Feliu, A.; Inigo, P.M.; Mestre, L.; Carrillo-Salinas, F.J.; Guaza, C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: A role for A2A receptors. Neurobiol. Dis. 2013, 59, 141–150. [Google Scholar] [CrossRef]
- Gomes, F.V.; Llorente, R.; Del Bel, E.A.; Viveros, M.P.; Lopez-Gallardo, M.; Guimaraes, F.S. Decreased glial reactivity could be involved in the antipsychotic-like effect of cannabidiol. Schizophr. Res. 2015, 164, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Watt, G.; Karl, T. In vivo evidence for therapeutic properties of cannabidiol (CBD) for Alzheimer’s disease. Front. Pharmacol. 2017, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Arencibia, M.; Gonzalez, S.; de Lago, E.; Ramos, J.A.; Mechoulam, R.; Fernandez-Ruiz, J. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: Importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 2007, 1134, 162–170. [Google Scholar] [CrossRef]
- Silveira, J.W.; Issy, A.C.; Castania, V.A.; Salmon, C.E.; Nogueira-Barbosa, M.H.; Guimaraes, F.S.; Defino, H.L.; Del Bel, E. Protective effects of cannabidiol on lesion-induced intervertebral disc degeneration. PLoS ONE 2014, 9, e113161. [Google Scholar]
- Mori, M.A.; Meyer, E.; Soares, L.M.; Milani, H.; Guimaraes, F.S.; de Oliveira, R.M.W. Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 75, 94–105. [Google Scholar] [CrossRef]
- Zuardi, A.W. Cannabidiol: From an inactive cannabinoid to a drug with wide spectrum of action. Braz. J. Psychiatry 2008, 30, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Zuardi, A.W.; Crippa, J.A.; Hallak, J.E.; Pinto, J.P.; Chagas, M.H.; Rodrigues, G.G.; Dursun, S.M.; Tumas, V. Cannabidiol for the treatment of psychosis in Parkinson’s disease. J. Psychopharmacol. 2009, 23, 979–983. [Google Scholar] [CrossRef]
- Chagas, M.H.; Zuardi, A.W.; Tumas, V.; Pena-Pereira, M.A.; Sobreira, E.T.; Bergamaschi, M.M.; dos Santos, A.C.; Teixeira, A.L.; Hallak, J.E.; Crippa, J.A. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: An exploratory double-blind trial. J. Psychopharmacol. 2014, 28, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Leehey, M.A.; Liu, Y.; Hart, F.; Epstein, C.; Cook, M.; Sillau, S.; Klawitter, J.; Newman, H.; Sempio, C.; Forman, L.; et al. Safety and tolerability of cannabidiol in Parkinson Disease: An open label, dose-escalation study. Cannabis Cannabinoid Res. 2020, 5, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal route of drug administration: Should it be used in experimental animal studies? Pharm. Res. 2019, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.; Youssef, I.; Utvik, J.K.; Florent-Bechard, S.; Barthelemy, V.; Malaplate-Armand, C.; Kriem, B.; Stenger, C.; Koziel, V.; Olivier, J.L.; et al. Ciliary neurotrophic factor cell-based delivery prevents synaptic impairment and improves memory in mouse models of Alzheimer’s disease. J. Neurosci. 2010, 30, 7516–7527. [Google Scholar] [CrossRef]
- Jaber, M.; Robinson, S.W.; Missale, C.; Caron, M.G. Dopamine receptors and brain function. Neuropharmacology 1996, 35, 1503–1519. [Google Scholar] [CrossRef]
- Iuvone, T.; Esposito, G.; Esposito, R.; Santamaria, R.; Di Rosa, M.; Izzo, A.A. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004, 89, 134–141. [Google Scholar] [CrossRef]
- Esposito, G.; De Filippis, D.; Carnuccio, R.; Izzo, A.A.; Iuvone, T. The marijuana component cannabidiol inhibits beta-amyloid-induced tau protein hyperphosphorylation through Wnt/beta-catenin pathway rescue in PC12 cells. J. Mol. Med. 2006, 84, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Mishima, K.; Irie, K.; Hazekawa, M.; Mishima, S.; Fujioka, M.; Orito, K.; Egashira, N.; Katsurabayashi, S.; Takasaki, K.; et al. Cannabidiol prevents a post-ischemic injury progressively induced by cerebral ischemia via a high-mobility group box1-inhibiting mechanism. Neuropharmacology 2008, 55, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, A.P.; Soares, L.M.; Bonato, J.M.; Milani, H.; Guimaraes, F.S.; Weffort de Oliveira, R.M. Protective effects of cannabidiol against hippocampal cell death and cognitive impairment induced by bilateral common carotid artery occlusion in mice. Neurotox. Res. 2014, 26, 307–316. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo, A.; Tolon, M.R.; Fernandez-Ruiz, J.; Romero, J.; Martinez-Orgado, J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol. Dis. 2010, 37, 434–440. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, S.E.; Sun, Y.; Bennett, A.J.; Randall, M.D.; Kendall, D.A. Time-dependent vascular actions of cannabidiol in the rat aorta. Eur. J. Pharmacol. 2009, 612, 61–68. [Google Scholar] [CrossRef]
- Giacoppo, S.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. Target regulation of PI3K/Akt/mTOR pathway by cannabidiol in treatment of experimental multiple sclerosis. Fitoterapia 2017, 116, 77–84. [Google Scholar] [CrossRef]
- Rajan, T.S.; Giacoppo, S.; Iori, R.; De Nicola, G.R.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E. Anti-inflammatory and antioxidant effects of a combination of cannabidiol and moringin in LPS-stimulated macrophages. Fitoterapia 2016, 112, 104–115. [Google Scholar] [CrossRef]
- Stockli, K.A.; Lottspeich, F.; Sendtner, M.; Masiakowski, P.; Carroll, P.; Gotz, R.; Lindholm, D.; Thoenen, H. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature 1989, 342, 920–923. [Google Scholar] [CrossRef]
- Kang, S.S.; Keasey, M.P.; Cai, J.; Hagg, T. Loss of neuron-astroglial interaction rapidly induces protective CNTF expression after stroke in mice. J. Neurosci. 2012, 32, 9277–9287. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Wang, J.; Wang, L.; Yao, X.; Liu, Y.; Li, Y.; Chen, S.; Yue, T.; Wang, X.; Yu, W.; et al. Capsaicin protects against oxidative insults and alleviates behavioral deficits in rats with 6-OHDA-induced Parkinson’s disease via activation of TRPV1. Neurochem. Res. 2017, 42, 3431–3438. [Google Scholar] [CrossRef]
- Hagg, T.; Varon, S.; Louis, J.C. Ciliary neurotrophic factor (CNTF) promotes low-affinity nerve growth factor receptor and CD4 expression by rat CNS microglia. J. Neuroimmunol. 1993, 48, 177–187. [Google Scholar] [CrossRef]
- Lee, M.Y.; Hofmann, H.D.; Kirsch, M. Expression of ciliary neurotrophic factor receptor-alpha messenger RNA in neonatal and adult rat brain: An in situ hybridization study. Neuroscience 1997, 77, 233–246. [Google Scholar] [CrossRef]
- Staff, P.O. Correction: Activation of CNTF/CNTFRalpha signaling pathway by hRheb(S16H) transduction of dopaminergic neurons in vivo. PLoS ONE 2015, 10, e0126450. [Google Scholar]
- Monville, C.; Torres, E.M.; Dunnett, S.B. Comparison of incremental and accelerating protocols of the rotarod test for the assessment of motor deficits in the 6-OHDA model. J. Neurosci. Methods 2006, 158, 219–223. [Google Scholar] [CrossRef]
- Young, K.; Morrison, H. Quantifying microglia morphology from photomicrographs of immunohistochemistry prepared tissue using ImageJ. J. Vis. Exp. 2018, 136, 57648. [Google Scholar] [CrossRef] [Green Version]
- Stauffer, W.; Sheng, H.; Lim, H.N. EzColocalization: An ImageJ plugin for visualizing and measuring colocalization in cells and organisms. Sci. Rep. 2018, 8, 15764. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliano, C.; Francavilla, M.; Ongari, G.; Petese, A.; Ghezzi, C.; Rossini, N.; Blandini, F.; Cerri, S. Neuroprotective and Symptomatic Effects of Cannabidiol in an Animal Model of Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 8920. https://doi.org/10.3390/ijms22168920
Giuliano C, Francavilla M, Ongari G, Petese A, Ghezzi C, Rossini N, Blandini F, Cerri S. Neuroprotective and Symptomatic Effects of Cannabidiol in an Animal Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2021; 22(16):8920. https://doi.org/10.3390/ijms22168920
Chicago/Turabian StyleGiuliano, Claudio, Miriam Francavilla, Gerardo Ongari, Alessandro Petese, Cristina Ghezzi, Nora Rossini, Fabio Blandini, and Silvia Cerri. 2021. "Neuroprotective and Symptomatic Effects of Cannabidiol in an Animal Model of Parkinson’s Disease" International Journal of Molecular Sciences 22, no. 16: 8920. https://doi.org/10.3390/ijms22168920
APA StyleGiuliano, C., Francavilla, M., Ongari, G., Petese, A., Ghezzi, C., Rossini, N., Blandini, F., & Cerri, S. (2021). Neuroprotective and Symptomatic Effects of Cannabidiol in an Animal Model of Parkinson’s Disease. International Journal of Molecular Sciences, 22(16), 8920. https://doi.org/10.3390/ijms22168920





