Theoretical Analysis for Using Pulsed Heating Power in Magnetic Hyperthermia Therapy of Breast Cancer
Abstract
1. Introduction
2. Results
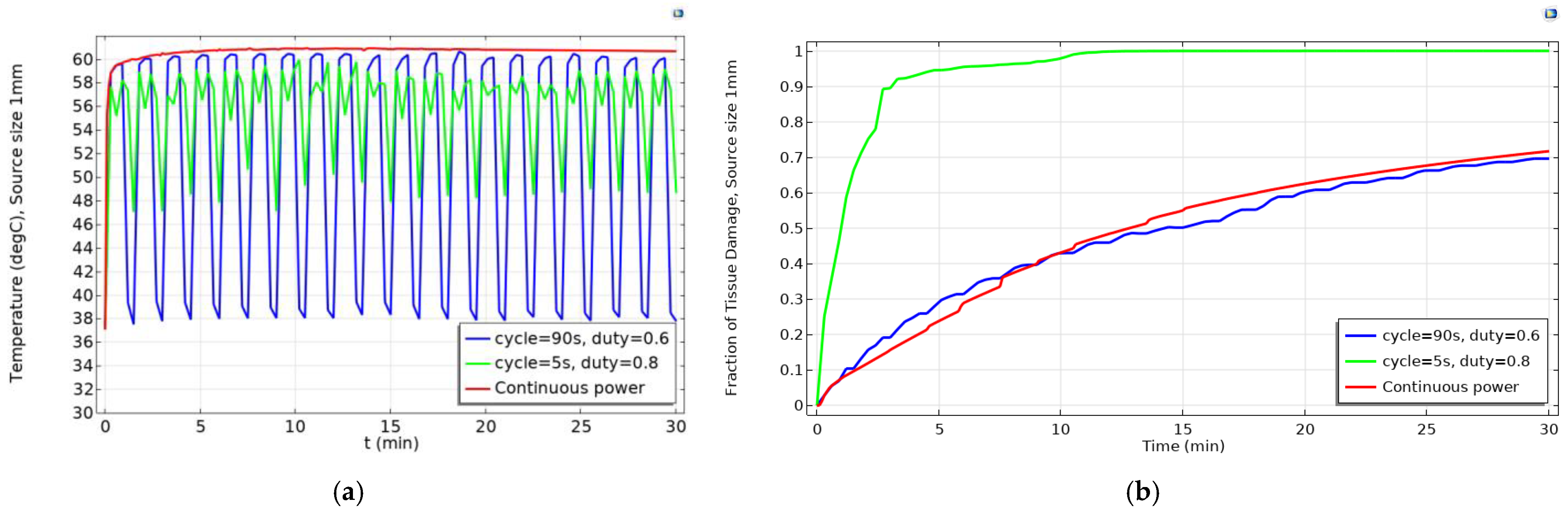
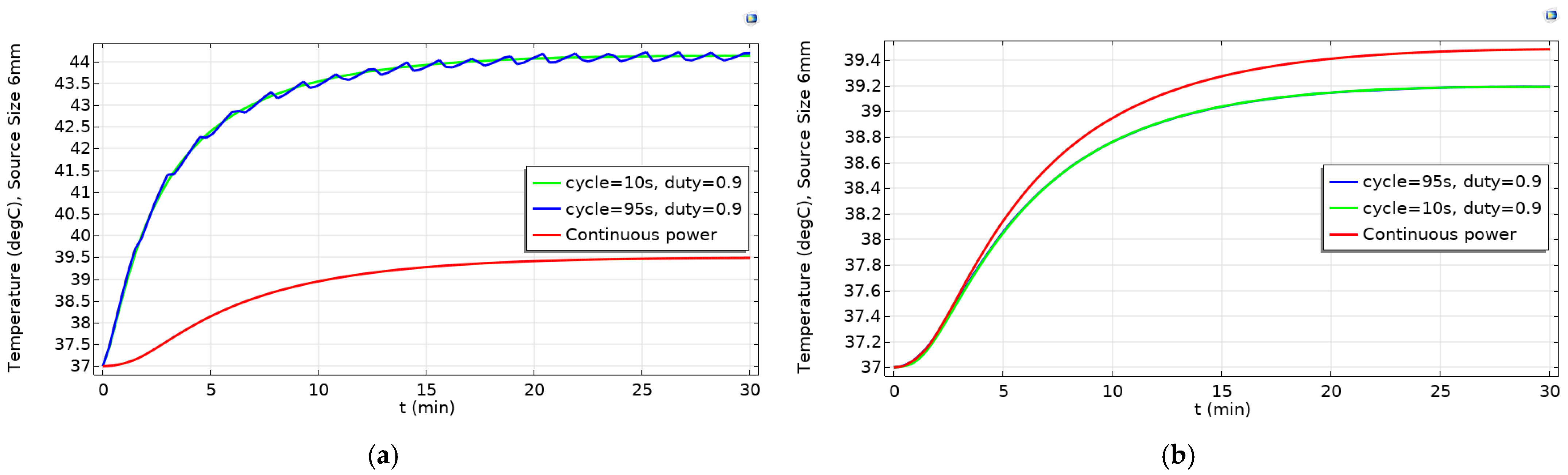
2.1. Determination of the Heating Power Necessary for MNPs in the Presence of Blood Flow and Metabolism to Effectively Damage the Tumor Tissues
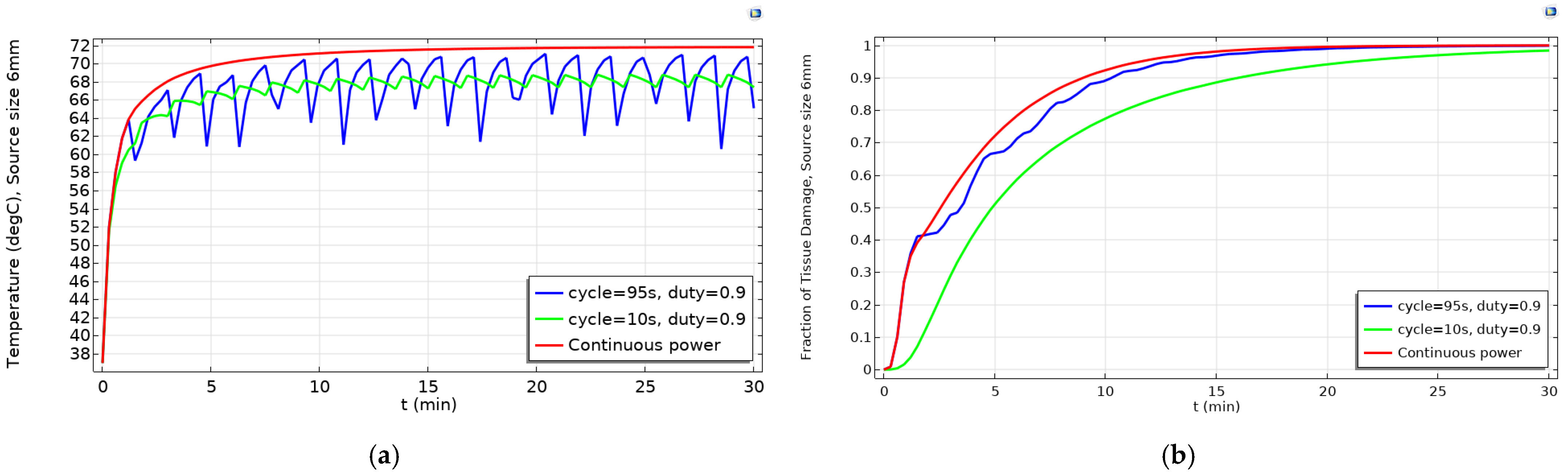
2.2. Determination of Optimized Pulsed Powers for Various Source Diameters to Get High Fraction of Tumor Damage
3. Discussion
4. Material and Methods
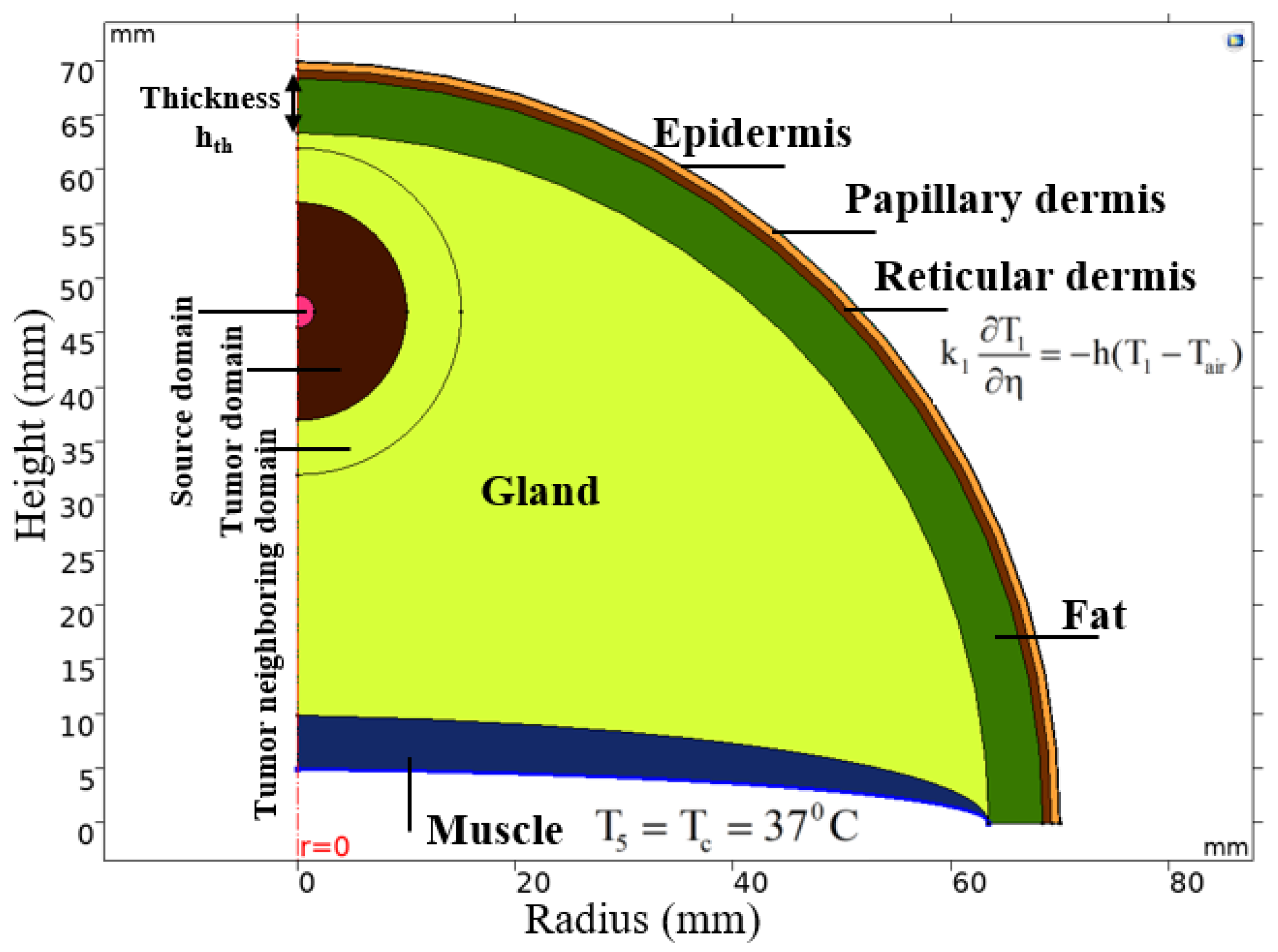
4.1. Breast Cancer Model and Bio-Heat Transfer Model
4.2. Fourier’s Law for Estimating Heating Power Necessary for Magnetic Nanoparticles
4.3. Arrhenius Equation for Fraction of Tissue Damage
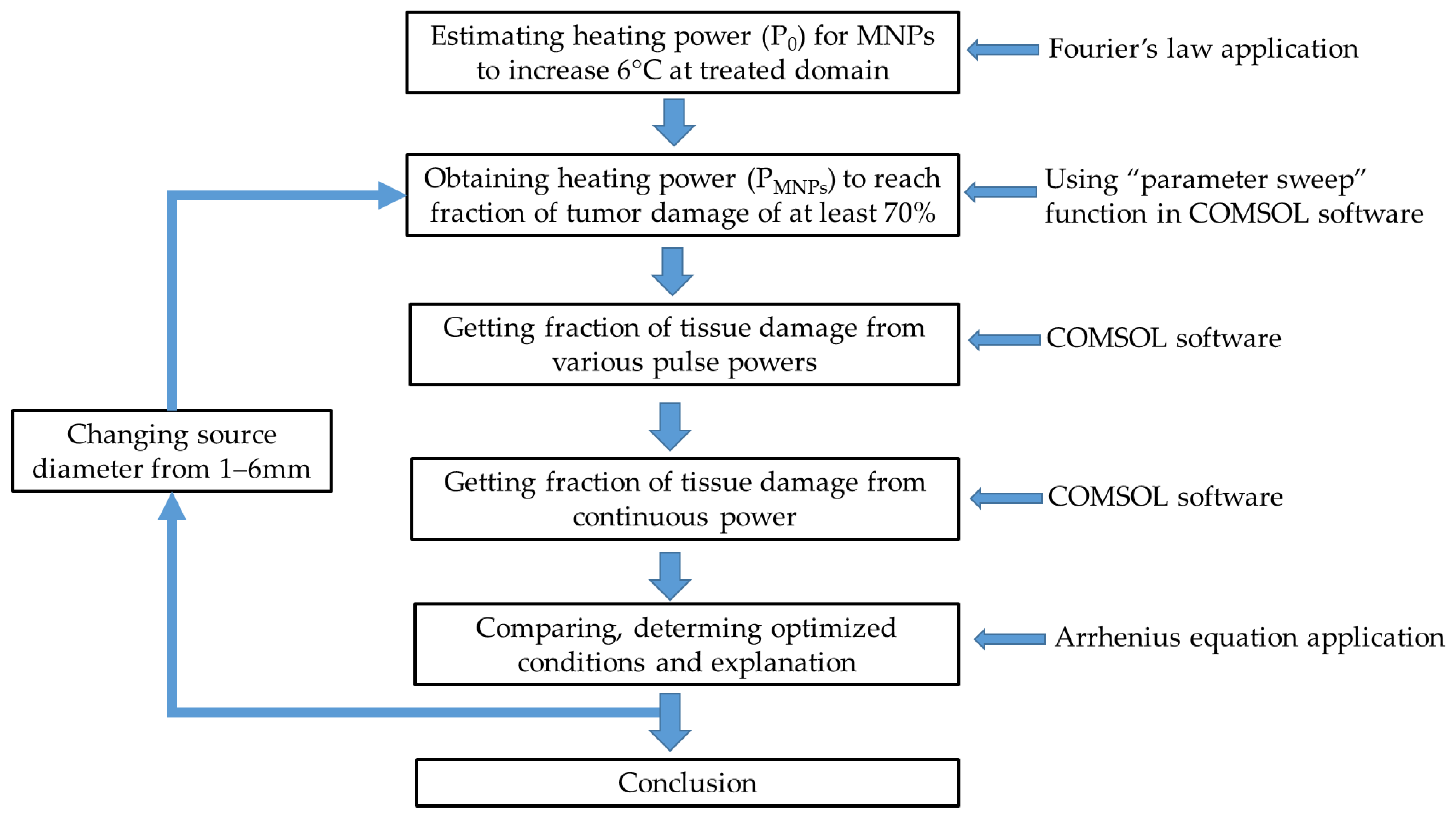
4.4. Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alphandery, E. Perspectives of breast cancer thermotherapies. J. Cancer 2014, 5, 472–479. [Google Scholar] [CrossRef][Green Version]
- General Information about Breast Cancer. Available online: https://www.cancer.org/ (accessed on 6 April 2021).
- Onitilo, A.; Engel, J.; Stankowski, R.; Doi, S. Survival Comparisons for Breast Conserving Surgery and Mastectomy Revisited: Community Experience and the Role of Radiation Therapy. Clin. Med. Res. 2014, 13. [Google Scholar] [CrossRef]
- Moo, T.A.; Sanford, R.; Dang, C.; Morrow, M. Overview of Breast Cancer Therapy. PET Clin. 2018, 13, 339–354. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN); American Cancer Society (ACS). Breast Cancer Treatment Guidelines for Patients. Diunduh Tanggal 2006, 15, 33–47. [Google Scholar]
- Hammer, C.; Fanning, A.; Crowe, J. Overview of breast cancer staging and surgical treatment options. Cleve. Clin. J. Med. 2008, 75 (Suppl. 1), S10–S16. [Google Scholar] [CrossRef]
- Mendecki, J.; Friedenthal, E.; Botstein, C.; Sterzer, F.; Paglione, R.; Nowogrodzki, M.; Beck, E. Microwave-induced hyperthermia in cancer treatment: Apparatus and preliminary results. Int. J. Radiat. Oncol. Biol. Phys. 1978, 4, 1095–1103. [Google Scholar] [CrossRef]
- Sun, M.; Kiourti, A.; Wang, H.; Zhao, S.; Zhao, G.; Lu, X.; Volakis, J.L.; He, X. Enhanced Microwave Hyperthermia of Cancer Cells with Fullerene. Mol. Pharm. 2016, 13, 2184–2192. [Google Scholar] [CrossRef]
- Chung, H.J.; Lee, H.K.; Kwon, K.B.; Kim, H.J.; Hong, S.T. Transferrin as a thermosensitizer in radiofrequency hyperthermia for cancer treatment. Sci. Rep. UK 2018, 8. [Google Scholar] [CrossRef]
- Ware, M.J.; Krzykawska-Serda, M.; Chak-Shing Ho, J.; Newton, J.; Suki, S.; Law, J.; Nguyen, L.; Keshishian, V.; Serda, M.; Taylor, K.; et al. Optimizing non-invasive radiofrequency hyperthermia treatment for improving drug delivery in 4T1 mouse breast cancer model. Sci. Rep. 2017, 7, 43961. [Google Scholar] [CrossRef]
- Guthkelch, A.N.; Carter, L.P.; Cassady, J.R.; Hynynen, K.H.; Iacono, R.P.; Johnson, P.C.; Obbens, E.A.; Roemer, R.B.; Seeger, J.F.; Shimm, D.S.; et al. Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: Results of a phase I trial. J. Neurooncol. 1991, 10, 271–284. [Google Scholar] [CrossRef]
- Zhu, L.; Altman, M.B.; Laszlo, A.; Straube, W.; Zoberi, I.; Hallahan, D.E.; Chen, H. Ultrasound Hyperthermia Technology for Radiosensitization. Ultrasound Med. Biol. 2019, 45, 1025–1043. [Google Scholar] [CrossRef]
- Udagawa, Y.; Nagasawa, H.; Kiyokawa, S. Inhibition by whole-body hyperthermia with far-infrared rays of the growth of spontaneous mammary tumours in mice. Anticancer Res. 1999, 19, 4125–4130. [Google Scholar] [PubMed]
- Ishibashi, J.; Yamashita, K.; Ishikawa, T.; Hosokawa, H.; Sumida, K.; Nagayama, M.; Kitamura, S. The effects inhibiting the proliferation of cancer cells by far-infrared radiation (FIR) are controlled by the basal expression level of heat shock protein (HSP) 70 A. Med. Oncol. 2008, 25, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, C.; Yamamoto, D.; Tsubota, Y.; Sueoka, N.; Kawakami, K.; Yamamoto, M. The synergistic effect of local microwave hyperthermia and chemotherapy for advanced or recurrent breast cancer. Gan Kagaku Ryoho Cancer Chemother. 2014, 41, 1921–1923. [Google Scholar]
- Linthorst, M.; Baaijens, M.; Wiggenraad, R.; Creutzberg, C.; Ghidey, W.; van Rhoon, G.C.; van der Zee, J. Local control rate after the combination of re-irradiation and hyperthermia for irresectable recurrent breast cancer: Results in 248 patients. Radiother. Oncol. 2015, 117, 217–222. [Google Scholar] [CrossRef]
- Takeda, T.; Takeda, T.; Etani, M.; Kobayashi, S.; Takeda, H. The effect of immunotherapy and hyperthermia on patients with advanced or recurrent breast cancer. Gan Kagaku Ryoho Cancer Chemother. 2013, 40, 1596–1599. [Google Scholar]
- Ito, A.; Kuga, Y.; Honda, H.; Kikkawa, H.; Horiuchi, A.; Watanabe, Y.; Kobayashi, T. Magnetite nanoparticle-loaded anti-HER2 immunoliposomes for combination of antibody therapy with hyperthermia. Cancer Lett. 2004, 212, 167–175. [Google Scholar] [CrossRef]
- Kikumori, T.; Kobayashi, T.; Sawaki, M.; Imai, T. Anti-cancer effect of hyperthermia on breast cancer by magnetite nanoparticle-loaded anti-HER2 immunoliposomes. Breast Cancer Res. Treat. 2009, 113, 435–441. [Google Scholar] [CrossRef]
- Bornstein, B.A.; Zouranjian, P.S.; Hansen, J.L.; Fraser, S.M.; Gelwan, L.A.; Teicher, B.A.; Svensson, G.K. Local hyperthermia, radiation therapy, and chemotherapy in patients with local-regional recurrence of breast carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 79–85. [Google Scholar] [CrossRef]
- Chang, D.; Lim, M.; Goos, J.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharmacol. 2018, 9, 831. [Google Scholar] [CrossRef]
- Ahmed, M.; Douek, M. The role of magnetic nanoparticles in the localization and treatment of breast cancer. Biomed. Res. Int. 2013, 2013, 281230. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrott, J.C.; Taylor, C.B. Selective inductive heating of lymph nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Hilger, I.; Hiergeist, R.; Hergt, R.; Winnefeld, K.; Schubert, H.; Kaiser, W.A. Thermal ablation of tumors using magnetic nanoparticles: An in vivo feasibility study. Investig. Radiol. 2002, 37, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Hilger, I.; Andrä, W.; Hergt, R.; Hiergeist, R.; Schubert, H.; Kaiser, W.A. Electromagnetic Heating of Breast Tumors in Interventional Radiology: In Vitro and in Vivo Studies in Human Cadavers and Mice. Radiology 2001, 218, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Motomura, K.; Ishitobi, M.; Komoike, Y.; Koyama, H.; Inaji, H.; Inoue, M.; Nagae, H.; Nagano, I. Novel thermal tumor ablation for breast cancer in mice using magnetic nanoparticles. Cancer Res. 2009, 69, 203s. [Google Scholar]
- Salimi, M.; Sarkar, S.; Hashemi, M.; Saber, R. Treatment of Breast Cancer-Bearing BALB/c Mice with Magnetic Hyperthermia using Dendrimer Functionalized Iron-Oxide Nanoparticles. Nanomaterials 2020, 10, 2310. [Google Scholar] [CrossRef]
- Atkinson, W.J.; Brezovich, I.A.; Chakraborty, D.P. Usable Frequencies in Hyperthermia with Thermal Seeds. IEEE Trans. Biomed. Eng. 1984, BME-31, 70–75. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2919–S2934. [Google Scholar] [CrossRef]
- Ivkov, R.; DeNardo, S.J.; Daum, W.; Foreman, A.R.; Goldstein, R.C.; Nemkov, V.S.; DeNardo, G.L. Application of high amplitude alternating magnetic fields for heat induction of nanoparticles localized in cancer. Clin. Cancer Res. 2005, 11, 7093s–7103s. [Google Scholar] [CrossRef]
- DeNardo, S.J.; DeNardo, G.L.; Natarajan, A.; Miers, L.A.; Foreman, A.R.; Gruettner, C.; Adamson, G.N.; Ivkov, R. Thermal Dosimetry Predictive of Efficacy of 111In-ChL6 Nanoparticle AMF–Induced Thermoablative Therapy for Human Breast Cancer in Mice. J. Nucl. Med. 2007, 48, 437–444. [Google Scholar]
- Tsiapla, A.-R.; Kalimeri, A.-A.; Maniotis, N.; Myrovali, E.; Samaras, T.; Angelakeris, M.; Kalogirou, O. Mitigation of magnetic particle hyperthermia side effects by magnetic field controls. Int. J. Hyperth. 2021, 38, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.A.; Nguyen, U.D. Thermal modeling of lesion growth with radiofrequency ablation devices. Biomed. Eng. Online 2004, 3. [Google Scholar] [CrossRef] [PubMed]
- Le, T.A.; Bui, M.P.; Yoon, J. Optimal Design and Implementation of a Novel Two-Dimensional Electromagnetic Navigation System That Allows Focused Heating of Magnetic Nanoparticles. IEEE/ASME Trans. Mechatron. 2021, 26, 551–562. [Google Scholar] [CrossRef]
- Tay, Z.W.; Chandrasekharan, P.; Chiu-Lam, A.; Hensley, D.W.; Dhavalikar, R.; Zhou, X.Y.; Yu, E.Y.; Goodwill, P.W.; Zheng, B.; Rinaldi, C.; et al. Magnetic Particle Imaging-Guided Heating in Vivo Using Gradient Fields for Arbitrary Localization of Magnetic Hyperthermia Therapy. ACS Nano 2018, 12, 3699–3713. [Google Scholar] [CrossRef]
- Balivada, S.; Rachakatla, R.S.; Wang, H.; Samarakoon, T.N.; Dani, R.K.; Pyle, M.; Kroh, F.O.; Walker, B.; Leaym, X.; Koper, O.B.; et al. A/C magnetic hyperthermia of melanoma mediated by iron(0)/iron oxide core/shell magnetic nanoparticles: A mouse study. BMC Cancer 2010, 10, 119. [Google Scholar] [CrossRef]
- Huang, H.S.; Hainfeld, J.F. Intravenous magnetic nanoparticle cancer hyperthermia. Int. J. Nanomed. 2013, 8, 2521–2532. [Google Scholar] [CrossRef]
- Bui, M.P.; Le, T.A.; Yoon, J. A Magnetic Particle Imaging-based Navigation Platform for Magnetic Nanoparticles using Interactive Manipulation of a Virtual Field Free Point to Ensure Targeted Drug Delivery. IEEE Trans. Ind. Electron. 2020. [Google Scholar] [CrossRef]
- Park, M.; Le, T.A.; Eizad, A.; Yoon, J. A Novel Shared Guidance Scheme for Intelligent Haptic Interaction Based Swarm Control of Magnetic Nanoparticles in Blood Vessels. IEEE Access 2020, 8, 106714–106725. [Google Scholar] [CrossRef]
- Le, T.A.; Bui, M.P.; Yoon, J. Electromagnetic Actuation System for Focused Capturing of Magnetic Particles with a Half of Static Saddle Potential Energy Configuration. IEEE Trans. Biomed. Eng. 2021, 68, 869–880. [Google Scholar] [CrossRef]
- Sefidgar, M.; Bashooki, E.; Shojaee, P. Numerical simulation of the effect of necrosis area in systemic delivery of magnetic nanoparticles in hyperthermia cancer treatment. J. Therm. Biol. 2020, 94, 102742. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-D.; Jin, T.; Flesch, R. Numerical Temperature Analysis of Magnetic Hyperthermia Considering Nanoparticle Clustering and Blood Vessels. IEEE Trans. Magn. 2017, 53, 10. [Google Scholar] [CrossRef]
- Fortin, J.P.; Gazeau, F.; Wilhelm, C. Intracellular heating of living cells through Néel relaxation of magnetic nanoparticles. Eur. Biophys. J. 2008, 37, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.B.; Rauwerdink, A.M.; Hansen, E.W. Magnetic nanoparticle temperature estimation. Med. Phys. 2009, 36, 1822–1829. [Google Scholar] [CrossRef]
- Salamon, J.; Dieckhoff, J.; Kaul, M.G.; Jung, C.; Adam, G.; Möddel, M.; Knopp, T.; Draack, S.; Ludwig, F.; Ittrich, H. Visualization of spatial and temporal temperature distributions with magnetic particle imaging for liver tumor ablation therapy. Sci. Rep. UK 2020, 10, 7480. [Google Scholar] [CrossRef]
- Hadadian, Y.; Uliana, J.H.; Carneiro, A.A.O.; Pavan, T.Z. A Novel Theranostic Platform: Integration of Magnetomotive and Thermal Ultrasound Imaging With Magnetic Hyperthermia. IEEE Trans. Biomed. Eng. 2021, 68, 68–77. [Google Scholar] [CrossRef]
- Chanmugam, A.; Hatwar, R.; Herman, C. Thermal analysis of cancerous breast model. Int. Mech. Eng. Congress Expo 2012, 2012, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Cetingül, M.P.; Herman, C. A heat transfer model of skin tissue for the detection of lesions: Sensitivity analysis. Phys. Med. Biol. 2010, 55, 5933–5951. [Google Scholar] [CrossRef]
- Sudharsan, N.M.; Ng, E.Y. Parametric optimization for tumour identification: Bioheat equation using ANOVA and the Taguchi method. Proc. Inst. Mech. Eng. H 2000, 214, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.Y.K.; Sudharsan, N.M. An improved three-dimensional direct numerical modelling and thermal analysis of a female breast with tumour. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2001, 215, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Amri, A.; Saidane, A.; Pulko, S. Thermal analysis of a three-dimensional breast model with embedded tumour using the transmission line matrix (TLM) method. Comput. Biol. Med. 2011, 41, 76–86. [Google Scholar] [CrossRef]
- Jiang, L.; Zhan, W.; Loew, M.H. Modeling static and dynamic thermography of the human breast under elastic deformation. Phys. Med. Biol. 2011, 56, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Périgo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and advances in magnetic hyperthermia. Appl. Phys. Rev. 2015, 2, 041302. [Google Scholar] [CrossRef]
- Liu, K.-C.; Tu, F.-J. Numerical Solution of Bioheat Transfer Problems with Transient Blood Temperature. Int. J. Comput. Methods 2018, 16, 1843001. [Google Scholar] [CrossRef]
- Deka, K.; Bhanja, D.; Nath, S. Fundamental solution of steady and transient bio heat transfer equations especially for skin burn and hyperthermia treatments. Heat Transf. Asian Res. 2019, 48, 361–378. [Google Scholar] [CrossRef]
- Rabin, Y. Is intracellular hyperthermia superior to extracellular hyperthermia in thermal sense? Int. J. Hyperth. 2009, 18, 194–202. [Google Scholar] [CrossRef]
- Laidler, K.J. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494. [Google Scholar] [CrossRef]
- Paruch, M. Mathematical Modeling of Breast Tumor Destruction Using Fast Heating during Radiofrequency Ablation. Materials 2020, 13, 136. [Google Scholar] [CrossRef]
- Chang, I.A. Considerations for thermal injury analysis for RF ablation devices. Open Biomed. Eng. J. 2010, 4, 3–12. [Google Scholar] [CrossRef]
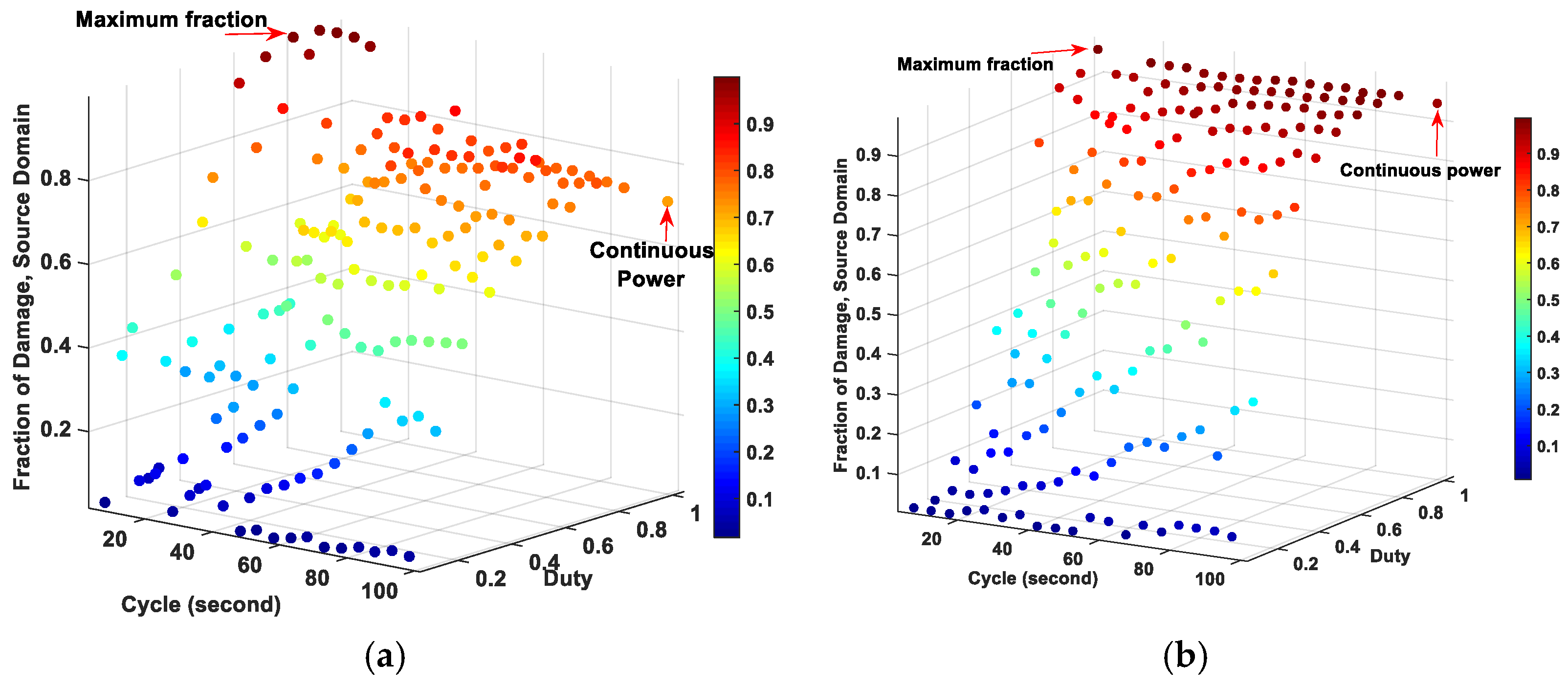
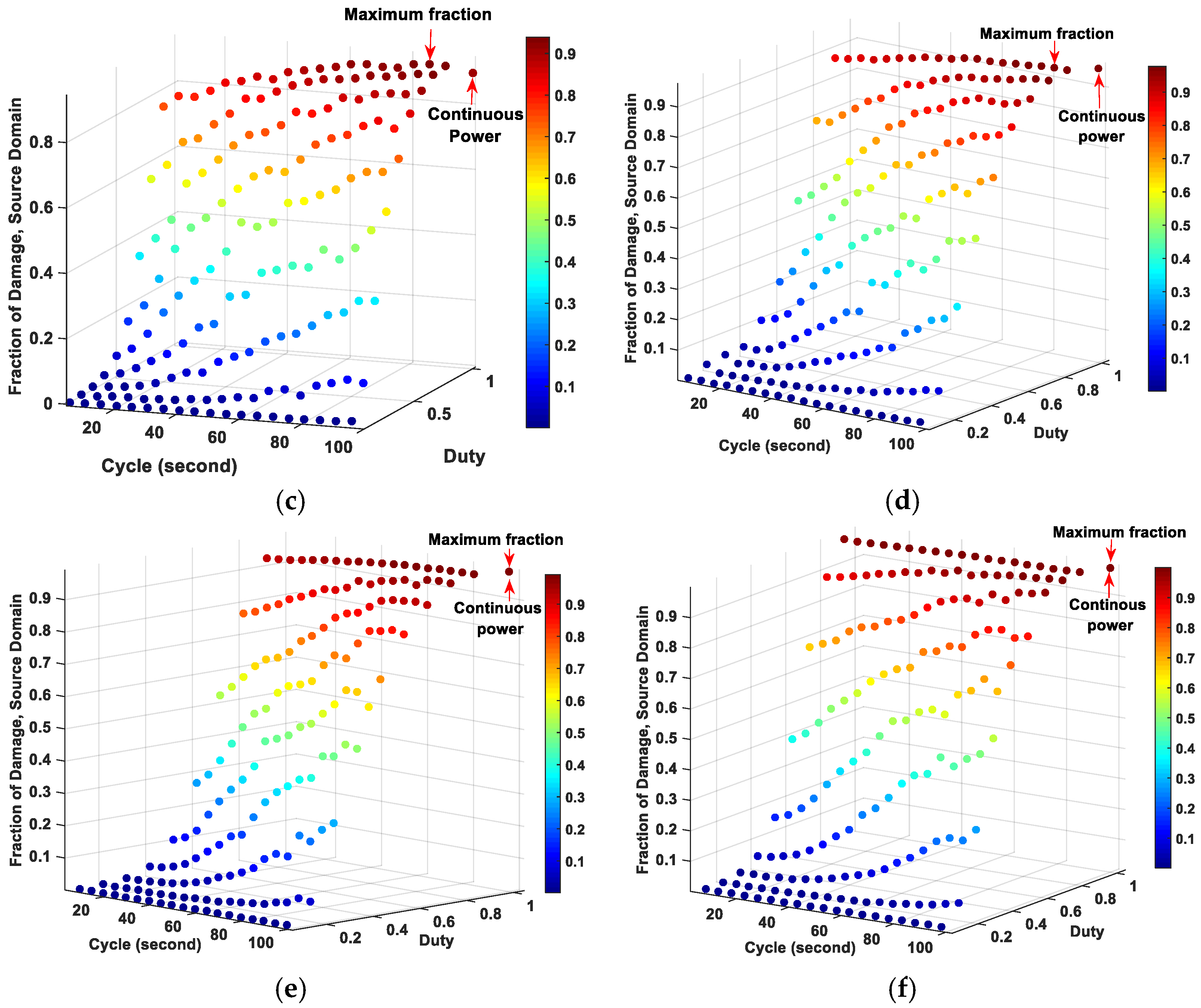
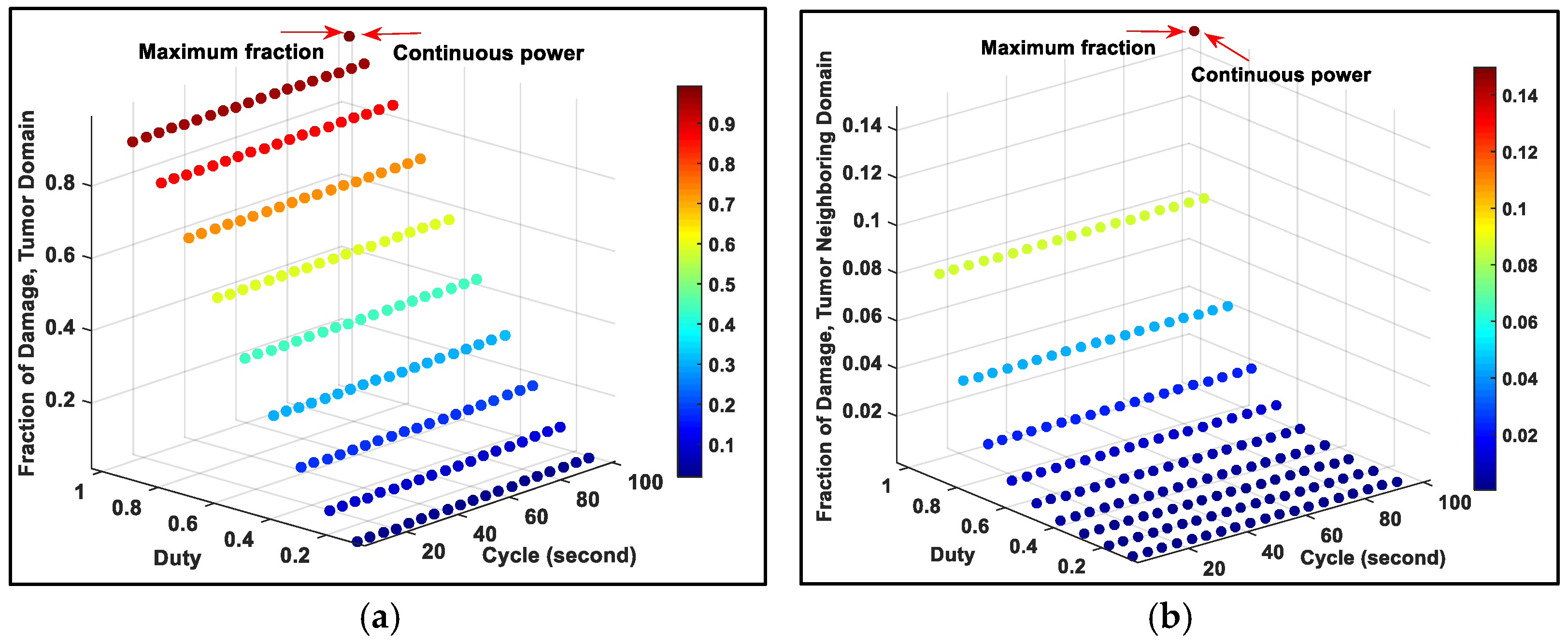
| Diameter of Source Domain (mm) | P0 (W/m3) to Increase 6 °C at Treated Domain | PMNPs (W/m3) to Reach Fraction of Tumor Damage of at Least 70% |
|---|---|---|
| 1 | 2.30 × 107 | 4.8 × 107 |
| 2 | 0.57 × 107 | 3.6 × 107 |
| 3 | 0.25 × 107 | 2.2 × 107 |
| 4 | 0.14 × 107 | 1.9 × 107 |
| 5 | 0.92 × 107 | 1.8 × 107 |
| 6 | 0.64 × 107 | 1.7 × 107 |
| Source Diameter (mm) | Pulsed Powers | Fraction of Damage of Pulsed Power | Fraction of Damage of Continuous Power | |
|---|---|---|---|---|
| Duty | Cycle (second) | |||
| 1 | 0.8 | 5 | 0.99983 | 0.71695 |
| 2 | 0.9 | 10 | 0.99627 | 0.9543 |
| 3 | 0.9 | 90 | 0.93884 | 0.89937 |
| 4 | 0.9 | 90 | 0.97700 | 0.96749 |
| 5 | 0.9 | 95 | 0.99130 | 0.99213 |
| 6 | 0.9 | 95 | 0.99903 | 0.99972 |
| Epidermis | Papillary Dermis | Reticular Dermis | Fat | Gland | Muscle | Tumor | Air | |
|---|---|---|---|---|---|---|---|---|
| Thickness hth (mm) | 0.1 [48] | 0.7 [48] | 0.8 [48] | 5.0 [49] | 43.4 [49] | 15 [49] | 20 | -- |
| k (W/(m.K)) | 0.235 [48] | 0.445 [48] | 0.445 [48] | 0.21 [50] | 0.48 [50] | 0.48 [50] | 0.48 [50] | -- |
| ρ (kg/m3) | 1200 [48] | 1200 [48] | 1200 [48] | 930 [51] | 1050 [51] | 1100 [51] | 1050 [51] | -- |
| c (J/(kg.K)) | 3589 [48] | 3300 [48] | 3300 [48] | 2770 [51] | 3770 [51] | 3800 [48] | 3852 [48] | -- |
| Q0 (W/m3) | 0 [48] | 368.1 [48] | 368.1 [48] | 400 [50] | 700 [50] | 700 [50] | 5000 [52] | -- |
| ωb (m3/((s.m3)) | 0 [48] | 0.0002 [48] | 0.0013 [48] | 0.0002 [47] | 0.0006 [47] | 0.0009 [47] | 0.012 [47] | -- |
| Initial temperature T0 (°C) | 37 | 37 | 37 | 37 | 37 | 37 | 37 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, T.-L.; Le, T.-A.; Hadadian, Y.; Yoon, J. Theoretical Analysis for Using Pulsed Heating Power in Magnetic Hyperthermia Therapy of Breast Cancer. Int. J. Mol. Sci. 2021, 22, 8895. https://doi.org/10.3390/ijms22168895
Cao T-L, Le T-A, Hadadian Y, Yoon J. Theoretical Analysis for Using Pulsed Heating Power in Magnetic Hyperthermia Therapy of Breast Cancer. International Journal of Molecular Sciences. 2021; 22(16):8895. https://doi.org/10.3390/ijms22168895
Chicago/Turabian StyleCao, Thanh-Luu, Tuan-Anh Le, Yaser Hadadian, and Jungwon Yoon. 2021. "Theoretical Analysis for Using Pulsed Heating Power in Magnetic Hyperthermia Therapy of Breast Cancer" International Journal of Molecular Sciences 22, no. 16: 8895. https://doi.org/10.3390/ijms22168895
APA StyleCao, T.-L., Le, T.-A., Hadadian, Y., & Yoon, J. (2021). Theoretical Analysis for Using Pulsed Heating Power in Magnetic Hyperthermia Therapy of Breast Cancer. International Journal of Molecular Sciences, 22(16), 8895. https://doi.org/10.3390/ijms22168895








