Role of Activating Transcription Factor 4 in Murine Choroidal Neovascularization Model
Abstract
:1. Introduction
2. Results
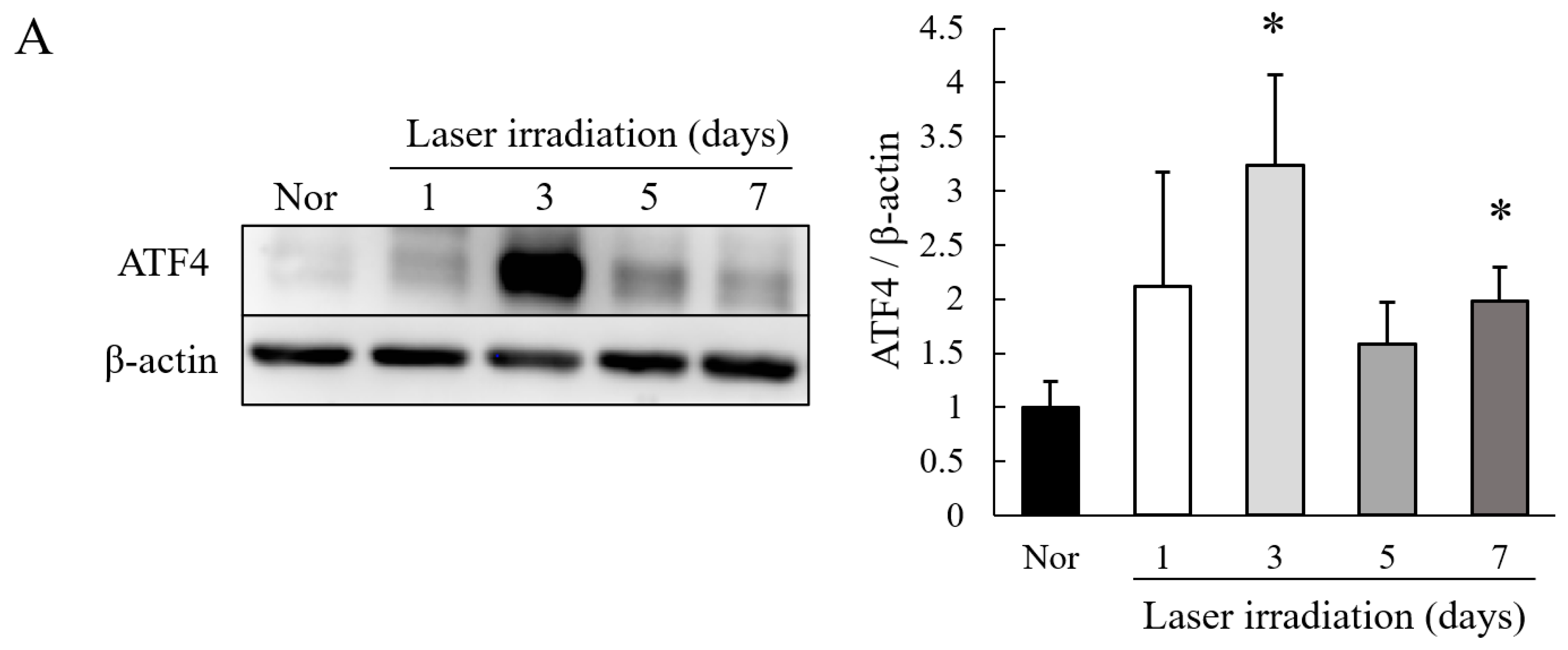
2.1. Level of Expression of ATF4 Is Increased in Choroidal Neovascularization (CNV) Lesions
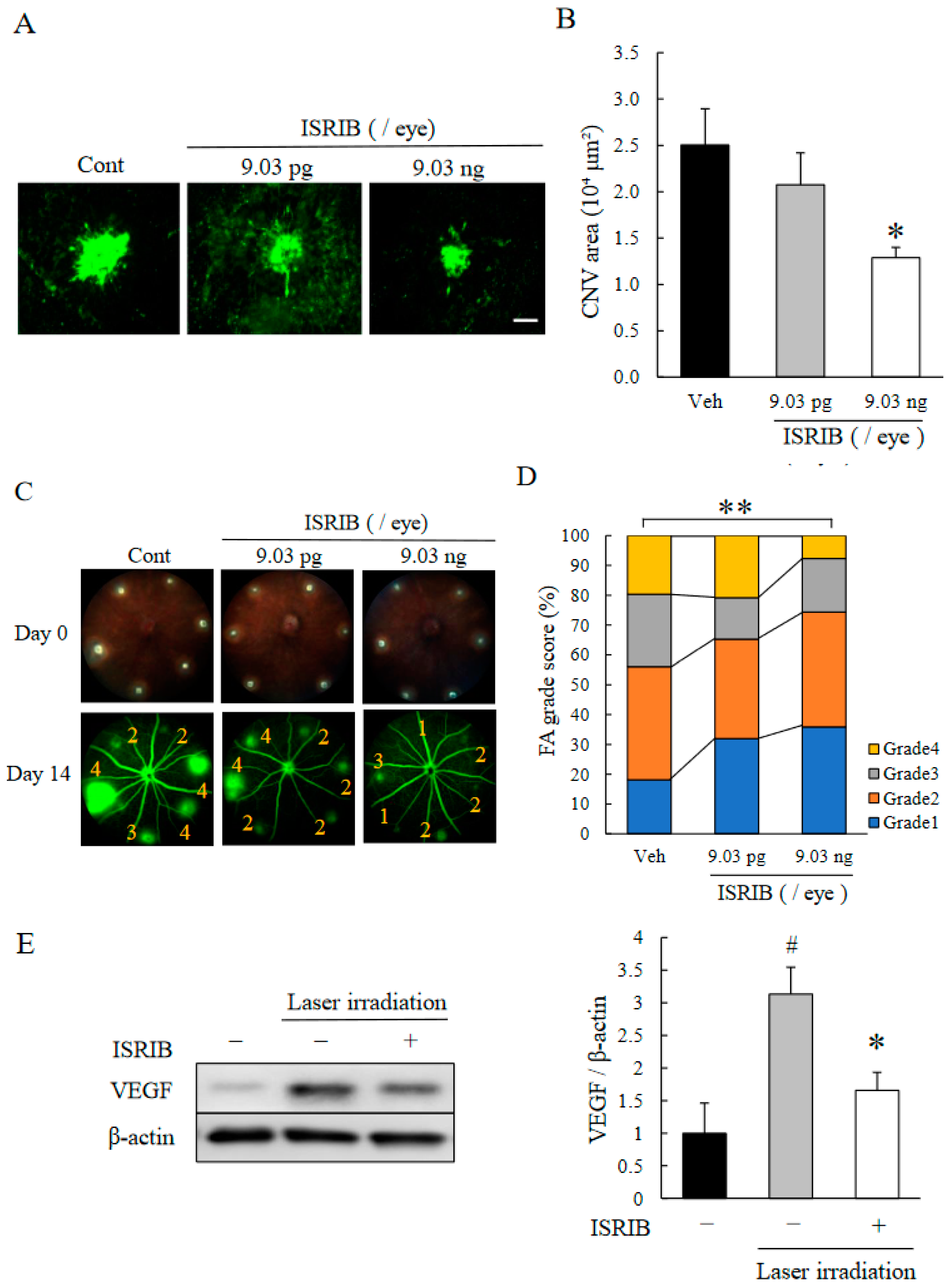
2.2. ISR Inhibitor, ISRIB, Suppressed CNV Formation
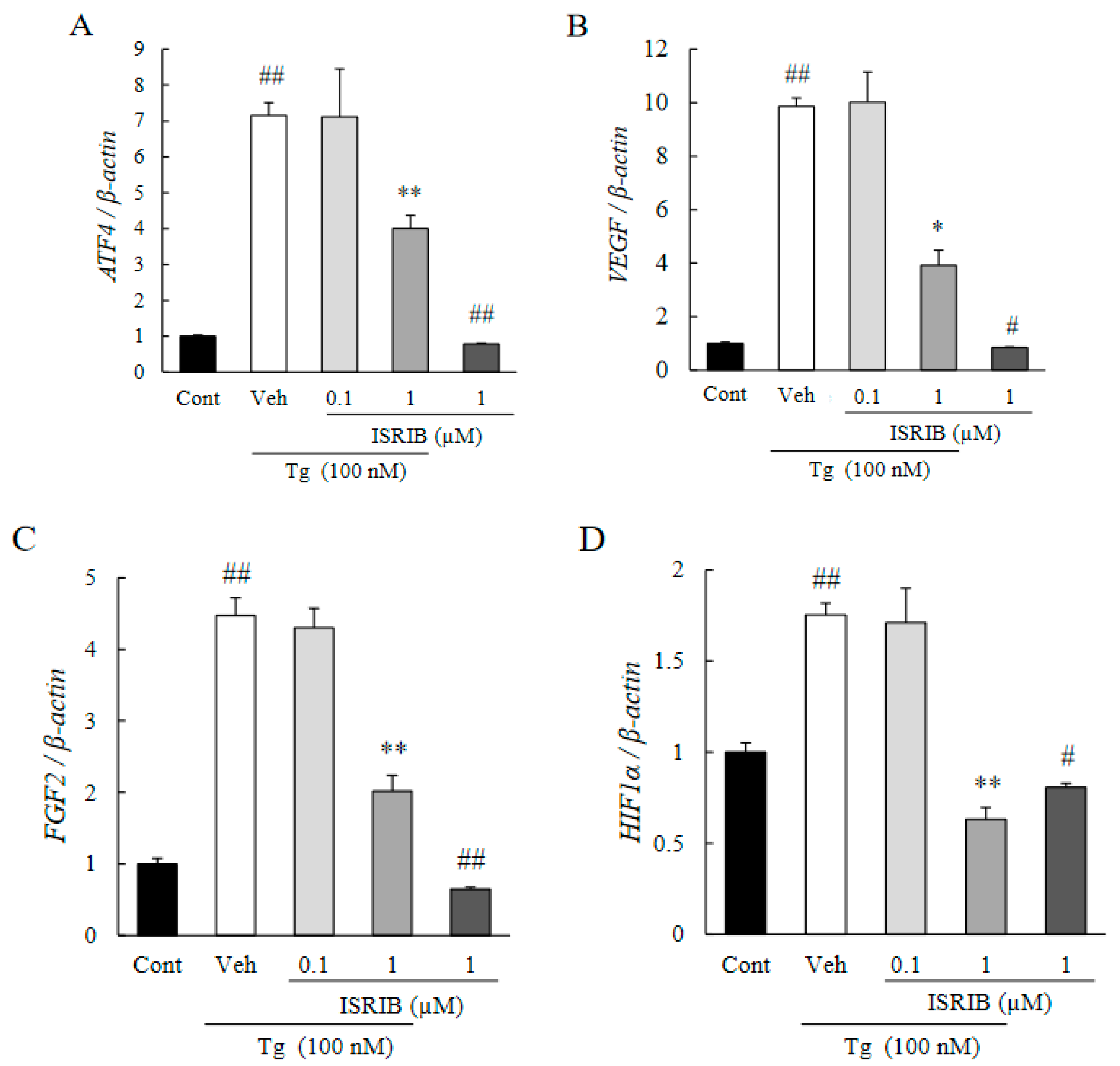
2.3. ISRIB Suppressed Expression of Pro-Angiogenic Factors Induced by ER Stress
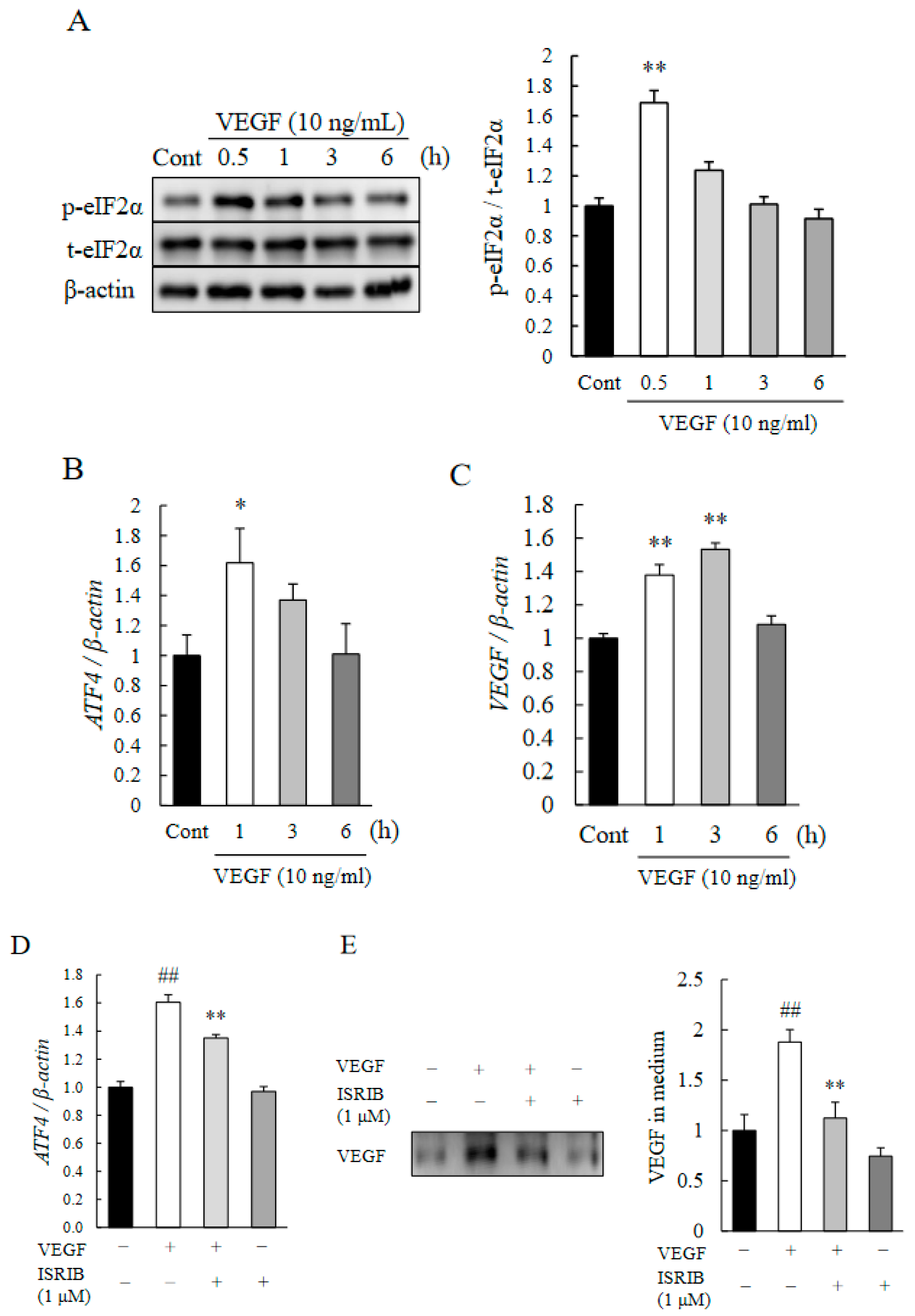
2.4. eIF2α-ATF4 Pathway Regulated Autocrine Loop of VEGF in HRMECs
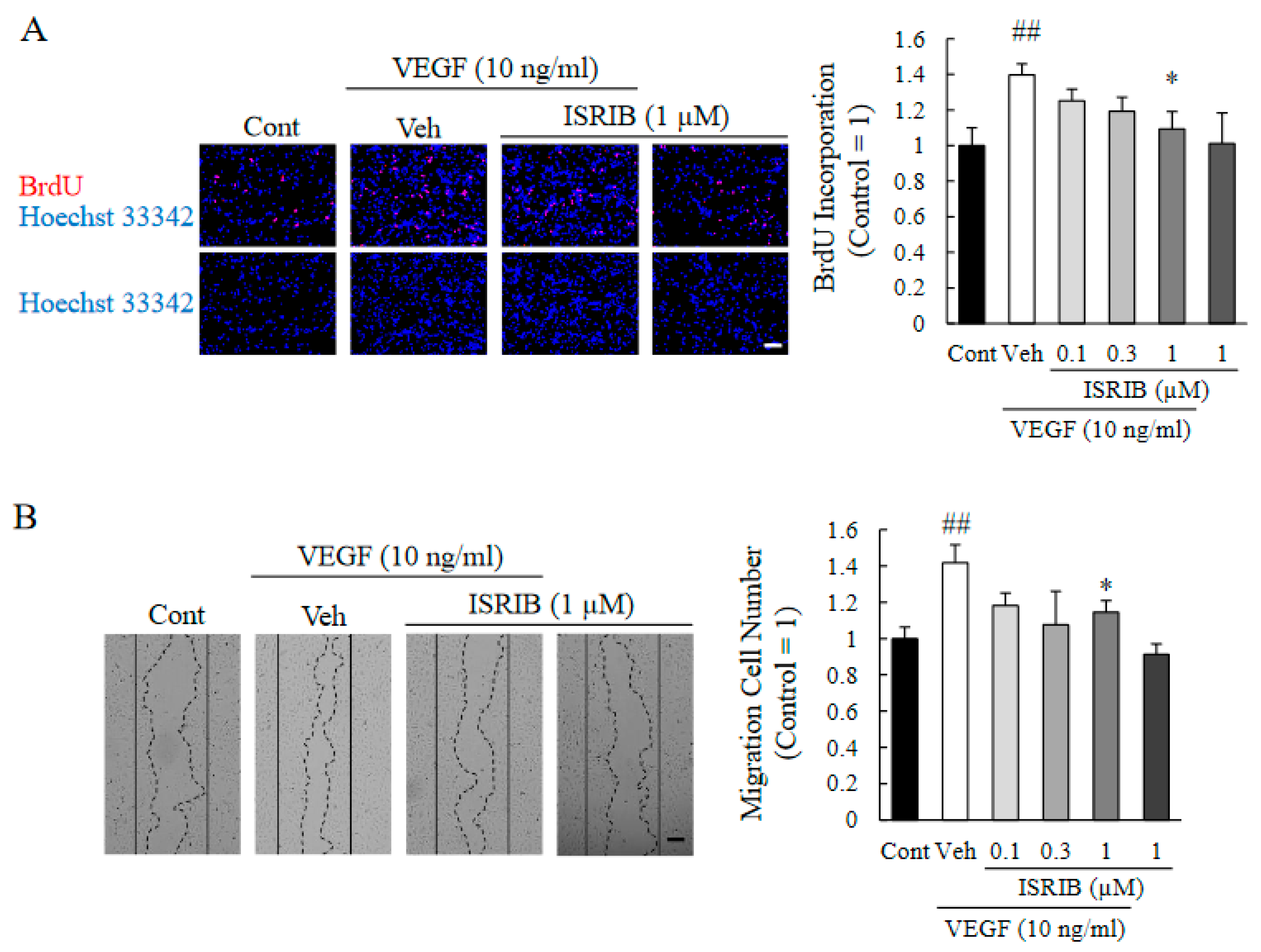
2.5. ISRIB Inhibited Proliferation and Migration of HRMECs
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Laser-Induced CNV Model
4.3. Intravitreal Injections
4.4. Fluorescein Angiography
4.5. Measurements of CNV Area
4.6. Histologic Analysis
4.7. Cell Cultures
4.8. Western Blots
4.9. Measurements of Endogenous VEGF Secreted from HRMECs
4.10. RNA Isolation and qRT-PCR
4.11. Cell Proliferation Assay
4.12. Wound-Healing Assay
4.13. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gheorghe, A.; Mahdi, L.; Musat, O. Age-Related Macular Degeneration. Rom. J. Ophthalmol. 2015, 59, 74–77. [Google Scholar]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Kvanta, A.; Algvere, P.V.; Berglin, L.; Seregard, S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1929–1934. [Google Scholar] [CrossRef]
- Lopez, P.F.; Sippy, B.D.; Lambert, H.M.; Thach, A.B.; Hinton, D.R. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Investig. Ophthalmol. Vis. Sci. 1996, 37, 855–868. [Google Scholar]
- Schmidt-Erfurth, U.; Chong, V.; Loewenstein, A.; Larsen, M.; Souied, E.; Schlingemann, R.; Eldem, B.; Monés, J.; Richard, G.; Bandello, F. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br. J. Ophthalmol. 2014, 98, 1144–1167. [Google Scholar] [CrossRef]
- Kim, L.A.; D’Amore, P.A. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am. J. Pathol. 2012, 181, 376–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mettu, P.S.; Allingham, M.J.; Cousins, S.W. Incomplete response to Anti-VEGF therapy in neovascular AMD: Exploring disease mechanisms and therapeutic opportunities. Prog. Retin. Eye Res. 2021, 82, 100906. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, S.; Zhao, J. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: A comprehensive review. Drug Des. Dev. Ther. 2016, 10, 1857–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlich, R.; Harris, A.; Kheradiya, N.S.; Winston, D.M.; Ciulla, T.A.; Wirostko, B. Age-related macular degeneration and the aging eye. Clin. Interv. Aging 2008, 3, 473–482. [Google Scholar] [PubMed] [Green Version]
- Krukowski, K.; Nolan, A.; Frias, E.S.; Boone, M.; Ureta, G.; Grue, K.; Paladini, M.-S.; Elizarraras, E.; Delgado, L.; Bernales, S.; et al. Small molecule cognitive enhancer reverses age-related memory decline in mice. eLife 2020, 9, 1–22. [Google Scholar] [CrossRef]
- Sharma, V.; Ounallah-Saad, H.; Chakraborty, D.; Hleihil, M.; Sood, R.; Barrera, I.; Edry, E.; Chandran, S.K.; Leon, S.B.T.D.; Kaphzan, H.; et al. Local Inhibition of PERK Enhances Memory and Reverses Age-Related Deterioration of Cognitive and Neuronal Properties. J. Neurosci. 2018, 38, 648–658. [Google Scholar] [CrossRef] [Green Version]
- Hoozemans, J.; van Haastert, E.; Eikelenboom, P.; de Vos, R.; Rozemuller, J.; Scheper, W. Activation of the unfolded protein response in Parkinson’s disease. Biochem. Biophys. Res. Commun. 2007, 354, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Kasetti, R.B.; Patel, P.D.; Maddineni, P.; Patil, S.; Kiehlbauch, C.; Millar, J.C.; Searby, C.C.; Raghunathan, V.; Sheffield, V.C.; Zode, G.S. ATF4 leads to glaucoma by promoting protein synthesis and ER client protein load. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, S.; Zhu, Y.; Shen, X. Elevated Activating Transcription Factor 4 and Glucose-Regulated 78 Kda Protein Levels Correlate with Inflammatory Cytokines in the Aqueous Humor and Vitreous of Proliferative Diabetic Retinopathy. Curr. Eye Res. 2017, 42, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Costa-Mattioli, M.; Walter, P. The integrated stress response: From mechanism to disease. Science 2020, 368, 5314. [Google Scholar] [CrossRef] [PubMed]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef] [Green Version]
- Harding, H.; Novoa, I.; Zhang, Y.; Zeng, H.; Wek, R.; Schapira, M.; Ron, D. Regulated Translation Initiation Controls Stress-Induced Gene Expression in Mammalian Cells. Mol. Cell 2000, 6, 1099–1108. [Google Scholar] [CrossRef]
- Lange, P.S.; Chavez, J.C.; Pinto, J.T.; Coppola, G.; Sun, C.-W.; Townes, T.M.; Geschwind, D.H.; Ratan, R.R. ATF4 is an oxidative stress–inducible, prodeath transcription factor in neurons in vitro and in vivo. J. Exp. Med. 2008, 205, 1227–1242. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, N.; Gorman, A.; Gupta, S.; Samali, A. The eIF2α kinases: Their structures and functions. Cell. Mol. Life Sci. 2013, 70, 3493–3511. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Wu, M.; Zhang, L.; Thompson, R.; Nath, A.; Chan, C. Signaling dynamics of palmitate-induced ER stress responses mediated by ATF4 in HepG2 cells. BMC Syst. Biol. 2013, 7, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, K.; Jiao, H.; Li, S.; Cao, H.; Galson, D.L.; Zhao, Z.; Zhao, X.; Lai, Y.; Fan, J.; Im, H.-J.; et al. ATF4 promotes bone angiogenesis by increasing vegf expression and release in the bone environment. J. Bone Miner. Res. 2013, 28, 1870–1884. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, G.; Kunte, M.; Shinde, V.; Gorbatyuk, M. Modulation of angiogenesis by genetic manipulation of atf4 in a mouse modulation of angiogenesis by genetic manipulation. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5995–6002. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ning, Y.; Alam, G.N.; Jankowski, B.M.; Dong, Z.; Nör, J.E.; Polverini, P.J. Amino Acid Deprivation Promotes Tumor Angiogenesis through the GCN2/ATF4 Pathway. Neoplasia 2013, 15, 989–997. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, N. Vascular Endothelial Growth Factor: Basic Science and Clinical Progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Zhao, X.; Yuan, M.; Chen, Y. Comparison of cytokine levels in the aqueous humor of polypoidal choroidal vasculopathy and neovascular age-related macular degeneration patients. BMC Ophthalmol. 2020, 20, 2788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, Y.; Oguchi, Y.; Omori, T.; Shintake, H.; Tomita, R.; Kasai, A.; Ogasawara, M.; Sugano, Y.; Itagaki, K.; Ojima, A.; et al. Complement Activation Products and Cytokines in Pachychoroid Neovasculopathy and Neovascular Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 39. [Google Scholar] [CrossRef]
- Karali, E.; Bellou, S.; Stellas, D.; Klinakis, A.; Murphy, C.; Fotsis, T. VEGF Signals through ATF6 and PERK to Promote Endothelial Cell Survival and Angiogenesis in the Absence of ER Stress. Mol. Cell 2014, 54, 559–572. [Google Scholar] [CrossRef] [Green Version]
- Zyryanova, A.F.; Kashiwagi, K.; Rato, C.; Harding, H.P.; Crespillo-Casado, A.; Perera, L.A.; Sakamoto, A.; Nishimoto, M.; Yonemochi, M.; Shirouzu, M.; et al. ISRIB Blunts the Integrated Stress Response by Allosterically Antagonising the Inhibitory Effect of Phosphorylated eIF2 on eIF2B. Mol. Cell 2021, 81, 88–103. [Google Scholar] [CrossRef]
- Sidrauski, C.; Acosta-Alvear, D.; Khoutorsky, A.; Vedantham, P.; Hearn, B.R.; Li, H.; Gamache, K.; Gallagher, C.M.; Ang, K.K.H.; Wilson, C.; et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. eLife 2013, 2013, 1–22. [Google Scholar] [CrossRef]
- Halliday, M.J.; Radford, H.; Sekine, Y.; Moreno, J.J.; Verity, N.; Le Quesne, J.P.C.; Ortori, C.A.; Barrett, D.; Fromont, C.; Fischer, P.; et al. Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity. Cell Death Dis. 2015, 6, e1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbink, T.E.M.; Wisse, L.E.; Jaku, E.; Thiecke, M.J.; Voltolini-González, D.; Fritsen, H.; Bobeldijk, S.; Ter Braak, T.J.; Polder, E.; Postma, N.L.; et al. Vanishing white matter: Deregulated integrated stress response as therapy target. Ann. Clin. Transl. Neurol. 2019, 6, 1407–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.G.; Liu, X.; Kiss, S.; Connolly, E.; Gragoudas, E.S.; Michaud, N.A.; Bulgakov, O.V.; Adamian, M.; DeAngelis, M.M.; Miller, J.; et al. Increased Choroidal Neovascularization following Laser Induction in Mice Lacking Lysyl Oxidase-like 1. Investig. Opthalmol. Vis. Sci. 2008, 49, 2599–2605. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Sene, A.; Santeford, A.; Gdoura, A.; Kubota, S.; Zapata, N.; Apte, R.S. IL10-driven STAT3 signalling in senescent macrophages promotes pathological eye angiogenesis. Nat. Commun. 2015, 6, 7847. [Google Scholar] [CrossRef] [Green Version]
- Ueta, T.; Ishihara, K.; Notomi, S.; Lee, J.-J.; Maidana, D.E.; Efstathiou, N.E.; Murakami, Y.; Hasegawa, E.; Azuma, K.; Toyono, T.; et al. RIP1 kinase mediates angiogenesis by modulating macrophages in experimental neovascularization. Proc. Natl. Acad. Sci. USA 2019, 116, 23705–23713. [Google Scholar] [CrossRef] [Green Version]
- Salminen, A.; Kauppinen, A.; Hyttinen, J.M.T.; Toropainen, E.; Kaarniranta, K. Endoplasmic Reticulum Stress in Age-Related Macular Degeneration: Trigger for Neovascularization. Mol. Med. 2010, 16, 535–542. [Google Scholar] [CrossRef]
- Remtulla, S.; Hallett, P.E. A schematic eye for the mouse, and comparisons with the rat. Vision Res. 1985, 25, 21–31. [Google Scholar] [CrossRef]
- Chang, T.-K.; Lawrence, D.A.; Lu, M.; Tan, J.; Harnoss, J.M.; Marsters, S.A.; Liu, P.; Sandoval, W.; Martin, S.E.; Ashkenazi, A. Coordination between Two Branches of the Unfolded Protein Response Determines Apoptotic Cell Fate. Mol. Cell 2018, 71, 629–636.e5. [Google Scholar] [CrossRef] [Green Version]
- Bugallo, R.; Marlin, E.; Baltanás, A.; Toledo, E.; Ferrero, R.; Vinueza-Gavilanes, R.; Larrea, L.; Arrasate, M.; Aragón, T. Fine tuning of the unfolded protein response by ISRIB improves neuronal survival in a model of amyotrophic lateral sclerosis. Cell Death Dis. 2020, 11, 397. [Google Scholar] [CrossRef]
- Wang, Y.; Alam, G.N.; Ning, Y.; Visioli, F.; Dong, Z.; Nör, J.E.; Polverini, P.J. The Unfolded Protein Response Induces the Angiogenic Switch in Human Tumor Cells through the PERK/ATF4 Pathway. Cancer Res. 2012, 72, 5396–5406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, H.-F.; Zhu, J.-Y.; Wang, J.-N.; Ren, J.-G.; Cai, Y.; Wang, F.-Q.; Zhang, W.; Chen, G.; Zhao, Y.-F.; Zhao, J.-H. Association of ATF4 Expression with Tissue Hypoxia and M2 Macrophage Infiltration in Infantile Hemangioma. J. Histochem. Cytochem. 2017, 65, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Gargalovic, P.S.; Imura, M.; Zhang, B.; Gharavi, N.M.; Clark, M.J.; Pagnon, J.; Yang, W.-P.; He, A.; Truong, A.; Patel, S.; et al. Identification of inflammatory gene modules based on variations of human endothelial cell responses to oxidized lipids. Proc. Natl. Acad. Sci. USA 2006, 103, 12741–12746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Jing, G.; Wang, J.J.; Sheibani, N.; Zhang, S.X. ATF4 is a novel regulator of MCP-1 in microvascular endothelial cells. J. Inflamm. 2015, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Jha, P.; Lyzogubov, V.V.; Tytarenko, R.G.; Bora, N.S.; Bora, P.S. Relationship between Complement Membrane Attack Complex, Chemokine (C-C Motif) Ligand 2 (CCL2) and Vascular Endothelial Growth Factor in Mouse Model of Laser-induced Choroidal Neovascularization. J. Biol. Chem. 2011, 286, 20991–21001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiose, S.; Hata, Y.; Noda, Y.; Sassa, Y.; Takeda, A.; Yoshikawa, H.; Fujisawa, K.; Kubota, T.; Ishibashi, T. Fibrinogen stimulates in vitro angiogenesis by choroidal endothelial cells via autocrine VEGF. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 242, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Oskolkova, O.; Afonyushkin, T.; Leitner, A.; Von Schlieffen, E.; Gargalovic, P.S.; Lusis, A.J.; Binder, B.R.; Bochkov, V.N. ATF4-dependent transcription is a key mechanism in VEGF up-regulation by oxidized phospholipids: Critical role of oxidized sn-2 residues in activation of unfolded protein response. Blood 2008, 112, 330–339. [Google Scholar] [CrossRef]
- Wang, H.; Han, X.; Wittchen, E.S.; Hartnett, M.E. TNF-α mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent β-catenin activation. Mol. Vis. 2016, 22, 116–128. [Google Scholar]
- Hou, H.-Y.; Wang, Y.-S.; Xu, J.-F.; Wang, Y.-C.; Liu, J.-P. The Dynamic Conduct of Bone Marrow-Derived Cells in the Choroidal Neovascularization Microenvironment. Curr. Eye Res. 2006, 31, 1051–1061. [Google Scholar] [CrossRef]
- Song, J.; Lee, K.; Park, S.W.; Chung, H.; Jung, D.; Na, Y.R.; Quan, H.; Cho, C.S.; Che, J.-H.; Kim, J.H.; et al. Lactic Acid Upregulates VEGF Expression in Macrophages and Facilitates Choroidal Neovascularization. Investig. Opthalmol. Vis. Sci. 2018, 59, 3747–3754. [Google Scholar] [CrossRef] [Green Version]
- Masuda, T.; Shimazawa, M.; Takata, S.; Nakamura, S.; Tsuruma, K.; Hara, H. Edaravone is a free radical scavenger that protects against laser-induced choroidal neovascularization in mice and common marmosets. Exp. Eye Res. 2016, 146, 196–205. [Google Scholar] [CrossRef]
- Takata, S.; Masuda, T.; Nakamura, S.; Kuchimaru, T.; Tsuruma, K.; Shimazawa, M.; Nagasawa, H.; Kizaka-Kondoh, S.; Hara, H. The effect of triamcinolone acetonide on laser-induced choroidal neovascularization in mice using a hypoxia visualization bio-imaging probe. Sci. Rep. 2015, 5, 9898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Nakamura, S.; Maekawa, M.; Higashiyama, S.; Hara, H. ANKFY1 is essential for retinal endothelial cell proliferation and migration via VEGFR2/Akt/eNOS pathway. Biochem. Biophys. Res. Commun. 2020, 533, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Takizawa, H.; Shimazawa, M.; Hashimoto, Y.; Sugitani, S.; Tsuruma, K.; Hara, H. Mild Endoplasmic Reticulum Stress Promotes Retinal Neovascularization via Induction of BiP/GRP78. PLoS ONE 2013, 8, e60517. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuda, H.; Tanaka, M.; Nishinaka, A.; Nakamura, S.; Shimazawa, M.; Hara, H. Role of Activating Transcription Factor 4 in Murine Choroidal Neovascularization Model. Int. J. Mol. Sci. 2021, 22, 8890. https://doi.org/10.3390/ijms22168890
Yasuda H, Tanaka M, Nishinaka A, Nakamura S, Shimazawa M, Hara H. Role of Activating Transcription Factor 4 in Murine Choroidal Neovascularization Model. International Journal of Molecular Sciences. 2021; 22(16):8890. https://doi.org/10.3390/ijms22168890
Chicago/Turabian StyleYasuda, Hiroto, Miruto Tanaka, Anri Nishinaka, Shinsuke Nakamura, Masamitsu Shimazawa, and Hideaki Hara. 2021. "Role of Activating Transcription Factor 4 in Murine Choroidal Neovascularization Model" International Journal of Molecular Sciences 22, no. 16: 8890. https://doi.org/10.3390/ijms22168890
APA StyleYasuda, H., Tanaka, M., Nishinaka, A., Nakamura, S., Shimazawa, M., & Hara, H. (2021). Role of Activating Transcription Factor 4 in Murine Choroidal Neovascularization Model. International Journal of Molecular Sciences, 22(16), 8890. https://doi.org/10.3390/ijms22168890




