Sex-Specific Effects of Plastic Caging in Murine Viral Myocarditis
Abstract
:1. Introduction
2. Results
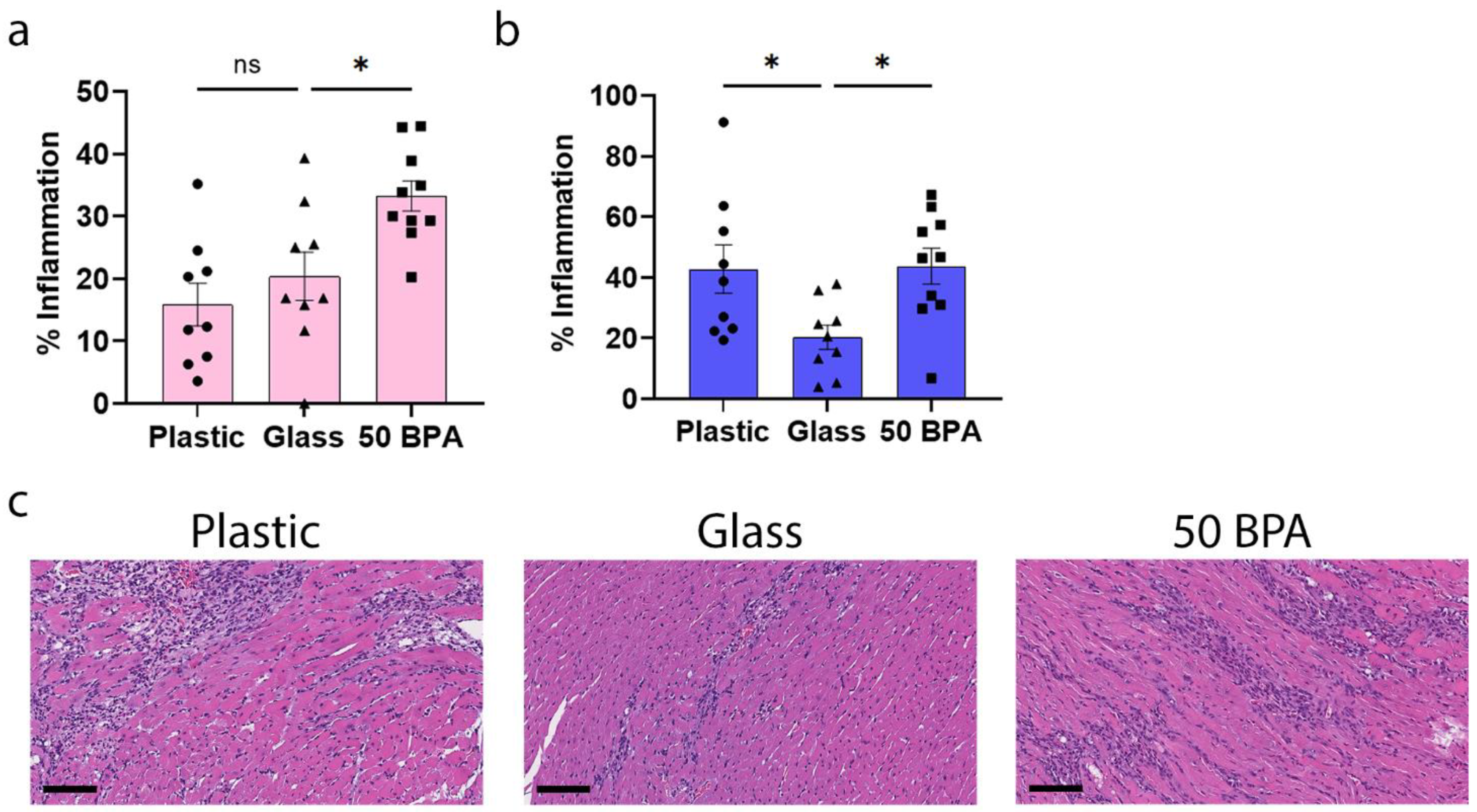
2.1. Plastic Caging Increases Myocarditis in Male BALB/c Mice during Viral Myocarditis
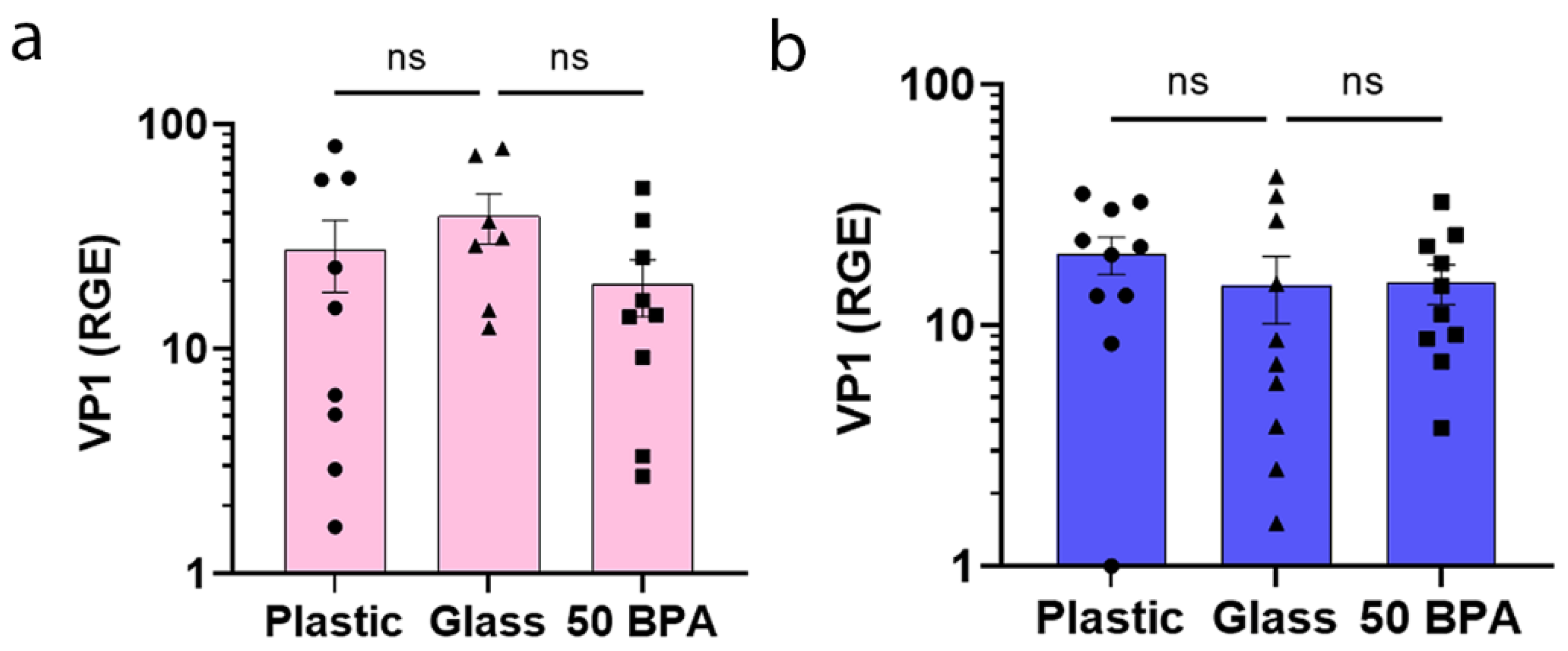
2.2. Plastic Caging and BPA Exposure Has No Significant Affect on VP1 Viral Gene Expression in Males or Females during Myocarditis
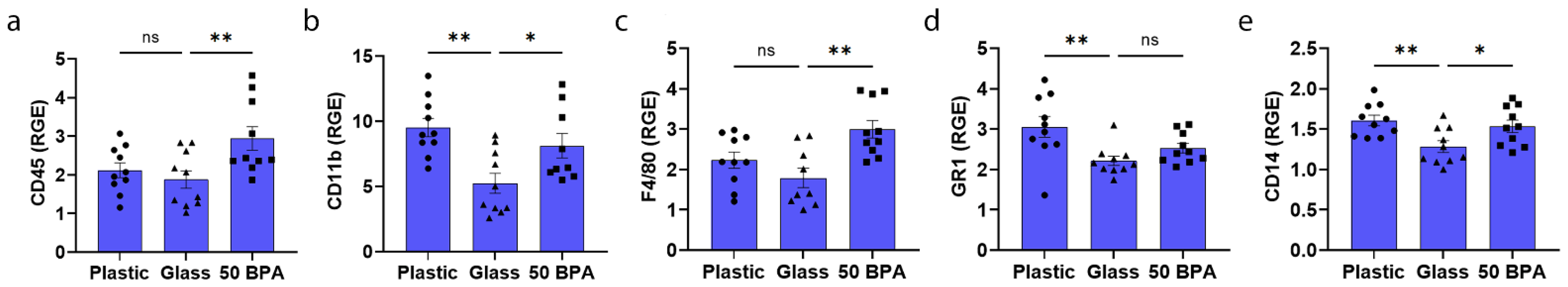
2.3. Plastic Caging Increases Macrophage and Neutrophil Markers in Males during Myocarditis by qRT-PCR
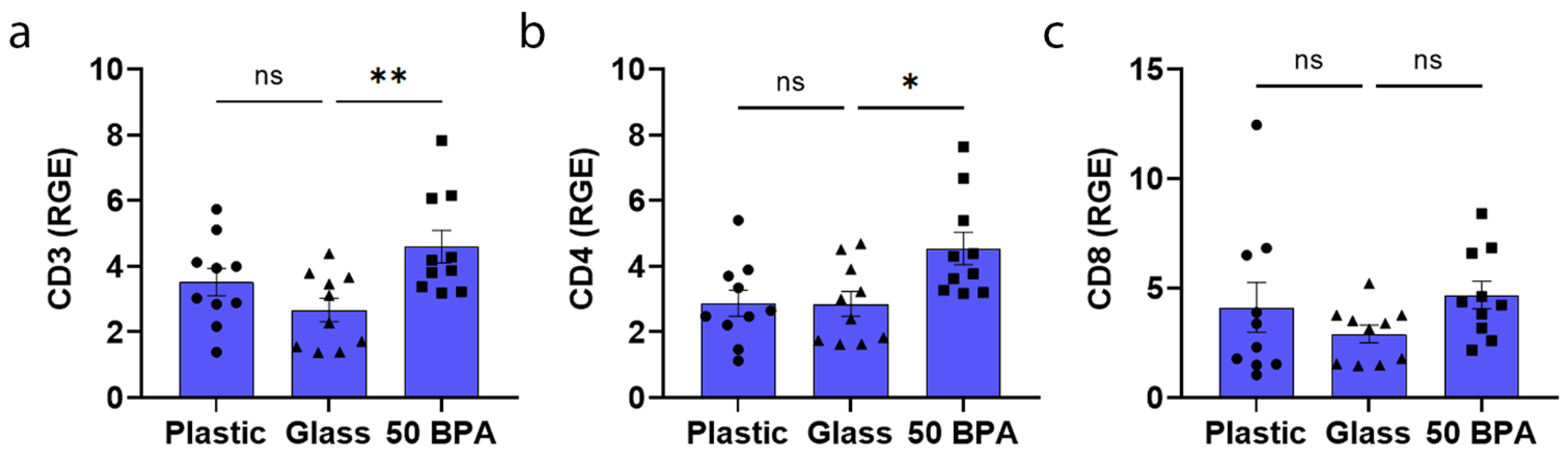
2.4. Plastic Caging Has No Significant Effect on T-Cell Markers in Males and Females during Myocarditis
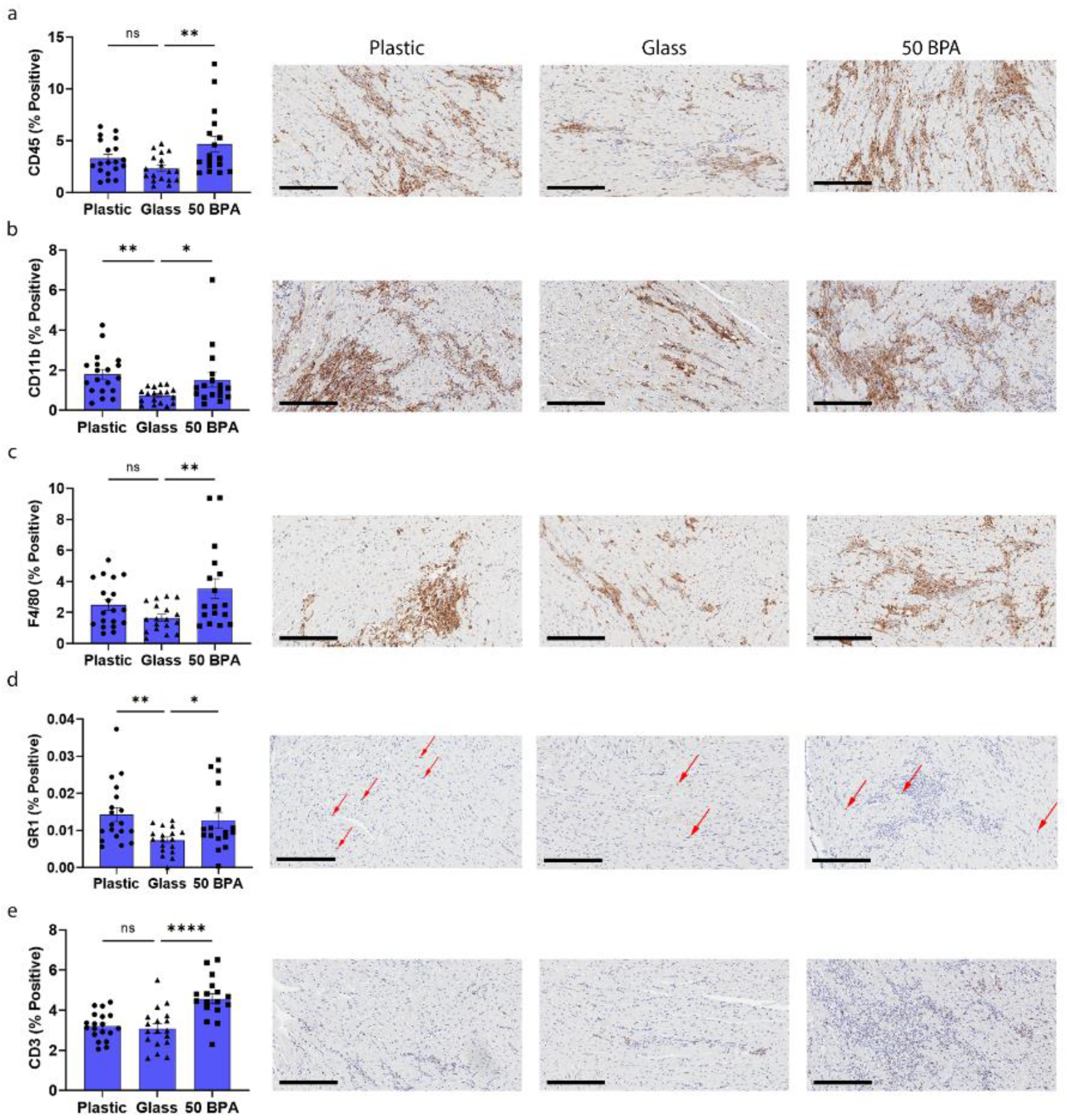
2.5. Plastic Caging Increases Macrophages and Neutrophils in Males during Myocarditis by IHC
2.6. Plastic Caging Increases the Mast Cell Marker cKit in the Heart of Females with Myocarditis, but Decreases it in Males
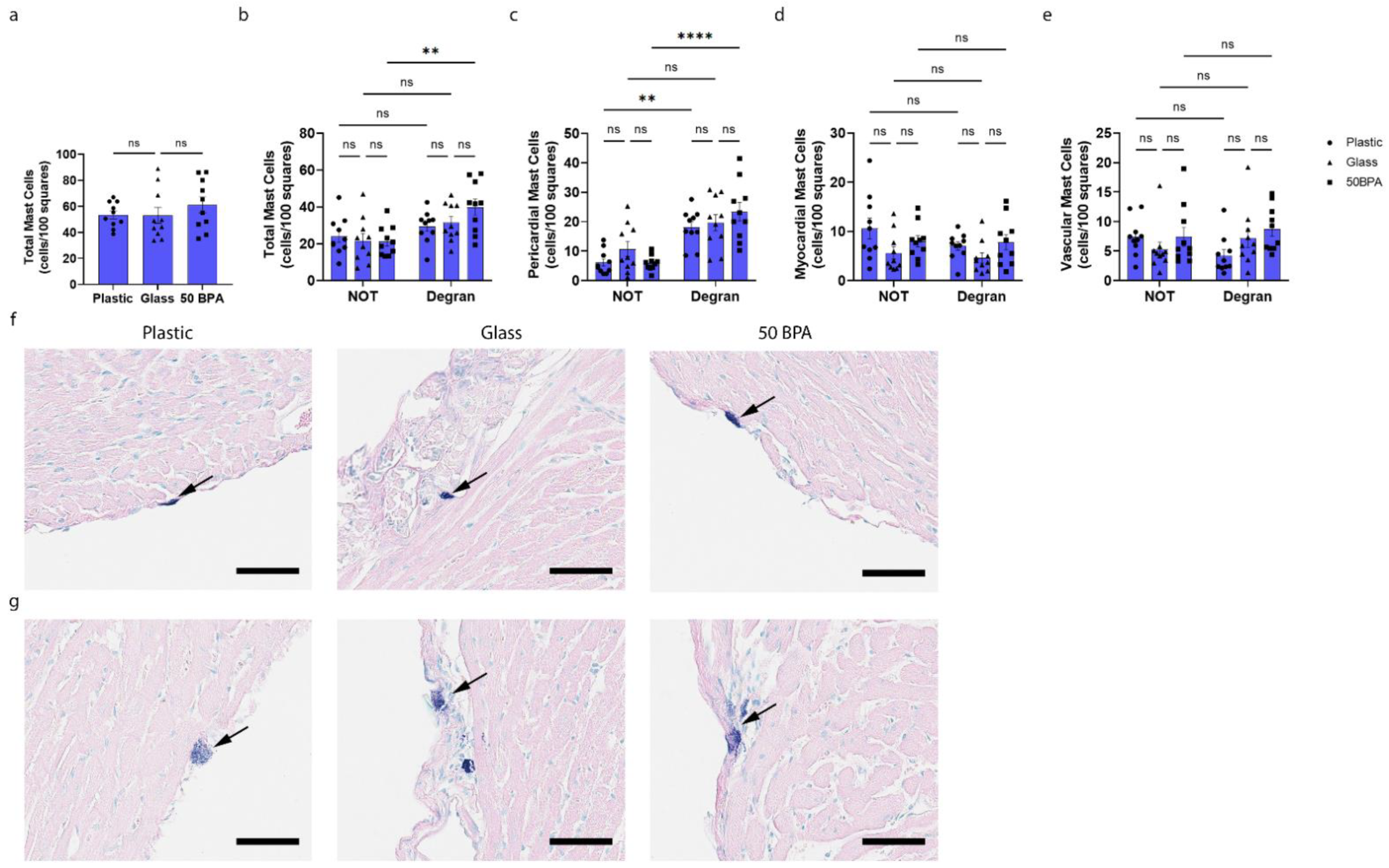
2.7. Plastic Caging Increases Pericardial Mast Cell Numbers and Degranulation in Females during Myocarditis
2.8. Plastic Caging Has No Effect on Mast Cell Numbers or Degranulation in Males during Myocarditis, but Males Have More Degranulating Pericardial Mast Cells Than Females Regardless of Plastic Exposure
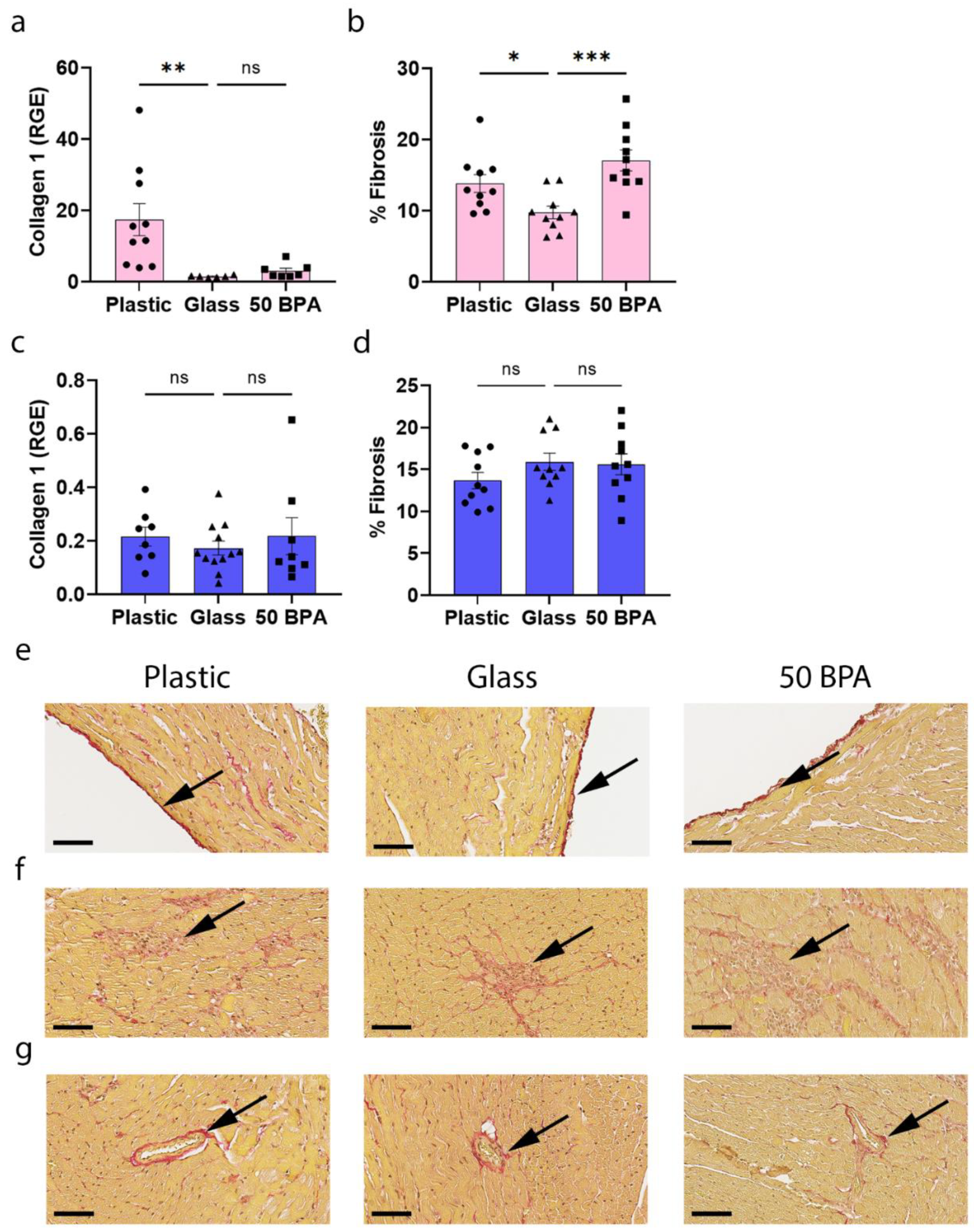
2.9. Plastic Caging Increases Fibrosis in the Heart during Myocarditis in Females, but Has No Significant Affect in Males
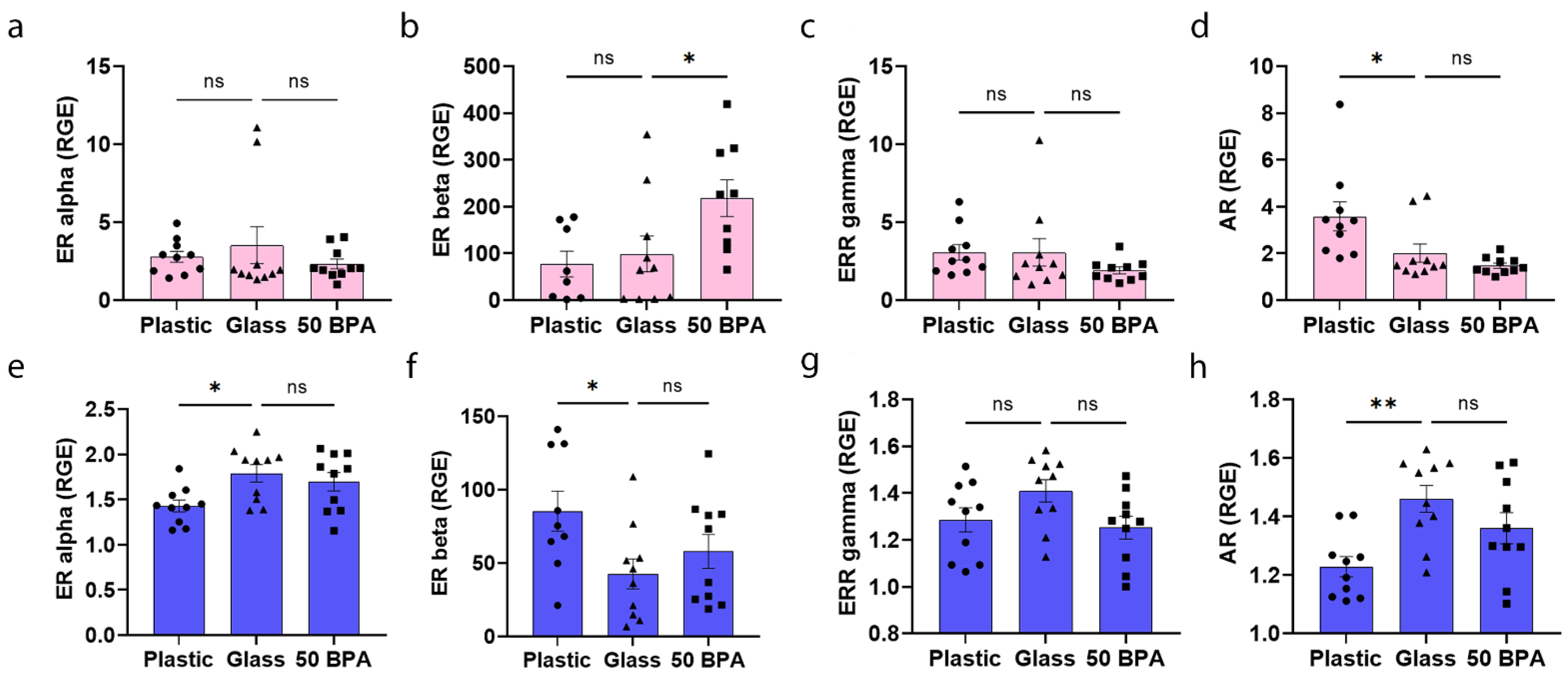
2.10. Plastic Caging Decreases ERα and AR and Increases ERβ Expression in the Heart of Males during Myocarditis, but Has No Affect on ERs in Females
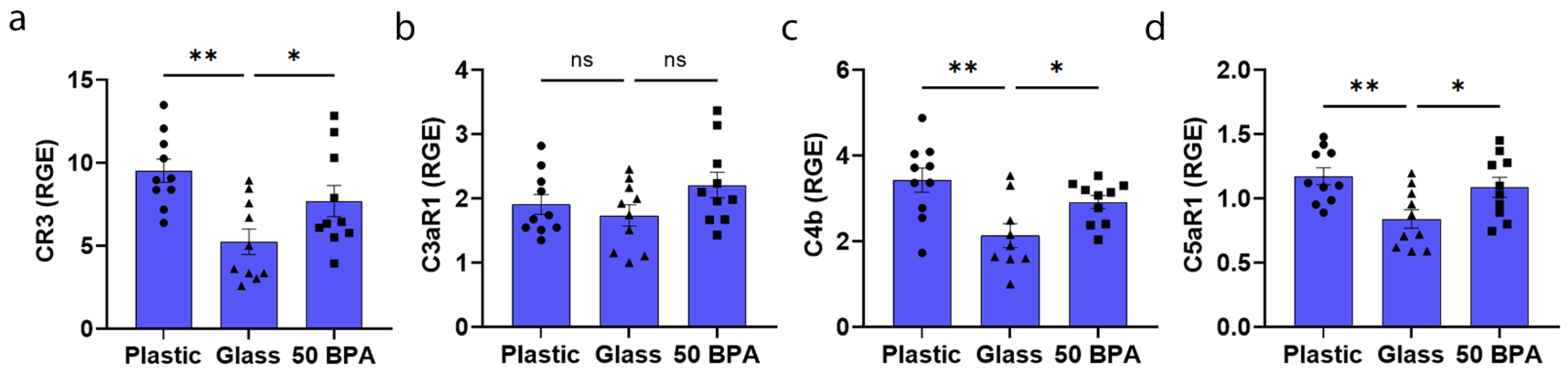
2.11. Plastic Caging Increases Complement Gene Expression in the Heart of Males with Myocarditis, but Not Females
3. Discussion
4. Materials and Methods
4.1. Animal Care Ethics Statement
4.2. CVB3-Induced Myocarditis Model
4.3. Bisphenol A and Bedding
4.4. Histology
4.5. Quantitative Real-Time PCR
4.5.1. RNA Isolation of Heart Tissue
4.5.2. qRT-PCR Method
4.5.3. Measurement of CVB3 Genome VP1 Levels by qRT-PCR
4.6. Immunohistochemistry
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pauschinger, M.; Phan, M.D.; Doerner, A.; Kuehl, U.; Schwimmbeck, P.L.; Poller, W.; Kandolf, R.; Schultheiss, H.P. Enteroviral RNA replication in the myocardium of patients with left ventricular dysfunction and clinically suspected myocarditis. Circulation 1999, 99, 889–895. [Google Scholar] [CrossRef] [Green Version]
- Kuhl, U.; Pauschinger, M.; Seeberg, B.; Lassner, D.; Noutsias, M.; Poller, W.; Schultheiss, H.P. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 2005, 112, 1965–1970. [Google Scholar] [CrossRef] [Green Version]
- Fairweather, D.; Stafford, K.A.; Sung, Y.K. Update on coxsackievirus B3 myocarditis. Curr. Opin. Rheumatol. 2012, 24, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Sawalha, K.; Abozenah, M.; Kadado, A.J.; Battisha, A.; Al-Akchar, M.; Salerno, C.; Hernandez-Montfort, J.; Islam, A.M. Systematic review of COVID-19 related myocarditis: Insights on management and outcome. Cardiovasc Revasc. Med. 2021, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020, 38, 1504–1507. [Google Scholar] [CrossRef] [PubMed]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T., Jr.; Chahal, C.A.A. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020, 17, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, H.; Escher, F.; Aleshcheva, G.; Baumeier, C.; Morawietz, L.; Elsaesser, A.; Schultheiss, H.P. Proof of SARS-CoV-2 genomes in endomyocardial biopsy with latency after acute infection. Int. J. Infect. Dis. 2021, 102, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Escher, F.; Pietsch, H.; Aleshcheva, G.; Bock, T.; Baumeier, C.; Elsaesser, A.; Wenzel, P.; Hamm, C.; Westenfeld, R.; Schultheiss, M.; et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020, 7, 2440–2447. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K. Inflammation in myocardial disease: From myocarditis to dilated cardiomyopathy. Pathol. Int. 2020, 70, 1–11. [Google Scholar] [CrossRef]
- Bracamonte-Baran, W.; Cihakova, D. Cardiac Autoimmunity: Myocarditis. Adv. Exp. Med. Biol. 2017, 1003, 187–221. [Google Scholar]
- Fairweather, D.; Cooper, L.T.; Blauwet, L.A.; Blauwet, L.A. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr. Probl. Cardiol. 2013, 38, 7–46. [Google Scholar] [CrossRef] [Green Version]
- Schultheiss, H.P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Primers 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Cocker, M.S.; Abdel-Aty, H.; Strohm, O.; Friedrich, M.G. Age and gender effects on the extent of myocardial involvement in acute myocarditis: A cardiovascular magnetic resonance study. Heart (Br. Card. Soc.) 2009, 95, 1925–1930. [Google Scholar] [CrossRef]
- McNamara, D.M.; Starling, R.C.; Cooper, L.T.; Boehmer, J.P.; Mather, P.J.; Janosko, K.M.; Gorcsan, J.; Kip, K.E.; Dec, G.W. Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: Results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 study. J. Am. Coll. Cardiol. 2011, 58, 1112–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tschöpe, C.; Van Linthout, S. New insights in (inter)cellular mechanisms by heart failure with preserved ejection fraction. Curr. Heart Fail. Rep. 2014, 11, 436–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Martinez, E.; Brugnolaro, C.; Ibarrola, J.; Ravassa, S.; Buonafine, M.; Lopez, B.; Fernandez-Celis, A.; Querejeta, R.; Santamaria, E.; Fernandez-Irigoyen, J.; et al. CT-1 (Cardiotrophin-1)-Gal-3 (Galectin-3) Axis in cardiac fibrosis and inflammation. Hypertension 2019, 73, 602–611. [Google Scholar] [CrossRef]
- Mack, M. Inflammation and fibrosis. Matrix Biol. 2018, 68, 106–121. [Google Scholar] [CrossRef]
- Baldeviano, G.C.; Barin, J.G.; Talor, M.V.; Srinivasan, S.; Bedja, D.; Zheng, D.; Gabrielson, K.; Iwakura, Y.; Rose, N.R.; Cihakova, D. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ. Res. 2010, 106, 1646–1655. [Google Scholar] [CrossRef] [Green Version]
- Myers, J.M.; Cooper, L.T.; Kem, D.C.; Stavrakis, S.; Kosanke, S.D.; Shevach, E.M.; Fairweather, D.; Stoner, J.A.; Cox, C.J.; Cunningham, M.W. Cardiac myosin-Th17 responses promote heart failure in human myocarditis. JCI Insight 2016, 1, e85851. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, Y.; Zhang, C.; Li, P.; Cui, W.; Hao, J.; Ma, X.; Yin, Z.; Du, J. gammadeltaT Cell-derived interleukin-17A via an interleukin-1beta-dependent mechanism mediates cardiac injury and fibrosis in hypertension. Hypertension 2014, 64, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Gieseck, R.L., 3rd; Wilson, M.S.; Wynn, T.A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 2018, 18, 62–76. [Google Scholar] [CrossRef]
- Coronado, M.J.; Brandt, J.E.; Kim, E.; Bucek, A.; Bedja, D.; Abston, E.D.; Shin, J.; Gabrielson, K.L.; Mitzner, W.; Fairweather, D. Testosterone and interleukin-1β increase cardiac remodeling during coxsackievirus B3 myocarditis via serpin A 3n. Am. J. Physiology Heart Circ. Physiol. 2012, 302, H1726–H1736. [Google Scholar] [CrossRef] [PubMed]
- Coronado, M.J.; Bruno, K.A.; Blauwet, L.A.; Tschöpe, C.; Cunningham, M.W.; Pankuweit, S.; van Linthout, S.; Jeon, E.S.; McNamara, D.M.; Krejci, J.; et al. Elevated sera sST2 is associated with heart failure in men ≤ 50 years old with myocarditis. J. Am. Heart Assoc. 2019, 8, e008968. [Google Scholar] [CrossRef] [Green Version]
- Huber, S. ERbeta and ERalpha differentially regulate NKT and Vgamma4 (+) T-cell activation and T-regulatory Cell Response in Coxsackievirus B3 Infected Mice. J. Clin. Cell. Immunol. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Frisancho-Kiss, S.; Davis, S.E.; Nyland, J.F.; Frisancho, J.A.; Cihakova, D.; Barrett, M.A.; Rose, N.R.; Fairweather, D. Cutting edge: Cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J. Immunol. (Baltim. Md. 1950) 2007, 178, 6710–6714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omur, O.; Erdogan, M.; Ozkilic, H.; Yilmaz, C. Scintigraphic methods to evaluate alterations of gastric and esophageal functions in female obesity. Mol. Imaging Radionucl. Ther. 2014, 23, 5–11. [Google Scholar] [CrossRef]
- Frisancho-Kiss, S.; Nyland, J.F.; Davis, S.E.; Barrett, M.A.; Gatewood, S.J.; Njoku, D.B.; Cihakova, D.; Silbergeld, E.K.; Rose, N.R.; Fairweather, D. Cutting edge: T cell Ig mucin-3 reduces inflammatory heart disease by increasing CTLA-4 during innate immunity. J. Immunol. 2006, 176, 6411–6415. [Google Scholar] [CrossRef] [Green Version]
- Fairweather, D.; Frisancho-Kiss, S.; Njoku, D.B.; Nyland, J.F.; Kaya, Z.; Yusung, S.A.; Davis, S.E.; Frisancho, J.A.; Barrett, M.A.; Rose, N.R. Complement receptor 1 and 2 deficiency increases coxsackievirus B3-induced myocarditis, dilated cardiomyopathy, and heart failure by increasing macrophages, IL-1beta, and immune complex deposition in the heart. J. Immunol. (Baltim. Md. 1950) 2006, 176, 3516–3524. [Google Scholar] [CrossRef] [Green Version]
- Oldring, P.K.; Castle, L.; O’Mahony, C.; Dixon, J. Estimates of dietary exposure to bisphenol A (BPA) from light metal packaging using food consumption and packaging usage data: A refined deterministic approach and a fully probabilistic (FACET) approach. Food Addit. Contam. Part. A Chem Anal. Control. Expo. Risk Assess. 2014, 31, 466–489. [Google Scholar] [CrossRef] [Green Version]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global assessment of bisphenol A in the environment: Review and analysis of its occurrence and bioaccumulation. Dose Response 2015, 13, 1559325815598308. [Google Scholar] [CrossRef] [Green Version]
- Lorber, M.; Schecter, A.; Paepke, O.; Shropshire, W.; Christensen, K.; Birnbaum, L. Exposure assessment of adult intake of bisphenol A (BPA) with emphasis on canned food dietary exposures. Environ. Int. 2015, 77, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndaw, S.; Remy, A.; Jargot, D.; Robert, A. Occupational exposure of cashiers to Bisphenol A via thermal paper: Urinary biomonitoring study. Int. Arch. Occup. Environ. Health 2016, 89, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, L.S. State of the science of endocrine disruptors. Environ. Health Perspect 2013, 121, A107. [Google Scholar] [CrossRef]
- Gerona, R.R.; Pan, J.; Zota, A.R.; Schwartz, J.M.; Friesen, M.; Taylor, J.A.; Hunt, P.A.; Woodruff, T.J. Direct measurement of Bisphenol A (BPA), BPA glucuronide and BPA sulfate in a diverse and low-income population of pregnant women reveals high exposure, with potential implications for previous exposure estimates: A cross-sectional study. Environ. Health 2016, 15, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haq, M.E.U.; Akash, M.S.H.; Sabir, S.; Mahmood, M.H.; Rehman, K. Human exposure to bisphenol A through dietary sources and development of diabetes mellitus: A cross-sectional study in Pakistani population. Environ. Sci. Pollut. Res. Int. 2020, 27, 26262–26275. [Google Scholar] [CrossRef]
- Kim, J.K.; Khan, A.; Cho, S.; Na, J.; Lee, Y.; Bang, G.; Yu, W.J.; Jeong, J.S.; Jee, S.H.; Park, Y.H. Effect of developmental exposure to bisphenol A on steroid hormone and vitamin D3 metabolism. Chemosphere 2019, 237, 124469. [Google Scholar] [CrossRef]
- Amin, M.M.; Ebrahim, K.; Hashemi, M.; Shoshtari-Yeganeh, B.; Rafiei, N.; Mansourian, M.; Kelishadi, R. Association of exposure to Bisphenol A with obesity and cardiometabolic risk factors in children and adolescents. Int. J. Environ. Health Res. 2019, 29, 94–106. [Google Scholar] [CrossRef]
- Gonzalez, N.; Marques, M.; Cunha, S.C.; Fernandes, J.O.; Domingo, J.L.; Nadal, M. Biomonitoring of co-exposure to bisphenols by consumers of canned foodstuffs. Environ. Int. 2020, 140, 105760. [Google Scholar] [CrossRef]
- Bae, S.; Kim, J.H.; Lim, Y.H.; Park, H.Y.; Hong, Y.C. Associations of bisphenol A exposure with heart rate variability and blood pressure. Hypertension 2012, 60, 786–793. [Google Scholar] [CrossRef]
- Shankar, A.; Teppala, S.; Sabanayagam, C. Bisphenol A and peripheral arterial disease: Results from the NHANES. Environ. Health Perspect. 2012, 120, 1297–1300. [Google Scholar] [CrossRef]
- Han, C.; Hong, Y.-C. Bisphenol A, hypertension, and cardiovascular diseases: Epidemiological, laboratory, and clinical trial evidence. Curr. Hypertens. Rep. 2016, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Lind, P.M.; Lind, L. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atheroscler. 2011, 218, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Ning, B.; Waqar, A.B.; Niimi, M.; Li, S.; Satoh, K.; Shiomi, M.; Ye, T.; Dong, S.; Fan, J. Bisphenol A exposure enhances atherosclerosis in WHHL rabbits. PLoS ONE 2014, 9, e110977. [Google Scholar]
- Gao, X.; Wang, H.S. Impact of bisphenol A on the cardiovascular system–Epidemiological and experimental evidence and molecular mechanisms. Int. J. Environ. Res. Public Health 2014, 11, 8399–8413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasneci, A.; Lee, J.S.; Yun, T.J.; Shang, J.; Lampen, S.; Gomolin, T.; Cheong, C.C.; Chalifour, L.E. From the cover: Lifelong exposure of C57bl/6n male mice to Bisphenol A or Bisphenol S reduces recovery from a myocardial infarction. Toxicol. Sci. 2017, 159, 189–202. [Google Scholar] [CrossRef]
- Liang, Q.; Gao, X.; Chen, Y.; Hong, K.; Wang, H.S. Cellular mechanism of the nonmonotonic dose response of bisphenol A in rat cardiac myocytes. Environ. Health Perspect. 2014, 122, 601–608. [Google Scholar] [CrossRef]
- Belcher, S.M.; Gear, R.B.; Kendig, E.L. Bisphenol A alters autonomic tone and extracellular matrix structure and induces sex-specific effects on cardiovascular function in male and female CD-1 mice. Endocrinology 2015, 156, 882–895. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Q.; Liu, X.; Shen, Y.; Yu, P.; Chen, S.; Hu, J.; Yu, J.; Li, J.; Wang, H.S.; Cheng, X.; et al. Elevated serum Bisphenol A level in patients with dilated cardiomyopathy. Int. J. Environ. Res. Public Health 2015, 12, 5329–5337. [Google Scholar] [CrossRef] [Green Version]
- Weinstock-Guttman, B.; Jacobs, L.; Brownscheidle, C.; Baier, M.; Rea, D.; Apatoff, B.; Blitz, K.; Coyle, P.; Frontera, A.; Goodman, A.; et al. Multiple sclerosis characteristics in African American patients in the New York State Multiple Sclerosis Consortium. Mult. Scler. 2003, 9, 293–298. [Google Scholar] [CrossRef]
- Kendziorski, J.A.; Kendig, E.L.; Gear, R.B.; Belcher, S.M. Strain specific induction of pyometra and differences in immune responsiveness in mice exposed to 17α-ethinyl estradiol or the endocrine disrupting chemical bisphenol A. Reprod. Toxicol. (Elmsford N.Y.) 2012, 34, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Melzer, D.; Osborne, N.J.; Henley, W.E.; Cipelli, R.; Young, A.; Money, C.; McCormack, P.; Luben, R.; Khaw, K.T.; Wareham, N.J.; et al. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 2012, 125, 1482–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, E.; Dolinoy, D.C.; Mancuso, P. Perinatal bisphenol A exposures increase production of pro-inflammatory mediators in bone marrow-derived mast cells of adult mice. J. Immunotoxicol. 2014, 11, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Jochmanová, I.; Lazúrová, Z.; Rudnay, M.; Bačová, I.; Mareková, M.; Lazúrová, I. Environmental estrogen bisphenol A and autoimmunity. Lupus 2015, 24, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Rancière, F.; Lyons, J.G.; Loh, V.H.Y.; Botton, J.; Galloway, T.; Wang, T.; Shaw, J.E.; Magliano, D.J. Bisphenol A and the risk of cardiometabolic disorders: A systematic review with meta-analysis of the epidemiological evidence. Environ. Health A Glob. Access Sci. Source 2015, 14, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.; Ichinose, T.; Yoshida, S.; Takano, H.; Nishikawa, M.; Shibamoto, T.; Sun, G. Exposure to bisphenol A enhanced lung eosinophilia in adult male mice. Allergy Asthma Clin. Immunol. 2016, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Orton, S.-M.; Herrera, B.M.; Yee, I.M.; Valdar, W.; Ramagopalan, S.V.; Sadovnick, A.D.; Ebers, G.C. Sex ratio of multiple sclerosis in Canada: A longitudinal study. Lancet. Neurol. 2006, 5, 932–936. [Google Scholar] [CrossRef]
- Cai, S.; Rao, X.; Ye, J.; Ling, Y.; Mi, S.; Chen, H.; Fan, C.; Li, Y. Relationship between urinary bisphenol A levels and cardiovascular diseases in the U.S. adult population, 2003–2014. Ecotoxicol. Environ. Saf. 2020, 192, 110300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Shan, C.; Wang, Y.; Qian, L.L.; Jia, D.D.; Zhang, Y.F.; Hao, X.D.; Xu, H.M. Cardiovascular toxicity and mechanism of bisphenol A and emerging risk of bisphenol S. Sci. Total Environ. 2020, 723, 137952. [Google Scholar] [CrossRef]
- Bruno, K.A.; Mathews, J.E.; Yang, A.L.; Frisancho, J.A.; Scott, A.J.; Greyner, H.D.; Molina, F.A.; Greenaway, M.S.; Cooper, G.M.; Bucek, A.; et al. BPA alters estrogen receptor expression in the heart after viral infection activating cardiac mast cells and T cells leading to perimyocarditis and fibrosis. Front. Endocrinol. 2019, 10, 598. [Google Scholar] [CrossRef]
- Vitku, J.; Sosvorova, L.; Chlupacova, T.; Hampl, R.; Hill, M.; Sobotka, V. Differences in Bisphenol A and estrogen levels in the plasma and seminal plasma of men with different degrees of infertility. Physiol. Res. 2015, 64, S303–S311. [Google Scholar] [CrossRef] [PubMed]
- Rebuli, M.E.; Gibson, P.; Rhodes, C.L.; Cushing, B.S.; Patisaul, H.B. Sex differences in microglial colonization and vulnerabilities to endocrine disruption in the social brain. Gen. Comp. Endocrinol. 2016, 238, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Molina-Molina, J.; Amaya, E.; Grimaldi, M.; Sáenz, J.M.; Real, M.; Fernández, M.F.; Balaguer, P.; Olea, N. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol. Appl. Pharmacol. 2013, 272, 127–136. [Google Scholar] [CrossRef]
- Miao, S.; Gao, Z.; Kou, Z.; Xu, G.; Su, C.; Liu, N. Influence of bisphenol A on developing rat estrogen receptors and some cytokines in rats: A two-generational study. J. Toxicol. Environ. Health Part A 2008, 71, 1000–1008. [Google Scholar] [CrossRef]
- Cipelli, R.; Harries, L.; Okuda, K.; Yoshihara, S.I.; Melzer, D.; Galloway, T. Bisphenol A modulates the metabolic regulator oestrogen-related receptor-alpha in T-cells. Reproduction 2014, 147, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, S.; Wang, J.; Eltoum, I.; Desmond, R.; Lamartiniere, C.A. Chronic oral exposure to bisphenol A results in a nonmonotonic dose response in mammary carcinogenesis and metastasis in MMTV-erbB2 mice. Environ. Health Perspect. 2011, 119, 1604–1609. [Google Scholar] [CrossRef] [Green Version]
- Antoniak, S.; Iii, A.P.O.; Baunacke, M.; Williams, J.C.; Lee, R.D.; Weithauser, A.; Sheridan, P.A.; Malz, R.; Luyendyk, J.P.; Esserman, D.A.; et al. PAR-1 contributes to the innate immune response during viral infection. J. Clin. Investig. 2013, 123, 1310–1322. [Google Scholar] [CrossRef] [Green Version]
- Regitz-Zagrosek, V.; Becher, E.; Mahmoodzadeh, S.; Schubert, C. Sex steroid hormones. In Cardiovascular Hormone Systems: From Molecular Mechanisms to Novel Therapeutics, 1st ed.; Bader, M., Ed.; Wiley-Blackwell: Weinheim, Germany, 2008; pp. 39–64. [Google Scholar]
- Vitale, C.; Mendelsohn, M.E.; Rosano, G.M.C. Gender differences in the cardiovascular effect of sex hormones. Nat. Reviews Cardiol. 2009, 6, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Petri, M.A.; Coronado, M.J.; Cooper, L.T. Autoimmune heart disease: Role of sex hormones and autoantibodies in disease pathogenesis. Expert Rev. Clin. Immunol. 2012, 8, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Buskiewicz, I.A.; Huber, S.A.; Fairweather, D. Sex hormone receptor expression in the immune system. In Sex Differences in Physiology; Neigh, G., Mitzelfelt, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1, pp. 45–60. [Google Scholar]
- Frisancho-Kiss, S.; Nyland, J.F.; Davis, S.E.; Frisancho, J.A.; Barrett, M.A.; Rose, N.R.; Fairweather, D. Sex differences in coxsackievirus B3-induced myocarditis: IL-12Rbeta1 signaling and IFN-gamma increase inflammation in males independent from STAT4. Brain Res. 2006, 1126, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Howdeshell, K.L.; Peterman, P.H.; Judy, B.M.; Taylor, J.A.; Orazio, C.E.; Ruhlen, R.L.; Vom Saal, F.S.; Welshons, W.V. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ. Health Perspect. 2003, 111, 1180–1187. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, H.; Nagake, Y.; Makino, H. Determination of bisphenol a in effluents of hemodialyzers. Nephron 2001, 88, 376–378. [Google Scholar] [CrossRef]
- Draganov, D.I.; Markham, D.A.; Beyer, D.; Waechter, J.M., Jr.; Dimond, S.S.; Budinsky, R.A.; Shiotsuka, R.N.; Snyder, S.A.; Ehman, K.D.; Hentges, S.G. Extensive metabolism and route-dependent pharmacokinetics of bisphenol A (BPA) in neonatal mice following oral or subcutaneous administration. Toxicology 2015, 333, 168–178. [Google Scholar] [CrossRef]
- Huang, F.M.; Chang, Y.C.; Lee, S.S.; Yang, M.L.; Kuan, Y.H. Expression of pro-inflammatory cytokines and mediators induced by Bisphenol A via ERK-NFkappaB and JAK1/2-STAT3 pathways in macrophages. Environ. Toxicol. 2019, 34, 486–494. [Google Scholar] [CrossRef]
- Cimmino, I.; Oriente, F.; D’Esposito, V.; Liguoro, D.; Liguoro, P.; Ambrosio, M.R.; Cabaro, S.; D’Andrea, F.; Beguinot, F.; Formisano, P.; et al. Low-dose Bisphenol-A regulates inflammatory cytokines through GPR30 in mammary adipose cells. J. Mol. Endocrinol. 2019, 63, 273–283. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Luo, D.; Zhai, L.; Bai, Y.; Wei, W.; Sun, Q.; Jia, L. Bisphenol A increases TLR4–mediated inflammatory response by up-regulation of autophagy-related protein in lung of adolescent mice. Chemosphere 2021, 268, 128837. [Google Scholar] [CrossRef]
- Yang, D.; Xiao, C.; Long, F.; Su, Z.; Jia, W.; Qin, M.; Huang, M.; Wu, W.; Suguro, R.; Liu, X.; et al. HDAC4 regulates vascular inflammation via activation of autophagy. Cardiovasc. Res. 2018, 114, 1016–1028. [Google Scholar] [CrossRef] [Green Version]
- Fairweather, D.; Kaya, Z.; Shellam, G.R.; Lawson, C.M.; Rose, N.R. From infection to autoimmunity. J. Autoimmun. 2001, 16, 175–186. [Google Scholar] [CrossRef]
- Cooper, L.T., Jr.; Onuma, O.K.; Sagar, S.; Oberg, A.L.; Mahoney, D.W.; Asmann, Y.W.; Liu, P. Genomic and proteomic analysis of myocarditis and dilated cardiomyopathy. Heart Fail. Clin. 2010, 6, 75–85. [Google Scholar] [CrossRef]
- Takeuchi, T.; Tsutsumi, O. Serum bisphenol a concentrations showed gender differences, possibly linked to androgen levels. Biochem. Biophys. Res. Commun. 2002, 291, 76–78. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Frisancho-Kiss, S.; Coronado, M.J.; Frisancho, J.A.; Lau, V.M.; Rose, N.R.; Klein, S.L.; Fairweather, D. Gonadectomy of male BALB/c mice increases Tim-3 (+) alternatively activated M2 macrophages, Tim-3 (+) T cells, Th2 cells and Treg in the heart during acute coxsackievirus–induced myocarditis. Brain Behav. Immun. 2009, 23, 649–657. [Google Scholar] [CrossRef] [Green Version]
- Onyimba, J.A.; Coronado, M.J.; Garton, A.E.; Kim, J.B.; Bucek, A.; Bedja, D.; Gabrielson, K.L.; Guilarte, T.R.; Fairweather, D. The innate immune response to coxsackievirus B3 predicts progression to cardiovascular disease and heart failure in male mice. Biol. Sex Differ. 2011, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- De Coster, S.; Van Larebeke, N. Endocrine-disrupting chemicals: Associated disorders and mechanisms of action. J. Environ. Public Health 2012, 2012, 713696. [Google Scholar] [CrossRef]
- Peretz, J.; Flaws, J.A. Bisphenol A down-regulates rate-limiting Cyp11a1 to acutely inhibit steroidogenesis in cultured mouse antral follicles. Toxicol. Appl. Pharmacol. 2013, 271, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Acconcia, F.; Pallottini, V.; Marino, M. Molecular mechanisms of action of BPA. Dose-Respons A Publ. Int. Hormesis. Soc. 2015, 13, 1559325815610582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairweather, D.; Coronado, M.J.; Garton, A.E.; Dziedzic, J.L.; Bucek, A.; Cooper, L.T.; Brandt, J.E.; Alikhan, F.S.; Wang, H.; Endres, C.J.; et al. Sex differences in translocator protein 18 kDa (TSPO) in the heart: Implications for imaging myocardial inflammation. J. Cardiovasc. Transl. Res. 2014, 7, 192–202. [Google Scholar] [CrossRef]
- Yan, S.; Chen, Y.; Dong, M.; Song, W.; Belcher, S.M.; Wang, H.S. Bisphenol A and 17beta-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS ONE 2011, 6, e25455. [Google Scholar] [CrossRef] [Green Version]
- Koenig, A.; Buskiewicz, I.; Huber, S.A. Age-Associated changes in estrogen receptor ratios correlate with increased female susceptibility to Coxsackievirus B3-induced myocarditis. Front. Immunol. 2017, 8, 1585. [Google Scholar] [CrossRef] [Green Version]
- Weihua, Z.; Makela, S.; Andersson, L.C.; Salmi, S.; Saji, S.; Webster, J.I.; Jensen, E.V.; Nilsson, S.; Warner, M.; Gustafsson, J.A. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc. Natl. Acad. Sci. USA 2001, 98, 6330–6335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirmira, P.; Evans-Molina, C. Bisphenol A, obesity, and type 2 diabetes mellitus: Genuine concern or unnecessary preoccupation? Transl. Res. 2014, 164, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairweather, D.; Rose, N.R. Coxsackievirus-induced myocarditis in mice: A model of autoimmune disease for studying immunotoxicity. Methods 2007, 41, 118–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, J.M.; Fairweather, D.; Huber, S.A.; Cunningham, M.W. Autoimmune myocarditis, valvulitis, and cardiomyopathy. Curr. Protoc. Immunol. 2013, 101, 14–15. [Google Scholar] [CrossRef] [Green Version]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Cagen, S.Z.; Waechter, J.M., Jr.; Dimond, S.S.; Breslin, W.J.; Butala, J.H.; Jekat, F.W.; Joiner, R.L.; Shiotsuka, R.N.; Veenstra, G.E.; Harris, L.R. Normal reproductive organ development in CF-1 mice following prenatal exposure to bisphenol A. Toxicol. Sci. 1999, 50, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairweather, D.; Yusung, S.; Frisancho, S.; Barrett, M.; Gatewood, S.; Steele, R.; Rose, N.R. IL-12 receptor beta 1 and Toll-like receptor 4 increase IL-1 beta- and IL-18-associated myocarditis and coxsackievirus replication. J. Immunol. 2003, 170, 4731–4737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abston, E.D.; Coronado, M.J.; Bucek, A.; Bedja, D.; Shin, J.; Kim, J.B.; Kim, E.; Gabrielson, K.L.; Georgakopoulos, D.; Mitzner, W.; et al. Th2 regulation of viral myocarditis in mice: Different roles for TLR3 versus TRIF in progression to chronic disease. Clin. Dev. Immunol. 2012, 2012, 129486. [Google Scholar] [CrossRef]



| Cell Marker | Description | Glass | Plastic | p-Value |
|---|---|---|---|---|
| Complement | ||||
| CR3 | complement receptor 3 | 6.2 ± 1.1 | 5.5 ± 1.3 | 0.34 |
| C3aR1 | complement component 3a receptor 1 | 3.5 ± 0.6 | 4.7 ± 0.8 | 0.13 |
| C4b | complement component 4b | 4.6 ± 0.7 | 6.0 ± 1.2 | 0.15 |
| C5aR1 | complement component 5a receptor 1 | 3.3 ± 0.5 | 3.4 ± 0.5 | 0.46 |
| Fibro-inflammatory IL-1R2 | interleukin 1 receptor, type II | 1.9 ± 0.2 | 2.4 ± 0.4 | 0.08 |
| Timp-1 | tissue inhibitor matrix metalloproteinase 1 | 7.5 ± 1.6 | 9.6 ± 2.3 | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, K.A.; Macomb, L.P.; Morales-Lara, A.C.; Mathews, J.E.; Frisancho, J.A.; Yang, A.L.; Di Florio, D.N.; Edenfield, B.H.; Whelan, E.R.; Salomon, G.R.; et al. Sex-Specific Effects of Plastic Caging in Murine Viral Myocarditis. Int. J. Mol. Sci. 2021, 22, 8834. https://doi.org/10.3390/ijms22168834
Bruno KA, Macomb LP, Morales-Lara AC, Mathews JE, Frisancho JA, Yang AL, Di Florio DN, Edenfield BH, Whelan ER, Salomon GR, et al. Sex-Specific Effects of Plastic Caging in Murine Viral Myocarditis. International Journal of Molecular Sciences. 2021; 22(16):8834. https://doi.org/10.3390/ijms22168834
Chicago/Turabian StyleBruno, Katelyn A., Logan P. Macomb, A. Carolina Morales-Lara, Jessica E. Mathews, J. Augusto Frisancho, Alex L. Yang, Damian N. Di Florio, Brandy H. Edenfield, Emily R. Whelan, Gary R. Salomon, and et al. 2021. "Sex-Specific Effects of Plastic Caging in Murine Viral Myocarditis" International Journal of Molecular Sciences 22, no. 16: 8834. https://doi.org/10.3390/ijms22168834
APA StyleBruno, K. A., Macomb, L. P., Morales-Lara, A. C., Mathews, J. E., Frisancho, J. A., Yang, A. L., Di Florio, D. N., Edenfield, B. H., Whelan, E. R., Salomon, G. R., Hill, A. R., Hewa-Rahinduwage, C. C., Scott, A. J., Greyner, H. D., Molina, F. A., Greenaway, M. S., Cooper, G. M., & Fairweather, D. (2021). Sex-Specific Effects of Plastic Caging in Murine Viral Myocarditis. International Journal of Molecular Sciences, 22(16), 8834. https://doi.org/10.3390/ijms22168834






