Abstract
Xenoestrogens and phytoestrogens are referred to as “foreign estrogens” that are produced outside of the human body and have been shown to exert estrogen-like activity. Xenoestrogens are synthetic industrial chemicals, whereas phytoestrogens are chemicals present in the plant. Considering that these environmental estrogen mimics potentially promote hormone-related cancers, an understanding of how they interact with estrogenic pathways in human cells is crucial to resolve their possible impacts in cancer. Here, we conducted an extensive literature evaluation on the origins of these chemicals, emerging research techniques, updated molecular mechanisms, and ongoing clinical studies of estrogen mimics in human cancers. In this review, we describe new applications of patient-derived xenograft (PDX) models and single-cell RNA sequencing (scRNA-seq) techniques in shaping the current knowledge. At the molecular and cellular levels, we provide comprehensive and up-to-date insights into the mechanism of xenoestrogens and phytoestrogens in modulating the hallmarks of cancer. At the systemic level, we bring the emerging concept of window of susceptibility (WOS) into focus. WOS is the critical timing during the female lifespan that includes the prenatal, pubertal, pregnancy, and menopausal transition periods, during which the mammary glands are more sensitive to environmental exposures. Lastly, we reviewed 18 clinical trials on the application of phytoestrogens in the prevention or treatment of different cancers, conducted from 2002 to the present, and provide evidence-based perspectives on the clinical applications of phytoestrogens in cancers. Further research with carefully thought-through concepts and advanced methods on environmental estrogens will help to improve understanding for the identification of environmental influences, as well as provide novel mechanisms to guide the development of prevention and therapeutic approaches for human cancers.
1. Introduction
Estrogens are classified as either endogenous or exogenous, according to their origins []. Yet, both can bind to estrogen receptors (ERs), and/or many other nuclear receptors, simultaneously triggering genomic and transcriptomic changes in various organ systems. These changes can consequently contribute to the initiation and progression of multiple types of cancers, including the classical hormone-related breast and prostate cancer [,], as well as the non-classical hormone-related cancers, such as lung cancer [], colorectal cancer [], and gastric cancer [].
Endogenous estrogens (estradiol/E2, estrone/E1, and estriol/E3) in humans are produced by endocrine glands and/or by extra-glandular tissues through steroidogenesis enzymes, such as cytochrome P450 oxidases (CYPs), hydroxysteroid dehydrogenases (HSDs), and aromatase (CYP19) []. Although the sex gonads (ovaries and testes) and adrenal cortex are the primary sites of estrogen synthesis, extra-gonadal estrogens are also produced in the mammary glands, lungs, liver, and intestine, and play an equally important role in controlling biological activities []. The important roles of endogenous estrogens in the etiology of breast cancer have been extensively studied, leading to the development of well-tolerated endocrine therapy for breast cancer [].
Exogenous estrogens are those which are produced outside of the human body. In addition to synthetic estrogens developed for pharmacological purposes, a group of chemicals have been found to have estrogen-like activities, such as the ability to bind to ERs and to modulate the expression of estrogen-regulated genes. These exogenous and unexpected estrogen mimics include synthetic industrial compounds (xenoestrogens) and phytochemicals (phytoestrogens) []. They can alter the activities of ERs and send false signals, disrupting the normal estrogen response, changing physiological functions, and promoting diseases, including cancer []. Xenoestrogens include synthetic industrial chemicals used as solvents/lubricants and their byproducts such as plastics (bisphenol A, BPA), plasticizers (phthalates), flame retardants (polybrominated diphenyl ethers, PBDEs), pesticides (dichlorodiphenyltrichloroethane, DDT), and pharmaceutical agents (diethylstilbestrol, DES). The scientific consensus on xenoestrogens characterizes them as serious environmental hazards that have hormone-disruptive effects on both wildlife and humans []. Phytoestrogens are plant-produced compounds found in a wide variety of herbs and foods, most notably, soy-containing foods. Phytoestrogens, made naturally, often share structural features with endogenous E2, allowing phytoestrogens to cause estrogenic and/or anti-estrogenic effects []. They have been suggested to have a large spectrum of beneficial effects, including the reduction of cancer risk and postmenopausal symptoms []. However, there is also concern that phytoestrogens may act as endocrine disruptors that adversely affect health []. Based on available research findings, it is not clear whether the potential health benefits of phytoestrogens outweigh their risks. The potential for endocrine disruption by phytoestrogens needs to be considered as well []. Compared with endogenous estrogens, exogenous estrogens represent an under-recognized contributor to the development and progression of cancers. Further research on exogenous estrogens will help to provide insights for the identification of environmental influences, as well as provide new perspectives in the development of prevention and therapeutic approaches against human cancers.
At the molecular and cellular levels, xenoestrogens/phytoestrogens can imitate endogenous estrogens by enhancing and/or interrupting endogenous estrogen signaling pathways. They may exert either beneficial or harmful activities in humans depending on a set of complex factors such as exposure dose, time, intracellular signal transduction, and tissue complexity []. The binding of estrogens to ERs results in the activation of estrogen signaling pathways. There are intracellular ERs, including ER-alpha (ERα) and ER-beta (ERβ), as well as membrane-associated ERs, such as membrane ERs (mERs) and G Protein-Coupled Estrogen Receptors (GPER/GPR30) []. In addition to binding to ERs, exogenous estrogens can exert estrogenic activity by cross-talk with many other pathways, including pathways related to membrane-associated growth factor receptors, such as human epidermal growth factor receptor (EGFR/HER) and insulin-like growth factor 1-receptor (IGF1R) [], as well as nuclear receptors, including aryl hydrocarbon receptor (AhR) [], peroxisome proliferator-activated receptors (PPARs) [], and estrogen-related receptor alpha/gamma (ERRα/γ) []. Multiple synergistic signaling pathways may contribute to the outcome of exogenous estrogen exposure on overall health and/or cancer cells. At the tissue level, exogenous estrogens may exhibit another dimension of complexity by influencing both cancer cells and cancer-associated stromal cells, including immune cells, fibroblasts, and adipocytes []. At the systemic level, exposure to exogenous estrogens has been linked to increased breast cancer risk during certain life stages known as the windows of susceptibility (WOS) including the prenatal, pubertal, pregnancy, and menopausal transition periods, during which the mammary glands undergo anatomical and functional transformations. Therefore, environmental hormones (e.g., endocrine-disrupting chemicals/EDC) and certain therapeutics (e.g., prescribed for the coexisting medical conditions or in the form of the hormone replacement therapy) can influence breast cancer risk, development, or outcome []. Considering the spatial heterogeneity (variety of cell types) and temporal heterogeneity (various stages of differentiation) of cancer, xenoestrogens/phytoestrogens could display integrated activities in a tumor-selective and/or life stage(s)-specific manner.
The growing concerns of the exogenous estrogenic influence on health, especially towards cancer, have prompted considerable public attention and scientific interest. Knowledge of how these exogenous estrogens mimic endogenous estrogens, and how they exert their impacts on overall health, is crucial to resolve their impacts in the etiology of varying cancers. In this review, we conducted an exhaustive evaluation on the advanced research technology, molecular mechanisms, and ongoing translational studies in the development of prevention and therapeutic approaches towards human cancers. Here, we aim to provide thorough, updated understandings of xenoestrogens/phytoestrogens and their biological activities and mechanisms in cancer.
2. Xenoestrogens and Phytoestrogens: Definitions and Origins
2.1. Xenoestrogens: Synthetic Industrial Chemicals
Xenoestrogens are synthetic industrial chemicals found in various plastics, sealants, consumer goods, preservatives, and pesticides. They have unexpected activities by acting as either estrogen, triggering receptor pathways, or anti-estrogens, blocking normal estrogenic activity. These synthetic industrial chemicals can affect health and possibly trigger cancer []. The impact of these estrogen mimics is dictated by their binding affinities towards different types of ERs, predominantly ERα and ERβ, with ERα binding playing a pro-oncogenic role and ERβ typically playing a tumor-suppressive role [] (Table 1).

Table 1.
Various xenoestrogens and their implications for cancer. Compilation of ten types of xenoestrogens and their sources, biological and experimental evidence from pre-clinical studies, and implications towards cancer. Relative binding affinities were adapted from Kuiper et al. [], unless otherwise noted, with E2 set as 100.
An extensively studied xenoestrogen is bisphenol A (BPA). BPA was first used as a pharmaceutical estrogen in the 1930s but is now commonly used in the manufacture of polycarbonate plastics and epoxy resins used in food containers, water bottles, and other protective coatings []. BPA has been shown to disrupt ER activity by mimicking, enhancing, or inhibiting endogenous estrogens, causing a direct impact on the intracellular signal transduction pathways []. It has a relative binding affinity of 0.01 for both ERα and ERβ and has been strongly correlated with an increased risk for breast, prostate, and uterine cancer []. Because of this, many organizations concerned with the environment have suggested that the public avoid using items made with BPA [].
Another xenoestrogen of interest is the estrogenic pesticide DDT which has been banned in the US for almost 50 years. DDT was a commonly used pesticide sprayed across many agricultural fields and homes, acting as an insect neurotoxin to kill mosquitoes and other insect vectors that carry malaria, typhus, and other insect-borne diseases. It is still widely used, particularly in India and southern Africa []. Only later would it be known that DDT accumulates in adipose tissue and continues to persist in the environment []. Adverse environmental effects on non-insect species led to DDT being banned in many countries. Since then, scientists have continued to study its estrogenic activity and its impacts on gene expression and hormone synthesis through transgenerational studies. With a relative binding affinity of 0–0.01 and 0–0.02 to ERα and ERβ, respectively, DDT was previously not associated with increased cancer risk. However, DDT has been linked to increased breast cancer, especially if the tissue is exposed during certain WOS []. Following the banning of DDT, methoxychlor (DMDT) was synthesized as an alternative for vector control. It was used to protect pets, crops, and livestock from pests such as mosquitoes, cockroaches, and other insects. Despite growing evidence that DMDT is an ERα agonist and ERβ antagonist, with relative binding affinities of <0.01% for both, resulting in increased inhibition of estrogen binding, it is still currently being used today. DMDT has been associated with increased ovarian cancer risk, but not with other human cancers [].
Although numerous studies indicating the toxic effects of both BPA and DDT, these two xenoestrogens are still being used today. BPA has continued to be used in plastics despite epidemiological studies correlating its exposure with decreased sperm quality in males []. On the other hand, while DDT has been banned in the US for a half-century, it is still used in regions where malaria is endemic, is concerning as both epidemiological and clinical data have reported a decrease in semen volume, concentration, motility, and normal morphology to be associated with DDT exposure [].
Polychlorinated biphenyls (PCBs) are well-known xenoestrogens that are widely used to make various electrical equipment, such as transformers and capacitors, and are also found in hydraulic fluids and plasticizers. These materials eventually make their way into landfills, where PCBs can re-enter the environment by being released into the soil and air []. PCBs include many compounds that have relative binding affinities for ERα and ERβ between 0.01 and 3.4 and <0.01 and 7.2, respectively. These relative binding affinities were adapted from Kuiper et al. who used solid-phase competition experiments to calculate binding affinities by setting E2 as 100 []. Despite the almost two-fold difference in ERβ binding affinity, compared to ERα, PCBs are associated with an increased breast cancer risk, making them a significant topic of research [].
PBDEs are used as flame retardants, electrical equipment coating, construction materials, textiles, and furniture padding [,]. PBDEs encompass a large umbrella of compounds that have a relative binding affinity range of 1.3–20 to ERs []. Despite PBDEs exhibiting a higher binding affinity to ERs than that of PCBs, there has been no clear conclusion between PBDE exposure and breast cancer risk. Studies from our group recently demonstrated in breast cancer PDX models that PBDEs induced the expression of estrogen-responsive genes, especially genes related to cell proliferation in cancer cells [,].
Unlike the previously mentioned xenoestrogens, DES was synthesized as an “estrogen” and was previously prescribed to women to prevent miscarriages, premature labor, and pregnancy complications, before it, too, was realized to be carcinogenic. However, DES is no longer used to treat pregnancies at risk for miscarriage and menopausal symptoms but is still rarely used to treat prostate and breast cancer []. DES was the first synthetic estrogen and the first carcinogen to be shown to cross the placenta to cause cancer in the offspring. It has a potent relative binding affinity of 236% and 221% to ERα and ERβ, respectively, due to the additional hydrophobic interactions causing DES to be a potent transcriptional activator through genomic signaling [].
Ethinyl estradiol (EE2) is a xenoestrogen synthetically derived from E2. It works as an ovulation inhibitor and is mostly found in hormonal contraceptives. It has a strong relative binding affinity of 190% for ER and has been shown to increase cell proliferation, but at a lower rate than E2. There have been controversial data regarding EE2’s effects on cancer risk, but more recent studies have suggested that EE2 has little/no breast cancer risk, while having decreased ovarian, endometrial, colorectal, lymphatic cancer risks [].
Other xenoestrogens of interest include phthalates, nonylphenols (NP), and parabens. Phthalates are found in soft packaging plastic materials and can competitively inhibit E2 binding to ER [,]. Meanwhile, NP is used in various industrial processes and is found in consumer goods, such as laundry detergents, personal hygiene, automotive, and lawn care products. NP has a low relative binding affinity to ER of 0.0032–0.037, compared to the relative binding affinities of other xenoestrogens. Even so, they can exhibit an estrogen-like activity on ER+ breast cancer cells [,]. On the other hand, parabens are preservatives used in many consumable items such as beer, sauce, soda, and several cosmetics. They have a relative binding affinity range of 0.011–0.11 and 0.011–0.123 for ERα and ERβ, respectively, and can increase breast cancer cell proliferation and tumor size in animals [,]. These three types of xenoestrogens have all been implicated with breast cancer risk and their continued presence jeopardizes future health standards.
The presence of xenoestrogens in our environment and our everyday products warrants more research into their implications concerning cancer. Although some compounds have lower binding affinities than others, their impact on ERα, as well as their increased cancer risks, necessitates more attention to understanding the exact mechanisms and route of exposure by which they function.
2.2. Phytoestrogens: Plant-Derived Chemicals
Phytoestrogens are a group of estrogen mimics present in plants. They are becoming subjects of interest due to their estrogenic potentials and constant exposure to humans (Table 2).

Table 2.
Various phytoestrogens and their implications for cancer. Compilation of ten types of phytoestrogens and their sources, biological and experimental evidence from pre-clinical studies, and implications for cancer. Relative binding affinities were adapted from Kuiper et al. [], unless otherwise noted, with E2 set as 100.
Soybeans, a staple in many Asian cuisines, contain two major isoflavones: genistein (GEN) and daidzein (DAI). Although similar in structure and function, GEN has both stronger binding to ERβ than ERα. For GEN, the difference is 20-fold, and for DAI the difference is five-fold. This stronger binding affinity for ERβ, combined with the observation that GEN results in a decrease of ERα mRNA and protein levels [,,,,,], has led to clinical trials in cancer prevention and treatment. To date, GEN and DAI have been shown to reduce breast cancer-related gene expression [,], and reduce the increase in serum PSA during prostate cancer development []. Both GEN and DAI are well tolerated with minimal toxicity.
Other phytoestrogens, such as quercetin (QUE) [,,,,,,], apigenin (APE) [,,,,,], resveratrol (RES) [,,,,], myricetin (MYR) [,,,,,], and are found in many berries, leafy greens, and wine. Although their relative binding affinity differences between ERα and ERβ are not as great as in GEN and DAI, these compounds have been investigated due to their widespread presence in plants and extensive human consumption. More specifically, QUE, APE, and even RES have been noted to exhibit a biphasic effect; at low concentrations, these phytoestrogens display estrogenic activity, whereas, at higher concentrations, they display more protective anti-estrogenic activity [,,,,,,,,,,,]. Like GEN, RES has been extensively studied in many clinical trials. It has been shown that RES can significantly decrease epigenetic gene methylation in women at high risk for breast cancer and suppresses the important WNT signaling pathway [,,,,]. These findings support the chemo-preventive effects of RES as possible cancer therapeutic. However, health beneficial effects of RES have not been established due to non-physiological research designs.
Kaempferol (KPF), found in tea, pollen, and garlic, has been shown to decrease breast cancer risk possibly due to its 30-fold difference in ERα and ERβ relative binding affinities. KPF, although fairly novel, is an exciting phytoestrogen due to its ability to decrease cancer cell growth and increase apoptosis [,,,,,]. On the other hand, luteolin (LUT) is another more recently studied phytoestrogen found in seasonings that exhibits similar results as KPF: increasing cell cycle arrest, apoptosis, and decreasing proliferation [].
Of all the reviewed phytoestrogens, curcumin (CUR) has been the most evaluated in terms of both pre-clinical and clinical investigations. CUR is derived from the plant Curcuma longa, otherwise known as turmeric. In breast cancer cells and tissues exposed to CUR, it has been shown to decrease ER expression, leading to decreased cell proliferation, migration, invasion, and angiogenesis, while increasing apoptosis, cell cycle arrest, and senescence in breast cancer cell lines [,,]. In clinical trials, CUR has been shown to slightly reduce fatigue in women with advanced, metastatic breast cancer and can be used as an anti-oxidation, anti-cancer agent that does not compromise the therapeutic efficacy of radiotherapy [,,,].
Meanwhile, coumestrol (COU), found in various beans, leafy greens, and sunflower seeds, has the strongest relative binding affinity for ERβ at 140. Dietary COU intake has been shown to decrease ERα mRNA and protein levels like GEN, indicating possible usage as an anti-cancer therapeutic [,,,].
In summary, the literature suggests that phytoestrogens can act as anti-cancer agents by competing with endogenous estrogens, particularly with differences in relative binding to different ERs. While outcomes vary with tissue location and cancer types, the physiologically relevant research into phytoestrogens seems promising and will help to better understand the biological activities of these plant-produced estrogen mimics.
3. Advanced Methodology in Studying the Biological Effects of Xenoestrogens and Phytoestrogens
While population-based studies have defined correlations with environmental estrogen exposure and cancer, and cell/molecular studies have revealed some mechanisms for these effects, several novels approach to investigating the estrogen-cancer link are revealing more sophisticated insights [,]. Recently, nonbiased “multi-omic” approaches, including genomics, transcriptomics, proteomics, and metabolomics, have been widely applied to reveal the mechanisms at the molecular level [,,]. In addition to these in vitro studies, the use of in vivo rodent models has been useful for studying the phenotypic changes and mechanisms of exogenous estrogen exposure. The main advantage of in vivo models is the ability to test a given chemical in a more relevant setting to humans so that the results can be more reasonably extrapolated at the tissue and systemic levels []. A better understanding of the “pros and cons” of each methodology and proper exploitation, or a transdisciplinary approach, will better progress the study of the causal relationship between exogenous estrogen exposure and human cancers. The Breast Cancer and the Environment Research Program (BCERP), funded by the US government, is a multi-institutional, multi-disciplinary group of teams of laboratory-based scientists, epidemiologists, social scientists, and clinicians with various specialties and from different perspectives. Our group is included in this program (https://bcerp.org/ (accessed on 16 May 2021). In this section, we describe our experience within the program context in developing new ways to study the biological effects of xenoestrogens and phytoestrogens in breast cancer.
3.1. In Vitro Models with Cultured Cells
In vitro models with cultured cells are powerful tools for screening and identifying the estrogenic activity of chemicals existing in the living environment [,]. E-screen is a cell-proliferation assay that uses estrogen-dependent cancer cell lines to elucidate the estrogenic effects of these environmental chemicals [,]. Additionally, many gene reporter assays have been developed using human cancer cell lines transfected with reporter genes to assess whether a given compound could induce ER-mediated gene expressions [,]. We previously generated a model cell line by stable transfection with the estrogen-responsive element (ERE)-driven luciferase reporter into an aromatase-overexpressing MCF7 human breast cancer cell line, named MCF7aro-ERE []. We successfully developed the AroER Tri-screen assay system with MCF7aro-ERE, which is an improved model that is suitable for a high-throughput screening system []. AroER Tri-screen assay shows luciferase activity when estrogen-bound ERs induce gene expression by binding to the ERE promoter region. The AroER Tri-screen is a robust bioanalytical assay that has a high signal-to-background ratio, enabling the application of a high-throughput format of up to 1536 wells in a single experiment. In addition, this system is a multiplex assay, used not only for the screening of ER-agonistic chemicals but also for screening of ER-antagonistic or an aromatase inhibitor (AI)-like compound []. The AroER Tri-screen system has been adopted into a collaborative project called “Tox21” (https://tox21.gov/ (accessed on 16 May 2021)), which aims to develop target-specific, mechanism-based, and biologically relevant in vitro assays to screen for health-hazard chemicals. In this Tox21 program, we have utilized AroER Tri-screen to test a library of 10,000 compounds for anti-aromatase activity. The screen revealed 10 novel inhibitors. For example, imazalil, a widely used agricultural fungicide, showed irreversible and long-lasting anti-aromatase activity []. These high-throughput screening assays remain important for exploring exogenous estrogens among a large collection of chemicals, in newly developed consumer products, industrial chemicals, and drugs, as well as in unknown phytoestrogens from foods or plants, in a timely and reproducibly manner.
3.2. In Vivo Models with Rodents
In vivo models with rodents have been useful for studying the phenotypic changes and mechanisms of exogenous estrogen exposure. These models include carcinogenesis models and therapeutic models, with the former consisting of healthy or genetically engineered mice upon long-term exposure and the latter using established tumor xenografts. Conventional xenograft models using human cell lines or spontaneous mouse tumors have the limitations that they do not necessarily recapitulate the nature of original human cancers, leading to a lack of predictive value of the results in a clinical setting [,]. More specific and cancer-relevant PDX models, generated by the direct implantation of tumor fragments from human patients into immune-deficient mice, are increasingly being utilized for translational cancer research because they have been proven to maintain many of the biological properties of human cancers, such as genetic features, histology, and tumor cell population heterogeneity [,,]. Many studies have reported that the response to treatments in PDX models correlates well with the results of treatment in the patients whose tumors supplied the PDX cancer. Therefore, PDX models provide a suitable option for studying the effects of exogenous estrogens on human cancers [,,]. For example, the xenoestrogen methylparaben was shown to promote tumor growth and stem-like features using an ER+ breast cancer PDX model []. Our group has also recently performed bulk RNA-seq analysis on an ER+ breast cancer PDX treated with PBDEs, concluding that PBDEs induced the expression of estrogen-responsive genes, especially those related to cell proliferation []. Other groups also reported the effect of GEN [,] and DES [] on prostate cancer PDX models. Additionally, another study investigated the potential chemo-enhancing effects and mechanisms of GEN and its analog AXP107-11, which showed an improved bioavailability of AXP107-11 for clinical use compared to GEN []. These findings suggest that PDX models would help further the understanding of the biological effects of exogenous estrogens as relevant models of human cancers.
In addition to its advantage in mimicking the natural situation of tumor development, PDX models include all the cells in the surrounding tissues, rather than just the cancer cells, enabling the assessment of the biological effects on the whole population in a tissue and the specific cell-to-cell interactions []. Furthermore, we can observe various phenotypical changes, such as tumor invasion, metastasis, or immunomodulation [], beyond simple cell proliferation or gene expression that can easily be observed in in vitro models. In contrast, there are also several disadvantages of in vivo models against in vitro models. First, animal models are often time- and cost-consuming, which limits their usefulness for exploratory studies as discussed in the in vitro screening assays []. Second, the experimental dosage used in animal studies is often much higher than typical human exposures, making the extrapolation to the human situation problematic []. In the real situation in human tumor development, exposure to low doses of xenoestrogens may result in subtle effects that accumulate over time. These are difficult to observe in animal studies. In addition, ethical considerations of animal use must be considered, especially when testing compounds in the cosmetic or consumer product industry [].
3.3. Single-Cell RNA-Sequencing (scRNA-seq)
Tumor development and progression are widely recognized as complicated processes in which tumor cells, and many other contributors such as fibroblasts, immune cells, and other stromal cells from the tumor microenvironment, play distinct roles by their interactions with one another. Thus, the heterogeneity of cell populations within tissues of interest has been one of the major limitations of previous, especially with in vitro models. Additionally, even in the in vivo models, it is sometimes a challenge to capture the effect of estrogenic compounds in each type of cell, especially when those cells are too minor to cause apparent phenotypic changes. The recent development of scRNA-seq provides transcriptomic information at a single-cell resolution, enabling the ability to profile each isolated cell’s characteristics from a given tissue or organ [,]. This unprecedented capability of scRNA-seq technology allows us to capture subtle changes caused by xenoestrogens/phytoestrogens and their targeted cells, not only in the tumor cells of interest but also in the surrounding stromal cells (e.g., fibroblasts or immune cells), furthering the understanding of the potential interactions between these heterogeneous cell populations. Thus, this information can greatly help to reveal the mechanisms of cancer-initiating and/or promoting the effects of exogenous estrogens.
We have demonstrated that this state-of-art technology can overcome some of the limitations of the pre-existing in vitro and in vivo models. We previously reported a study using scRNA-seq analysis on normal mouse mammary glands of a surgically menopaused mouse model treated with estrogen and PBDEs []. Our results suggest that PBDEs enhance estrogen-mediated mammary gland regrowth through the up-regulation of Areg expression in mammary epithelial cells, which in turn affects its cognate receptor, EGFR expressed on mammary fibroblasts and further modulates the recruitment of tumor-promoting M2 macrophages. These findings support the hypothesis that PBDE exposure with estrogen treatment increases the risk of breast cancer development during a critical period, menopause. ScRNA-seq analysis also provides fundamental insights into the regulatory activity of PBDEs on distinct populations in normal mammary glands in the presence of estrogen. Furthermore, we expanded our scRNA-seq analysis to study the effect of PBDEs on the differentiation of mammary epithelial cells by integrating human and mouse datasets from our and others’ studies, thereby constructing a mammary cell gene expression atlas []. One group utilized scRNA-seq technology, although not directly related to cancer research, to investigate the transcriptomic changes induced by a known xenoestrogen, di (2-Ethylhexyl) phthalate (DEHP), exposure. They revealed the reproductive toxicity of DEHP in murine germ cells and pre-granulosa cells at a single-cell level []. Although scRNA-seq has some limitations, such as technical noise from the cell preparation process, loss of spatial information, higher costs than other models, and requirement for freshly prepared samples [,,], it serves as an excellent option for studying the complicated activity of xenoestrogens/phytoestrogens in heterogeneous cell populations of target tissues.
4. Biological Activities and Mechanisms of Xenoestrogens and Phytoestrogens in Cancers
4.1. Effects of Xenoestrogens and Phytoestrogens on the Bioavailability and Formation of Endogenous Estrogens
Human sex hormone-binding globulin (hSHBG) is a high-affinity binding protein in the bloodstream for endogenous estrogens, modulating the bioactivity of estrogens by limiting their diffusion into target tissues and cells []. By binding to hSHBG, xenoestrogens and phytoestrogens could modulate the bioavailability of endogenous estrogens []. Meanwhile, extra-glandular tissues can also synthesize estrogens from adrenal dehydroepiandrosterone (DHEA) and androstenedione (4-dione) by steroidogenesis enzymes, such as aromatase and 3beta- and 17beta-hydroxysteroid dehydrogenases (3β-HSDs and 17β-HSDs) []. These exogenous estrogens can also disrupt extra-glandular estrogen formation via interruption of steroidogenesis enzymes (Figure 1).
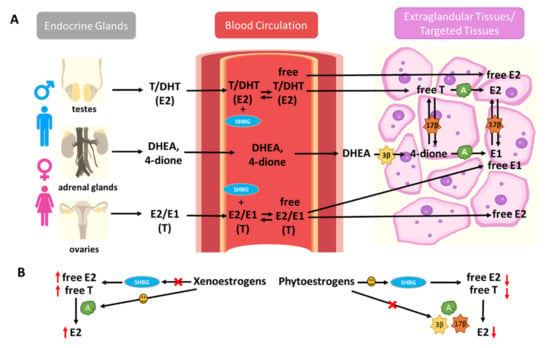
Figure 1.
Xenoestrogens and phytoestrogens modify endogenous estrogen bioavailability and formation. (A) Endogenous estrogens are produced by endocrine glands (ovaries, testes, and adrenal glands) and transported to endocrine-responsive tissues through blood circulation. Human sex hormone-binding globulin (hSHBG) is a high-affinity binding protein in the bloodstream for endogenous estrogens, modulating the bioactivity of estrogens by limiting their diffusion into target tissues and cells. Extra-glandular tissues can also synthesize estrogens from adrenal dehydroepiandrosterone (DHEA) and androstenedione (4-dione) by steroidogenesis enzymes, such as aromatase (CYP19) and 3beta- and 17beta-hydroxysteroid dehydrogenases (3β-HSDs and 17β-HSDs). (B) Xenoestrogens and phytoestrogens can modify the bioavailability of circulating endogenous estrogens by interfering with hSHBG binding. Xenoestrogens can also disrupt extra-glandular estrogen formation via interruption of steroidogenesis enzymes (A, aromatase, 3β, 3β-HSDs, and 17β, 17β-HSDs). Xenoestrogens are more likely to displace endogenous E2 from hSHBG binding sites, enhance E2 formation by inducing the steroidogenesis enzyme expressions, such as aromatase, consequently promoting the estrogenic responses in humans. However, phytoestrogens may lead to a decrease in plasma E2 levels via interaction with hSHBG levels and interruption of estrogen metabolism.
Xenoestrogens, such as BPA, NP, and monobutyl phthalate (MBP), have displayed a high binding affinity for hSHBG, with reversible and competitive binding activity for both testosterone and E2. Therefore, xenoestrogens may displace endogenous testosterone and E2 from hSHBG binding sites, leading to an increased level of free-form E2 in circulation. On the other hand, hSHBG may transport these xenoestrogens to target tissues and facilitate their diffusion into target cells []. Moreover, studies have found that xenoestrogens, such as BPA, exert their impacts on steroidogenesis by promoting aromatase expression in the adrenal cortex and ovaries; the increase of aromatase expression is responsible for the E2 increase [,]. This effect promotes the activation of ERα, which plays a pivotal role in the regulation of endocrine disorders such as cancer.
The flavonoid phytoestrogens, such as GEN and naringenin, have also been identified as hSHBG ligands []. Several studies in women have suggested a significant positive correlation between the intake of phytoestrogens and the concentration of plasma hSHBG []. Studies have also shown that the intake of phytoestrogens is negatively correlated with the plasma percentage of free-form E2 []. Such observations were further validated in large cross-sectional studies in postmenopausal women. Results have shown that phytoestrogen exposure is associated with lower plasma E2 in postmenopausal women and interacts with hSHBG levels and estrogen metabolism []. Dietary phytoestrogens suppress adrenal and ovarian 3β-HSDs and aromatase gene expression, therefore, decreasing estrogen formation []. Isoflavones have also been shown to exert inhibitory effects on 17β-HSD1 []. Amongst the phytoestrogens, isoflavones are the most potent inhibitors of aromatase []. Many phytoestrogens decrease the plasma estrogen levels, pointing towards a possible inhibitory effect in the regulation of E2 synthesis via suppressing the expression and activity of aromatase [,,].
In summary, xenoestrogens and phytoestrogens may have distinct effects on the bioavailability and formation of endogenous estrogens. Xenoestrogens are more likely to displace endogenous E2 from hSHBG binding sites, enhance E2 formation by inducing steroidogenesis enzyme expression, such as aromatase, consequently promoting estrogenic responses in humans. Meanwhile, supplementation with phytoestrogens may lead to decreased plasma E2 levels via interaction with hSHBG levels and interruption of estrogen metabolism (Figure 1).
4.2. Effects of Xenoestrogens and Phytoestrogens on Estrogen Receptor Activation and Signaling
The variety of ERs reflects the diversity of receptor mechanisms involved with xenoestrogen and phytoestrogen effects on cells. This has relevance to the effects on these estrogenic molecules in cancer. There are two types of ERs: intracellular ERα and ERβ and membrane-associated mERs and GPER []. The intracellular ERα and ERβ belong to a group of nuclear receptors that act as ligand-activated transcription factors. They are also the primary receptors for both endogenous and exogenous estrogens. ERs are activated in four ways (Figure 2): (1) the classical genomic pathway where estrogens are bound to ERs that will activate the transcription of target genes, (2) the non-classical genomic pathway involving ER interactions with other transcription factors such as activator protein 1 (AP-1), including c-Fos, c-Jun, and c-myc, (3) the E2-independent pathway which activates ERs through phosphorylation induced by growth factor (EGFR/IGFR/Her2/3) signaling cascades [], and (4) the non-genomic pathway involving membrane-associated ERs such as mERs and GPER [].
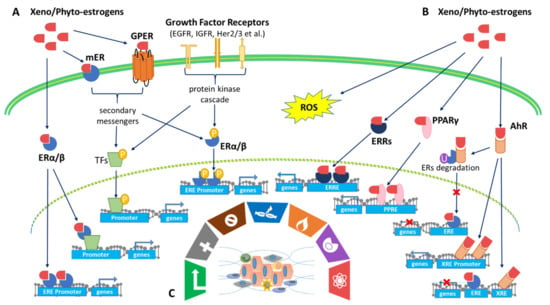
Figure 2.
Xenoestrogens and phytoestrogens
modulate multiple estrogen-mediated signaling pathways to shape the hallmarks
of cancer. (A) Activation of estrogen receptor signaling. There are two
types of ERs: intracellular ERα and ERβ and membrane-associated mERs and GPER []. ERs are activated in four manners: (1) the
classical genomic pathway where estrogens are bound to ERs that will activate
the transcription of target genes, (2) the non-classical genomic pathway
involving ERs interactions with other transcription factors (TFs) such as
activator protein 1 (AP-1), including c-Fos, c-Jun, c-myc, (3) the
E2-independent pathway which activates ERs through phosphorylation induced by
growth factors (EGFR/IGFR/Her2/3) signaling cascades [],
and (4) the non-genomic pathway involving membrane-associated ERs such as mERs and
GPER. (B) Co-activation of AhR/PPARγ/ERRγ/ROS pathways.
Xenoestrogens/phytoestrogens activate AhR signaling pathways and cross-talk
with ER pathways: (1) AhR competes with ERs for promoter binding, leading to
inhibition of ER signaling, (2) activation of AhR signaling regulates E2
production by controlling the gene expression of CYP19, also known as
aromatase, and (3) activation of AhR signaling ubiquitinates ERs for
degradation via the proteasome, leading to inhibition of ER signaling.
Xenoestrogens/phytoestrogens activate peroxisome proliferator-activated
receptors (PPARs) and estrogen-related receptor gamma (ERRγ).
Xenoestrogens/phytoestrogens could also induce oxidative stress-mediated
signaling by generating reactive oxygen species (ROS). (C) Shaping the hallmarks
of cancer. These features are linked to cell cycle and checkpoint disruption ( ), cell apoptosis and death
reprogramming (
), cell apoptosis and death
reprogramming ( ), growth suppressor evading (
), growth suppressor evading ( ), genome instability and
mutation (
), genome instability and
mutation ( ), tumor inflammation-promoting
(
), tumor inflammation-promoting
( ), immune response destruction
(
), immune response destruction
( ), redox homeostasis
interrupting, and metabolic rewiring (
), redox homeostasis
interrupting, and metabolic rewiring ( ).
).
The activation of ER signaling pathways plays a vital role in the malignant progression of multiple cancers by comprehensively regulating downstream genes. ERα activation has been shown to exert pro-oncogenic responses while ERβ activation has been shown to exert tumor-suppressive responses. These differences play a large role in the overall prognosis of patients with cancers [,]. Most xenoestrogens, including PBDE congeners and BPA, are agonists of both ERα and ERβ. They can mimic endogenous estrogens by interacting with ERα and ERβ, leading to phenotypic changes in cell proliferation, apoptosis, or migration []. These cellular changes contribute to the development and progression of hormone-related cancers in the breast, ovaries, and prostate []. In recent studies, many lines of evidence have also revealed that BPA exerts its function via activation of human estrogen-related receptor gamma (ERRγ), which behaves as a constitutive activator of transcription []. BPA preserves ERRγ’s basal constitutive activity and protects the selective ER modulator, 4-hydroxytamoxifen from its deactivation of ERRγ. This provides possible support that BPA exposure from the environment may potentially induce tamoxifen resistance to breast cancer treatment [].
However, according to the literature, phytoestrogens such as GEN, DAI, and COU, along with others, exert a much stronger binding affinity for ERβ than for ERα []. For instance, GEN is a full ERβ agonist and, to a much lesser extent (~20-fold) of ERα []. Therefore, it is believed that the anti-cancer effects of these phytoestrogens may be due to their interactions with ERβ. ERβ in MCF7 breast cancer cells increases the anti-cancer efficacy of GEN by affecting cell cycle transition []. Several studies have also reported that GEN inhibits the cell cycle division of human prostate cancer cells via ERβ activation []. It is worth noting that the ERα and ERβ may mediate distinct biological effects in many tissues such as the mammary glands, prostate, lungs, and intestine in both males and females. Therefore, the ERα/ERβ ratio is an important factor to consider when predicting the response of cancer cells to phytoestrogen treatment []. In addition to ERα/ERβ, flavone and isoflavone phytoestrogens were also ligands of estrogen-related receptors (ERRα/ERRγ). These phytoestrogens induced the activity of ERRs [].
Although the majority of xenoestrogens/phytoestrogens are believed to exert their biological effects through ERα and ERβ modulation, many of these compounds also activate ERs via a non-genomic pathway which involves mERs and GPER [,]. Especially in cancer cells, exogenous estrogens can bind to mERs and/or GPER and activate signaling cascades (Akt, MAPK) through the recruitment of protein kinases (Src and PI3K), therefore mediating rapid transcriptional events []. Xenoestrogens/phytoestrogens also activate ERs through phosphorylation induced by growth factor signaling cascades, for instance, the crosstalk between the EGFR/IGFR/Her2/3 growth factor signaling pathways []. Phytoestrogens such as GEN can inhibit MCF7 cell proliferation by inactivating the IGF-1R-PI3K/Akt pathway and decreasing the Bcl-2/Bax mRNA and protein expressions []. However, xenoestrogens such as BPA and NP can mediate EGFR signaling activation in lung cancer, causing an increase in proliferation, clonogenic growth, and tumor spheroid formation [].
In conclusion, xenoestrogens/phytoestrogens mimic endogenous estrogens by binding to and activating different types of ERs (ERα, ERβ, mER, and GPER), orphan nuclear receptors (such as ERRα and ERRγ), and cross-talking with many other membrane-associated growth factor receptors (Figure 2). Xenoestrogens/phytoestrogens could act as either an agonist or display antagonistic activity, when endogenous estrogen is present, in a tissue-selective and spatiotemporal manner in human cancers.
4.3. Effect of Xenoestrogens/Phytoestrogens on Activation of AhR/PPARγ/ROS Pathways
AhR (aryl hydrocarbon receptor), binds many types of molecules, including phytoestrogens and xenoestrogens, entering the nucleus and acting as a transcription factor. Because it is also activated by many environmental pollutants. AhR has been called a “xenobiotic sensor”. A major action for activated AhR is enhanced transcription of genes encoding CYPs, some of which are involved in estrogen biosynthesis []. In addition, there are interactions between the AhR and ER signaling pathways, with AhR agonists having anti-estrogenic activities. The mechanisms involve (1) AhR competes with ERs for promoter binding, leading to inhibition of ERs signaling, (2) activation of AhR signaling regulates E2 production by controlling the gene expression of CYP19, and (3) activation of AhR signaling ubiquitinates ERs for degradation via the proteasome, leading to inhibition of ER signaling (Figure 2) []. Phytoestrogens from soy (GEN, DAI, and S-equol) and licorice roots (liquidities) negatively regulate ERs activation via binding to AhR []. However, xenoestrogens, like PCBs and BPA, act selectively through AhR xenobiotic response element (XRE) and enhance AhR target-gene expression, including CYP19, therefore increasing endogenous E2 production []. Both ERs and AhR should be considered mediators of the biology, toxicology, and pharmacology of exogenous estrogens.
In addition to the AhR signaling pathway, PPARs can also be activated by exogenous estrogens. PPARs belong to a family of nuclear receptors that act as transcription factors. They have comprehensive impacts on diabetes, adipocyte differentiation, inflammation, and cancer []. PPARα stimulation appears to inhibit the proliferation of human colon cancer cell lines and reduce polyp formation in the mouse model of familial adenomatous. PPARβ (also referred to as PPARδ) has been described in the regulation of keratinocyte differentiation, apoptosis, inflammation, and wound healing. PPARγ not only controls the expression of genes involved in differentiation but also negatively regulates the cell cycle []. BPA analogs have been reported to be ligands of ERs and PPARs; the greater their capability to activate PPARγ, the weaker their estrogenic potential is []. Meanwhile, the activation of PPARγ by GEN can down-regulate the transcriptional activity of ERα or ERβ in breast cancer cells [,]. Xenoestrogens/phytoestrogens concurrently activate ERs and PPARs, which may exert opposite biological effects. As a result, the balance between activated ERs and PPARs determines the biological effects of exogenous estrogens and estrogen-like mimics on cells and tissues (Figure 2).
In addition to regulating cell functions through interactions with estrogen signaling, xenoestrogens and phytoestrogens can affect cells through oxidative stress signaling by generating reactive oxygen species (ROS) within healthy cells or cancer cells (Figure 2). Oxidative stress-mediated signaling is a double-edged sword in cancer cell behavior. Oxidative stresses are suggested to play important roles in estrogen-induced breast carcinogenesis []. There is growing evidence that the induction of ROS by BPA may contribute significantly to its genomic toxicity and carcinogenic potential [,]. On the contrary, many chemotherapeutic strategies are designed to significantly increase cellular ROS levels, leading to tumor cell apoptosis []. As noted above, the phytoestrogen COU is a potential chemotherapeutic agent for breast cancer. Evidence indicates that COU acts by inducing intracellular ROS, coupled with DNA fragmentation, up-regulation of p53/p21, cell cycle arrest, mitochondrial membrane depolarization, and caspases 9/3 activation [].
4.4. Effects of Xenoestrogens/Phytoestrogens on Modulating the Hallmarks of Cancer
Xenoestrogens/phytoestrogens primarily modulate the hallmarks of cancer cells by inappropriately activating ERs, cross-talking with membrane-associated growth factor receptors (EGFR/IGFR/Her2/3), and many other nuclear receptors (AhR/PPARs/ERRα/γ). In the presence of active signaling, the hallmarks acquired by cancer cells are modulated and linked to cell cycle and checkpoint disruption, metabolic rewiring, regulation of apoptosis, and redox homeostasis []. In addition to cancer cells, tumors exhibit another dimension of complexity by recruiting heterogeneous cell types and creating a “tumor microenvironment”. These cells include tumor-infiltrating immune cells, cancer-associated fibroblasts (CAFs), cancer-associated adipocytes (CAAs), and more []. The impact of exogenous estrogens on tumor-associated cells is significant (Figure 3).
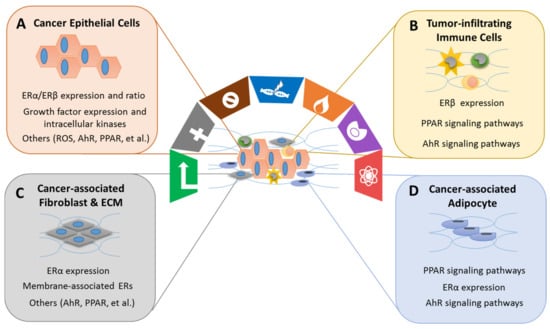
Figure 3.
Xenoestrogens/phytoestrogens modulate the cancer cells and cancer-associated cells. Xenoestrogens/phytoestrogens modulate the cancer cells and cancer-associated cells by inappropriately activating ERs, cross-talking with membrane-associated growth factor receptors (EGFR/IGFR/Her2/3) [], and many other transcriptional factors (AhR/PPARs/ERRα/γ). In the presence of active signaling, the hallmarks acquired by cancer have been are modulated and linked to (A) cancer epithelial cells, (B) tumor-infiltrating immune cells, (C) cancer-associated fibroblasts and extracellular matrix (ECM), and (D) cancer-associated adipocytes.
Immune modulation has been recognized as an emerging hallmark feature of cancer, including tumor-promoting inflammation and evading immune destruction []. Tumor-promoting inflammation is mainly characterized by the activation of innate immune cells, such as monocytes, macrophages, and natural killer cells (NK) often within the tumor environment. The innate immune cells subsequently increase the release of pro-inflammatory mediators such as TNF-α, IL-6, and IL-1β, which in turn stimulates the production of cyclooxygenase products and promote cancer progression. Evading immune destruction involves mechanisms of the adaptive immune cells (cytotoxic T cells, T helper cells, and B cells) by modulating certain immune checkpoint pathways []. The receptors (ERs, PPARs, and AhR) that bind xenoestrogens/phytoestrogens are present in lymphocytes, macrophages, neutrophils, and other immune cells []. Exposure to xenoestrogens increases the incidence of inflammation by activation of AhR and PPARγ []. Considerable research has found that GEN, a natural PPARγ agonist found in soy foods, exhibits anti-inflammatory activities via TNFα-induced NF-κB-dependent IL-6 gene expression by interfering with the mitogen- and stress-activated protein kinase 1 activation pathways [,].
However, the mechanisms of xenoestrogens/phytoestrogens via ER pathways in human immune cells have not been well studied. Their molecular mechanisms are based on interactions with ERα and ERβ, as well as with membrane associated GPER []. The expression of ERs in immune cells has various levels. For example, human CD4+ T cells and macrophages express higher levels of ERα than ERβ []. Xenoestrogens (BPA, DEHP, and PBDE) tend to stimulate M2-like tumor-associated macrophage (TAM) polarization and migration via simultaneously activating ERα or ERβ signaling pathways []. ERβ is involved in mediating estrogen action on NK cell activity []. Isoflavones such as GEN decrease IL-12/IL-18-induced IFN-γ production in NK cells without altering NK cell cytotoxicity. The regulation of NK cells via ERβ may be linked as a benefit of the anti-inflammatory and anti-cancer process of phytoestrogens [].
Cancer-associated fibroblasts (CAFs) and cancer-associated adipocytes (CAAs) within the tumor environment have recently been implicated in important aspects of epithelial cancer biology such as neoplastic progression, tumor growth, angiogenesis, and metastasis. CAAs from adipose tissue may contribute to breast cancer development and progression by altering neighboring epithelial cell behavior and phenotype through paracrine signaling []. Many xenoestrogens have been shown to cause obesity in animals at low-level exposures during critical periods of development. More specifically, DES and BPA have been implicated as environmental chemicals that increase fat accumulation by increasing the number of adipocytes, storage of fat within adipocytes, and facilitating obesity []. BPA is reported to exert estrogen-like activity on CAFs, particularly through the GPER. BPA induces the expression of GPER target genes, c-FOS, EGR-1, and CTGF, through the GPER/EGFR/ERK transduction pathway in CAFs, leading to their growth and migration in breast cancer []. On the contrary, dietary exposure to soy foods is associated with lower mammary tumor risk and a reduction in body weight and adiposity in human and rodent breast cancer models []. GEN has been shown to lower mammary adiposity and increase mammary tumor suppressor expressions, such as PTEN and E-cadherin, in female mice. These modulations mediate through ERβ and PPARγ by promoting the differentiation of stromal fibroblasts into mature adipocytes []. These results suggest that the direct regulation of mammary adiposity by GEN could be useful for breast cancer prevention.
The effects of xenoestrogens and phytoestrogens on the tumor microenvironment are challenging to study. Traditional animal models that use homogeneous cancer cells do not mimic the actual dynamic, multicellular environment of a human tumor. Therefore, advanced research models, such as PDXs and scRNA-seq technology, allow scientists to capture changes caused by xenoestrogens/phytoestrogens in both cancer cells and the surrounding stromal cells, ultimately improving the understanding of the interactions among these heterogeneous cell populations.
4.5. Effects of Xenoestrogens/Phytoestrogens Determines on Critical Timing of Exposure
Endogenous estrogen flux has been linked to increased breast cancer risk through critical estrogen exposure during certain events and time points during the life cycle such as nulliparity, older age at first birth, early menarche, and late menopause []. By the same principle, there is a consensus that the influence of environmental estrogens on breast cancer risk may be greater during certain WOS in a woman’s life. WOS are key life stages in which mammary glands undergo anatomical or molecular transformations and are most vulnerable to environmental exposures. The risk of breast cancer development increases if xenoestrogen/phytoestrogen exposure occurs during WOS, including prenatal development, puberty, pregnancy, and menopausal transition []. Exposures to xenoestrogens such as BPA and triclosan can change the timing of puberty and cause early breast development []. Menopause is a critical WOS because of its hypersensitivity to endocrine-disrupting chemicals due to the decline of endogenous estrogen []. Studies from our group have discovered that PBDEs, the flame retardants in household products, enhance E2-mediated regrowth of mammary glands, augment E2-facilitated gene expression, and modulate immune regulation, thus increasing the risk of developing breast cancer [,,,]. Importantly, like the WOS in female breast cancer, there appears to be a heightened sensitivity of the prostate to these exogenous estrogens during the critical developmental windows, such as in utero, the neonatal period and puberty. Thus, it is suggested that infants and children may be considered a highly susceptible population for exogenous estrogenic exposure with increased prostate cancer risk with aging [].
The biological effects of phytoestrogens on breast cancer have also been linked to age and critical time points in a woman’s life []. In premenopausal women, who are at high risk for early breast cancer, dietary isoflavone intake has been associated to increase breast cell cancer risk by promoting cancer cell growth. However, isoflavone intake appears to have a protective impact on later breast cancer recurrence and mortality among postmenopausal breast cancer patients []. On the other hand, some phytoestrogens appear to reduce breast cancer throughout life. Asian diets, with abundant soy products, include phytoestrogens that appear to be chemo-preventive for breast cancer in Asian women, who consume more soy than women who consume a Western diet []. However, the relevant research on phytoestrogens in breast cancer is complicated, inconsistent, and inconclusive [].
In addition to their influences on the etiology of hormone-related cancers, the impacts of xenoestrogens/phytoestrogens on reproductive health are manifested and determined based on the critical timing of exposure. Early life exposure alters the development of both female and male reproductive systems. The greatest risk may be during the prenatal (fetus) and early postnatal (infant) developmental windows when the organs are forming and developing []. Xenoestrogenic/phytoestrogenic exposure in young children may lead to early activation or interference with the hypothalamic-pituitary-gonadal (HPG) axis and therefore contribute to the early onset of puberty []. In adults, BPA, phthalates, pesticides, etc. have been shown to decrease the number of primordial follicles in female ovaries [] and decrease the number and motility of sperm in male semen [].
Xenoestrogens/phytoestrogens can also influence non-reproductive tissues and are involved in the etiology of disorders including obesity and diabetes mellitus [], cardiovascular and respiratory disease [], neurological effects [], and thyroid disease []. It is not surprising that the influences of xenoestrogens/phytoestrogens on these disorders are also associated to the critical timing of exposure. For instance, BPA exposure in women of reproductive age, including pregnant women, has been linked to an increased risk of insulin resistance and type 2 diabetes []. For women with BPA exposure during pregnancy, their offspring have a greater chance of having a higher diastolic blood pressure at an early age []. There is also correlative evidence suggesting that xenoestrogenic exposure during pregnancy, breastfeeding, and early in childhood may interfere with normal brain development, either directly or indirectly, by disrupting the thyroid hormone signaling axis []. More specifically, current literature has shown that many xenoestrogens disrupt thyroid functions through their influence on the thyroidal hormones, triiodothyronine (T3) and thyroxine (T4). These disruptions can lead to their indirect downstream effects in various developmental windows or human life stages. For instance, GEN and PCBs can disrupt thyroid transport proteins, resulting in hormone fluctuations that have been associated with impaired neurodevelopment in offspring [], whereas PBDE exposure has been associated with hypothyroidism [].
5. Application of Phytoestrogens in the Prevention or Treatment of Cancers: Evidence from Clinical Trials
Phytoestrogens such as soy isoflavones DAI, GEN, and glycitein are dietary components that are thought to reduce the incidence and severity of various cancers []. The assumed benefits of this soy diet have led to numerous clinical studies on phytoestrogen efficacies to determine a suitable amount for human consumption without any adverse effects. Additionally, clinical studies of phytoestrogens combined with cancer treatments are underway to observe if there is a synergistic effect to treat cancer. Here, we have reviewed 18 clinical trials [,,,,,,,,,,,,,,,,,,], conducted between 2002 to present, focused on breast cancer (seven trials) [,,,,,,,], prostate cancer (eight trials) [,,,,,,,], endometrial cancer (two trials) [,], and colon cancer (two trials) [,], combined with two categories of phytoestrogens treatments: fruits/whole grains/seeds such as resveratrol and curcumin (eight trials) and soy isoflavones such as GEN (10 trials) (Table 3 and Supplementary Table S1).

Table 3.
Clinical trials of phytoestrogens used as cancer prevention and/or cancer treatments.
Of the 18 trials, in terms of safety, four trials have shown that phytoestrogens are well-tolerated, safe to use, and/or have no major safety concerns. One trial studies prostate and colon cancer in phase 1 (NCT02138955) while two trials studies breast cancer in phase 2 (Nr 5592-17-02-23) and 3 (NCT00513916). In terms of the efficacy, seven trials showed little or no evidence that phytoestrogens were antagonistic to breast cancer (four trials, NCT01219075, NCT00597532, NCT00612560, and NCT00290758), or prostate cancer (two trials, NCT00255125 and NCT02724618), or endometrial cancer (one trial, NCT00118846). Meanwhile, a total of six clinical trials have shown no significant differences between the treatment and placebo groups, including two breast cancer trials (NCT00290758, NCT00597532), three prostate cancer trials (NCT01009736, NCT01917890, NCT02724618), and one endometrial cancer trial (NCT00118846). Additionally, four clinical trials stated that the conclusions were not statistically significant, including one breast cancer trial (NCT00597532), two prostate cancer trials (NCT00255125, NCT0191789), and one endometrial cancer trial (NCT00118846). Lastly, five clinical trials consisting of two breast cancer studies (University of North Dakota School of Medicine and Health Sciences and NCT00513916), one prostate cancer study (NCT00546039), one endometrial cancer study (NCT02017353, phase 2), and one colon cancer study (NCT00256334, phase 1) suggested the need for larger and/or longer studies.
While the clinical rials of phytoestrogens noted above gave few promising results, combinations of a phytoestrogen with an established chemotherapy drug may be a more promising approach. For example, patients receiving CUR and Paclitaxel to treat metastatic breast cancers had a greater objective response rate (p < 0.05 16 weeks after starting treatment, and p < 0.01 after completed treatment) compared to patients receiving Paclitaxel alone (Ministry of Health Republic of Armenia Registration No.: Nr 5592-17-02-23). Moreover, some men observed a slow rise of serum PSA after consuming 141 mg of isoflavones per day (NCT00596895). This prostate cancer trial has also shown that GEN may have an inhibitory effect on androgen-related biomarkers and supports GEN as a chemo-preventive agent in prostate cancer (NCT00546039).
While tumor response has been used to evaluate the effectiveness of phytoestrogens in cancer treatment, more recent clinical trials have added gene expression analysis. Phytoestrogens alter cancer-related gene expression profiles in breast cancer (NCT00597532, NCT00290758, and University of North Dakota School of Medicine and Health Sciences trail), prostate cancer (NCT00546039), endometrial cancer (NCT02017353), and colon cancer (NCT00256334). More interestingly, some of the trials have shown that phytoestrogens are altering the cancer-related gene expression profiles [,,,,]. Under the concept of personalized medicine, gene expression analyses could be an alternative and cost-effective way to predict the effectiveness of phytoestrogens in cancer prevention and treatments. However, a larger number of clinical trial participants and more studies of phytoestrogens and their impact on cancers are still needed to better define their anti-cancer potentials.
6. Future Directions and Conclusions
According to Global Cancer Statistics 2020, the burden of cancer incidence and mortality is rapidly growing worldwide. The epidemiological features of cancer reflect both the aging and growth of the population, as well as the changes in the prevalence and distribution of the main cancer risk factors, several of which are particularly associated with the environment [,]. Exogenous estrogens, such as synthetic industrial estrogenic compounds (xenoestrogens) and estrogenic molecules from plants (phytoestrogens), are a group of environmental factors that potentially cause various cancers through their interactions with cellular signaling processes involving estrogen signaling pathways.
Current knowledge of environmental health, oncology, and epidemiology gives new insight into the etiology of human cancers because of the gene-environmental interactions []. However, available epidemiological assessments of the risk of human cancers, which are multifactorial and multistage diseases, do not reflect the complex interactions between the biology of humans and/or their chemical exposure, and any consequent adverse health effects []. Moreover, models for the risk assessment of cancers are often based on single-agent causality. Such approaches may miss the possibility of a relationship with exposure to multiple hazardous compounds []. For this reason, the effects of the mixture of xenoestrogens/phytoestrogens have not been adequately addressed. While in vitro models with cultured cancer cells provide an advantageous method to interpret the single-agent causality of exposure and disease. However, these models also fail to consider a multifactorial analysis to explore the causal relationship between exposure and cancer development/progression. A novel approach to investigate the complexity of cancer with advanced modes and emerging techniques will be helpful to interpret measurable environmental and biological parameters simultaneously. These emerging approaches include in vivo models with rodents, PDX models, multi-omics-based unbiased analyses, and single-cell analyses [,]. Using multidisciplinary approaches, the etiology of human cancer can be more thoroughly investigated.
The Breast Cancer and the Environment Program (BCERP), launched by the US National Institute of Environmental Health Science (NIEHS) and National Cancer Institute (NCI), is a representative multidisciplinary research program that explores the environmental factors that may contribute to breast cancer (https://bcerp.org/ (accessed on 16 May 2021)). The BCERP involves a network of lab-based biologists, clinicians, epidemiologists, and community partners to examine the effects of environmental exposures that may predispose a woman to breast cancer throughout her life. Our team is a member of this project. By taking advantage of the PDX-breast cancer model and scRNA-seq analysis in surgically menopausal (ovariectomized/OVX) mouse models, our group has identified the response to exposure to the xenoestrogen PBDE by various types of cells within mammary tumors and normal breast tissue []. At the single-cell level, by integrating mouse and human datasets, we also describe the landscape of transcriptional changes in mammary glands upon endogenous and PBDE at different WOS in a woman’s life [,,]. Other key findings from the BCERP include (1) proteins produced by the developing mammary tissue may change after BPA exposure, which may alter the cell behavior in ways that contribute to breast cancer []. (2) DDT exposure during pregnancy may change the pattern of gene expression, leading to an increased chance of developing breast cancer in female offspring []. (3) The BCERP overarches a concept that the influence of environmental chemicals on breast cancer risk may be greater during certain WOS in a woman’s life, including prenatal development, puberty, pregnancy, and menopausal transition, during which the mammary glands undergo anatomical and functional transformations. Therefore, environmental hormones (e.g., endocrine-disrupting chemicals/EDC), and certain therapeutics (e.g., prescribed for the coexisting medical conditions or in the form of the hormone replacement therapy) can influence breast cancer risk, development, or outcome []. WOS is different from the well-known concept of “Sensitive Windows of Development”, which is referred to the period of fetal development and childhood when hormones regulate the formation and maturation of organs. Therefore, early-life exposures have been linked to developmental abnormalities and may increase the risk for a variety of diseases later in life []. (4) Finally, data from the BCERP have described the biological activities and molecular mechanisms of xenoestrogens on mammary gland biology and neoplasia, providing a scientific consensus with an integrated source of information and technology, of the development, function, and pathology of the mammary gland upon xenoestrogen exposure (https://bcerp.org/ (accessed on 16 May 2021)).
In addition to female breast cancer, there is increasing evidence from both epidemiology studies and animal models that environmental exposure to exogenous estrogens may influence the development or progression of prostate cancer, by interfering with estrogen signaling, either through interacting with ERs or by influencing steroid metabolism and altering estrogen levels within the body []. In humans, epidemiological evidence links specific pesticides such as the banned but still environmentally present PCBs exposures to elevated prostate cancer risk []. Studies in animal models also show augmentation of prostate carcinogenesis with several other environmental estrogenic compounds including BPA []. Recently, endogenous and exogenous estrogens have also been postulated as a contributor to non-classical hormone-related tumors, including lung cancer [], colorectal cancer [], and gastric cancer []. For instance, the etiology of lung cancer is mainly related to environmental exposure such as cigarette smoking and airborne genotoxic carcinogens. However, even correcting for carcinogen exposure, there appears to be an increased risk for lung cancer in women as compared to men. This suggests that sex hormones may be involved with lung carcinogenesis []. Several agents commonly present in the living environment can have dual biological effects: acting as genotoxic/carcinogenic and hormonally active xenoestrogens. The dualism of these environmental chemicals may contribute to the development and progression of lung cancer []. However, there has been a lack of solid evidence to prove the causal relationships between exogenous estrogen exposure and the increased risk of non-classical hormonal-related cancers.
Different from the xenoestrogens which are widely accepted as carcinogens, a wide range of beneficial effects of phytoestrogens on the cardiovascular, metabolic, and central nervous systems, as well as a reduction of cancer risk and postmenopausal symptoms, has been claimed []. The benefits of phytoestrogens such as the soy diet have led to numerous clinical studies on phytoestrogen efficacies to prevent or treat cancer [,,,,,,,,,,,,,,,,,,]. However, there is also concern that phytoestrogens may act as endocrine disruptors that adversely affect health []. Thus, clinical trials are underway to evaluate the safety and efficacy of phytoestrogens with breast, prostate, endometrial, and colon cancer, and more. Our review has included many phases I and II trials that have indicated the safety of phytoestrogens in humans. Existing data generally supports the safety of small doses of purified phytoestrogen consumption as a medication for breast cancer [,,,,,,,]. However, for the entire general population, including women with benign breast disorders, those at risk for breast cancer, and even survivors of cancer, the prescription of phytoestrogens is still not recommended due to insufficient evidence []. Under the framework of personalized medicine, several clinical trials [,,,,] have suggested that phytoestrogens have been shown to change the cancer-related gene expression profiles, providing a perspective that gene expression analyses may help to better predict the effectiveness of phytoestrogens in cancer prevention and treatments. Moving forward, continued research into phase II and III trials with larger participant cohorts and more studies into phytoestrogens are needed to fully elucidate their anti-cancer benefits.
In conclusion, exogenous estrogens, particularly xenoestrogens and phytoestrogens are an important contributor to the development and progression of cancers. Future studies on etiology of human cancers related to environmental exogenous estrogen exposure should focus on synthesizing various perspectives: (1) at the molecular and cellular level, looking at different types of ERs (ERα, ERβ, mER, and GPER) and cross-talk with other signaling pathways, (2) at the tissue level, considering the spatial heterogeneity of tissue composition and temporal heterogeneity of cancer progression, (3) at the systematic level, studying the exposure time at critical developmental windows, and (4) at the individual or population level, considering gene-environment interactions. Incorporated analysis of all the data in a clearly understood fashion allows for the modeling of prevention and therapy on an individual basis and the potential for developing new diagnostic biomarkers and drugs. Moreover, in the future, closer collaboration among oncology, systems biology, and environmental health may provide a significant qualitative and quantitative leap forward in the elucidation of human cancer etiology. The information gained from such collaborations could be applied in the introduction of preventive measures, personalized medicine, and more relevant public health intervention, ultimately, improving the knowledge and management of the complex environmental interactions underlying this life-threatening disease.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22168798/s1, Table S1 Additional information on the clinical trials from Table 3.
Author Contributions
Conceptualization: X.W. and S.C.; writing—original draft preparation: X.W., D.H., R.Y., and Y.S.C.; writing—review, and editing: X.W., D.H., D.S., and S.C.; supervision: S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by NIH grants U01ES026137 and R01CA227230. It was also supported by NIH P30CA033572 and the Lester M. and Irene C. Finkelstein Chair endowment to Dr. Shiuan Chen.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tazuke, S.; Khaw, K.-T.; Barrett-Connor, E. Exogenous estrogen and endogenous sex hormones. Medicine 1992, 71, 44–51. [Google Scholar] [CrossRef]
- Wendy, Y.; Chen, M.D. Exogenous and endogenous hormones and breast cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 573–585. [Google Scholar]
- Hu, W.Y.; Shi, G.B.; Hu, D.P.; Nelles, J.L.; Prins, G.S. Actions of estrogens and endocrine disrupting chemicals on human prostate stem/progenitor cells and prostate cancer risk. Mol. Cell Endocrinol. 2012, 354, 63–73. [Google Scholar] [CrossRef]
- Hsu, L.H.; Chu, N.M.; Kao, S.H. Estrogen, Estrogen Receptor and Lung Cancer. Int. J. Mol. Sci. 2017, 18, 1713. [Google Scholar] [CrossRef]
- Barzi, A.; Lenz, A.M.; LaBonte, M.J.; Lenz, H.J. Molecular pathways: Estrogen pathway in colorectal cancer. Clin. Cancer Res. 2013, 19, 5842–5848. [Google Scholar] [CrossRef]
- Ur Rahman, M.S.; Cao, J. Estrogen receptors in gastric cancer: Advances and perspectives. World J. Gastroenterol. 2016, 22, 2475–2482. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Barakat, R.; Oakley, O.; Kim, H.; Jin, J.; Ko, C.J. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2016, 49, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Ingle, J.N. Estrogen as therapy for breast cancer. Breast Cancer Res. 2002, 4, 133–136. [Google Scholar] [CrossRef][Green Version]
- Kerdivel, G.; Habauzit, D.; Pakdel, F. Assessment and molecular actions of endocrine-disrupting chemicals that interfere with estrogen receptor pathways. Int. J. Endocrinol. 2013, 2013, 501851. [Google Scholar] [CrossRef] [PubMed]
- Fucic, A.; Gamulin, M.; Ferencic, Z.; Katic, J.; von Kauss, M.K.; Bartonova, A.; Merlo, D.F. Environmental exposure to xenoestrogens and oestrogen related cancers: Reproductive system, breast, lung, kidney, pancreas, and brain. Environ. Health 2012, 11, S8. [Google Scholar] [CrossRef] [PubMed]
- Paterni, I.; Granchi, C.; Minutolo, F. Risks and benefits related to alimentary exposure to xenoestrogens. Crit. Rev. Food Sci. Nutr. 2017, 57, 3384–3404. [Google Scholar] [CrossRef]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef]
- Virk-Baker, M.K.; Nagy, T.R.; Barnes, S. Role of phytoestrogens in cancer therapy. Planta Med. 2010, 76, 1132–1142. [Google Scholar] [CrossRef]
- Torrens-Mas, M.; Roca, P. Phytoestrogens for Cancer Prevention and Treatment. Biology 2020, 9, 427. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [PubMed]
- Yaşar, P.; Ayaz, G.; User, S.D.; Güpür, G.; Muyan, M. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod. Med. Biol. 2016, 16, 4–20. [Google Scholar] [CrossRef]
- Márquez, D.C.; Lee, J.; Lin, T.; Pietras, R.J. Epidermal growth factor receptor and tyrosine phosphorylation of estrogen receptor. Endocrine 2001, 16, 73–81. [Google Scholar] [CrossRef]
- Gong, P.; Madak-Erdogan, Z.; Flaws, J.A.; Shapiro, D.J.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Estrogen receptor-α and aryl hydrocarbon receptor involvement in the actions of botanical estrogens in target cells. Mol. Cell Endocrinol. 2016, 437, 190–200. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, Q. Mediating Roles of PPARs in the Effects of Environmental Chemicals on Sex Steroids. PPAR Res. 2017, 2017, 3203161. [Google Scholar] [CrossRef]
- Kumari, K.; Adhya, A.K.; Rath, A.K.; Reddy, P.B.; Mishra, S.K. Estrogen-related receptors alpha, beta and gamma expression and function is associated with transcriptional repressor EZH2 in breast carcinoma. BMC Cancer 2018, 18, 690. [Google Scholar] [CrossRef]
- Rothenberger, N.J.; Somasundaram, A.; Stabile, L.P. The Role of the Estrogen Pathway in the Tumor Microenvironment. Int. J. Mol. Sci. 2018, 19, 611. [Google Scholar] [CrossRef]
- Terry, M.B.; Michels, K.B.; Brody, J.G.; Byrne, C.; Chen, S.; Jerry, D.J.; Malecki, K.M.C.; Martin, M.B.; Miller, R.L.; Neuhausen, S.L.; et al. Environmental exposures during windows of susceptibility for breast cancer: A framework for prevention research. Breast Cancer Res. 2019, 21, 96. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer and the Environment Research Program. Endocrine-Disrupting Chemicals. Available online: https://bcerp.org/health-professionals/endocrine-disrupting-chemicals/ (accessed on 16 May 2021).
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor Beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Wetherill, Y.B.; Akingbemi, B.T.; Kanno, J.; McLachlan, J.A.; Nadal, A.; Sonnenschein, C.; Watson, C.S.; Zoeller, R.T.; Belcher, S.M. In Vitro Molecular Mechanisms of Bisphenol a Action. Reprod. Toxicol. 2007, 24, 178–198. [Google Scholar] [CrossRef]
- Lee, H.J.; Chattopadhyay, S.; Gong, E.; Ahn, R.S.; Lee, K. Antiandrogenic Effects of Bisphenol A and Nonylphenol on the Function of Androgen Receptor. Toxicol. Sci. 2003, 75, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Seachrist, D.D.; Bonk, K.W.; Ho, S.; Prins, G.S.; Soto, A.M.; Keri, R.A. A Review of the Carcinogenic Potential of Bisphenol A. Reprod. Toxicol. 2016, 59, 167–182. [Google Scholar] [CrossRef] [PubMed]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisual, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the Key Characteristics of Endocrine-Disrupting Chemicals as a Basis for Hazard Identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, M.; Zhuang, S.; Yang, Y.; Yang, Y.; Liu, W. Low Concentrations of o,p’-DDT Inhibit Gene Expression and Prostaglandin Synthesis by Estrogen Receptor-Independent Mechanism in Rat Ovarian Cells. PLoS ONE 2012, 11, e49916. [Google Scholar] [CrossRef]
- Cohn, B.A.; Cirillo, P.M.; Terry, M.B. DDT and Breast Cancer: Prospective Study of Induction Time and Susceptibility Windows. J. Natl. Cancer Inst. 2019, 111, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Maness, S.C.; McDonnell, D.P.; Gaido, K.W. Inhibition of Androgen Receptor-Dependent Transcriptional Activity by DDT isomers and Methoxychlor in HepG2 Human Heptaoma Cells. Toxicol. Appl. Pharmacol. 1998, 151, 135–142. [Google Scholar] [CrossRef]
- Cariati, F.; D’Uonno, N.; Borrillo, F.; Iervolino, S.; Galdiero, G.; Tomaiuolo, R. Bisphenol a: An emerging threat to male fertility. Reprod. Biol. Endocrinol. 2019, 17, 6. [Google Scholar] [CrossRef]
- Perry, M.J.; Young, H.A.; Grandjean, P.; Halling, J.; Petersen, M.S.; Martenies, S.E.; Karimi, P.; Weihe, P. Sperm Aneuploidy in Faroese Men with Lifetime Exposure to Dichlorodiphenyldichloroethylene (p,p’-DDE) and Polychlorinated Biphenyl (PCB) Pollutants. Environ. Health Perspect. 2016, 124, 951–956. [Google Scholar] [CrossRef]
- Schrader, T.J.; Cooke, G.M. Effects of Aroclors and Individual PCB Congeners on Activation of the Human Androgen Receptor In Vitro. Reprod. Toxicol. 2003, 17, 15–23. [Google Scholar] [CrossRef]
- Connor, K.; Ramamoorthy, K.; Moore, M.; Mustain, M.; Chen, I.; Safe, S.; Zacharewski, T.; Gillesby, B.; Joyeux, A.; Balaguer, P. Hydroxylated Polychlorinated Biphenyls (PCBs) as Estrogens and Antiestrogens: Structure-Activity Relationships. Toxicol. Appl. Pharmacol. 1997, 145, 111–123. [Google Scholar] [CrossRef]
- Breast Cancer Prevention Partners. Polychlorinated Biphenyls (PCBs). Available online: https://www.bcpp.org/resource/polychlorinated-biphenyls/ (accessed on 20 May 2021).
- Hurley, S.; Goldberg, D.; Park, J.; Petreas, M.; Bernstein, L.; Anton-Culver, H.; Neuhausen, S.L.; Nelson, D.O.; Reynolds, P. A Breast Cancer Case-Control Study of Polybrominated Diphenyl Ether (PBDE) Serum Levels among California Women. Environ. Int. 2019, 127, 412–419. [Google Scholar] [CrossRef]
- Cao, L.; Ren, X.; Yang, Y.; Wan, B.; Guo, L.; Chen, D.; Fan, Y. Hydroxylated Polybrominated Biphenyl Ethers Exert Estrogenic Effects via Non-genomic G Protein-Coupled Estrogen Receptor Mediated Pathways. Environ. Health Perspect. 2018, 126, 057005. [Google Scholar] [CrossRef]
- Luthe, G.; Jacobus, J.A.; Robertson, L.W. Receptor Interactions by Polybrominated Diphenyl Ethers Versus Polychlorinated Biphenyls: A Theoretical Structure-Activity Assessment. Environ. Toxicol. Pharmacol. 2008, 25, 202–210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Z.; Liu, X.; Wang, N.; Chen, J.; Chen, Y.; Huang, J.; Su, C.; Xie, F.; Yu, B.; Chen, D. Effects of Decabrominated Diphenyl Ether (PBDE-209) in Regulation of Growth and Apoptosis of Breast, Ovarian, and Cervical Cancer Cells. Environ. Health Perspect. 2012, 120, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.R.; Cano-Sancho, G.; Mohamed, O.; Cervenka, I.; Omichessan, H.; Marchand, P.; Boutron-Ruault, M.; Arveux, P.; Severi, G.; Antignac, P.; et al. Plasma Concentration of Brominated Flame Retardants and Postmenopausal Breast Cancer Risk: A Nested Case-Control Study in the French E3N Cohort. Environ. Health 2020, 19, 54. [Google Scholar] [CrossRef]
- Hoover, R.N.; Hyer, M.; Pfeiffer, R.M.; Adam, E.; Bond, B.; Cheville, A.L.; Colton, T.; Hartge, P.; Hatch, E.E.; Herbst, A.L.; et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N. Engl. J. Med. 2011, 365, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.H.B.; de Haan, L.H.J.; Estruch, I.M.; Hooiveld, G.J.E.J.; Louisse, J.; Rietjens, I.M.C.M. Estrogen receptor alpha (ERα)-mediated coregulator binding and gene expression discriminates the toxic ERα agonist diethylstilbestrol (DES) from the endogenous ERα agonist 17β-estradiol (E2). Cell Biol. Toxicol. 2020, 36, 417–435. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Z.H.; Wang, H.; Dang, Z.; Liu, Y. A review of 17α-ethynylestradiol (EE2) in surface water across 32 countries: Sources, concentrations, and potential estrogenic effects. J. Environ. Manag. 2021, 292, 112804. [Google Scholar] [CrossRef] [PubMed]
- Ahern, T.P.; Broe, A.; Lash, T.L.; Cronin-Fenton, D.P.; Ulrichsen, S.P.; Christiansen, P.M.; Cole, B.F.; Tamimi, R.M.; Sorensen, H.T.; Damkier, P. Phthalate Exposure and Breast Cancer Incidence: A Danish Nationwide Cohort Study. J. Clin. Oncol. 2019, 37, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- López-Carrillo, L.; Hernández-Ramírez, R.U.; Calafat, A.M.; Torres-Sánchez, L.; Galván-Portillo, M.; Needham, L.L.; Ruiz-Ramos, R.; Cebrián, M.E. Exposure to Phthalates and Breast Cancer Risk in Northern Mexico. Environ. Health Perspect. 2010, 118, 539–544. [Google Scholar] [CrossRef]
- Crobeddu, B.; Ferraris, E.; Kolasa, E.; Plante, I. Di(2-ethylhexyl) Phthalate (DEHP) Increases Proliferation of Epithelial Breast Cancer Cells Through Progesterone Receptor Dysregulation. Environ. Res. 2019, 173, 165–173. [Google Scholar] [CrossRef]
- Gan, W.; Zhou, M.; Xiang, Z.; Han, X.; Li, D. Combined Effects of Nonylphenol and Bisphenol A on the Human Prostate Epithelial Cell Line RWPE-1. Int. J. Environ. Res. Public Health 2015, 12, 4141–4155. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Di Lorenzo, M.; Carrizzo, A.; Valiante, S.; Vecchione, C.; Laforgia, V.; De Falco, M. Nonylphenol Effects on Human Prostate non Tumorigenic Cells. Toxicology 2016, 357–358, 21–32. [Google Scholar] [CrossRef]
- Golden, R.; Gandy, J.; Vollmer, G. A Review of the Endocrine Activity of Parabens and Implications for Potential Risks to Human Health. Crit. Rev. Toxicol. 2005, 35, 435–458. [Google Scholar] [CrossRef] [PubMed]
- Varinska, L.; Gal, P.; Mojzisova, G.; Mirossay, L.; Majzis, J.; Srivastava, S.K. Soy and Breast Cancer: Focus on Angiogenesis. Int. J. Mol. Sci. 2015, 16, 11728–11749. [Google Scholar] [CrossRef]
- Miodini, P.; Fioravanti, L.; Di Fronzo, G.; Cappelletti, V. The Two Phyto-oestrogens Genistein and Quercetin Exert Different Effects on Oestrogen Receptor Function. Br. J. Cancer 1999, 80, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Obiorah, I.E.; Fan, P.; Jordan, V.C. Breast Cancer Cell Apoptosis with Phytoestrogens is Dependent on an Estrogen-deprived State. Cancer Prev. Res. (Phila) 2014, 7, 939–949. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Subramaniam, D.; Jensen, R.A.; Anant, S. Targeting Cancer Stem Cells and Signaling Pathways by Phytochemicals: Novel Approach for Breast Cancer Therapy. Semin. Cancer Biol. 2016, 40–41, 192–208. [Google Scholar] [CrossRef]
- Liu, R.; Yu, X.; Chen, X.; Zhong, H.; Liang, C.; Xu, X.; Xu, W.; Cheng, Y.; Wang, W.; Yu, L.; et al. Individual Factors Define the Overall Effects of Dietary Genistein Exposure on Breast Cancer Patients. Nutr. Res. 2019, 67, 1–16. [Google Scholar] [CrossRef]
- Chan, K.K.L.; Siu, M.K.Y.; Jiang, Y.; Wang, J.; Leung, T.H.Y.; Ngan, H.Y.S. Estrogen Receptor Modulators Genistein, Daidzein and ERB-041 Inhibit Cell Migration, Invasion, Proliferation and Sphere Formation via Modulation of FAK and PI3K/AKT Signaling in Ovarian Cancer. Cancer Cell Int. 2018, 18, 65. [Google Scholar] [CrossRef]
- Yu, M.; Qi, H.; Gao, X. Daidzein Promotes Milk Synthesis and Proliferation of Mammary Epithelial Cells via the Estrogen Receptor-dependent NF-B1 Activation. Anim. Biotechnol. 2020, 1–10. [Google Scholar]
- Staar, S.; Richter, D.; Makovitzky, J.; Briese, V.; Bergemann, C. Stimulation of Endometrial Glandular Cells with Genistein and Daidzein and Their Effects on ERα- and ERβ-mRNA and Protein Expresion. Anticancer Res. 2005, 25, 1713–1718. [Google Scholar] [PubMed]
- Pendleton, J.M.; Tan, W.W.; Anai, S.; Chang, M.; Hou, W.; Shiverick, K.T.; Rosser, C.J. Phase II Trial of Isoflavone in Prostate-Specific Antigen Recurrent Prostate Cancer after Previous Local Therapy. BMC Cancer 2008, 8, 1–10. [Google Scholar] [CrossRef]
- Couture, R.; Mora, N.; Al Bittar, S.; Najih, M.; Touaibia, M.; Martin, L.J. Luteolin Modulates Gene Expression Related to Steroidogenesis, Apoptosis, and Stress Response in Rat LC540 Tumor Leydig Cells. Cell Biol. Toxicol. 2020, 36, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Nordeen, S.K.; Bona, B.J.; Jones, D.N.; Lambert, J.R.; Jackson, T.A. Endocrine Disrupting Activities of the Flavonoid Nutraceuticals Luteolin and Quercetin. Horm. Cancer 2013, 4, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Maggiolini, M.; Bonofiglio, D.; Marsico, S.; Panno, M.L.; Cenni, B.; Picard, D.; Andó, S. Estrogen Receptor Alpha Mediates the Proliferation but not the Cytotoxic Dose-dependent Effects of Major Phytoestrogens on Human Breast Cancer Cells. Mol. Pharmacol. 2001, 60, 595–602. [Google Scholar]
- Wei, L.; Liu, T.; Wang, H.; Hong, H.; Yu, A.L.; Feng, H.; Chang, W. Hsp27 Participates in the Maintenance of Breast Cancer Stem Cells Through Regulation of Epithelial-mesenchymal Transition and Nuclear Factor-B. Breast Cancer Res. 2011, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, P.; Badgeley, A.; Murphy, P.; Anwar, H.; Sharma, U.; Lawrence, K.; Lakshmikuttyamma, A. Flavonoids and Other Polyphenols Act as Epigenetic Modifiers in Breast Cancer. Nutrients 2020, 12, 761. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, D.; Zheng, X.; Huang, B.; Xia, X.; Pan, X. Quercetin Exerts Bidirectional Regulation Effects on the Efficacy of Tamoxifen in Estrogen Receptor-positive Breast Cancer Therapy: An In Vitro Study. Environ. Toxicol. 2020, 35, 1179–1193. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and Apoptosis-inducing Effects of Quercetin In Vitro and In Vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef]
- Shankar, E.; Goel, A.; Gupta, K.; Gupta, S. Plant Flavone Apigenin: An Emerging Anticancer Agent. Curr. Pharmacol. Rep. 2017, 3, 423–446. [Google Scholar] [CrossRef]
- Dean, M.; Murphy, B.T.; Burdette, J.E. Phytosteroids Beyond Estrogens: Regulators of Reproductive and Endocrine Function in Natural Products. Mol. Cell Endocrinol. 2017, 442, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin: A Promising Molecule for Cancer Prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Long, X.; Fan, M.; Bigsby, R.M.; Nephew, K.P. Apigenin Inhibits Antiestrogen-resisitent Breast Cancer Cell Growth through Estrogen Receptor-α-dependent and -independent Mechanisms. Mol. Cancer Ther. 2008, 7, 2096–2108. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.; Záhoranska, Z.; Tarko, A.; Fabova, Z.; Alwasel, S.; Harrath, A.H. Plant Polyphenols Can Directly Affect Ovarian Cell Functions and Modify Toluene Effects. J. Anim. Physiol. Anim. Nutr. 2020, 105, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Park, C.J.; Perez, P.A.; Chiu, K.; Ko, C. Female Antiestrogens. Encycl. Reprod. 2018, 2, 740–747. [Google Scholar]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol Acts as a Mixed Agonist/Antagonist for Estrogen Receptors. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and Cancer: Focus on In Vivo Evidence. Endocr. Relat. Cancer 2014, 21, R209–R225. [Google Scholar] [CrossRef]
- Harada, N.; Murata, Y.; Yamaji, R.; Miura, T.; Inui, H.; Nakano, Y. Resvertrol Down-regulates the Androgen Receptor at the Post-translational Level in Prostate Cancer Cells. J. Nutr. Sci. Vitaminol. (Tokyo) 2007, 53, 556–560. [Google Scholar] [CrossRef]
- Wang, Y.; Romigh, T.; He, X.; Orloff, M.S.; Silverman, R.H.; Heston, W.D.; Eng, C. Resveratrol Regulates the PTEN/AKT Pathway Through Androgen Receptor-dependent and -independent Mechanisms in Prostate Cancer Cell Lines. Hum. Mol. Genet. 2010, 19, 4319–4329. [Google Scholar] [CrossRef] [PubMed]
- Waite, K.A.; Sinden, M.R.; Eng, C. Phytoestrogen Exposure Elevates PTEN Levels. Hum. Mol. Genet. 2005, 14, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Ci, Y.; Zhang, Y.; Liu, Y.; Lu, S.; Cao, J.; Li, H.; Zhang, J.; Huang, Z.; Zhu, X.; Gao, J.; et al. Myricetin Suppresses Breast Cancer Metastasis Through Down-regulating the Activity of Matrix Metalloproteinase (MMP)-2/9. Phytother. Res. 2018, 32, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Assanga, S.I.; Lujan, L.L. Flavones and Flavonols May Have Clinical Potential as CK2 Inhibitors in Cancer Therapy. Med. Hypotheses 2020, 141, 109723. [Google Scholar] [CrossRef]
- Maggiolini, M.; Recchia, A.G.; Bonofiglio, D.; Catalano, S.; Vivacqua, A.; Carpino, A.; Rago, V.; Rossi, R.; Andò, A. The Red Wine Phenolics Piceatannol and Myricetin Act as Agonists for Estrogen Receptor Alpha in Human Breast Cancer Cells. J. Mol. Endocrinol. 2005, 35, 269–281. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, Y.; Ye, X.; Xue, S.; Shi, J.; Pan, J.; Chen, Q. Inhibition Effects and Induction of Apoptosis of Flavonoids on the Prostate Cancer Cell Line PC-3 In Vitro. Food Chem. 2013, 138, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Sajedi, N.; Homayoun, M.; Mohammadi, F.; Soleimani, M. Myricetin Exerts its Apoptotic Effects on MCF-7 Breast Cancer Cells through Evoing the BRCA1-GADD45 Pathway. Asian Pac. J. Cancer Prev. 2020, 21, 3461–3468. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Chen, Y.C. A Review of the Dietary Flavnoid, Kaempferol on Human Health and Cancer Chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A Key Emphasis to its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef]
- Resende, F.A.; de Oliveira, A.P.S.; de Camargo, M.S.; Vilegas, W.; Varanda, E.A. Evaulation of Estrogenic Potential of Flavonoids Using a Recombinant Yeast Strain an dMCF7/BUS Cell Proliferation Assay. PLoS ONE 2013, 8, e74881. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Xu, H.; Fan, P.; Xie, J.; He, J.; Yu, J.; Gu, X.; Zhang, C. Kaempferol Blocks Neutrophil Extracellular Traps Formation and Reduces Tumour Metastasis by Inhibiting ROS-PAD4 Pathway. J. Cell Mol. Med. 2020, 24, 7590–7599. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; An, Y.; Fang, G. The Mechanism of Anticancer Action and Potential Clinical Use of Kaempferol in the Treatment of Breast Cancer. Biomed. Pharmacother. 2019, 117, 109086. [Google Scholar] [CrossRef]
- Pham, H.N.T.; Sakoff, J.A.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. Comparative Cytotoxic Activity Between Kaempferol and Gallic Acid Against Various Cancer Cell Lines. Data Brief 2018, 21, 1033–1036. [Google Scholar] [CrossRef]
- Seelinger, G.; Merfort, I.; Wolfle, U.; Schempp, C.M. Anti-carcinogenic Effects of the Flavonoid Luteolin. Molecules 2008, 13, 2628–2651. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.E.; Mirisola, V.; Romeo, F.; Generoso, L.; Esposito, A.; Dell’eva, R.; Blengio, F.; Killian, P.H.; Albini, A.; Ufeffer, U. Reference Profile Correlation Reveals Estrogen-like Transcriptional Activity of Curcumin. Cell Physiol. Biochem. 2010, 26, 471–482. [Google Scholar] [CrossRef]
- Hallman, K.; Aleck, K.; Dwyer, B.; Lloyd, V.; Quigley, M.; Sitto, N.; Siebert, A.E.; Dinda, S. The Effects of Tumeric (Curcumin) on Tumor Suppressor Protein (p53) and Estrogen Receptor in Breast Cancer Cells. Breast Cancer 2017, 9, 153–161. [Google Scholar] [PubMed]
- Mohajeri, M.; Vianconi, V.; Ávila-Rodriguez, M.F.; Barreto, G.E.; Jamialahmadi, T.; Pirro, M.; Sahebkar, A. Curcumin: A Phytochemical Modulator of Estrogens and Androgens in Tumors of the Reproductive System. Pharmacol. Res. 2020, 156, 104765. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.A.; González-Sarrias, A.; Martínez-Díaz, F.; Abellán, B.; Martínez-Torrano, A.J.; Fernández-López, A.J.; Giménez-Bastida, J.A.; Espín, J.C. Disposition of Dietary Polypenols in Breast Cancer Patients’ Tumors, and Their Associated Anticancer Activity: The Particular Case of Curcumin. Mol. Nutr. Food Res. 2021, 65, 2100163. [Google Scholar] [CrossRef] [PubMed]
- Dorai, T.; Dutcher, J.P.; Dempster, D.W.; Wiernik, P.H. Therapeutic Potential of Curcumin in Prostate Cancer-V: Interference with the Osteomimetic Properties of Hormone Refractory C4–2B Prostate Cancer Cells. Prostate 2004, 60, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Singh, S.; Satija, Y.K.; Saluja, D.; Naseem, I. Deciphering the Molecular Mechanism Underlying Anticancer Activity of Coumestrol in Triple-negative Breast Cancer Cells. Toxicol. Vitr. 2018, 46, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Singh, S.; Satija, Y.K.; Naseem, I. Cytotoxic Activity of Soy Phytoestrogen Coumetrol Against Human Breast Cancer MCF-7 Cells: Insights into the Molecular Mechanism. Food Chem. Toxicol. 2017, 99, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Jeong, M.; Bazar, F.W.; Song, G. Coumestrol Inhibits Proliferation and Migration of Prostate Cancer Cells by Regulating AKT, ERK1/2, and JNK MAPK Cell Signaling Cascades. J. Cell Physiol. 2017, 232, 862–871. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, C.; Lai, J.; Tsai, Y. Detection of a Negative Correlation Between Prescription of Chinese Herbal Products Containing Coumestrol, Genistein or Daidzein and Risk of Subsequent Endometrial Cancer Among Tamoxifen-treated Female Breast Cancer Survivors in Taiwan between 1998 and 2008: A Population-based Study. J. Ethnopharmacol. 2015, 169, 356–362. [Google Scholar] [PubMed]
- Gea, M.; Toso, A.; Schilirò, T. Estrogenic activity of biological samples as a biomarker. Sci. Total Environ. 2020, 740, 140050. [Google Scholar] [CrossRef]
- Wang, L.H.; Chen, L.R.; Chen, K.H. In vitro and vivo identification, metabolism and action of xenoestrogens: An overview. Int. J. Mol. Sci. 2021, 22, 4013. [Google Scholar] [CrossRef]
- Lin, H.; Li, H.; Lu, G.; Chen, Z.; Sun, W.; Shi, Y.; Fu, Z.; Huang, B.; Zhu, X.; Lu, W.; et al. Low dose of bisphenol a modulates ovarian cancer gene expression profile and promotes epithelial to mesenchymal transition via canonical wnt pathway. Toxicol. Sci. 2018, 164, 527–538. [Google Scholar]
- Chen, Z.J.; Zhang, K.S.; Ge, L.C.; Liu, H.; Chen, L.K.; Du, J.; Wang, H.S. Signals involved in the effects of bisphenol A (BPA) on proliferation and motility of Leydig cells: A comparative proteomic analysis. Toxicol. Res. 2016, 5, 1573–1584. [Google Scholar] [CrossRef]
- Warth, B.; Raffeiner, P.; Granados, A.; Huan, T.; Fang, M.; Forsberg, E.M.; Benton, H.P.; Goetz, L.; Johnson, C.H.; Siuzdak, G. Metabolomics Reveals that Dietary Xenoestrogens Alter Cellular Metabolism Induced by Palbociclib/Letrozole Combination Cancer Therapy. Cell Chem. Biol. 2018, 25, 291–300.e3. [Google Scholar] [CrossRef] [PubMed]
- Rudel, R.A.; Attfield, K.R.; Schifano, J.N.; Brody, J.G. Chemicals causing mammary gland tumors in animals signal new directions for epidemiology, chemicals testing, and risk assessment for breast cancer prevention. Cancer 2007, 109, 2635–2666. [Google Scholar] [CrossRef]
- Soto, A.M.; Sonnenschein, C.; Chung, K.L.; Fernandez, M.F.; Olea, N.; Olea Serrano, F. The E-SCREEN assay as a tool to identify estrogens: An update on estrogenic environmental pollutants. Environ. Health Perspect. 1995, 103, 113–122. [Google Scholar]
- Villalobos, M.; Olea, N.; Brotons, J.A.; Olea-Serrano, M.F.; de Almodovar, J.M.R.; Pedraza, V. The E-screen assay: A comparison of different MCF7 cell stocks. Environ. Health Perspect. 1995, 103, 844–850. [Google Scholar] [CrossRef]
- Sonneveld, E.; Jansen, H.J.; Riteco, J.A.C.; Brouwer, A.; van der Burg, B. Development of androgen- and estrogen-responsive bioassays members of a panel of human cell line-based highly selective steroid-responsive bioassays. Toxicol. Sci. 2005, 83, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Bonefeld-Jorgensen, E.C.; Grünfeld, H.T.; Gjermandsen, I.M. Effect of pesticides on estrogen receptor transactivation in vitro: A comparison of stable transfected MVLN and transient transfected MCF-7 cells. Mol. Cell. Endocrinol. 2005, 244, 20–30. [Google Scholar] [CrossRef]
- Wong, S.P.; Li, J.; Shen, P.; Gong, Y.; Yap, P.S.; Yong, L.E. Ultrasensitive cell-based bioassay for the measurement of global estrogenic activity of flavonoid mixtures revealing additive, restrictive, and enhanced actions in binary and higher order combinations. Assay Drug Dev. Technol. 2007, 5, 355–362. [Google Scholar] [CrossRef]
- Lui, K.; Tamura, T.; Mori, T.; Zhou, D.; Chen, S. MCF-7aro/ERE, a novel cell line for rapid screening of aromatase inhibitors, ERα ligands and ERRα ligands. Biochem. Pharmacol. 2008, 76, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, N.; Nguyen, D.M.; Lu, H.; Wang, Y.Z.; Hsin, L.Y.; Petreas, M.; Nelson, D.; Guo, W.; Reynolds, P.; Synold, T.; et al. AroER tri-screen™ is a novel functional assay to estimate both estrogenic and estrogen precursor activity of chemicals or biological specimens. Breast Cancer Res. Treat. 2015, 151, 335–345. [Google Scholar] [CrossRef]
- Chen, S.; Hsieh, J.H.; Huang, R.; Sakamuru, S.; Hsin, L.Y.; Xia, M.; Shockley, K.R.; Auerbach, S.; Kanaya, N.; Lu, H.; et al. Cell-based high-throughput screening for aromatase inhibitors in the Tox21 10K library. Toxicol. Sci. 2015, 147, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Pompili, L.; Porru, M.; Caruso, C.; Biroccio, A.; Leonetti, C. Patient-derived xenografts: A relevant preclinical model for drug development. J. Exp. Clin. Cancer Res. 2016, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinská, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Mælandsmo, G.M.; et al. Patient-derived Xenograft models: An emerging platform for translational cancer research. Cancer Discov. 2014, 4, 998–1013. [Google Scholar] [CrossRef]
- Fichtner, I.; Rolff, J.; Soong, R.; Hoffmann, J.; Hammer, S.; Sommer, A.; Becker, M.; Merk, J. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin. Cancer Res. 2008, 14, 6456–6468. [Google Scholar] [CrossRef]
- Derose, Y.S.; Wang, G.; Lin, Y.C.; Bernard, P.S.; Buys, S.S.; Ebbert, M.T.W.; Factor, R.; Matsen, C.; Milash, B.A.; Nelson, E.; et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat. Med. 2011, 17, 1514–1520. [Google Scholar] [CrossRef]
- Julien, S.; Merino-Trigo, A.; Lacroix, L.; Pocard, M.; Goeŕé, D.; Mariani, P.; Landron, S.; Bigot, L.; Nemati, F.; Dartigues, P.; et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin. Cancer Res. 2012, 18, 5314–5328. [Google Scholar] [CrossRef]
- Dong, X.; Guan, J.; English, J.C.; Flint, J.; Yee, J.; Evans, K.; Murray, N.; MacAulay, C.; Ng, R.T.; Gout, P.W.; et al. Patient-derived first generation xenografts of non-small cell lung cancers: Promising tools for predicting drug responses for personalized chemotherapy. Clin. Cancer Res. 2010, 16, 1442–1451. [Google Scholar] [CrossRef]
- Bertotti, A.; Migliardi, G.; Galimi, F.; Sassi, F.; Torti, D.; Isella, C.; Corà, D.; di Nicolantonio, F.; Buscarino, M.; Petti, C.; et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011, 1, 508–523. [Google Scholar] [CrossRef]
- Zhang, X.; Claerhout, S.; Prat, A.; Dobrolecki, L.E.; Petrovic, I.; Lai, Q.; Landis, M.D.; Wiechmann, L.; Schiff, R.; Giuliano, M.; et al. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res. 2013, 73, 4885–4897. [Google Scholar] [CrossRef]
- Lillo, M.A.; Nichols, C.; Perry, C.; Runke, S.; Krutilina, R.; Seagroves, T.N.; Miranda-Carboni, G.A.; Krum, S.A. Methylparaben stimulates tumor initiating cells in ER+ breast cancer models. J. Appl. Toxicol. 2017, 37, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, N.; Bernal, L.; Chang, G.; Yamamoto, T.; Nguyen, D.; Wang, Y.Z.; Park, J.S.; Warden, C.; Wang, J.; Wu, X.; et al. Molecular mechanisms of polybrominated diphenyl ethers (BDE-47, BDE-100, and BDE-153) in human breast cancer cells and patient-derived xenografts. Toxicol. Sci. 2019, 169, 380–398. [Google Scholar] [CrossRef]
- Nakamura, H.; Wang, Y.; Kurita, T.; Adomat, H.; Cunha, G.R.; Wang, Y. Genistein increases epidermal growth factor receptor signaling and promotes tumor progression in advanced human prostate cancer. PLoS ONE 2011, 6, e20034. [Google Scholar] [CrossRef]
- Nakamura, H.; Wang, Y.; Xue, H.; Romanish, M.T.; Mager, D.L.; Helgason, C.D.; Wang, Y. Genistein versus ICI 182, 780: An ally or enemy in metastatic progression of prostate cancer. Prostate 2013, 73, 1747–1760. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kiyota, H.; Nakata, D.; Masaki, T.; Kusaka, M.; Egawa, S. A novel androgen-dependent prostate cancer xenograft model derived from skin metastasis of a Japanese patient. Prostate 2009, 69, 1660–1667. [Google Scholar] [CrossRef]
- Mesmar, F.; Dai, B.; Ibrahim, A.; Hases, L.; Jafferali, M.H.; Augustine, J.J.; DiLorenzo, S.; Kang, Y.; Zhao, Y.; Wang, J.; et al. Clinical candidate and genistein analogue AXP107-11 has chemoenhancing functions in pancreatic adenocarcinoma through G protein-coupled estrogen receptor signaling. Cancer Med. 2019, 8, 7705–7719. [Google Scholar] [CrossRef]
- Mendes, N.; Carvalho, P.D.; Martins, F.; Mendonça, S.; Malheiro, A.R.; Ribeiro, A.; Carvalho, J.; Velho, S. Animal Models to Study Cancer and Its Microenvironment. In Tumor Microenvironment: The Main Driver of Metabolic Adaptation; Serpa, J., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 389–401. ISBN 978-3-030-34025-4. [Google Scholar]
- Day, C.P.; Merlino, G.; Van Dyke, T. Preclinical Mouse Cancer Models: A Maze of Opportunities and Challenges. Cell 2015, 163, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. The relevance of experimental carcinogenicity studies to human safety. Curr. Opin. Toxicol. 2017, 3, 6–11. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Regulation no 1223/2009 on cosmetic products. Nouv. Dermatologiques 2010, 29, 217–221. [Google Scholar]
- Regev, A.; Teichmann, S.A.; Lander, E.S.; Amit, I.; Benoist, C.; Birney, E.; Bodenmiller, B.; Campbell, P.; Carninci, P.; Clatworthy, M.; et al. The human cell atlas. eLife 2017, 6, 1–30. [Google Scholar] [CrossRef]
- Schaum, N.; Karkanias, J.; Neff, N.F.; May, A.P.; Quake, S.R.; Wyss-Coray, T.; Darmanis, S.; Batson, J.; Botvinnik, O.; Chen, M.B.; et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 2018, 562, 367–372. [Google Scholar]
- Kanaya, N.; Chang, G.; Wu, X.; Saeki, K.; Bernal, L.; Shim, H.J.; Wang, J.; Warden, C.; Yamamoto, T.; Li, J.; et al. Single-cell RNA-sequencing analysis of estrogen- and endocrine-disrupting chemical-induced reorganization of mouse mammary gland. Commun. Biol. 2019, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Saeki, K.; Chang, G.; Kanaya, N.; Wu, X.; Wang, J.; Bernal, L.; Ha, D.; Neuhausen, S.L.; Chen, S. Mammary cell gene expression atlas links epithelial cell remodeling events to breast carcinogenesis. Commun. Biol. 2021, 4, 660. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Tian, Y.; Li, M.-H.; Feng, Y.-Q.; Kong, L.; Zhang, F.-L.; Shen, W. Single-cell transcriptome dissection of the toxic impact of Di (2-ethylhexyl) phthalate on primordial follicle assembly. Theranostics 2021, 11, 4992–5009. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Saeki, K.; Mori, H.; Chen, S. Environmental carcinogenesis at the single-cell level. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.; Grzelak, M.; Hadfield, J. RNA sequencing: The teenage years. Nat. Rev. Genet. 2019, 20, 631–656. [Google Scholar] [CrossRef]
- Haque, A.; Engel, J.; Teichmann, S.A.; Lönnberg, T. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Med. 2017, 9, 1–12. [Google Scholar] [CrossRef]
- Hammond, G.L. Diverse roles for sex hormone-binding globulin in reproduction. Biol. Reprod. 2011, 85, 431–441. [Google Scholar] [CrossRef]
- Déchaud, H.; Ravard, C.; Claustrat, F.; de la Perrière, A.B.; Pugeat, M. Xenoestrogen interaction with human sex hormone-binding globulin (hSHBG). Steroids 1999, 64, 328–334. [Google Scholar] [CrossRef]
- Sheikh, I.A.; Tayubi, I.A.; Ahmad, E.; Ganaie, M.A.; Bajouh, O.S.; AlBasri, S.F.; Abdulkarim, I.M.J.; Beg, M.A. Computational insights into the molecular interactions of environmental xenoestrogens 4-tert-octylphenol, 4-nonylphenol, bisphenol A (BPA), and BPA metabolite, 4-methyl-2, 4-bis (4-hydroxyphenyl) pent-1-ene (MBP) with human sex hormone-binding globulin. Ecotoxicol. Environ. Saf. 2017, 135, 284–291. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, J.; Liao, L.; Han, S.; Liu, J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol. Cell Endocrinol. 2008, 283, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, S. Modulation of aromatase activity and expression by environmental chemicals. Front. Biosci. 2002, 7, 1712–1719. [Google Scholar] [CrossRef]
- Pino, A.M.; Valladares, L.E.; Palma, M.A.; Mancilla, A.M.; Yáñez, M.; Albala, C. Dietary isoflavones affect sex hormone-binding globulin levels in postmenopausal women. J. Clin. Endocrinol. Metab. 2000, 85, 2797–2800. [Google Scholar] [CrossRef] [PubMed]
- Low, Y.L.; Taylor, J.I.; Grace, P.B.; Dowsett, M.; Scollen, S.; Dunning, A.M.; Mulligan, A.A.; Welch, A.A.; Luben, R.N.; Khaw, K.T.; et al. Phytoestrogen exposure correlation with plasma estradiol in postmenopausal women in European Prospective Investigation of Cancer and Nutrition-Norfolk may involve diet-gene interactions. Cancer Epidemiol. Biomark. Prev. 2005, 14, 213–220. [Google Scholar]
- Low, Y.L.; Dunning, A.M.; Dowsett, M.; Folkerd, E.; Doody, D.; Taylor, J.; Bhaniani, A.; Luben, R.; Khaw, K.; Wareham, N.J.; et al. Phytoestrogen exposure is associated with circulating sex hormone levels in postmenopausal women and interact with ESR1 and NR1I2 gene variants. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- van Duursen, M.B.M. Modulation of estrogen synthesis and metabolism by phytoestrogens in vitro and the implications for women’s health. Toxicol. Res. 2017, 6, 772–794. [Google Scholar] [CrossRef]
- Deluca, D.; Krazeisen, A.; Breitling, R.; Prehn, C.; Möller, G.; Adamski, J. Inhibition of 17beta-hydroxysteroid dehydrogenases by phytoestrogens: Comparison with other steroid metabolizing enzymes. J. Steroid. Biochem. Mol. Biol. 2005, 93, 285–292. [Google Scholar] [CrossRef]
- Kao, Y.C.; Zhou, C.; Sherman, M.; Laughton, C.A.; Chen, S. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: A site-directed mutagenesis study. Environ. Health Perspect. 1998, 106, 85–92. [Google Scholar] [CrossRef]
- Chen, S.; Oh, S.R.; Phung, S.; Hur, G.; Ye, J.J.; Kwok, S.L.; Shrode, G.E.; Belury, M.; Adams, L.S.; Williams, D. Anti-aromatase activity of phytochemicals in white button mushrooms (Agaricus bisporus). Cancer Res. 2006, 66, 12026–12034. [Google Scholar] [CrossRef]
- Wang, Y.; Gho, W.M.; Chan, F.; Chen, S.; Leung, L.K. The red clover (Trifolium pratense) isoflavone biochanin A inhibits aromatase activity and expression. Br. J. Nutr. 2008, 99, 303–310. [Google Scholar] [CrossRef]
- Kijima, I.; Phung, S.; Hur, G.; Kwok, S.L.; Chen, S. Grape seed extract is an aromatase inhibitor and a suppressor of aromatase expression. Cancer Res. 2006, 66, 5960–5967. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chan, F.L.; Chen, S.; Leung, L.K. The plant polyphenol butein inhibits testosterone-induced proliferation in breast cancer cells expressing aromatase. Life Sci. 2005, 77, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-R.; Zhang, R.; Lian, Z.-X.; Deng, S.-L.; Yu, K. Estrogen-Receptor Expression and Function in Female Reproductive Disease. Cells 2019, 8, 1123. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, P.; Katchy, A.; Williams, C. Support of a bi-faceted role of estrogen receptor β (ERβ) in ERα-positive breast cancer cells. Endocr. Relat. Cancer 2014, 21, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Warner, M.; Gustafsson, J.Å. Estrogen receptors in breast carcinogenesis and endocrine therapy. Mol. Cell Endocrinol. 2015, 418, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Meerts, I.A.; Letcher, R.J.; Hoving, S.; Marsh, G.; Bergman, A.; Lemmen, J.G.; van der Burg, B.; Brouwer, A. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environ. Health Perspect. 2001, 109, 399–407. [Google Scholar] [CrossRef]
- Watson, C.S.; Koong, L.; Jeng, Y.J.; Vinas, R. Xenoestrogen interference with nongenomic signaling actions of physiological estrogens in endocrine cancer cells. Steroids 2019, 142, 84–93. [Google Scholar] [CrossRef]
- Takayanagi, S.; Tokunaga, T.; Liu, X.; Okada, H.; Matsushima, A.; Shimohigashi, Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor gamma (ERRgamma) with high constitutive activity. Toxicol. Lett. 2006, 167, 95–105. [Google Scholar] [CrossRef]
- Huang, B.; Luo, N.; Wu, X.; Xu, Z.; Wang, X.; Pan, X. The modulatory role of low concentrations of bisphenol A on tamoxifen-induced proliferation and apoptosis in breast cancer cells. Environ. Sci. Pollut. Res. Int. 2019, 26, 2353–2362. [Google Scholar] [CrossRef]
- Mueller, S.O.; Simon, S.; Chae, K.; Metzler, M.; Korach, K.S. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol. Sci. 2004, 80, 14–25. [Google Scholar] [CrossRef]
- Jiang, H.; Fan, J.; Cheng, L.; Hu, P.; Liu, R. The anticancer activity of genistein is increased in estrogen receptor beta 1-positive breast cancer cells. Onco Targets Ther. 2018, 11, 8153–8163. [Google Scholar] [CrossRef]
- Christoforou, P.; Christopoulos, P.F.; Koutsilieris, M. The role of estrogen receptor β in prostate cancer. Mol. Med. 2014, 20, 427–434. [Google Scholar] [CrossRef]
- Lecomte, S.; Demay, F.; Ferrière, F.; Pakdel, F. Phytochemicals Targeting Estrogen Receptors: Beneficial Rather Than Adverse Effects? Int. J. Mol. Sci. 2017, 18, 1381. [Google Scholar] [CrossRef]
- Suetsugi, M.; Su, L.; Karlsberg, K.; Yuan, Y.C.; Chen, S. Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors. Mol. Cancer Res. 2003, 1, 981–991. [Google Scholar]
- Prossnitz, E.R.; Barton, M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011, 12, 715–726. [Google Scholar] [CrossRef]
- Viñas, R.; Jeng, Y.J.; Watson, C.S. Non-genomic effects of xenoestrogen mixtures. Int. J. Environ. Res. Public Health 2012, 9, 2694–2714. [Google Scholar] [CrossRef] [PubMed]
- Bulayeva, N.N.; Watson, C.S. Xenoestrogen-induced ERK-1 and ERK-2 activation via multiple membrane-initiated signaling pathways. Environ. Health Perspect. 2004, 112, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Velloso, F.J.; Bianco, A.F.; Farias, J.O.; Torres, N.E.; Ferruzo, P.Y.; Anschau, V.; Jesus-Ferreira, H.C.; Chang, T.H.; Sogayar, M.C.; Zerbini, L.F.; et al. The crossroads of breast cancer progression: Insights into the modulation of major signaling pathways. Onco Targets Ther. 2017, 10, 5491–5524. [Google Scholar] [CrossRef]
- Chen, J.; Duan, Y.; Zhang, X.; Ye, Y.; Ge, B.; Chen, J. Genistein induces apoptosis by the inactivation of the IGF-1R/p-Akt signaling pathway in MCF-7 human breast cancer cells. Food Funct. 2015, 6, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Stolz, A.; Schönfelder, G.; Schneider, M.R. Endocrine Disruptors: Adverse Health Effects Mediated by EGFR? Trends Endocrinol. Metab. 2018, 29, 69–71. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Matthews, J.; Gustafsson, J.A. Estrogen receptor and aryl hydrocarbon receptor signaling pathways. Nucl. Recept. Signal. 2006, 4, e016. [Google Scholar] [CrossRef]
- Piasecka-Srader, J.; Sadowska, A.; Nynca, A.; Orlowska, K.; Jablonska, M.; Jablonska, O.; Petroff, B.K.; Ciereszko, R.E. The combined effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin and the phytoestrogen genistein on steroid hormone secretion, AhR and ERβ expression and the incidence of apoptosis in granulosa cells of medium porcine follicles. J. Reprod. Dev. 2016, 62, 103–113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Formosa, R.; Vassallo, J. The Complex Biology of the Aryl Hydrocarbon Receptor and Its Role in the Pituitary Gland. Horm. Cancer 2017, 8, 197–210. [Google Scholar] [CrossRef]
- Fanale, D.; Amodeo, V.; Caruso, S. The Interplay between Metabolism, PPAR Signaling Pathway, and Cancer. PPAR Res. 2017, 2017, 1830626. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef]
- Riu, A.; Grimaldi, M.; le Maire, A.; Bey, G.; Phillips, K.; Boulahtouf, A.; Perdu, E.; Zalko, D.; Bourguet, W.; Balaguer, P. Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ. Health Perspect. 2011, 119, 1227–1232. [Google Scholar] [CrossRef]
- Grygiel-Górniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Das, I.; Chandhok, D.; Saha, T. Redox regulation in cancer: A double-edged sword with therapeutic potential. Oxid. Med. Cell Longev. 2010, 3, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Gassman, N.R. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagen. 2017, 58, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Anet, A.; Olakkaran, S.; Purayil, A.K.; Puttaswamygowda, G.H. Bisphenol A induced oxidative stress mediated genotoxicity in Drosophila melanogaster. J. Hazard. Mater. 2019, 370, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.A.; Choi, K.C.; Hwang, K.A. Treatment with Phytoestrogens Reversed Triclosan and Bisphenol A-Induced Anti-Apoptosis in Breast Cancer Cells. Biomol. Ther. (Seoul) 2018, 26, 503–511. [Google Scholar] [CrossRef]
- Zafar, A.; Singh, S.; Naseem, I. Cu(II)-coumestrol interaction leads to ROS-mediated DNA damage and cell death: A putative mechanism for anticancer activity. J. Nutr. Biochem. 2016, 33, 15–27. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18, 84. [Google Scholar] [CrossRef]
- Cavallo, F.; De Giovanni, C.; Nanni, P.; Forni, G.; Lollini, P.L. 2011: The immune hallmarks of cancer. Cancer Immunol. Immunother. 2011, 60, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Nowak, K.; Jabłońska, E.; Ratajczak-Wrona, W. Immunomodulatory effects of synthetic endocrine disrupting chemicals on the development and functions of human immune cells. Environ. Int. 2019, 125, 350–364. [Google Scholar] [CrossRef]
- Medjakovic, S.; Mueller, M.; Jungbauer, A. Potential health-modulating effects of isoflavones and metabolites via activation of PPAR and AhR. Nutrients 2010, 2, 241–279. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.B.; Misyak, S.A.; Hontecillas, R.; Bassaganya-Riera, J. Dietary modulation of inflammation-induced colorectal cancer through PPARγ. PPAR Res. 2009, 2009, 498352. [Google Scholar] [CrossRef] [PubMed]
- Notas, G.; Kampa, M.; Castanas, E. G Protein-Coupled Estrogen Receptor in Immune Cells and Its Role in Immune-Related Diseases. Front. Endocrinol. (Lausanne) 2020, 11, 579420. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, A.; Rothenberger, N.J.; Stabile, L.P. The Impact of Estrogen in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1277, 33–52. [Google Scholar] [PubMed]
- Teixeira, D.; Marques, C.; Pestana, D.; Faria, A.; Norberto, S.; Calhau, C.; Monteiro, R. Effects of xenoestrogens in human M1 and M2 macrophage migration, cytokine release, and estrogen-related signaling pathways. Environ. Toxicol. 2015, 31, 1496–1509. [Google Scholar] [CrossRef]
- Curran, E.M.; Berghaus, L.J.; Vernetti, N.J.; Saporita, A.J.; Lubahn, D.B.; Estes, D.M. Natural killer cells express estrogen receptor-alpha and estrogen receptor-beta and can respond to estrogen via a non-estrogen receptor-alpha-mediated pathway. Cell Immunol. 2001, 214, 12–20. [Google Scholar] [CrossRef]
- Mace, T.A.; Ware, M.B.; King, S.A.; Loftus, S.; Farren, M.R.; McMichael, E.; Scoville, S.; Geraghty, C.; Young, G.; Carson, W.E.; et al. Soy isoflavones and their metabolites modulate cytokine-induced natural killer cell function. Sci. Rep. 2019, 9, 5068. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, B.; Li, Z.; Li, J.; Sun, S.; Sun, S. Cancer-associated adipocytes: Key players in breast cancer progression. J. Hematol. Oncol. 2019, 12, 95. [Google Scholar] [CrossRef]
- De Filippis, E.; Li, T.; Rosen, E.D. Exposure of adipocytes to bisphenol-A in vitro interferes with insulin action without enhancing adipogenesis. PLoS ONE 2018, 13, e0201122. [Google Scholar] [CrossRef]
- Pupo, M.; Pisano, A.; Lappano, R.; Santolla, M.F.; De Francesco, E.M.; Abonante, S.; Rosano, C.; Maggiolini, M. Bisphenol A induces gene expression changes and proliferative effects through GPER in breast cancer cells and cancer-associated fibroblasts. Environ. Health Perspect. 2012, 120, 1177–1182. [Google Scholar] [CrossRef]
- Hakkak, R.; Shaaf, S.; Jo, C.H.; Macleod, S.; Korourian, S. Effects of high-isoflavone soy diet vs. casein protein diet and obesity on DMBA-induced mammary tumor development. Oncol. Lett. 2011, 2, 29–36. [Google Scholar] [CrossRef]
- Montales, M.T.; Rahal, O.M.; Nakatani, H.; Matsuda, T.; Simmen, R.C. Repression of mammary adipogenesis by genistein limits mammosphere formation of human MCF-7 cells. J. Endocrinol. 2013, 218, 135–149. [Google Scholar] [CrossRef]
- Bernstein, L.; Ross, R.K. Endogenous hormones and breast cancer risk. Epidemiol. Rev. 1993, 15, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, L.C.; Lee, M.M. Endocrine disrupters and pubertal timing. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 49–54. [Google Scholar] [CrossRef]
- Santoro, N.; Randolph, J.F., Jr. Reproductive hormones and the menopause transition. Obstet. Gynecol. Clin. N. Am. 2011, 38, 455–466. [Google Scholar] [CrossRef]
- Prins, G.S. Endocrine disruptors and prostate cancer risk. Endocr. Relat. Cancer 2008, 15, 649–656. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.R.; Ko, N.Y.; Chen, K.H. Isoflavone Supplements for Menopausal Women: A Systematic Review. Nutrients 2019, 11, 2649. [Google Scholar] [CrossRef]
- Bilal, I.; Chowdhury, A.; Davidson, J.; Whitehead, S. Phytoestrogens and prevention of breast cancer: The contentious debate. World J. Clin. Oncol. 2014, 5, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.; Whitehead, S.A. Phytoestrogens and breast cancer--promoters or protectors? Endocr. Relat. Cancer 2006, 13, 995–1015. [Google Scholar] [CrossRef]
- Padmanabhan, V.; Song, W.; Puttabyatappa, M. Praegnatio Perturbatio-Impact of Endocrine-Disrupting Chemicals. Endocr. Rev. 2021, 42, 295–353. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Jung, H.W.; Lee, Y.J.; Lee, Y.A. Early-life exposure to endocrine-disrupting chemicals and pubertal development in girls. Ann. Pediatric Endocrinol. Metab. 2019, 24, 78–91. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Keating, A.F. Impact of environmental exposures on ovarian function and role of xenobiotic metabolism during ovotoxicity. Toxicol. Appl. Pharmacol. 2012, 261, 227–235. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Legler, J.; Fletcher, T.; Govarts, E.; Porta, M.; Blumberg, B.; Heindel, J.J.; Trasande, L. Obesity, diabetes, and associated costs of exposure to endocrine-disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab. 2015, 100, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Rancière, F.; Lyons, J.G.; Loh, V.H.Y.; Botton, J.; Galloway, T.; Wang, T.; Shaw, J.E.; Magliano, D.J. Bisphenol A and the risk of cardiometabolic disorders: A systematic review with meta-analysis of the epidemiological evidence. Environ. Health 2015, 14, 46. [Google Scholar] [CrossRef]
- Kajta, M.; Wójtowicz, A.K. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol. Rep. 2013, 65, 1632–1639. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Mínguez-Alarcón, L.; Ford, J.B.; Keller, M.; Seely, E.W.; Messerlian, C.; Petrozza, J.; Williams, P.L.; Ye, X.; Calafat, A.M.; et al. Trimester-Specific Urinary Bisphenol A Concentrations and Blood Glucose Levels Among Pregnant Women from a Fertility Clinic. J. Clin. Endocrinol. Metab. 2017, 102, 1350–1357. [Google Scholar] [CrossRef]
- Bae, S.; Lim, Y.H.; Lee, Y.A.; Shin, C.H.; Oh, S.Y.; Hong, Y.C. Maternal Urinary Bisphenol A Concentration During Midterm Pregnancy and Children’s Blood Pressure at Age 4. Hypertension 2017, 69, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; Okosieme, O.E.; Murphy, R.; Hales, C.; Chiusano, E.; Maina, A.; Joomun, M.; Bestwick, J.P.; Smyth, P.; Paradice, R.; et al. Maternal perchlorate levels in women with borderline thyroid function during pregnancy and the cognitive development of their offspring: Data from the Controlled Antenatal Thyroid Study. J. Clin. Endocrinol. Metab. 2014, 99, 4291–4298. [Google Scholar] [CrossRef]
- Marchesini, G.R.; Meimaridou, A.; Haasnoot, W.; Meulenberg, E.; Albertus, F.; Mizuguchi, M.; Takeuchi, M.; Irth, H.; Murk, A.J. Biosensor discovery of thyroxine transport disrupting chemicals. Toxicol. Appl. Pharmacol. 2008, 232, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Oulhote, Y.; Chevrier, J.; Bouchard, M.F. Exposure to Polybrominated Diphenyl Ethers (PBDEs) and Hypothyroidism in Canadian Women. J. Clin. Endocrinol. Metab. 2016, 101, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, K.; Akhtar, M.H. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1280–1293. [Google Scholar] [CrossRef]
- Shike, M.; Doane, A.S.; Russo, L.; Cabal, R.; Reis-Filho, J.S.; Gerald, W.; Cody, H.; Khanin, R.; Bromberg, J.; Norton, L. The Effects of Soy Supplementation on Gene Expression in Breast Cancer: A Randomized Placebo-Controlled Study. J. Natl. Cancer Inst. 2014, 106, 189. [Google Scholar] [CrossRef]
- Maskarinec, G.; Suzuki, S.; Pagano, I.S.; Morimoto, Y.; Franke, A.A.; Ehya, H. Cytology in Nipple Aspirate Fluid during a Randomized Soy Food Intervention among Premenopausal Women. Nutr. Cancer 2013, 65, 1116–1121. [Google Scholar] [CrossRef]
- Sen, C.; Morimoto, Y.; Heak, S.; Cooney, R.V.; Franke, A.A.; Maskarinec, G. Soy Foods and Urinary Isoprostanes: Results from a Randomized Study in Premenopausal Women. Food Funct. 2012, 3, 517. [Google Scholar] [CrossRef]
- McCann, S.E.; Edge, S.B.; Hicks, D.G.; Thompson, L.U.; Morrison, C.D.; Fetterly, G.; Andrews, C.; Clark, K.; Wilton, J.; Kulkarni, S. A Pilot Study Comparing the Effect of Flaxseed, Aromatase Inhibitor, and the Combination on Breast Tumor Biomarkers. Nutr. Cancer 2014, 66, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Chatterton, R.T.; Michel, N.; Bryk, M.; Lee, O.; Ivancic, D.; Heinz, R.; Zalles, C.M.; Helenowski, I.B.; Jovanovic, B.D.; et al. Soy Isoflavone Supplementation for Breast Cancer Risk Reduction: A Randomized Phase II Trial. Cancer Prev. Res. (Phila) 2012, 5, 309–319. [Google Scholar] [CrossRef]
- Wu, A.H.; Spicer, D.; Garcia, A.; Tseng, C.-C.; Hovanessian-Larsen, L.; Sheth, P.; Martin, S.E.; Hawes, D.; Russell, C.; MacDonald, H.; et al. Double-Blind Randomized 12-Month Soy Intervention Had No Effects on Breast MRI Fibroglandular Tissue Density or Mammographic Density. Cancer Prev. Res. (Phila) 2015, 8, 942–951. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.-C.; Kliethermes, B.; Sauter, E.R. Trans-Resveratrol Alters Mammary Promoter Hypermethylation in Women at Increased Risk for Breast Cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef]
- Saghatelyan, T.; Tananyan, A.; Janoyan, N.; Tadevosyan, A.; Petrosyan, H.; Hovhannisyan, A.; Hayrapetyan, L.; Arustamyan, M.; Arnhold, J.; Rotmann, A.-R.; et al. Efficacy and Safety of Curcumin in Combination with Paclitaxel in Patients with Advanced, Metastatic Breast Cancer: A Comparative, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Phytomedicine 2020, 70, 153218. [Google Scholar] [CrossRef] [PubMed]
- Grainger, E.M.; Moran, N.E.; Francis, D.M.; Schwartz, S.J.; Wan, L.; Thomas-Ahner, J.; Kopec, R.E.; Riedl, K.M.; Young, G.S.; Abaza, R.; et al. A Novel Tomato-Soy Juice Induces a Dose-Response Increase in Urinary and Plasma Phytochemical Biomarkers in Men with Prostate Cancer. J. Nutr. 2019, 149, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Reeves, J.M.; Banerjee, S.; Banerjee, S.K.; Holzbeierlein, J.M.; Thrasher, J.B.; Kambhampati, S.; Keighley, J.; Van Veldhuizen, P. Short-Term Soy Isoflavone Intervention in Patients with Localized Prostate Cancer: A Randomized, Double-Blind, Placebo-Controlled Trial. PLoS ONE 2013, 8, e68331. [Google Scholar] [CrossRef] [PubMed]
- Bosland, M.C.; Enk, E.; Schmoll, J.; Schlicht, M.J.; Randolph, C.; Deaton, R.J.; Xie, H.; Zeleniuch-Jacquotte, A.; Kato, I. Soy Protein Supplementation in Men Following Radical Prostatectomy: A 2-Year Randomized, Placebo-Controlled Clinical Trial. Am. J. Clin. Nutr. 2021, 113, 821–831. [Google Scholar] [CrossRef]
- Lazarevic, B.; Hammarström, C.; Yang, J.; Ramberg, H.; Diep, L.M.; Karlsen, S.J.; Kucuk, O.; Saatcioglu, F.; Taskèn, K.A.; Svindland, A. The Effects of Short-Term Genistein Intervention on Prostate Biomarker Expression in Patients with Localised Prostate Cancer before Radical Prostatectomy. Br. J. Nutr. 2012, 108, 2138–2147. [Google Scholar] [CrossRef] [PubMed]
- Saadipoor, A.; Razzaghdoust, A.; Simforoosh, N.; Mahdavi, A.; Bakhshandeh, M.; Moghadam, M.; Abdollahi, H.; Mofid, B. Randomized, Double-Blind, Placebo-Controlled Phase II Trial of Nanocurcumin in Prostate Cancer Patients Undergoing Radiotherapy: Nanocurcumin for Prostate Cancer Patients Undergoing Radiotherapy. Phytother. Res. 2019, 33, 370–378. [Google Scholar] [CrossRef]
- Greil, R.; Greil-Ressler, S.; Weiss, L.; Schönlieb, C.; Magnes, T.; Radl, B.; Bolger, G.T.; Vcelar, B.; Sordillo, P.P. A Phase 1 Dose-Escalation Study on the Safety, Tolerability and Activity of Liposomal Curcumin (LipocurcTM) in Patients with Locally Advanced or Metastatic Cancer. Cancer Chemother. Pharmacol. 2018, 82, 695–706. [Google Scholar] [CrossRef]
- Hejazi, J.; Rastmanesh, R.; Taleban, F.-A.; Molana, S.-H.; Hejazi, E.; Ehtejab, G.; Hara, N. Effect of Curcumin Supplementation during Radiotherapy on Oxidative Status of Patients with Prostate Cancer: A Double Blinded, Randomized, Placebo-Controlled Study. Nutr. Cancer 2016, 68, 77–85. [Google Scholar] [CrossRef]
- Quaas, A.M.; Kono, N.; Mack, W.J.; Hodis, H.N.; Felix, J.C.; Paulson, R.J.; Shoupe, D. Effect of Isoflavone Soy Protein Supplementation on Endometrial Thickness, Hyperplasia, and Endometrial Cancer Risk in Postmenopausal Women: A Randomized Controlled Trial. Menopause 2013, 20, 840–844. [Google Scholar] [CrossRef]
- Tuyaerts, S.; Rombauts, K.; Everaert, T.; Van Nuffel, A.M.T.; Amant, F. A Phase 2 Study to Assess the Immunomodulatory Capacity of a Lecithin-Based Delivery System of Curcumin in Endometrial Cancer. Front. Nutr. 2019, 5, 138. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C.; Holcombe, R.F. Results of a Phase I Pilot Clinical Trial Examining the Effect of Plant-Derived Resveratrol and Grape Powder on Wnt Pathway Target Gene Expression in Colonic Mucosa and Colon Cancer. Cancer Manag. Res. 2009, 1, 25–37. [Google Scholar] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wogan, G.N.; Hecht, S.S.; Felton, J.S.; Conney, A.H.; Loeb, L.A. Environmental and chemical carcinogenesis. Semin. Cancer Biol. 2004, 14, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Mbemi, A.; Khanna, S.; Njiki, S.; Yedjou, C.G.; Tchounwou, P.B. Impact of Gene-Environment Interactions on Cancer Development. Int. J. Environ. Res. Public Health 2020, 17, 8089. [Google Scholar] [CrossRef] [PubMed]
- Clavel, J. Progress in the epidemiological understanding of gene-environment interactions in major diseases: Cancer. Comptes Rendus Biol. 2007, 330, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Kriebel, D. Causal models in epidemiology: Past inheritance and genetic future. Environ. Health 2006, 5, 21. [Google Scholar] [CrossRef]
- Goodspeed, A.; Heiser, L.M.; Gray, J.W.; Costello, J.C. Tumor-Derived Cell Lines as Molecular Models of Cancer Pharmacogenomics. Mol. Cancer Res. 2016, 14, 3–13. [Google Scholar] [CrossRef]
- Amato, R.; Pinelli, M.; D’Andrea, D.; Miele, G.; Nicodemi, M.; Raiconi, G.; Cocozza, S. A novel approach to simulate gene-environment interactions in complex diseases. BMC Bioinform. 2010, 11, 8. [Google Scholar] [CrossRef]
- Perrot-Applanat, M.; Kolf-Clauw, M.; Michel, C.; Beausoleil, C. Alteration of mammary gland development by bisphenol a and evidence of a mode of action mediated through endocrine disruption. Mol. Cell Endocrinol. 2018, 475, 29–53. [Google Scholar] [CrossRef]
- Wu, H.C.; Cohn, B.A.; Cirillo, P.M.; Santella, R.M.; Terry, M.B. DDT exposure during pregnancy and DNA methylation alterations in female offspring in the Child Health and Development Study. Reprod. Toxicol. 2020, 92, 138–147. [Google Scholar] [CrossRef]
- Ali, I.; Julin, B.; Glynn, A.; Högberg, J.; Berflund, M.; Johansson, J.; Andersson, S.; Andrén, O.; Giovannucci, E.; Wolk, A.; et al. Exposure to polychlorinated biphenyls and prostate cancer: Population-based prospective cohort and experimental studies. Carcinogenesis 2016, 37, 1144–1151. [Google Scholar] [CrossRef]
- Ho, S.M.; Tang, W.Y.; de Frausto, J.B.; Prins, G.S. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006, 66, 5624–5632. [Google Scholar] [CrossRef] [PubMed]
- Prins, G.S.; Birch, L.; Tang, W.Y.; Ho, S.M. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod. Toxicol. 2007, 23, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Yang, Z.; Li, J.; Wen, Y.; Wu, Z.; Zheng, Y.; Yu, Y.; Xu, Y.; Gao, S.; Tan, F.; et al. Associations between female lung cancer risk and sex steroid hormones: A systematic review and meta-analysis of the worldwide epidemiological evidence on endogenous and exogenous sex steroid hormones. BMC Cancer 2021, 21, 690. [Google Scholar] [CrossRef]
- Wolf, L.A.; Terry, P.D.; Potter, J.D.; Bostick, R.M. Do factors related to endogenous and exogenous estrogens modify the relationship between obesity and risk of colorectal adenomas in women? Cancer Epidemiol. Biomark. Prev. 2007, 16, 676–683. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Butler, L.M.; Wu, A.H.; Koh, W.; Jin, A.; Wang, R.; Yuan, J. Reproductive factors, hormone use and gastric cancer risk: The Singapore Chinese Health Study. Int. J. Cancer 2016, 138, 2837–2845. [Google Scholar] [CrossRef]
- Fucic, A.; Gamulin, M.; Ferencic, Z.; Rokotov, D.S.; Katic, J.; Bartonova, A.; Lovasic, I.B.; Merlo, D.F. Lung cancer and environmental chemical exposure: A review of our current state of knowledge with reference to the role of hormones and hormone receptors as an increased risk factor for developing lung cancer in man. Toxicol. Pathol. 2010, 38, 849–855. [Google Scholar] [CrossRef]
- Desmawati, D.; Sulastri, D. Phytoestrogens and Their Health Effect. Open Access Maced. J. Med. Sci. 2019, 7, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Hilakivi-Clarke, L. Estrogens, BRCA1 and breast cancer. Cancer Res. 2000, 60, 4993–5000. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).