Toxic and Teratogenic Effects of Prenatal Alcohol Exposure on Fetal Development, Adolescence, and Adulthood
Abstract
1. Introduction
2. Candidate Mechanisms for Immediate and Persistent Effect of PAE
2.1. Epigenetic Mechanisms
2.2. miRNAs and Translational Regulation
2.3. Cytokine and Immune Response
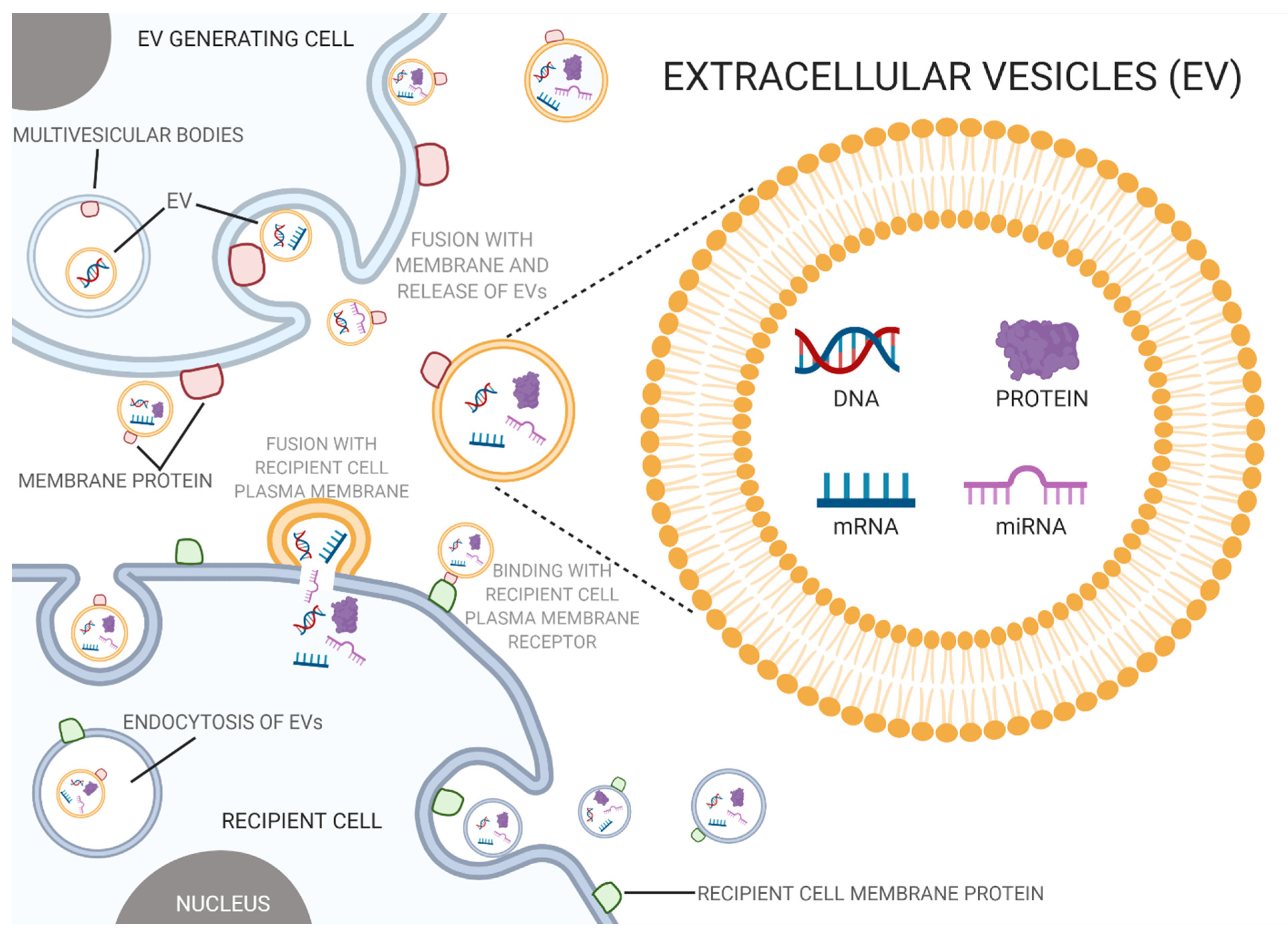
2.4. EVs and Intercellular Transfer of Toxicity
3. Effects of PAE beyond Child Development
3.1. Puberty
3.1.1. Hypothalamic-Pituitary-Gonadal Axis and PAE
3.1.2. Animal Studies on HPG Axis and PAE
3.1.3. Human Studies on HPG Axis and PAE
3.1.4. Reproductive Health and PAE
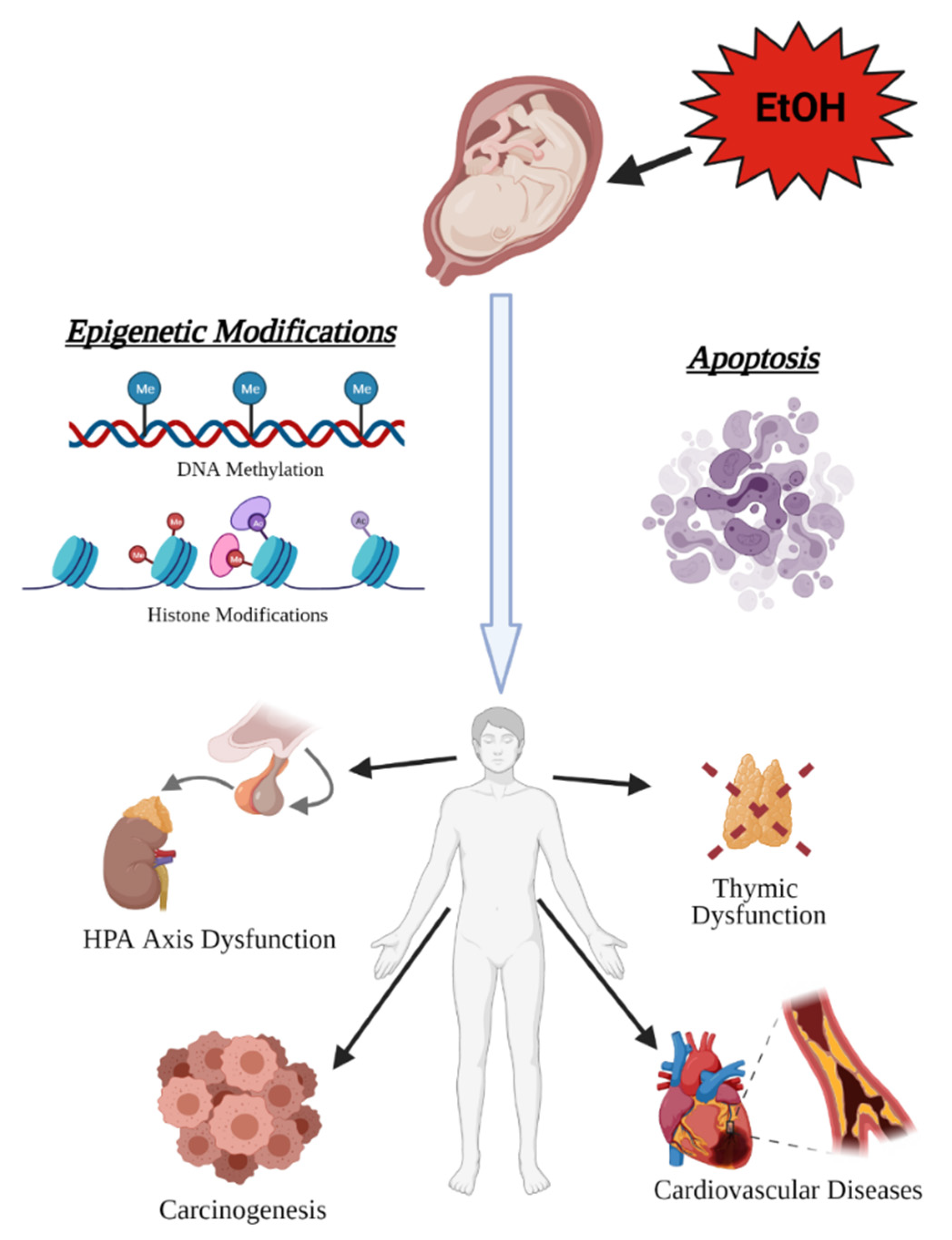
3.2. Prenatal Alcohol as a Contributor to Disease Burden in Adulthood
3.2.1. Cancer
3.2.2. Hypothalamic-Pituitary-Adrenal (HPA) Axis
3.2.3. Neural Crest and the Emergence of Heart Disease and Immune System Dysfunction
3.2.4. Congenital Heart Disease
3.2.5. Immune System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrelli, B.; Weinberg, J.; Hicks, G.G. Effects of prenatal alcohol exposure (PAE): Insights into FASD using mouse models of PAE. Biochem. Cell Biol. 2018, 96, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.D.; Warren, K.R.; Hewitt, B.G. Fetal alcohol spectrum disorders: From research to policy. Alcohol Res. Health 2010, 33, 118–126. [Google Scholar] [PubMed]
- Lipinski, R.J.; Hammond, P.; O’Leary-Moore, S.K.; Ament, J.J.; Pecevich, S.J.; Jiang, Y.; Budin, F.; Parnell, S.E.; Suttie, M.; Godin, E.A.; et al. Ethanol-Induced Face-Brain Dysmorphology Patterns Are Correlative and Exposure-Stage Dependent. PLoS ONE 2012, 7, e43067. [Google Scholar] [CrossRef] [PubMed]
- Mattson, S.N.; Crocker, N.; Nguyen, T.T. Fetal Alcohol Spectrum Disorders: Neuropsychological and Behavioral Features. Neuropsychol. Rev. 2011, 21, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.M.; Chung, D.D.; Pinson, M.R.; Salem, N.A.; Eaves, S.E.; Miranda, R.C. Ethanol Exposure Increases miR-140 in Extracellular Vesicles: Implications for Fetal Neural Stem Cell Proliferation and Maturation. Alcohol. Clin. Exp. Res. 2019, 43, 1414–1426. [Google Scholar] [CrossRef]
- Bhave, S.V.; Hoffman, P.L. Ethanol Promotes Apoptosis in Cerebellar Granule Cells by Inhibiting the Trophic Effect of NMDA. J. Neurochem. 2002, 68, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, M.M.; Tessmer, L.L.; Smith, S.M. Ethanol-Induced Neural Crest Apoptosis Is Coincident with Their Endogenous Death, But Is Mechanistically Distinct. Alcohol. Clin. Exp. Res. 1998, 22, 142–149. [Google Scholar] [CrossRef]
- Light, K.E.; Belcher, S.M.; Pierce, D.R. Time course and manner of Purkinje neuron death following a single ethanol exposure on postnatal day 4 in the developing rat. Neuroscience 2002, 114, 327–337. [Google Scholar] [CrossRef]
- Monte, S.M.; Wands, J.R. Mitochondrial DNA Damage and Impaired Mitochondrial Function Contribute to Apoptosis of Insulin-Stimulated Ethanol-Exposed Neuronal Cells. Alcohol. Clin. Exp. Res. 2001, 25, 898–906. [Google Scholar] [CrossRef]
- May, P.A.; Gossage, J.P.; Kalberg, W.O.; Robinson, L.K.; Buckley, D.; Manning, M.; Hoyme, H.E. Prevalence and epidemio-logic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev. Disabil. Res. Rev. 2009, 15, 176–192. [Google Scholar] [CrossRef]
- May, P.; Chambers, C.D.; Kalberg, W.O.; Zellner, J.; Feldman, H.; Buckley, D.; Kopald, D.; Hasken, J.M.; Xu, R.; Honerkamp-Smith, G.; et al. Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. JAMA 2018, 319, 474–482. [Google Scholar] [CrossRef]
- Dejong, K.; Olyaei, A.; Lo, J.O. Alcohol Use in Pregnancy. Clin. Obstet. Gynecol. 2019, 62, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Finer, L.B.; Zolna, M.R. Declines in Unintended Pregnancy in the United States, 2008–2011. N. Engl. J. Med. 2016, 374, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Benz, J.; Rasmussen, C.; Andrew, G. Diagnosing fetal alcohol spectrum disorder: History, challenges and future directions. Paediatr. Child Health 2009, 14, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.J.; Payne, J.; Haan, E.; Bower, C. Diagnosis of foetal alcohol syndrome and alcohol use in pregnancy: A survey of paediatricians’ knowledge, attitudes and practice. J. Paediatr. Child Health 2006, 42, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Nevin, A.C.; Parshuram, C.; Nulman, I.; Koren, G.; Einarson, A. A survey of physicians knowledge regarding awareness of maternal alcohol use and the diagnosis of FAS. BMC Fam. Pr. 2002, 3, 2. [Google Scholar] [CrossRef]
- Streissguth, A.P.; O’Malley, K. Neuropsychiatric implications and long-term consequences of fetal alcohol spectrum disorders. Semin. Clin Neuropsychiatry 2000, 5, 177–190. [Google Scholar] [CrossRef]
- Chudley, A.E.; Kilgour, A.R.; Cranston, M.; Edwards, M. Challenges of diagnosis in fetal alcohol syndrome and fetal alcohol spectrum disorder in the adult. Am. J. Med. Genet. C Semin. Med. Genet. 2007, 145c, 261–272. [Google Scholar] [CrossRef]
- Astley, S.J.; Clarren, S.K. Diagnosing the Full Spectrum of Fetal Alcohol-Exposed Individuals: Introducing The 4-Digit Diagnostic Code. Alcohol Alcohol. 2000, 35, 400–410. [Google Scholar] [CrossRef]
- Guerri, C. Neuroanatomical and Neurophysiological Mechanisms Involved in Central Nervous System Dysfunctions Induced by Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 1998, 22, 304–312. [Google Scholar] [CrossRef]
- Resnicoff, M.; Sell, C.; Ambrose, D.; Baserga, R.; Rubin, R. Ethanol inhibits the autophosphorylation of the insulin-like growth factor 1 (IGF-1) receptor and IGF-1-mediated proliferation of 3T3 cells. J. Biol. Chem. 1993, 268, 21777–21782. [Google Scholar] [CrossRef]
- Ramanathan, R.; Wilkemeyer, M.F.; Mittal, B.; Perides, G.; Charness, M.E. Alcohol inhibits cell-cell adhesion mediated by human L1. J. Cell Biol. 1996, 133, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.W.; Robertson, S. Prenatal exposure to ethanol alters the postnatal development and transformation of radial glia to astrocytes in the cortex. J. Comp. Neurol. 1993, 337, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Goodlett, C.R.; Horn, K.H. Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Res. Health 2001, 25, 175–184. [Google Scholar]
- Uysal, F.; Ozturk, S.; Akkoyunlu, G. DNMT1, DNMT3A and DNMT3B proteins are differently expressed in mouse oocytes and early embryos. J. Mol. Histol. 2017, 48, 417–426. [Google Scholar] [CrossRef]
- Jambhekar, A.; Dhall, A.; Shi, Y. Roles and regulation of histone methylation in animal development. Nat. Rev. Mol. Cell Biol. 2019, 20, 625–641. [Google Scholar] [CrossRef]
- Kramer, J.M. Regulation of cell differentiation and function by the euchromatin histone methyltranserfases G9a and GLP. Biochem. Cell Biol. 2016, 94, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Auta, J.; Zhang, H.; Pandey, S.C.; Guidotti, A. Chronic Alcohol Exposure Differentially Alters One-Carbon Metabolism in Rat Liver and Brain. Alcohol. Clin. Exp. Res. 2017, 41, 1105–1111. [Google Scholar] [CrossRef]
- Gatta, E.; Auta, J.; Gavin, D.P.; Bhaumik, D.K.; Grayson, D.R.; Pandey, S.C.; Guidotti, A. Emerging Role of One-Carbon Metabolism and DNA Methylation Enrichment on delta-Containing GABAA Receptor Expression in the Cerebellum of Subjects with Alcohol Use Disorders (AUD). Int. J. Neuropsychopharmacol. 2017, 20, 1013–1026. [Google Scholar] [CrossRef]
- Perkins, A.; Lehmann, C.; Lawrence, R.C.; Kelly, S.J. Alcohol exposure during development: Impact on the epigenome. Int. J. Dev. Neurosci. 2013, 31, 391–397. [Google Scholar] [CrossRef]
- Veazey, K.J.; Carnahan, M.N.; Muller, D.; Miranda, R.C.; Golding, M.C. Alcohol-Induced Epigenetic Alterations to Develop-mentally Crucial Genes Regulating Neural Stemness and Differentiation. Alcohol. Clin. Exp. Res. 2013, 37, 1111–1122. [Google Scholar] [CrossRef]
- Tyler, C.R.; Allan, A.M. Prenatal alcohol exposure alters expression of neurogenesis-related genes in an ex vivo cell culture model. Alcohol 2014, 48, 483–492. [Google Scholar] [CrossRef]
- Nagre, N.N.; Subbanna, S.; Shivakumar, M.; Psychoyos, D.; Basavarajappa, B.S. CB1-receptor knockout neonatal mice are protected against ethanol-induced impairments of DNMT1, DNMT3A, and DNA methylation. J. Neurochem. 2014, 132, 429–442. [Google Scholar] [CrossRef]
- Subbanna, S.; Shivakumar, M.; Umapathy, N.S.; Saito, M.; Mohan, P.S.; Kumar, A.; Nixon, R.A.; Verin, A.D.; Psychoyos, D.; Basavarajappa, B.S. G9a-mediated histone methylation regulates ethanol-induced neurodegeneration in the neonatal mouse brain. Neurobiol. Dis. 2013, 54, 475–485. [Google Scholar] [CrossRef]
- Subbanna, S.; Nagre, N.N.; Shivakumar, M.; Umapathy, N.S.; Psychoyos, D.; Basavarajappa, B.S. Ethanol induced acety-lation of histone at G9a exon1 and G9a-mediated histone H3 dimethylation leads to neurodegeneration in neonatal mice. Neuroscience 2014, 258, 422–432. [Google Scholar] [CrossRef]
- Dasmahapatra, A.K.; Khan, I.A. Modulation of DNA methylation machineries in Japanese rice fish (Oryzias latipes) embryo-genesis by ethanol and 5-azacytidine. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 179, 174–183. [Google Scholar] [CrossRef]
- Chen, Y.; Öztürk, N.C.; Zhou, F.C. DNA Methylation Program in Developing Hippocampus and Its Alteration by Alcohol. PLoS ONE 2013, 8, e60503. [Google Scholar] [CrossRef]
- Autti-Rämö, I.; Autti, T.; Korkman, M.; Kettunen, S.; Salonen, O.; Valanne, L. MRI findings in children with school problems who had been exposed prenatally to alcohol. Dev. Med. Child. Neurol. 2002, 44, 98–106. [Google Scholar] [CrossRef]
- Willoughby, K.A.; Sheard, E.D.; Nash, K.; Rovet, J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J. Int. Neuropsychol. Soc. 2008, 14, 1022–1033. [Google Scholar] [CrossRef]
- Norman, A.L.; Crocker, N.; Mattson, S.; Riley, E.P. Neuroimaging and fetal alcohol spectrum disorders. Dev. Disabil. Res. Rev. 2009, 15, 209–217. [Google Scholar] [CrossRef]
- Sarkola, T.; Iles, M.R.; Kohlenberg-Mueller, K.; Eriksson, C.J.P. Ethanol, Acetaldehyde, Acetate, and Lactate Levels After Alcohol Intake in White Men and Women: Effect of 4-Methylpyrazole. Alcohol. Clin. Exp. Res. 2002, 26, 239–245. [Google Scholar] [CrossRef]
- Soliman, M.L.; Rosenberger, T.A. Acetate supplementation increases brain histone acetylation and inhibits histone deacetylase activity and expression. Mol. Cell. Biochem. 2011, 352, 173–180. [Google Scholar] [CrossRef]
- Mews, P.; Egervari, G.; Nativio, R.; Sidoli, S.; Donahue, G.; Lombroso, S.I.; Alexander, D.C.; Riesche, S.L.; Heller, E.A.; Nestler, E.J.; et al. Alcohol metabolism contributes to brain histone acetylation. Nat. Cell Biol. 2019, 574, 717–721. [Google Scholar] [CrossRef]
- Pan, B.; Zhu, J.; Lv, T.; Sun, H.; Huang, X.; Tian, J. Alcohol Consumption during Gestation Causes Histone3 Lysine9 Hy-peracetylation and an Alternation of Expression of Heart Development-Related Genes in Mice. Alcohol. Clin. Exp. Res. 2014, 38, 2396–2402. [Google Scholar] [CrossRef]
- Park, P.-H.; Lim, R.W.; Shukla, S.D. Involvement of histone acetyltransferase (HAT) in ethanol-induced acetylation of histone H3 in hepatocytes: Potential mechanism for gene expression. Am. J. Physiol. Liver Physiol. 2005, 289, G1124–G1136. [Google Scholar] [CrossRef]
- Choudhury, M.; Shukla, S.D. Surrogate Alcohols and Their Metabolites Modify Histone H3 Acetylation: Involvement of Histone Acetyl Transferase and Histone Deacetylase. Alcohol. Clin. Exp. Res. 2008, 32, 829–839. [Google Scholar] [CrossRef]
- Peng, C.; Zhu, J.; Sun, H.-C.; Huang, X.-P.; Zhao, W.-A.; Zheng, M.; Liu, L.-J.; Tian, J. Inhibition of Histone H3K9 Acetylation by Anacardic Acid Can Correct the Over-Expression of Gata4 in the Hearts of Fetal Mice Exposed to Alcohol during Pregnancy. PLoS ONE 2014, 9, e104135. [Google Scholar] [CrossRef]
- Kleiber, M.L.; Mantha, K.; Stringer, R.L.; Singh, S.M. Neurodevelopmental alcohol exposure elicits long-term changes to gene expression that alter distinct molecular pathways dependent on timing of exposure. J. Neurodev. Disord. 2013, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Lucia, D.; Burgess, D.; Cullen, C.L.; Dorey, E.S.; Rawashdeh, O.; Moritz, K.M. Periconceptional maternal alcohol consumption leads to behavioural changes in adult and aged offspring and alters the expression of hippocampal genes associated with learning and memory and regulators of the epigenome. Behav. Brain Res. 2019, 362, 249–257. [Google Scholar] [CrossRef]
- Kim, P.; Park, J.H.; Choi, C.S.; Choi, I.; Joo, S.H.; Kim, M.K.; Kim, S.Y.; Kim, K.C.; Park, S.H.; Kwon, K.J.; et al. Effects of Ethanol Exposure During Early Pregnancy in Hyperactive, Inattentive and Impulsive Behaviors and MeCP2 Expression in Rodent Offspring. Neurochem. Res. 2013, 38, 620–631. [Google Scholar] [CrossRef]
- Bekdash, R.A.; Zhang, C.; Sarkar, D.K. Gestational Choline Supplementation Normalized Fetal Alcohol-Induced Altera-tions in Histone Modifications, DNA Methylation, and Proopiomelanocortin (POMC) Gene Expression in β-Endorphin-Producing POMC Neurons of the Hypothalamus. Alcohol. Clin. Exp. Res. 2013, 37, 1133–1142. [Google Scholar] [CrossRef]
- Govorko, D.; Bekdash, R.A.; Zhang, C.; Sarkar, D.K. Male Germline Transmits Fetal Alcohol Adverse Effect on Hypothalamic Proopiomelanocortin Gene Across Generations. Biol. Psychiatry 2012, 72, 378–388. [Google Scholar] [CrossRef]
- Sathyan, P.; Golden, H.B.; Miranda, R.C. Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: Evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J. Neurosci. 2007, 27, 8546–8557. [Google Scholar] [CrossRef]
- Tal, T.L.; Franzosa, J.A.; Tilton, S.C.; Philbrick, K.; Iwaniec, U.T.; Turner, R.T.; Waters, K.M.; Tanguay, R.L. MicroRNAs control neurobehavioral development and function in zebrafish. FASEB J. 2011, 26, 1452–1461. [Google Scholar] [CrossRef]
- Wang, L.L.; Zhang, Z.; Li, Q.; Yang, R.; Pei, X.; Xu, Y.; Wang, J.; Zhou, S.F.; Li, Y. Ethanol exposure induces differential mi-croRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Hum. Reprod. 2009, 24, 562–579. [Google Scholar] [CrossRef]
- Tsai, P.-C.; Bake, S.; Balaraman, S.; Rawlings, J.; Holgate, R.R.; Dubois, D.; Miranda, R.C. MiR-153 targets the nuclear factor-1 family and protects against teratogenic effects of ethanol exposure in fetal neural stem cells. Biol. Open 2014, 3, 741–758. [Google Scholar] [CrossRef]
- Krichevsky, A.M.; King, K.S.; Donahue, C.P.; Khrapko, K.; Kosik, K.S. A microRNA array reveals extensive regulation of mi-croRNAs during brain development. RNA 2003, 9, 1274–1281. [Google Scholar] [CrossRef]
- Vreugdenhil, E.; Berezikov, E. Fine-tuning the brain: MicroRNAs. Front. Neuroendocr. 2010, 31, 128–133. [Google Scholar] [CrossRef]
- Smalheiser, N.R.; Lugli, G. microRNA Regulation of Synaptic Plasticity. NeuroMolecular Med. 2009, 11, 133–140. [Google Scholar] [CrossRef]
- Balaraman, S.; Tingling, J.D.; Tsai, P.-C.; Miranda, R. Dysregulation of microRNA Expression and Function Contributes to the Etiology of Fetal Alcohol Spectrum Disorders. Alcohol Res. Curr. Rev. 2013, 35, 18–24. [Google Scholar]
- Salero, E.; Hatten, M.E. Differentiation of ES cells into cerebellar neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 2997–3002. [Google Scholar] [CrossRef]
- Akamatsu, W.; Okano, H.J.; Osumi, N.; Inoue, T.; Nakamura, S.; Sakakibara, S.-I.; Miura, M.; Matsuo, N.; Darnell, R. Mammalian ELAV-like neuronal RNA-binding proteins HuB and HuC promote neuronal development in both the central and the peripheral nervous systems. Proc. Natl. Acad. Sci. USA 1999, 96, 9885–9890. [Google Scholar] [CrossRef]
- Mahnke, A.H.; Sideridis, G.D.; Salem, N.A.; Tseng, A.M.; Carter, R.C.; Dodge, N.C.; Rathod, A.B.; Molteno, C.D.; Meintjes, E.M.; Jacobson, S.W.; et al. Infant circulating MicroRNAs as biomarkers of effect in fetal alcohol spectrum disorders. Sci. Rep. 2021, 11, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Saha, B.; Kodys, K.; Catalano, D.; Satishchandran, A.; Szabo, G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J. Transl. Med. 2015, 13, 1–13. [Google Scholar] [CrossRef]
- Drew, P.D.; Johnson, J.W.; Douglas, J.C.; Phelan, K.D.; Kane, C.J. Pioglitazone blocks ethanol induction of microglial acti-vation and immune responses in the hippocampus, cerebellum, and cerebral cortex in a mouse model of fetal alcohol spectrum disorders. Alcohol Clin. Exp. Res. 2015, 39, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, T.S.; Hill, L.A.; Weinberg, J. Evidence for an immune signature of prenatal alcohol exposure in female rats. Brain Behav. Immun. 2016, 58, 130–141. [Google Scholar] [CrossRef]
- Terasaki, L.S.; Schwarz, J.M. Effects of Moderate Prenatal Alcohol Exposure during Early Gestation in Rats on Inflammation across the Maternal-Fetal-Immune Interface and Later-Life Immune Function in the Offspring. J. Neuroimmune Pharmacol. 2016, 11, 680–692. [Google Scholar] [CrossRef]
- Crews, F.T.; Bechara, R.; Brown, L.A.; Guidot, D.M.; Mandrekar, P.; Oak, S.; Qin, L.; Szabo, G.; Wheeler, M.; Zou, J. Cytokines and Alcohol. Alcohol. Clin. Exp. Res. 2006, 30, 720–730. [Google Scholar] [CrossRef]
- Megyeri, P.; Ábrahám, C.S.; Temesvári, P.; Kovács, J.; Vas, T.; Speer, C.P. Recombinant human tumor necrosis factor α constricts pial arterioles and increases blood-brain barrier permeability in newborn piglets. Neurosci. Lett. 1992, 148, 137–140. [Google Scholar] [CrossRef]
- Pang, Y.; Cai, Z.; Rhodes, P.G. Effect of tumor necrosis factor-α on developing optic nerve oligodendrocytes in culture. J. Neurosci. Res. 2005, 80, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Curatolo, L.; Valsasina, B.; Caccia, C.; Raimondi, G.L.; Orsini, G.; Bianchetti, A. Recombinant human IL-2 is cytotoxic to oli-godendrocytes after in vitro self aggregation. Cytokine 1997, 9, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.L.; Giuliani, F.; Power, C.; Imai, Y.; Yong, V.W. Interleukin-1beta promotes oligodendrocyte death through glutamate excitotoxicity. Ann. Neurol. 2003, 53, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Raineki, C.; Bodnar, T.; Holman, P.J.; Baglot, S.; Lan, N.; Weinberg, J. Effects of early-life adversity on immune function are mediated by prenatal environment: Role of prenatal alcohol exposure. Brain Behav. Immun. 2017, 66, 210–220. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Qian, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered Extracellular Vesicles for Cancer Therapy. Adv. Mater. 2021, 2005709. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, F.; Wang, L.; Zhang, L.; Zhang, S.; Fang, M.; Zhou, F. The function and clinical application of extracellular vesicles in innate immune regulation. Cell. Mol. Immunol. 2020, 17, 323–334. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Kanninen, K.M.; Bister, N.; Koistinaho, J.; Malm, T. Exosomes as new diagnostic tools in CNS diseases. Biochim. Biophys. Acta 2016, 1862, 403–410. [Google Scholar] [CrossRef]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef]
- Karpman, D.; Ståhl, A.L.; Arvidsson, I. Extracellular vesicles in renal disease. Nat. Rev. Nephrol. 2017, 13, 545–562. [Google Scholar] [CrossRef]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro-Oncology 2013, 113, 1–11. [Google Scholar] [CrossRef]
- Crescitelli, R.; Lässer, C.; Szabó, T.G.; Kittel, A.; Eldh, M.; Dianzani, I.; Buzás, E.I.; Lötvall, J. Distinct RNA profiles in subpop-ulations of extracellular vesicles: Apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles 2013, 2, 20677. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.R.; Sangani, N.B.; Fernandes, T.G.; Diogo, M.M.; Curfs, L.M.G.; Reutelingsperger, C.P. Extracellular Vesicles in CNS Developmental Disorders. Int. J. Mol. Sci. 2020, 21, 9428. [Google Scholar] [CrossRef]
- Sun, Y.-Z.; Ruan, J.-S.; Jiang, Z.-S.; Wang, L.; Wang, S.-M. Extracellular Vesicles: A New Perspective in Tumor Therapy. BioMed Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Yang, C.; Robbins, P.D. Immunosuppressive Exosomes: A New Approach for Treating Arthritis. Int. J. Rheumatol. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yanqiao, Z.; Zhao, Q.; Zhan, F.; Wang, R.; Wang, L.; Zhang, Y.; Huang, X. Circulating exosomal miR-125a-3p as a novel biomarker for early-stage colon cancer. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Bala, S.; Kodys, K.; Szabo, G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci. Rep. 2015, 5, 9991. [Google Scholar] [CrossRef]
- Graykowski, D.R.; Wang, Y.-Z.; Upadhyay, A.; Savas, J.N. The Dichotomous Role of Extracellular Vesicles in the Central Nervous System. iScience 2020, 23, 101456. [Google Scholar] [CrossRef]
- Morton, M.C.; Neckles, V.N.; Seluzicki, C.M.; Holmberg, J.C.; Feliciano, D.M. Neonatal Subventricular Zone Neural Stem Cells Release Extracellular Vesicles that Act as a Microglial Morphogen. Cell Rep. 2018, 23, 78–89. [Google Scholar] [CrossRef]
- Mahnke, A.H.; Adams, A.M.; Wang, A.Z.; Miranda, R.C. Toxicant and teratogenic effects of prenatal alcohol. Curr. Opin. Toxicol. 2019, 14, 29–34. [Google Scholar] [CrossRef]
- Komada, M.; Hara, N.; Kawachi, S.; Kawachi, K.; Kagawa, N.; Nagao, T.; Ikeda, Y. Mechanisms underlying neuroinflammation and neurodevelopmental toxicity in the mouse neocortex following prenatal exposure to ethanol. Sci. Rep. 2017, 7, 4934. [Google Scholar] [CrossRef]
- Saha, B.; Momen-Heravi, F.; Kodys, K.; Szabo, G. MicroRNA Cargo of Extracellular Vesicles from Alcohol-exposed Monocytes Signals Naive Monocytes to Differentiate into M2 Macrophages*. J. Biol. Chem. 2016, 291, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Roitbak, T.; Thomas, K.; Martin, A.; Allan, A.; Cunningham, L.A. Moderate fetal alcohol exposure impairs neurogenic capacity of murine neural stem cells isolated from the adult subventricular zone. Exp. Neurol. 2011, 229, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Finniss, S.; Cazacu, S.; Xiang, C.; Brodie, C. Mesenchymal Stem Cells Deliver Exogenous miRNAs to Neural Cells and Induce Their Differentiation and Glutamate Transporter Expression. Stem Cells Dev. 2014, 23, 2851–2861. [Google Scholar] [CrossRef] [PubMed]
- Heaton, M.B.; Moore, D.B.; Paiva, M.; Madorsky, I.; Mayer, J.; Shaw, G. The Role of Neurotrophic Factors, Apoptosis-Related Proteins, and Endogenous Antioxidants in the Differential Temporal Vulnerability of Neonatal Cerebellum to Ethanol. Alcohol. Clin. Exp. Res. 2003, 27, 657–669. [Google Scholar] [CrossRef]
- Heaton, M.B.; Mitchell, J.; Paiva, M.; Walker, D.W. Ethanol-induced alterations in the expression of neurotrophic factors in the developing rat central nervous system. Dev. Brain Res. 2000, 121, 97–107. [Google Scholar] [CrossRef]
- Caputo, C.; Wood, E.; Jabbour, L. Impact of fetal alcohol exposure on body systems: A systematic review. Birth Defects Res. Part C Embryo Today Rev. 2016, 108, 174–180. [Google Scholar] [CrossRef]
- Moore, E.M.; Riley, E.P. What Happens When Children with Fetal Alcohol Spectrum Disorders Become Adults? Curr. Dev. Disord. Rep. 2015, 2, 219–227. [Google Scholar] [CrossRef]
- Macedo, D.B.; Kaiser, U.B. DLK1, Notch Signaling and the Timing of Puberty. Semin. Reprod. Med. 2019, 37, 174–181. [Google Scholar] [CrossRef]
- Sliwowska, J.H.; Comeau, W.L.; Bodnar, T.S.; Ellis, L.; Weinberg, J. Prenatal Alcohol Exposure and Pair Feeding Differentially Impact Puberty and Reproductive Development in Female Rats: Role of the Kisspeptin System. Alcohol. Clin. Exp. Res. 2016, 40, 2368–2376. [Google Scholar] [CrossRef][Green Version]
- Brix, N.; Ernst, A.; Lauridsen, L.L.B.; Parner, E.; Arah, O.A.; Olsen, J.; Henriksen, T.B.; Ramlau-Hansen, C.H. Maternal pre-pregnancy body mass index, smoking in pregnancy, and alcohol intake in pregnancy in relation to pubertal timing in the children. BMC Pediatr. 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lan, N.; Vogl, A.W.; Weinberg, J. Prenatal Ethanol Exposure Delays the Onset of Spermatogenesis in the Rat. Alcohol. Clin. Exp. Res. 2013, 37, 1074–1081. [Google Scholar] [CrossRef]
- Esquifino, A.I.; Sanchis, R.; Guerri, C. Effect of Prenatal Alcohol Exposure on Sexual Maturation of Female Rat Offspring. Neuroendocrinology 1986, 44, 483–487. [Google Scholar] [CrossRef] [PubMed]
- McGivern, R.F.; Raum, W.J.; Handa, R.J.; Sokol, R.Z. Comparison of two weeks versus one week of prenatal ethanol ex-posure in the rat on gonadal organ weights, sperm count, and onset of puberty. Neurotoxicol. Teratol. 1992, 14, 351–358. [Google Scholar] [CrossRef]
- McGivern, R.F.; Yellon, S. Delayed onset of puberty and subtle alterations in GnRH neuronal morphology in female rats exposed prenatally to ethanol. Alcohol 1992, 9, 335–340. [Google Scholar] [CrossRef]
- Carter, R.C.; Jacobson, J.L.; Dodge, N.C.; Granger, U.A.; Jacobson, S.W. Effects of prenatal alcohol exposure on testosterone and pubertal development. Alcohol. Clin. Exp. Res. 2014, 38, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Nohr, E.A.; Bech, B.H.; Ramlau-Hansen, C.H.; Olsen, J. Smoking and alcohol use during pregnancy and age of menarche in daughters. Hum. Reprod. 2010, 26, 259–265. [Google Scholar] [CrossRef]
- Wehkalampi, K.; Silventoinen, K.; Kaprio, J.; Dick, D.M.; Rose, R.J.; Pulkkinen, L.; Dunkel, L. Genetic and environmental in-fluences on pubertal timing assessed by height growth. Am. J. Hum. Biol. 2008, 20, 417–423. [Google Scholar] [CrossRef]
- Håkonsen, L.B.; Brath-Lund, M.L.; Hounsgaard, M.L.; Olsen, J.; Ernst, A.; Thulstrup, A.M.; Bech, B.H.; Ramlau-Hansen, C.H. In utero exposure to alcohol and puberty in boys: A pregnancy cohort study. BMJ Open 2014, 4, e004467. [Google Scholar] [CrossRef]
- May, P.A.; Hamrick, K.J.; Corbin, K.D.; Hasken, J.M.; Marais, A.-S.; Brooke, L.E.; Blankenship, J.; Hoyme, H.; Gossage, J.P. Dietary intake, nutrition, and fetal alcohol spectrum disorders in the Western Cape Province of South Africa. Reprod. Toxicol. 2014, 46, 31–39. [Google Scholar] [CrossRef]
- Nassau, D.E.; Best, J.C.; Cohen, J.; Gonzalez, D.C.; Alam, A.; Ramasamy, R. Androgenization in Klinefelter syndrome: Clinical spectrum from infancy through young adulthood. J. Pediatr. Urol. 2021, 17, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, L.L.; Arendt, L.H.; Stovring, H.; Olsen, J.; Ramlau-Hansen, C.H. Is age at puberty associated with semen quality and reproductive hormones in young adult life? Asian J. Androl. 2017, 19, 625–632. [Google Scholar] [CrossRef]
- Ramlau-Hansen, C.H.; Toft, G.; Jensen, M.S.; Strandberg-Larsen, K.; Hansen, M.L.; Olsen, J.; Lindhard, M.S. Maternal alcohol consumption during pregnancy and semen quality in the male offspring: Two decades of follow-up. Hum. Reprod. 2010, 25, 2340–2345. [Google Scholar] [CrossRef]
- McReight, E.K.; Liew, S.H.; Steane, S.E.; Hutt, K.J.; Moritz, K.M.; Akison, L.K. Moderate episodic prenatal alcohol does not impact female offspring fertility in rats. Reproduction 2020, 159, 615–626. [Google Scholar] [CrossRef]
- Ni, Y.; Xu, D.; Lv, F.; Wan, Y.; Fan, G.; Zou, W.; Chen, Y.; Pei, L.G.; Yang, J.; Wang, H. Prenatal ethanol exposure induces sus-ceptibility to premature ovarian insufficiency. J. Endocrinol. 2019, 243, 43–58. [Google Scholar] [CrossRef]
- Barker, D.; Godfrey, K.; Gluckman, P.; Harding, J.; Owens, J.; Robinson, J. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993, 341, 938–941. [Google Scholar] [CrossRef]
- Barker, D.J.P. Fetal origins of coronary heart disease. BMJ 1995, 311, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, P.; Buss, C.; Entringer, S.; Swanson, J. Developmental Origins of Health and Disease: Brief History of the Approach and Current Focus on Epigenetic Mechanisms. Semin. Reprod. Med. 2009, 27, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Lunde, E.R.; Washburn, S.E.; Golding, M.C.; Bake, S.; Miranda, R.C.; Ramadoss, J. Alcohol-Induced Developmental Origins of Adult-Onset Diseases. Alcohol. Clin. Exp. Res. 2016, 40, 1403–1414. [Google Scholar] [CrossRef]
- Veazey, K.J.; Parnell, S.E.; Miranda, R.C.; Golding, M.C. Dose-dependent alcohol-induced alterations in chromatin structure persist beyond the window of exposure and correlate with fetal alcohol syndrome birth defects. Epigenetics Chromatin 2015, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Gavin, D.P.; Grayson, D.R.; Varghese, S.P.; Guizzetti, M. Chromatin Switches during Neural Cell Differentiation and Their Dysregulation by Prenatal Alcohol Exposure. Genes 2017, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Garic, A.; Flentke, G.R.; Berres, M.E. Neural crest development in fetal alcohol syndrome. Birth Defects Res. Part C Embryo Today Rev. 2014, 102, 210–220. [Google Scholar] [CrossRef]
- Thanh, N.; Jonsson, E. Life Expectancy of People with Fetal Alcohol Syndrome. J. Popul. Ther. Clin. Pharmacol. 2016, 23, e53–e59. [Google Scholar]
- Popova, S.; Lange, S.; Shield, K.; Mihic, A.; Chudley, A.E.; Mukherjee, R.; Bekmuradov, D.; Rehm, J. Comorbidity of fetal alcohol spectrum disorder: A systematic review and meta-analysis. Lancet 2016, 387, 978–987. [Google Scholar] [CrossRef]
- Himmelreich, M.; Lutke, C.J.; Hargrove, E.T. The lay of the land: Fetal alcohol spectrum disorder (FASD) as a whole-body diagnosis. In The Routledge Handbook of Social Work and Addictive Behaviors; Begun, A.L., Murray, M.M., Eds.; Routledge: New York, NY, USA, 2020. [Google Scholar]
- Cook, J.C.; Lynch, M.E.; Coles, C.D. Association Analysis: Fetal Alcohol Spectrum Disorder and Hypertension Status in Children and Adolescents. Alcohol. Clin. Exp. Res. 2019, 43, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Kable, J.A.; Mehta, P.K.; Coles, C.D. Alterations in Insulin Levels in Adults with Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2021, 45, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Baylin, S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002, 3, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Scotting, P.J.; Walker, D.A.; Perilongo, G. Childhood solid tumours: A developmental disorder. Nat. Rev. Cancer 2005, 5, 481–488. [Google Scholar] [CrossRef]
- Bellacosa, A. Developmental disease and cancer: Biological and clinical overlaps. Am. J. Med Genet. Part A 2013, 161, 2788–2796. [Google Scholar] [CrossRef]
- Cobben, J.M.; Krzyzewska, I.M.; Venema, A.; Mul, A.N.; Polstra, A.; Postma, A.V.; Smigiel, R.; Pesz, K.; Niklinski, J.; Chomczyk, M.A.; et al. DNA methylation abundantly associates with fetal alcohol spectrum disorder and its subphenotypes. Epigenomics 2019, 11, 767–785. [Google Scholar] [CrossRef]
- Ehrlich, M. DNA methylation in cancer: Too much, but also too little. Oncogene 2002, 21, 5400–5413. [Google Scholar] [CrossRef]
- Banik, A.; Kandilya, D.; Ramya, S.; Stünkel, W.; Chong, Y.S.; Dheen, S.T. Maternal Factors that Induce Epigenetic Changes Contribute to Neurological Disorders in Offspring. Genes 2017, 8, 150. [Google Scholar] [CrossRef]
- Sarkar, D.K. Male germline transmits fetal alcohol epigenetic marks for multiple generations: A review. Addict. Biol. 2015, 21, 23–34. [Google Scholar] [CrossRef]
- Latino-Martel, P.; Chan, D.S.; Druesne-Pecollo, N.; Barrandon, E.; Hercberg, S.; Norat, T. Maternal Alcohol Consumption during Pregnancy and Risk of Childhood Leukemia: Systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1238–1260. [Google Scholar] [CrossRef]
- Polanco, T.A.; Crismale-Gann, C.; Reuhl, K.R.; Sarkar, D.K.; Cohick, W.S. Fetal Alcohol Exposure Increases Mammary Tumor Susceptibility and Alters Tumor Phenotype in Rats. Alcohol. Clin. Exp. Res. 2010, 34, 1879–1887. [Google Scholar] [CrossRef]
- Murugan, S.; Zhang, C.; Mojtahedzadeh, S.; Sarkar, D.K. Alcohol exposure in utero increases susceptibility to prostate tumor-igenesis in rat offspring. Alcohol. Clin. Exp. Res. 2013, 37, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Plass, C.; Oakes, C.; Blum, W.; Marcucci, G. Epigenetics in Acute Myeloid Leukemia. Semin. Oncol. 2008, 35, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, D.F.; Daniels, K.; Grunwald, J.T.; Ramos, S.A.; Propper, C.R.; Selmin, O.I. Epigenetics of breast cancer: Modifying role of environmental and bioactive food compounds. Mol. Nutr. Food Res. 2016, 60, 1310–1329. [Google Scholar] [CrossRef] [PubMed]
- Albany, C.; Alva, A.S.; Aparicio, A.M.; Singal, R.; Yellapragada, S.; Sonpavde, G.; Hahn, N.M. Epigenetics in Prostate Cancer. Prostate Cancer 2011, 2011, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yajima, F.; Suda, T.; Tomori, N.; Sumitomo, T.; Nakagami, Y.; Ushiyama, T.; Demura, H.; Shizume, K. Effects of opioid peptides on immunoreactive corticotropin-releasing factor release from the rat hypothalamus in vitro. Life Sci. 1986, 39, 181–186. [Google Scholar] [CrossRef]
- Buckingham, J.C. Stimulation and inhibition of corticotrophin releasing factor secretion by beta endorphin. Neuroendocrinology 1986, 42, 148–152. [Google Scholar] [CrossRef]
- Harno, E.; Ramamoorthy, T.G.; Coll, A.P.; White, A. POMC: The Physiological Power of Hormone Processing. Physiol. Rev. 2018, 98, 2381–2430. [Google Scholar] [CrossRef]
- Sarkar, D.K.; Kuhn, P.; Marano, J.; Chen, C.; Boyadjieva, N. Alcohol Exposure during the Developmental Period Induces β-Endorphin Neuronal Death and Causes Alteration in the Opioid Control of Stress Axis Function. Endocrinology 2007, 148, 2828–2834. [Google Scholar] [CrossRef]
- Hellemans, K.G.C.; Sliwowska, J.H.; Verma, P.; Weinberg, J. Prenatal alcohol exposure: Fetal programming and later life vul-nerability to stress, depression and anxiety disorders. Neurosci. Biobehav. Rev. 2010, 34, 791–807. [Google Scholar] [CrossRef]
- Johnson, S.; Knight, R.; Marmer, D.J.; Steele, R.W. Immune Deficiency in Fetal Alcohol Syndrome. Pediatr. Res. 1981, 15, 908–911. [Google Scholar] [CrossRef]
- Al-Yasari, A.; Jabbar, S.; Cabrera, M.A.; Rousseau, B.; Sarkar, D.K. Preconception Alcohol Exposure Increases the Susceptibility to Diabetes in the Offspring. Endocrinology 2020, 162, bqaa188. [Google Scholar] [CrossRef]
- Board, F.; Wadeson, R.; Persky, H. Depressive affect and endocrine functions; blood levels of adrenal cortex and thyroid hormones in patients suffering from depressive reactions. A.M.A. Arch. Neurol. Psychiatry 1957, 78, 612–620. [Google Scholar] [CrossRef]
- Gibbons, J.L. Cortisol Secretion Rate in Depressive Illness. Arch. Gen. Psychiatry 1964, 10, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.E. Steroids and depression. J. Steroid. Biochem. Mol. Biol. 1991, 38, 537–559. [Google Scholar] [CrossRef]
- Deuschle, M.; Gotthardt, U.; Schweiger, U.; Weber, B.; Körner, A.; Schmider, J.; Standhardt, H.; Lammers, C.-H.; Heuser, I. With aging in humans the activity of the hypothalamus-pituitary-adrenal system increases and its diurnal amplitude flattens. Life Sci. 1997, 61, 2239–2246. [Google Scholar] [CrossRef]
- Pfohl, B.; Sherman, B.; Schlechte, J.; Stone, R. Pituitary-adrenal axis rhythm disturbances in psychiatric depression. Arch. Gen. Psychiatry 1985, 42, 897–903. [Google Scholar] [CrossRef]
- Yehuda, R.; Teicher, M.H.; Trestman, R.; Levengood, R.A.; Siever, L.J. Cortisol regulation in posttraumatic stress disorder and major depression: A chronobiological analysis. Biol. Psychiatry 1996, 40, 79–88. [Google Scholar] [CrossRef]
- Iob, E.; Kirschbaum, C.; Steptoe, A. Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: The role of cognitive-affective and somatic symptoms. Mol. Psychiatry 2019, 25, 1130–1140. [Google Scholar] [CrossRef]
- Streissguth, A.P.; Bookstein, F.L.; Barr, H.M.; Sampson, P.D.; O’Malley, K.; Young, J.K. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J. Dev. Behav. Pediatr. 2004, 25, 228–238. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.J.; Kasari, C. Prenatal alcohol exposure and depressive features in children. Alcohol Clin. Exp. Res. 2000, 24, 1084–1092. [Google Scholar] [PubMed]
- Winston, N.J.; Maro, B. Calmodulin-Dependent Protein Kinase II Is Activated Transiently in Ethanol-Stimulated Mouse Oocytes. Dev. Biol. 1995, 170, 350–352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Debelak-Kragtorp, K.A.; Armant, D.R.; Smith, S.M. Ethanol-Induced Cephalic Apoptosis Requires Phospholipase C-Dependent Intracellular Calcium Signaling. Alcohol. Clin. Exp. Res. 2003, 27, 515–523. [Google Scholar] [CrossRef]
- Garic, A.; Flentke, G.R.; Amberger, E.; Hernandez, M.; Smith, S.M. CaMKII activation is a novel effector of alcohol’s neurotoxicity in neural crest stem/progenitor cells. J. Neurochem. 2011, 118, 646–657. [Google Scholar] [CrossRef]
- Flentke, G.R.; Garic, A.; Hernandez, M.; Smith, S.M. CaMKII represses transcriptionally active β-catenin to mediate acute ethanol neurodegeneration and can phosphorylate β-catenin. J. Neurochem. 2013, 128, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Flentke, G.R.; Garic, A.; Amberger, E.; Hernandez, M.; Smith, S.M. Calcium-mediated repression of β-catenin and its tran-scriptional signaling mediates neural crest cell death in an avian model of fetal alcohol syndrome. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 591–602. [Google Scholar] [CrossRef]
- Ellies, D.L.; Church, V.; Francis-West, P.; Lumsden, A. The WNT antagonist cSFRP2 modulates programmed cell death in the developing hindbrain. Developement 2000, 127, 5285–5295. [Google Scholar]
- Debelak, K.A.; Smith, S.M. Avian Genetic Background Modulates the Neural Crest Apoptosis Induced by Ethanol Exposure. Alcohol. Clin. Exp. Res. 2000, 24, 307–314. [Google Scholar] [CrossRef]
- Shyamala, K.; Yanduri, S.; Girish, H.C.; Murgod, S. Neural crest: The fourth germ layer. J. Oral Maxillofac. Pathol. 2015, 19, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qiu, H.; Qu, P.; Zhang, R.; Zeng, L.; Yan, H. Prenatal Alcohol Exposure and Congenital Heart Defects: A Meta-Analysis. PLoS ONE 2015, 10, e0130681. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Z.; Li, S.; Guo, L.; Peng, X.; Liu, Y. Prenatal alcohol exposure induced congenital heart diseases: From bench to bedside. Birth Defects Res. 2021, 113, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Ewald, S.J.; Frost, W.W. Effect of prenatal exposure to ethanol on development of the thymus. Thymus 1987, 9, 211–215. [Google Scholar]
- Keyte, A.; Hutson, M.R. The neural crest in cardiac congenital anomalies. Differ. Res. Biol. Divers. 2012, 84, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Backs, J.; Olson, E.N. Control of Cardiac Growth by Histone Acetylation/Deacetylation. Circ. Res. 2006, 98, 15–24. [Google Scholar] [CrossRef]
- Guo, T.; Chung, J.H.; Wang, T.; McDonald-McGinn, D.M.; Kates, W.R.; Hawuła, W.; Coleman, K.; Zackai, E.; Emanuel, B.S.; Morrow, B.E. Histone Modifier Genes Alter Conotruncal Heart Phenotypes in 22q11.2 Deletion Syndrome. Am. J. Hum. Genet. 2015, 97, 869–877. [Google Scholar] [CrossRef]
- Zhong, L.; Zhu, J.; Lv, T.; Chen, G.; Sun, H.; Yang, X.; Huang, X.; Tian, J. Ethanol and Its Metabolites Induce Histone Lysine 9 Acetylation and an Alteration of the Expression of Heart Development-Related Genes in Cardiac Progenitor Cells. Cardiovasc. Toxicol. 2010, 10, 268–274. [Google Scholar] [CrossRef]
- Raissadati, A.; Nieminen, H.; Haukka, J.; Sairanen, H.; Jokinen, E. Late Causes of Death After Pediatric Cardiac Surgery: A 60-Year Population-Based Study. J. Am. Coll. Cardiol. 2016, 68, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Brickner, M.E.; Hillis, L.D.; Lange, R.A. Congenital heart disease in adults. First of two parts. N. Engl. J. Med. 2000, 342, 256–263. [Google Scholar] [CrossRef]
- Kaemmerer, H.; Bauer, U.; de Haan, F.; Flesch, J.; Gohlke-Bärwolf, C.; Hagl, S.; Hess, J.; Hofbeck, M.; Kallfelz, H.C.; Lange, P.E.; et al. Recommendations for improving the quality of the interdisciplinary medical care of grown-up with congenital heart disease (GUCH). Int. J. Cardiol. 2011, 150, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Mandalenakis, Z.; Rosengren, A.; Lappas, G.; Eriksson, P.; Hansson, P.; Dellborg, M. Ischemic Stroke in Children and Young Adults with Congenital Heart Disease. J. Am. Hear. Assoc. 2016, 5, e003071. [Google Scholar] [CrossRef]
- Billett, J.; Cowie, M.; Gatzoulis, M.A.; Muhll, I.F.V.; Majeed, A. Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: Cross-sectional, population-based study with case-control analysis. Heart 2008, 94, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Moons, P.; Van Deyk, K.; Dedroog, D.; Troost, E.; Budts, W. Prevalence of cardiovascular risk factors in adults with congenital heart disease. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 612–616. [Google Scholar] [CrossRef]
- Bokma, J.P.; Zegstroo, I.; Kuijpers, J.M.; Konings, T.; Van Kimmenade, R.R.J.; Van Melle, J.P.; Kiès, P.; Mulder, B.J.M.; Bouma, B. Factors associated with coronary artery disease and stroke in adults with congenital heart disease. Heart 2017, 104, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Videbæk, J.; Laursen, H.B.; Olsen, M.; Høfsten, D.E.; Johnsen, S.P. Long-Term Nationwide Follow-Up Study of Simple Congenital Heart Disease Diagnosed in Otherwise Healthy Children. Cirulation 2016, 133, 474–483. [Google Scholar] [CrossRef]
- Schwartz, S.S.; Madsen, N.; Laursen, H.B.; Hirsch, R.; Olsen, M.S. Incidence and Mortality of Adults With Pulmonary Hyper-tension and Congenital Heart Disease. Am. J. Cardiol. 2018, 121, 1610–1616. [Google Scholar] [CrossRef]
- Olsen, M.; Marino, B.; Kaltman, J.; Laursen, H.; Jakobsen, L.; Mahle, W.; Pearson, G.; Madsen, N. Myocardial Infarction in Adults With Congenital Heart Disease. Am. J. Cardiol. 2017, 120, 2272–2277. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Liu, P.-H.; Wu, L.-S.; Chen, Y.-M.; Chang, C.-J.; Chu, P.-H. Major adverse cardiovascular events in adult congenital heart disease: A population-based follow-up study from Taiwan. BMC Cardiovasc. Disord. 2014, 14, 38. [Google Scholar] [CrossRef]
- Reid, N.; Akison, L.K.; Hoy, W.; Moritz, K.M. Adverse Health Outcomes Associated with Fetal Alcohol Exposure: A Systematic Review Focused on Cardio–Renal Outcomes. J. Stud. Alcohol Drugs 2019, 80, 515–523. [Google Scholar] [CrossRef]
- Gray, S.P.; Denton, K.; Cullen-McEwen, L.; Bertram, J.; Moritz, K.M. Prenatal Exposure to Alcohol Reduces Nephron Number and Raises Blood Pressure in Progeny. J. Am. Soc. Nephrol. 2010, 21, 1891–1902. [Google Scholar] [CrossRef]
- Bake, S.; Gardner, R.; Tingling, J.D.; Miranda, R.C.; Sohrabji, F. Fetal Alcohol Exposure Alters Blood Flow and Neurological Responses to Transient Cerebral Ischemia in Adult Mice. Alcohol. Clin. Exp. Res. 2016, 41, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.J.T.; Ritter, M. A Tissue Interaction in The Development Of Thymus Lymphocytes. J. Exp. Med. 1969, 129, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Petrie, H.T. Role of thymic organ structure and stromal composition in steady-state postnatal T-cell production. Immunol. Rev. 2002, 189, 8–20. [Google Scholar] [CrossRef]
- Davies, E.G. Immunodeficiency in DiGeorge Syndrome and Options for Treating Cases with Complete Athymia. Front. Immunol. 2013, 4, 322. [Google Scholar] [CrossRef]
- Wong, M.T.Y.; Schölvinck, E.H.; Lambeck, A.J.A.; van Ravenswaaij-Arts, C.M.A. CHARGE syndrome: A review of the immunological aspects. Eur. J. Hum. Genet. 2015, 23, 1451–1459. [Google Scholar] [CrossRef]
- Liu, Z.-Z.; Wang, Z.-L.; Choi, T.-I.; Huang, W.-T.; Wang, H.-T.; Han, Y.-Y.; Zhu, L.-Y.; Kim, H.-T.; Choi, J.-H.; Lee, J.-S.; et al. Chd7 Is Critical for Early T-Cell Development and Thymus Organogenesis in Zebrafish. Am. J. Pathol. 2018, 188, 1043–1058. [Google Scholar] [CrossRef]
- Yamazaki, H.; Sakata, E.; Yamane, T.; Yanagisawa, A.; Abe, K.; Yamamura, K.-I.; Hayashi, S.-I.; Kunisada, T. Presence and distribution of neural crest-derived cells in the murine developing thymus and their potential for differentiation. Int. Immunol. 2005, 17, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, M.A.; Cyster, J.G. Neural Crest-Derived Pericytes Promote Egress of Mature Thymocytes at the Corticomedullary Junction. Science 2010, 328, 1129–1135. [Google Scholar] [CrossRef]
- Komada, Y.; Yamane, T.; Kadota, D.; Isono, K.; Takakura, N.; Hayashi, S.-I.; Yamazaki, H. Origins and properties of dental, thymic, and bone marrow mesenchymal cells and their stem cells. PLoS ONE 2012, 7, e46436. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.; Sheridan, J.; Veiga-Fernandes, H.; Roderick, K.; Pachnis, V.; Adams, R.; Blackburn, C.; Kioussis, D.; Coles, M. Contribution of Neural Crest-Derived Cells in the Embryonic and Adult Thymus. J. Immunol. 2008, 180, 3183–3189. [Google Scholar] [CrossRef]
- Kuratani, S.; Bockman, D.E. The participation of neural crest derived mesenchymal cells in development of the epithelial primordium of the thymus. Arch. Histol. Cytol. 1990, 53, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Bockman, D.; Kirby, M.L. Dependence of thymus development on derivatives of the neural crest. Science 1984, 223, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Ewald, S.J.; Walden, S.M. Flow Cytometric and Histological Analysis of Mouse Thymus in Fetal Alcohol Syndrome. J. Leukoc. Biol. 1988, 44, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Ewald, S.J.; Shao, H. Ethanol Increases Apoptotic Cell Death of Thymocytes in Vitro. Alcohol. Clin. Exp. Res. 1993, 17, 359–365. [Google Scholar] [CrossRef]
- Bray, L.A.; Shao, H.; Ewald, S.J. Effect of Ethanol on Development of Fetal Mouse Thymocytes in Organ Culture. Cell. Immunol. 1993, 151, 12–23. [Google Scholar] [CrossRef]
- Ewald, S.J. T Lymphocyte Populations in Fetal Alcohol Syndrome. Alcohol. Clin. Exp. Res. 1989, 13, 485–489. [Google Scholar] [CrossRef]
- Taylor, A.N.; Tio, D.L.; Chiappelli, F. Thymocyte Development in Male Fetal Alcohol-Exposed Rats. Alcohol. Clin. Exp. Res. 1999, 23, 465–470. [Google Scholar] [CrossRef]
- Sanchez, J.J.; Noor, S.; Davies, S.; Savage, D.; Milligan, E.D. Prenatal alcohol exposure is a risk factor for adult neuropathic pain via aberrant neuroimmune function. J. Neuroinflammation 2017, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, J.; Jerrells, T.R. Suppression of immune responsiveness: Sex differences in prenatal ethanol effects. Alcohol. Clin. Exp. Res. 1991, 15, 525–531. [Google Scholar] [CrossRef]
- Seelig, L.L., Jr.; Steven, W.M.; Stewart, G.L. Effects of maternal ethanol consumption on the subsequent development of immunity to Trichinella spiralis in rat neonates. Alcohol. Clin. Exp. Res. 1996, 20, 514–522. [Google Scholar] [CrossRef] [PubMed]
- McGill, J.; Meyerholz, D.K.; Edsen-Moore, M.; Young, B.; Coleman, R.A.; Schlueter, A.J.; Waldschmidt, T.J.; Cook, R.T.; Legge, K.L. Fetal Exposure to Ethanol Has Long-Term Effects on the Severity of Influenza Virus Infections. J. Immunol. 2009, 182, 7803–7808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lan, N.; Bach, P.; Nordstokke, D.; Yu, W.; Ellis, L.; Meadows, G.G.; Weinberg, J. Prenatal alcohol exposure alters the course and severity of adjuvant-induced arthritis in female rats. Brain Behav. Immun. 2012, 26, 439–450. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, D.D.; Pinson, M.R.; Bhenderu, L.S.; Lai, M.S.; Patel, R.A.; Miranda, R.C. Toxic and Teratogenic Effects of Prenatal Alcohol Exposure on Fetal Development, Adolescence, and Adulthood. Int. J. Mol. Sci. 2021, 22, 8785. https://doi.org/10.3390/ijms22168785
Chung DD, Pinson MR, Bhenderu LS, Lai MS, Patel RA, Miranda RC. Toxic and Teratogenic Effects of Prenatal Alcohol Exposure on Fetal Development, Adolescence, and Adulthood. International Journal of Molecular Sciences. 2021; 22(16):8785. https://doi.org/10.3390/ijms22168785
Chicago/Turabian StyleChung, Dae D., Marisa R. Pinson, Lokeshwar S. Bhenderu, Michael S. Lai, Rhea A. Patel, and Rajesh C. Miranda. 2021. "Toxic and Teratogenic Effects of Prenatal Alcohol Exposure on Fetal Development, Adolescence, and Adulthood" International Journal of Molecular Sciences 22, no. 16: 8785. https://doi.org/10.3390/ijms22168785
APA StyleChung, D. D., Pinson, M. R., Bhenderu, L. S., Lai, M. S., Patel, R. A., & Miranda, R. C. (2021). Toxic and Teratogenic Effects of Prenatal Alcohol Exposure on Fetal Development, Adolescence, and Adulthood. International Journal of Molecular Sciences, 22(16), 8785. https://doi.org/10.3390/ijms22168785





