Revealing the Influences of Sex Hormones and Sex Differences in Atrial Fibrillation and Vascular Cognitive Impairment
Abstract
1. Introduction
2. Sex Differences in AF
2.1. Sex Differences in the Epidemiology and Clinical Outcomes of AF
2.2. Pathophysiology of Sex Differences in AF
2.2.1. Structural Remodeling
2.2.2. Electrical Remodeling
2.2.3. Autonomic Neural Control and Neuro-Humoral Modulation
3. Sex Differences in VCI
3.1. Sex Differences in the Epidemiology and Clinical Outcomes of VCI
3.2. Pathophysiology of Sex Differences in VCI
3.2.1. Sex Differences in Brain Structure and Function among Individuals with VCI
3.2.2. Sex Differences in Risk Factors for VCI
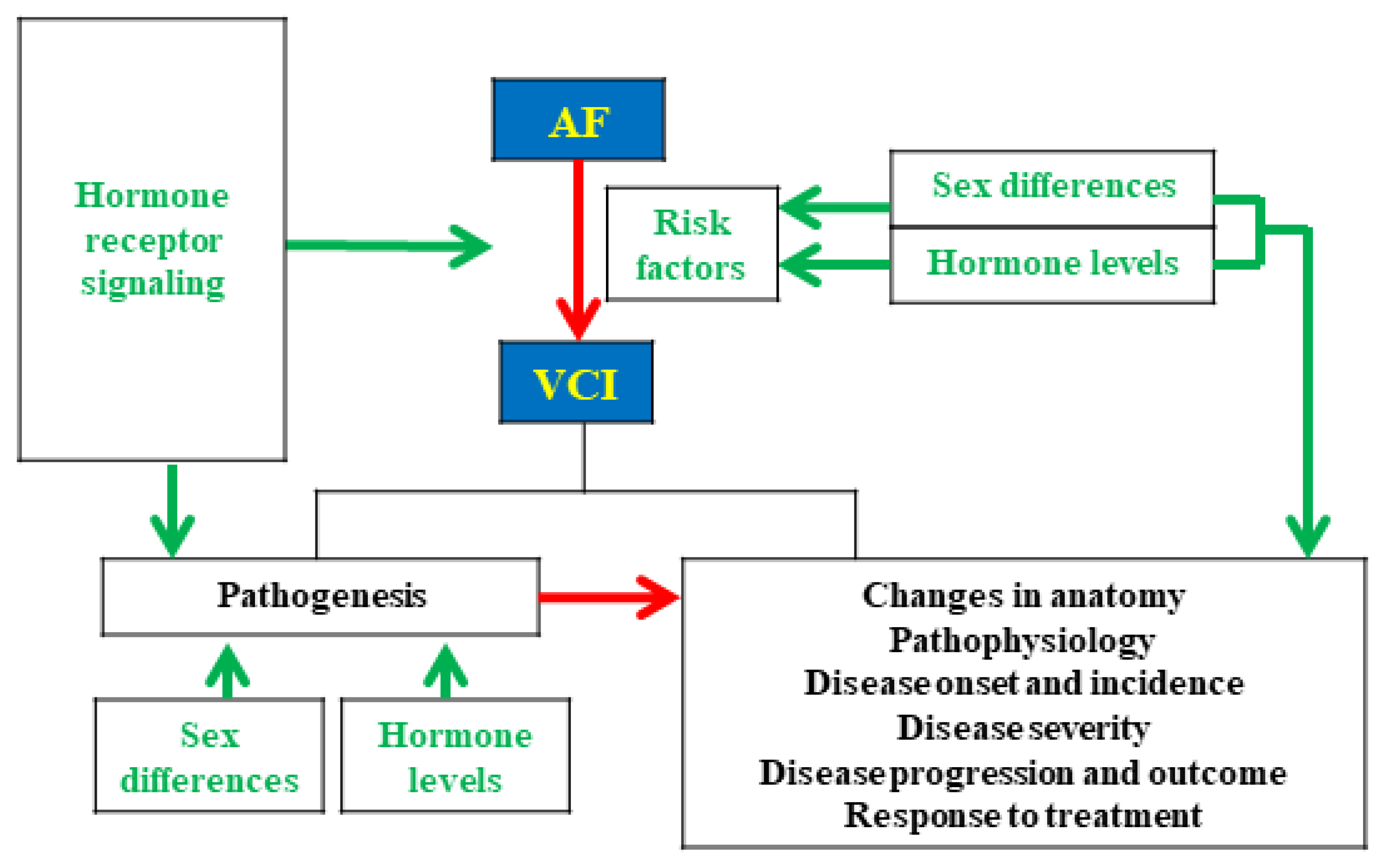
4. The Interactive Relationship of Sex Hormones and Sex Differences between AF and VCI
4.1. Effect of Sex Differences on Outcomes in Patients with AF-Related VCI
4.2. Pathophysiology of Sex Differences in AF and VCI
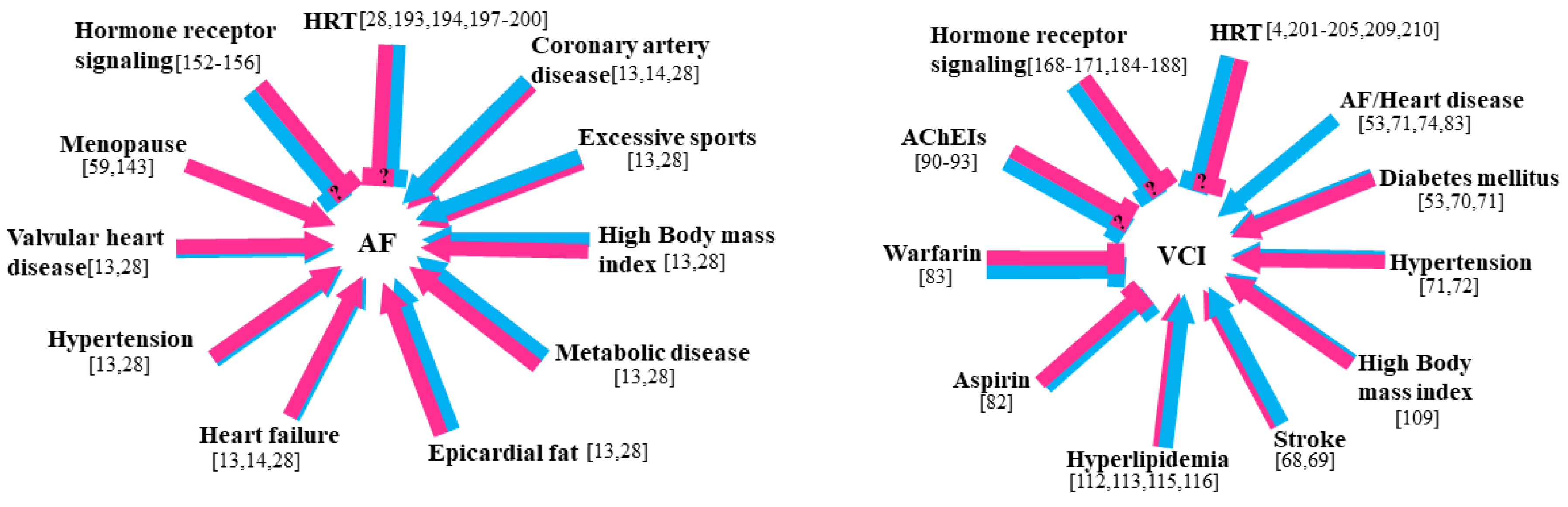
5. Effect of Sex Hormone Deficiency/Excess on AF and VCI
5.1. Sex Hormone Deficiency/Excess and AF
5.1.1. AF and Androgen Signaling
5.1.2. AF and Estrogen Signaling
5.2. Sex Hormone Deficiency/Excess and VCI
5.2.1. VCI and Androgen Signaling
5.2.2. VCI and Estrogen Signaling
6. Effects of Sex Hormone Therapy on AF and VCI
6.1. Effects of Sex Hormone Therapy on AF
6.2. Effects of Sex Hormone Therapy on VCI
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kang, H.-Y.; Tsai, M.-Y.; Chang, C.; Huang, K.-E. Mechanisms and clinical relevance of androgens and androgen receptor actions. Chang. Gung Med, J. 2003, 26, 388–402. [Google Scholar]
- Cornil, C.A.; Ball, G.; Balthazart, J. Functional significance of the rapid regulation of brain estrogen action: Where do the estrogens come from? Brain Res. 2006, 1126, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, J.; McEwen, B.S. Sex in the brain: Hormones and sex differences. Dialog-Clin. Neurosci. 2016, 18, 373–383. [Google Scholar]
- Arevalo, M.-A.; Azcoitia, I.; Garcia-Segura, L. The neuroprotective actions of oestradiol and oestrogen receptors. Nat. Rev. Neurosci. 2015, 16, 17–29. [Google Scholar] [CrossRef]
- Aryan, L.; Younessi, D.; Zargari, M.; Banerjee, S.; Agopian, J.; Rahman, S.; Borna, R.; Ruffenach, G.; Umar, S.; Eghbali, M. The Role of Estrogen Receptors in Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 4314. [Google Scholar] [CrossRef] [PubMed]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- Mendelsohn, M.E.; Karas, R.H. Molecular and cellular basis of cardiovascular gender differences. Science 2005, 308, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Hadjimarkou, M.M.; Vasudevan, N. GPER1/GPR30 in the brain: Crosstalk with classical estrogen receptors and implications for behavior. J. Steroid Biochem. Mol. Biol. 2018, 176, 57–64. [Google Scholar] [CrossRef]
- Kang, H.-Y. Beyond the male sex hormone: Deciphering the metabolic and vascular actions of testosterone. J. Endocrinol. 2013, 217, C1–C3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lizotte, E.; Grandy, S.A.; Tremblay, A.; Allen, B.G.; Fiset, C. Expression, Distribution and Regulation of Sex Steroid Hormone Receptors in Mouse Heart. Cell. Physiol. Biochem. 2009, 23, 075–086. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Herald, A.K.; Alves-Lopes, R.; Montezano, A.C.; Ahmed, S.F.; Touyz, R.M. Genomic and non-genomic effects of androgens in the cardiovascular system: Clinical implications. Clin. Sci. (Lond.) 2017, 131, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Linde, C.; Bongiorni, M.G.; Birgersdotter-Green, U.; Curtis, A.B.; Deisenhofer, I.; Furokawa, T.; Gillis, A.M.; Haugaa, K.H.; Lip, G.Y.H.; Van Gelder, I.; et al. Sex differences in cardiac arrhythmia: A consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace 2018, 20, 1565. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; Simon, D.N.; Steinberg, B.A.; Thomas, L.; Allen, L.A.; Fonarow, G.C.; Gersh, B.; Hylek, E.; Kowey, P.R.; Reiffel, J.A.; et al. Differences in Clinical and Functional Outcomes of Atrial Fibrillation in Women and Men: Two-Year Results From the ORBIT-AF Registry. JAMA Cardiol. 2016, 1, 282–291. [Google Scholar] [CrossRef]
- Rienstra, M.; Van Veldhuisen, D.J.; Hagens, V.E.; Ranchor, A.V.; Veeger, N.J.; Crijns, H.J.; Van Gelder, I.C. Gender-Related Differences in Rhythm Control Treatment in Persistent Atrial Fibrillation: Data of the Rate Control Versus Electrical Cardioversion (RACE) Study. J. Am. Coll. Cardiol. 2005, 46, 1298–1306. [Google Scholar] [CrossRef]
- Lehmann, M.H.; Timothy, K.W.; Frankovich, D.; Fromm, B.S.; Keating, M.; Locati, E.H.; Taggart, R.; A Towbin, J.; Moss, A.J.; Schwartz, P.J.; et al. Age-Gender Influence on the Rate-Corrected QT Interval and the QT-Heart Rate Relation in Families With Genotypically Characterized Long QT Syndrome. J. Am. Coll. Cardiol. 1997, 29, 93–99. [Google Scholar] [CrossRef]
- Di Carlo, A.; Bellino, L.; Consoli, D.; Mori, F.; Zaninelli, A.; Baldereschi, M.; Cattarinussi, A.; D’Alfonso, M.G.; Gradia, C.; Sgherzi, B.; et al. Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European Union: The FAI Project. Europace 2019, 21, 1468–1475. [Google Scholar] [CrossRef]
- Lip, G.Y.; Laroche, C.; Boriani, G.; Cimaglia, P.; Dan, G.-A.; Santini, M.; Kalarus, Z.; Rasmussen, L.H.; Popescu, M.I.; Tica, O.; et al. Sex-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: A report from the Euro Observational Research Programme Pilot survey on Atrial Fibrillation. Europace 2014, 17, 24–31. [Google Scholar] [CrossRef]
- Zylla, M.M.; Brachmann, J.; Lewalter, T.; Hoffmann, E.; Kuck, K.-H.; Andresen, D.; Willems, S.; Eckardt, L.; Tebbenjohanns, J.; Spitzer, S.G.; et al. Sex-related outcome of atrial fibrillation ablation: Insights from the German Ablation Registry. Heart Rhythm. 2016, 13, 1837–1844. [Google Scholar] [CrossRef]
- Patel, N.; Deshmukh, A.; Thakkar, B.; Coffey, J.O.; Agnihotri, K.; Patel, A.; Ainani, N.; Nalluri, N.; Patel, N.; Patel, N.; et al. Gender, Race, and Health Insurance Status in Patients Undergoing Catheter Ablation for Atrial Fibrillation. Am J Cardiol. 2016, 117, 1117–1126. [Google Scholar] [CrossRef]
- Nielsen, J.C.; Johannessen, A.; Raatikainen, P.; Hindricks, G.; Walfridsson, H.; Kongstad, O.; Pehrson, S.; Englund, A.; Hartikainen, J.; Mortensen, L.S.; et al. Radiofrequency Ablation as Initial Therapy in Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2012, 367, 1587–1595. [Google Scholar] [CrossRef]
- Bunch, T.J.; May, H.; Bair, T.L.; Weiss, J.P.; Crandall, B.G.; Osborn, J.S.; Mallender, C.; Anderson, J.L.; Muhlestein, B.J.; Lappe, D.L.; et al. Atrial fibrillation ablation patients have long-term stroke rates similar to patients without atrial fibrillation regardless of CHADS2 score. Heart Rhythm. 2013, 10, 1272–1277. [Google Scholar] [CrossRef]
- Morillo, C.A.; Verma, A.; Connolly, S.J.; Kuck, K.H.; Nair, G.M.; Champagne, J.; Sterns, L.D.; Beresh, H.; Healey, J.S.; Natale, A.; et al. Radiofrequency ablation vs. antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): A randomized trial. JAMA 2014, 311, 692–700. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Pecen, L.; Ojeda, F.M.; Lucerna, M.; Rzayeva, N.; Blankenberg, S.; Darius, H.; Kotecha, D.; De Caterina, R.; Kirchhof, P. Gender differences in clinical presentation and 1-year outcomes in atrial fibrillation. Heart 2017, 103, 1024–1030. [Google Scholar] [CrossRef]
- Kaiser, D.W.; Fan, J.; Schmitt, S.; Than, C.T.; Ullal, A.J.; Piccini, J.P.; Heidenreich, P.A.; Turakhia, M.P. Gender Differences in Clinical Outcomes After Catheter Ablation of Atrial Fibrillation. JACC Clin. Electrophysiol. 2016, 2, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Vallakati, A.; Reddy, M.; Sharma, A.; Kanmanthareddy, A.; Sridhar, A.; Pillarisetti, J.; Atkins, D.; Konda, B.; Bommana, S.; Di Biase, L.; et al. Impact of gender on outcomes after atrial fibrillation ablation. Int. J. Cardiol. 2015, 187, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yang, B.; Nattel, S. MicroRNAs and atrial fibrillation: Mechanisms and translational potential. Nat. Rev. Cardiol. 2014, 12, 80–90. [Google Scholar] [CrossRef]
- Odening, K.E.; Deiß, S.; Dilling-Boer, D.; Didenko, M.; Eriksson, U.; Nedios, S.; Ng, F.S.; Roca Luque, I.; Sanchez Borque, P.; Vernooy, K.; et al. Mechanisms of sex differences in atrial fibrillation: Role of hormones and differences in electrophysiology, structure, function, and remodelling. Ep Eur. 2019, 21, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Hudsmith, L.E.; Petersen, S.; Francis, J.M.; Robson, M.D.; Neubauer, S. Normal Human Left and Right Ventricular and Left Atrial Dimensions Using Steady State Free Precession Magnetic Resonance Imaging. J. Cardiovasc. Magn. Reson. 2005, 7, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, N.P.; Witte, K.K.A.; Thackray, S.D.R.; Goodge, L.J.; Clark, A.L.; Cleland, J.G.F. Effect of age and sex on left atrial morphology and function. Eur. J. Echocardiogr. 2003, 4, 36–42. [Google Scholar] [CrossRef]
- Forleo, G.B.; Tondo, C.; De Luca, L.; Russo, A.D.; Casella, M.; De Sanctis, V.; Clementi, F.; Fagundes, R.L.; Leo, R.; Romeo, F.; et al. Gender-related differences in catheter ablation of atrial fibrillation. Europace 2007, 9, 613–620. [Google Scholar] [CrossRef]
- Pfannmüller, B.; Boldt, A.; Reutemann, A.; Duerrschmidt, N.; Krabbes-Graube, S.; Mohr, F.W.; Dhein, S. Gender-specific remodeling in atrial fibrillation? Thorac. Cardiovasc. Surg. 2013, 61, 66–73. [Google Scholar] [CrossRef]
- Wong, C.X.; Sun, M.T.; Odutayo, A.; Emdin, C.A.; Mahajan, R.; Lau, D.H.; Pathak, R.; Wong, D.T.; Selvanayagam, J.B.; Sanders, P.; et al. Associations of Epicardial, Abdominal, and Overall Adiposity With Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2016, 9, e004378. [Google Scholar] [CrossRef] [PubMed]
- Canpolat, U.; Aytemir, K.; Yorgun, H.; Asil, S.; Dural, M.; Özer, N. The Impact of echocardiographic epicardial fat thickness on outcomes of cryoballoon-based atrial fibrillation ablation. Echocardiography 2016, 33, 821–829. [Google Scholar] [CrossRef]
- Keller, K.M.; Howlett, S.E. Sex Differences in the Biology and Pathology of the Aging Heart. Can. J. Cardiol. 2016, 32, 1065–1073. [Google Scholar] [CrossRef]
- Baker, A.R.; Da Silva, N.F.; Quinn, D.W.; Harte, A.L.; Pagano, D.; Bonser, R.S.; Kumar, S.; McTernan, P.G. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc. Diabetol. 2006, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, S.; Azarbal, F.; Stefanick, M.L.; LaMonte, M.J.; Li, W.; Tharp, K.M.; Martin, L.W.; Nassir, R.; Salmoirago-Blotcher, E.; Albert, C.; et al. The associations of leptin, adiponectin and resistin with incident atrial fibrillation in women. Heart 2016, 102, 1354–1362. [Google Scholar] [CrossRef]
- Tsai, W.C.; Chen, Y.C.; Kao, Y.H.; Lu, Y.Y.; Chen, S.A.; Chen, Y.J. Distinctive sodium and calcium regulation associated with sex differences in atrial electrophysiology of rabbits. Int. J. Cardiol. 2013, 168, 4658–4666. [Google Scholar] [CrossRef]
- Tsai, W.C.; Chen, Y.C.; Lin, Y.K.; Chen, S.A.; Chen, Y.J. Sex differences in the electrophysiological characteristics of pulmonary veins and left atrium and their clinical implication in atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2011, 4, 550–559. [Google Scholar] [CrossRef]
- Liu, X.K.; Katchman, A.; Whitfield, B.H.; Wan, G.; Janowski, E.M.; Woosley, R.L.; Ebert, S.N. In vivo androgen treatment shortens the QT interval and increases the densities of inward and delayed rectifier potassium currents in orchiectomized male rabbits. Cardiovasc. Res. 2003, 57, 28–36. [Google Scholar] [CrossRef]
- Furukawa, T.; Kurokawa, J. Non-genomic regulation of cardiac ion channels by sex hormones. Cardiovasc. Hematol. Disord. Drug Targets 2008, 8, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; DeKosky, S.T.; Barberger-Gateau, P.; Cummings, J.; Delacourte, A.; Galasko, D.; Gauthier, S.; Jicha, G.; et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007, 6, 734–746. [Google Scholar] [CrossRef]
- Odening, K.E. Another jigsaw piece in the complex picture of hormonal regulation of cardiac repolarization. Eur. Heart J. 2015, 37, 651–653. [Google Scholar] [CrossRef]
- Kurokawa, J.; Tamagawa, M.; Harada, N.; Honda, S.I.; Bai, C.X.; Nakaya, H.; Furukawa, T. Acute effects of oestrogen on the guinea pig and human IKr channels and drug-induced prolongation of cardiac repolarization. J. Physiol. 2008, 586, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Anneken, L.; Baumann, S.; Vigneault, P.; Biliczki, P.; Friedrich, C.; Xiao, L.; Girmatsion, Z.; Takac, I.; Brandes, R.; Kissler, S.; et al. Estradiol regulates human QT-interval: Acceleration of cardiac repolarization by enhanced KCNH2 membrane trafficking. Eur. Heart J. 2016, 37, 640–650. [Google Scholar] [CrossRef]
- Drici, M.D.; Burklow, T.R.; Haridasse, V.; Glazer, R.I.; Woosley, R.L. Sex Hormones Prolong the QT Interval and Downregulate Potassium Channel Expression in the Rabbit Heart. Circulation 1996, 94, 1471–1474. [Google Scholar] [CrossRef]
- El Gebeily, G.; El Khoury, N.; Mathieu, S.; Brouillette, J.; Fiset, C. Estrogen regulation of the transient outward K+ current involves estrogen receptor α in mouse heart. J. Mol. Cell. Cardiol. 2015, 86, 85–94. [Google Scholar] [CrossRef]
- Odening, K.E.; Choi, B.-R.; Liu, G.X.; Hartmann, K.; Ziv, O.; Chaves, L.; Schofield, L.; Centracchio, J.; Zehender, M.; Peng, X.; et al. Estradiol promotes sudden cardiac death in transgenic long QT type 2 rabbits while progesterone is protective. Heart Rhythm. 2012, 9, 823–832. [Google Scholar] [CrossRef]
- Odening, K.E.; Koren, G. How do sex hormones modify arrhythmogenesis in long QT syndrome? Sex hormone effects on arrhythmogenic substrate and triggered activity. Heart Rhythm. 2014, 11, 2107–2115. [Google Scholar] [CrossRef]
- Ambrosi, C.M.; Yamada, K.A.; Nerbonne, J.M.; Efimov, I.R. Gender Differences in Electrophysiological Gene Expression in Failing and Non-Failing Human Hearts. PLoS ONE 2013, 8, e54635. [Google Scholar] [CrossRef][Green Version]
- Coumel, P. Paroxysmal Atrial Fibrillation: A Disorder of Autonomic Tone? Eur. Heart J. 1994, 15, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Carlson, M.D.; Laurita, K.P. Cellular mechanisms of vagally mediated atrial tachyarrhythmia in isolated arterially perfused canine right atria. J. Cardiovasc. Electrophysiol. 2002, 13, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Dufouil, C.; Seshadri, S.; Chêne, G. Cardiovascular Risk Profile in Women and Dementia. J. Alzheimer’s Dis. 2014, 42, S353–S363. [Google Scholar] [CrossRef] [PubMed]
- Oberhauser, V.; Schwertfeger, E.; Rutz, T.; Beyersdorf, F.; Rump, L.C. Acetylcholine release in human heart atrium: Influence of muscarinic autoreceptors, diabetes, and age. Circulation 2001, 103, 1638–1643. [Google Scholar] [CrossRef]
- Harvey, R.D.; Belevych, A. Muscarinic regulation of cardiac ion channels. Br. J. Pharmacol. 2003, 139, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- . Insulander, P.; Juhlin-Dannfelt, A.; Freyschuss, U.; Vallin, H. Electrophysiologic effects of mental stress in healthy subjects: A comparison with epinephrine infusion. J. Electrocardiol. 2003, 36, 301–309. [Google Scholar] [CrossRef]
- Koenig, J.; Thayer, J.F. Sex differences in healthy human heart rate variability: A meta-analysis. Neurosci. Biobehav. Rev. 2016, 64, 288–310. [Google Scholar] [CrossRef]
- Yildirir, A.; Kabakci, G.; Akgul, E.; Tokgozoglu, L.; Oto, A. Effects of Menstrual Cycle on Cardiac Autonomic Innervation As Assessed By Heart Rate Variability. Ann. Noninvasive Electrocardiol. 2001, 7, 60–63. [Google Scholar] [CrossRef]
- Jensen-Urstad, K.; Storck, N.; Bouvier, F.; Ericson, M.; Lindbland, L.E.; Jensen-Urstad, M. Heart rate variability in healthy subjects is related to age and gender. Acta Physiol. Scand. 1997, 160, 235–241. [Google Scholar] [CrossRef]
- Snyder, H.M.; Corriveau, R.A.; Craft, S.; Faber, J.E.; Greenberg, S.M.; Knopman, D.; Lamb, B.T.; Montine, T.J.; Nedergaard, M.; Schaffer, C.B.; et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 710–717. [Google Scholar] [CrossRef]
- Skrobot, O.A.; Attems, J.; Esiri, M.; Hortobágyi, T.; Ironside, J.; Kalaria, R.N.; King, A.; Lammie, G.A.; Mann, D.; Neal, J.; et al. Vascular cognitive impairment neuropathology guidelines (VCING): The contribution of cerebrovascular pathology to cognitive impairment. Brain 2016, 139, 2957–2969. [Google Scholar] [CrossRef]
- van der Flier, W.M.; Skoog, I.; Schneider, J.A.; Pantoni, L.; Mok, V.; Chen, C.L.; Scheltens, P. Vascular cognitive impairment. Nat. Rev. Dis. Primers 2018, 4, 18003. [Google Scholar] [CrossRef]
- Cho, S.-J.; Yu, K.-H.; Oh, M.S.; Jung, S.; Lee, J.-H.; Koh, I.-S.; Bae, H.-J.; Kang, Y.; Lee, B.-C. Korean-Vascular Cognitive Impairment Harmonization Standards Study Group Post-stroke memory impairment among patients with vascular mild cognitive impairment. BMC Neurol. 2014, 14, 244. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ohara, T.; Nagakane, Y.; Tanaka, E.; Morii, F.; Koizumi, T.; Akiguchi, I. Chronic kidney disease, 24-h blood pressure and small vessel diseases are independently associated with cognitive impairment in lacunar infarct patients. Hypertens. Res. 2011, 34, 1276–1282. [Google Scholar] [CrossRef]
- Swardfager, W.; MacIntosh, B. Depression, Type 2 Diabetes, and Poststroke Cognitive Impairment. Neurorehabilit. Neural Repair 2016, 31, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Mellon, L.; on behalf of the ASPIRE-S Study Group; Brewer, L.; Hall, P.; Horgan, F.; Williams, D.; Hickey, A. Cognitive impairment six months after ischaemic stroke: A profile from the ASPIRE-S study. BMC Neurol. 2015, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Exalto, L.G.; Boomsma, J.M.F.; Mofrad, R.B.; Barkhof, F.; Groeneveld, O.N.; Heinen, R.; Kuijf, H.J.; Leeuwis, A.E.; Prins, N.D.; Biessels, G.J.; et al. Sex differences in memory clinic patients with possible vascular cognitive impairment. Alzheimer’s Dementia Diagn. Assess. Dis. Monit. 2020, 12, e12090. [Google Scholar] [CrossRef] [PubMed]
- Giroud, M.; Delpont, B.; Daubail, B.; Blanc, C.; Durier, J.; Giroud, M.; Béjot, Y. Temporal Trends in Sex Differences With Regard to Stroke Incidence: The Dijon Stroke Registry (1987–2012). Stroke 2017, 48, 846–849. [Google Scholar] [CrossRef]
- Appelros, P.; Stegmayr, B.; Terént, A. Sex differences in stroke epidemiology: A systematic review. Stroke 2009, 40, 1082–1090. [Google Scholar] [CrossRef]
- Chatterjee, S.; Peters, S.A.; Woodward, M.; Arango, S.M.; Batty, G.D.; Beckett, N.; Beiser, A.; Borenstein, A.R.; Crane, P.K.; Haan, M.; et al. Type 2 Diabetes as a Risk Factor for Dementia in Women Compared With Men: A Pooled Analysis of 2.3 Million People Comprising More Than 100,000 Cases of Dementia. Diabetes Care 2016, 39, 300–307. [Google Scholar] [CrossRef]
- Gannon, O.; Robison, L.; Custozzo, A.; Zuloaga, K. Sex differences in risk factors for vascular contributions to cognitive impairment & dementia. Neurochem. Int. 2019, 127, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.; Launer, L.J.; Dewey, M.; Letenneur, L.; Ott, A.; Copeland, J.R.M.; Dartigues, J.-F.; Kragh-Sorensen, P.; Baldereschi, M.; Brayne, C.; et al. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. Neurology 1999, 53, 1992. [Google Scholar] [CrossRef]
- Imfeld, P.; Brauchli Pernus, Y.B.; Jick, S.S.; Meier, C.R. Epidemiology, co-morbidities, and medication use of patients with Alzheimer’s disease or vascular dementia in the UK. J. Alzheimer’s Dis. 2013, 35, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Renoux, C.; Coulombe, J.; Li, L.; Ganesh, A.; Silver, L.; Rothwell, P.M. Confounding by Pre-Morbid Functional Status in Studies of Apparent Sex Differences in Severity and Outcome of Stroke. Stroke 2017, 48, 2731–2738. [Google Scholar] [CrossRef]
- Ruitenberg, A.; Ott, A.; van Swieten, J.; Hofman, A.; Breteler, M.M. Incidence of dementia: Does gender make a difference? Neurobiol. Aging 2001, 22, 575–580. [Google Scholar] [CrossRef]
- Di Carlo, A.; Baldereschi, M.; Amaducci, L.; Lepore, V.; Bracco, L.; Maggi, S.; Bonaiuto, S.; Perissinotto, E.; Scarlato, G.; Farchi, G.; et al. Incidence of dementia, Alzheimer’s disease, and vascular dementia in Italy. The ILSA Study. J. Am. Geriatr. Soc. 2002, 50, 41–48. [Google Scholar] [CrossRef]
- Corraini, P.; Henderson, V.W.; Ording, A.G.; Pedersen, L.; Horváth-Puhó, E.; Sørensen, H.T. Long-Term Risk of Dementia Among Survivors of Ischemic or Hemorrhagic Stroke. Stroke 2017, 48, 180–186. [Google Scholar] [CrossRef]
- Dhamoon, M.S.; McClure, L.A.; White, C.L.; Lakshminarayan, K.; Benavente, M.-F.; Elkind, M.S. Long-term disability after lacunar stroke: Secondary prevention of small subcortical strokes. Neurology 2015, 84, 1002–1008. [Google Scholar] [CrossRef]
- Rasquin, S.; Verhey, F.; Lousberg, R.; Winkens, I.; Lodder, J. Vascular cognitive disorders: Memory, mental speed and cognitive flexibility after stroke. J. Neurol. Sci. 2002, 203–204, 115–119. [Google Scholar] [CrossRef]
- Andersson, M.; Guo, X.; Börjesson-Hanson, A.; Liebetrau, M.; Östling, S.; Skoog, I. A population-based study on dementia and stroke in 97 year olds. Age Ageing 2012, 41, 529–533. [Google Scholar] [CrossRef]
- Lobo, A.; Launer, L.J.; Fratiglioni, L.; Andersen, K.; Di Carlo, A.; Breteler, M.M.; Copeland, J.R.; Dartigues, J.F.; Jagger, C.; Martinez-Lage, J.; et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000, 54, S4. [Google Scholar]
- Ridker, P.M.; Cook, N.R.; Lee, I.-M.; Gordon, D.; Gaziano, J.M.; Manson, J.E.; Hennekens, C.H.; Buring, J.E. A Randomized Trial of Low-Dose Aspirin in the Primary Prevention of Cardiovascular Disease in Women. N. Engl. J. Med. 2005, 352, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Poli, D.; Antonucci, E.; Grifoni, E.; Abbate, R.; Gensini, G.F.; Prisco, D. Gender differences in stroke risk of atrial fibrillation patients on oral anticoagulant treatment. Thromb. Haemost. 2009, 101, 938–942. [Google Scholar]
- Barha, C.K.; Hsiung, G.-Y.R.; Best, J.R.; Davis, J.C.; Eng, J.J.; Jacova, C.; Lee, P.E.; Munkacsy, M.; Cheung, W.; Liu-Ambrose, T. Sex Difference in Aerobic Exercise Efficacy to Improve Cognition in Older Adults with Vascular Cognitive Impairment: Secondary Analysis of a Randomized Controlled Trial. J. Alzheimer’s Dis. 2017, 60, 1397–1410. [Google Scholar] [CrossRef]
- Niewada, M.; Kobayashi, A.; Sandercock, P.A.; Kamiński, B.; Czlonkowska, A. Influence of Gender on Baseline Features and Clinical Outcomes among 17,370 Patients with Confirmed Ischaemic Stroke in the International Stroke Trial. Neuroepidemiology 2005, 24, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Lye, T.C.; Piguet, O.; A Grayson, D.; Creasey, H.; Ridley, L.J.; Bennett, H.P.; Broe, G.A. Hippocampal size and memory function in the ninth and tenth decades of life: The Sydney Older Persons Study. J. Neurol. Neurosurg. Psychiatry 2004, 75, 548–554. [Google Scholar] [CrossRef]
- Good, C.D.; Johnsrudeb, I.; Ashburner, J.; Henson, R.N.; Friston, K.J.; Frackowiak, R.S. Cerebral Asymmetry and the Effects of Sex and Handedness on Brain Structure: A Voxel-Based Morphometric Analysis of 465 Normal Adult Human Brains. NeuroImage 2001, 14, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Raz, N. Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neurosci. Biobehav. Rev. 2014, 42, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Sohn, M.K.; Jee, S.; Yang, S.S. The Characteristics of Cognitive Impairment and Their Effects on Functional Outcome After Inpatient Rehabilitation in Subacute Stroke Patients. Ann. Rehabil. Med. 2017, 41, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Lee, S.J.; Pyo, J.-S. Effect of acetylcholinesterase inhibitors on post-stroke cognitive impairment and vascular dementia: A meta-analysis. PLoS ONE 2020, 15, e0227820. [Google Scholar] [CrossRef]
- Román, G.C.; Kalaria, R.N. Vascular determinants of cholinergic deficits in Alzheimer disease and vascular dementia. Neurobiol. Aging 2006, 27, 1769–1785. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Hasselmo, M.; Bruno, J.P.; Givens, B. Unraveling the attentional functions of cortical cholinergic inputs: Interactions between signal-driven and cognitive modulation of signal detection. Brain Res. Rev. 2005, 48, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E.; Pepeu, G. Sex and Gender Differences in the Brain Cholinergic System and in the Response to Therapy of Alzheimer Disease with Cholinesterase Inhibitors. Curr. Alzheimer Res. 2018, 15, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, A.N.; Salimi-Khorshidi, G.; Lai, M.-C.; Baron-Cohen, S.; Lombardo, M.; Tait, R.J.; Suckling, J. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014, 39, 34–50. [Google Scholar] [CrossRef]
- Voyer, D.; Voyer, S.; Bryden, M.P. Magnitude of sex differences in spatial abilities: A meta-analysis and consideration of critical variables. Psychol. Bull. 1995, 117, 250–270. [Google Scholar] [CrossRef]
- Hyde, J.S.; Linn, M.C. Gender differences in verbal ability: A meta-analysis. Psychol. Bull. 1988, 104, 53–69. [Google Scholar] [CrossRef]
- Gur, R.C.; Mozley, L.H.; Mozley, P.D.; Resnick, S.M.; Karp, J.S.; Alavi, A.; E Arnold, S. Sex differences in regional cerebral glucose metabolism during a resting state. Science 1995, 267, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Valsasina, P.; Misci, P.; Falini, A.; Comi, G.; A Rocca, M. The organization of intrinsic brain activity differs between genders: A resting-state fMRI study in a large cohort of young healthy subjects. Hum. Brain Mapp. 2013, 34, 1330–1343. [Google Scholar] [CrossRef]
- Hjelmervik, H.; Hausmann, M.; Osnes, B.; Westerhausen, R.; Specht, K. Resting States Are Resting Traits—An fMRI Study of Sex Differences and Menstrual Cycle Effects in Resting State Cognitive Control Networks. PLoS ONE 2014, 9, e103492. [Google Scholar] [CrossRef]
- Allen, E.A.; Erhardt, E.B.; Damaraju, E.; Gruner, W.; Segall, J.M.; Silva, R.F.; Havlicek, M.; Rachakonda, S.; Fries, J.; Kalyanam, R.; et al. A Baseline for the Multivariate Comparison of Resting-State Networks. Front. Syst. Neurosci. 2011, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.S.; Damasio, H.; Grabowski, T.J. Normal neuroanatomical variation in the human brain: An MRI-volumetric study. Am. J. Phys. Anthropol. 2002, 118, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Carne, R.P.; Vogrin, S.; Litewka, L.; Cook, M.J. Cerebral cortex: An MRI-based study of volume and variance with age and sex. J. Clin. Neurosci. 2006, 13, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Im, K.; Lee, J.-M.; Lee, J.; Shin, Y.-W.; Kim, I.Y.; Kwon, J.S.; Kim, S.I. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. NeuroImage 2006, 31, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.M.; Seidman, L.J.; Horton, N.; Makris, N.; Kennedy, D.N.; Caviness, V.S.; Faraone, S.; Tsuang, M.T. Normal Sexual Dimorphism of the Adult Human Brain Assessed by In Vivo Magnetic Resonance Imaging. Cereb. Cortex 2001, 11, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, T. Structural differences in the cerebral cortex of healthy female and male subjects: A magnetic resonance imaging study. Psychiatry Res. Neuroimaging 1995, 61, 129–135. [Google Scholar] [CrossRef]
- Hsu, J.-L.; Leemans, A.; Bai, C.-H.; Lee, C.-H.; Tsai, Y.-F.; Chiu, H.-C.; Chen, W.-H. Gender differences and age-related white matter changes of the human brain: A diffusion tensor imaging study. NeuroImage 2008, 39, 566–577. [Google Scholar] [CrossRef]
- A Ahangar, A.; Saadat, P.; Heidari, B.; Taheri, S.T.; Alijanpour, S. Sex difference in types and distribution of risk factors in ischemic and hemorrhagic stroke. Int. J. Stroke 2018, 13, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Ahtiluoto, S.; Polvikoski, T.; Peltonen, M.; Solomon, A.; Tuomilehto, J.; Winblad, B.; Sulkava, R.; Kivipelto, M. Diabetes, Alzheimer disease, and vascular dementia: A population-based neuropathologic study. Neurology 2010, 75, 1195–1202. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef]
- Arnoldussen, I.A.; Kiliaan, A.J.; Gustafson, D.R. Obesity and dementia: Adipokines interact with the brain. Eur. Neuropsychopharmacol. 2014, 24, 1982–1999. [Google Scholar] [CrossRef]
- Kivimäki, M.; Luukkonen, R.; Batty, G.; Ferrie, J.E.; Pentti, J.; Nyberg, S.T.; Shipley, M.J.; Alfredsson, L.; Fransson, E.I.; Goldberg, M.; et al. Body mass index and risk of dementia: Analysis of individual-level data from 1.3 million individuals. Alzheimer’s Dement. 2018, 14, 601–609. [Google Scholar] [CrossRef]
- Ancelin, M.-L.; Ripoche, E.; Dupuy, A.-M.; Barberger-Gateau, P.; Auriacombe, S.; Rouaud, O.; Berr, C.; Carrière, I.; Ritchie, K. Sex Differences in the Associations Between Lipid Levels and Incident Dementia. J. Alzheimer’s Dis. 2013, 34, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.-G.; Wang, Q.-S.; Yu, K.; Wang, W.-W.; Lin, H.; Yang, Z.-H. Sex differences in associations between blood lipids and cerebral small vessel disease. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 28–34. [Google Scholar] [CrossRef]
- Heiss, W.-D.; Rosenberg, G.A.; Thiel, A.; Berlot, R.; De Reuck, J. Neuroimaging in vascular cognitive impairment: A state-of-the-art review. BMC Med. 2016, 14, 174. [Google Scholar] [CrossRef]
- Chuang, Y.F.; Hayden, K.M.; Norton, M.C.; Tschanz, J.; Breitner, J.C.; Welsh-Bohmer, K.A.; Zandi, P.P.; Cache County Investigators. Association between APOE epsilon4 allele and vascular dementia: The Cache County study. Dement. Geriatr. Cogn. Disord. 2010, 29, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.L.; Tybjærg-Hansen, A.; Nordestgaard, B.G.; Frikke-Schmidt, R. Absolute 10-year risk of dementia by age, sex and APOE genotype: A population-based cohort study. Can. Med Assoc. J. 2018, 190, E1033–E1041. [Google Scholar] [CrossRef]
- Wolf, P.A.; Dawber, T.R.; Thomas, E.; Kannel, W.B. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: The Framingham Study. Neurology 2011, 77, 1579. [Google Scholar] [CrossRef]
- Santangeli, P.; Di Biase, L.; Bai, R.; Mohanty, S.; Pump, A.; Brantes, M.C.; Horton, R.; Burkhardt, J.D.; Lakkireddy, D.; Reddy, Y.M.; et al. Atrial fibrillation and the risk of incident dementia: A meta-analysis. Heart Rhythm. 2012, 9, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Tatemichi, T.K.; Paik, M.; Bagiella, E.; Desmond, D.; Stern, Y.; Sano, M.; Hauser, W.A.; Mayeux, R. Risk of dementia after stroke in a hospitalized cohort: Results of a longitudinal study. Neurology 1994, 44, 1885. [Google Scholar] [CrossRef]
- Pohjasvaara, T.; Erkinjuntti, T.; Vataja, R.; Kaste, M. Dementia three months after stroke. Baseline frequency and effect of different definitions of dementia in the Helsinki Stroke Aging Memory Study (SAM) cohort. Stroke 1997, 28, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Desmond, D.W.; Moroney, J.T.; Sano, M.; Stern, Y. Incidence of dementia after ischemic stroke: Results of a longitudinal study. Stroke 2002, 33, 2254–2260. [Google Scholar] [CrossRef]
- Wändell, P.; Carlsson, A.C.; Sundquist, J.; Sundquist, K. The association between relevant comorbidities and dementia in patients with atrial fibrillation. GeroScience 2018, 40, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Chen, J.; Wang, H.-T.; Chang, Y.-T.; Chong, S.-Z.; Hsueh, S.; Chung, C.-M.; Lin, Y.-S. Sex Difference in the Risk of Dementia in Patients with Atrial Fibrillation. Diagnostics 2021, 11, 760. [Google Scholar] [CrossRef]
- Jin, M.N.; Kim, T.H.; Kang, K.W.; Yu, H.T.; Uhm, J.S.; Joung, B.; Lee, M.H.; Kim, E.; Pak, H.N. Atrial Fibrillation Catheter Ablation Improves 1-Year Follow-Up Cognitive Function, Especially in Patients With Impaired Cognitive Function. Circ. Arrhythm. Electrophysiol 2019, 12, e007197. [Google Scholar] [CrossRef]
- Goette, A.; Rocken, C. Atrial amyloidosis and atrial fibrillation: A gender-dependent “arrhythmogenic substrate”? Eur. Heart J. 2004, 25, 1185–1186. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Boriani, G.; Chiappini, B.; Pacini, D.; Cenacchi, G.; Suarez, S.M.; Rapezzi, C.; Reggiani, M.L.B.; Marinelli, G. Amyloid deposition as a cause of atrial remodelling in persistent valvular atrial fibrillation. Eur. Heart J. 2004, 25, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G.; Pearce, L.A.; McBride, R.; Rothbart, R.M.; Asinger, R.W. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: Analysis of 2012 participants in the SPAF I-III clinical trials. The Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Stroke 1999, 30, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Xu, B.; Xiang, Y.; Wu, L.; Zhang, Y.; Ma, X.; Tong, S.; Shu, M.; Song, Z.; Li, Y.; et al. Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: A meta-analysis. Int. J. Cardiol. 2013, 169, 62–72. [Google Scholar] [CrossRef]
- Jiang, W.; Gilkeson, G. Sex Differences in monocytes and TLR4 associated immune responses; implications for systemic lupus erythematosus (SLE). J. Immunother. Appl. 2014, 1, 1. [Google Scholar] [CrossRef]
- Harman, S.M.; Metter, E.J.; Tobin, J.D.; Pearson, J.; Blackman, M.R. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J. Clin. Endocrinol. Metab. 2001, 86, 724–731. [Google Scholar] [CrossRef]
- Mulligan, T.; Frick, M.F.; Zuraw, Q.C.; Stemhagen, A.; McWhirter, C. Prevalence of hypogonadism in males aged at least 45 years: The HIM study. Int. J. Clin. Pr. 2008, 60, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Magnani, J.W.; Moser, C.B.; Murabito, J.M.; Sullivan, L.M.; Wang, N.; Ellinor, P.T.; Vasan, R.S.; Benjamin, E.J.; Coviello, A.D. Association of sex hormones, aging, and atrial fibrillation in men: The Framingham Heart Study. Circ. Arrhythm. Electrophysiol. 2014, 7, 307–312. [Google Scholar] [CrossRef]
- Jiangtao, L.; Dongchen, Z.; Shudong, X.; Yunpeng, S.; Lihong, W.; Liangrong, Z.; Jianhua, Z. Reduced testosterone levels in males with lone atrial fibrillation. Clin. Cardiol. 2009, 32, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Zeller, T.; Schnabel, R.B.; Appelbaum, S.; Ojeda, F.; Berisha, F.; Schulte-Steinberg, B.; Brueckmann, B.-E.; Kuulasmaa, K.; Jousilahti, P.; Blankenberg, S.; et al. Low testosterone levels are predictive for incident atrial fibrillation and ischaemic stroke in men, but protective in women—Results from the FINRISK study. Eur. J. Prev. Cardiol. 2018, 25, 1133–1139. [Google Scholar] [CrossRef]
- O’Neal, W.T.; Nazarian, S.; Alonso, A.; Heckbert, S.R.; Vaccarino, V.; Soliman, E.Z. Sex hormones and the risk of atrial fibrillation: The Multi-Ethnic Study of Atherosclerosis (MESA). Endocrine 2017, 58, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Er, F.; Michels, G.; Brandt, M.C.; Khan, I.; Haase, H.; Eicks, M.; Lindner, M.; Hoppe, U.C. Impact of testosterone on cardiac L-type calcium channels and Ca2+ sparks: Acute actions antagonize chronic effects. Cell Calcium 2007, 41, 467–477. [Google Scholar] [CrossRef]
- Golden, K.L.; Marsh, J.D.; Jiang, Y. Testosterone regulates mRNA levels of calcium regulatory proteins in cardiac myocytes. Horm. Metab. Res. 2004, 36, 197–202. [Google Scholar]
- Lanfranco, F.; <monospace> </monospace>Kamischke, A.; Zitzmann, M.; Nieschlag, E. Klinefelter’s syndrome. Lancet 2004, 364, 273–283. [Google Scholar] [CrossRef]
- Cho, J.H.; Choi, E.K.; Moon, I.K.; Jung, J.H.; Han, K.D.; Choi, Y.J.; Park, J.; Lee, E.; Lee, S.R.; Cha, M.J.; et al. Chromosomal abnormalities and atrial fibrillation and ischemic stroke incidence: A nationwide population-based study. Sci. Rep. 2020, 10, 15872. [Google Scholar] [CrossRef]
- Bretagne, M.; Lebrun-Vignes, B.; Pariente, A.; Shaffer, C.M.; Malouf, G.G.; Dureau, P.; Potey, C.; Funck-Brentano, C.; Roden, D.M.; Moslehi, J.J.; et al. Heart failure and atrial tachyarrhythmia on abiraterone: A pharmacovigilance study. Arch. Cardiovasc. Dis. 2020, 113, 9–21. [Google Scholar] [CrossRef]
- Oliver-Williams, C.; Vassard, D.; Pinborg, A.; Schmidt, L. Polycystic ovary syndrome as a novel risk factor for atrial fibrillation: Results from a national Danish registry cohort study. Eur. J. Prev. Cardiol. 2020, 2047487320922927. [Google Scholar] [CrossRef]
- Ko, D.; Rahman, F.; Schnabel, R.B.; Yin, X.; Benjamin, E.; Christophersen, I.E. Atrial fibrillation in women: Epidemiology, pathophysiology, presentation, and prognosis. Nat. Rev. Cardiol. 2016, 13, 321–332. [Google Scholar] [CrossRef]
- Rossi, R.; Grimaldi, T.; Origliani, G.; Fantini, G.; Coppi, F.; Modena, M.G. Menopause and cardiovascular risk. Pathophysiol. Haemost. Thromb. 2002, 32, 325–328. [Google Scholar] [CrossRef]
- Lee, M.; Chen, W.; Zhang, Z.; Duan, L.; Ng, A.; Spencer, H.T.; Kwan, D.M.; Shen, A.Y. Atrial Fibrillation and Atrial Flutter in Pregnant Women—A Population-Based Study. J. Am. Heart Assoc. 2016, 5, e003182. [Google Scholar] [CrossRef]
- Salam, A.M.; Ertekin, E.; van Hagen, I.M.; Al Suwaidi, J.; Ruys, T.P.; Johnson, M.R.; Gumbiene, L.; Frogoudaki, A.A.; Sorour, K.A.; Iserin, L.; et al. Atrial fibrillation or flutter during pregnancy in patients with structural heart disease: Data from the ROPAC (Registry on Pregnancy and Cardiac Disease). JACC Clin. Electrophysiol. 2015, 1, 284–292. [Google Scholar] [CrossRef]
- Kirbas, O.; Biberoglu, E.H.; Kirbas, A.; Daglar, H.K.; Kurmus, O.; Uygur, D.; Danisman, N. P-wave duration changes and dispersion in preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 183, 141–145. [Google Scholar] [CrossRef]
- Parasuraman, R.; Gandhi, M.M.; Liversedge, N.H. Nifedipine tocolysis associated atrial fibrillation responds to DC cardioversion. BJOG 2006, 113, 844–845. [Google Scholar] [CrossRef]
- Carson, M.P.; Fisher, A.J.; Scorza, W.E. Atrial Fibrillation in Pregnancy Associated With Oral Terbutaline. Obstet. Gynecol. 2002, 100, 1096–1097. [Google Scholar] [CrossRef]
- Ntusi, N.B.; Badri, M.; Gumedze, F.; Sliwa, K.; Mayosi, B.M. Pregnancy-Associated Heart Failure: A Comparison of Clinical Presentation and Outcome between Hypertensive Heart Failure of Pregnancy and Idiopathic Peripartum Cardiomyopathy. PLoS ONE 2015, 10, e0133466. [Google Scholar]
- Yang, S.H.; Liu, R.; Perez, E.J.; Wen, Y.; Stevens, S.M.; Valencia, T.; Brun-Zinkernagel, A.M.; Prokai, L.; Will, Y.; Dykens, J.; et al. Mitochondrial localization of estrogen receptor beta. Proc. Natl. Acad. Sci. USA 2004, 101, 4130–4135. [Google Scholar] [CrossRef]
- Ishii, K.; Kano, T.; Ando, J. Sex differences in [3H]nitrendipine binding and effects of sex steroid hormones in rat cardiac and cerebral membranes. Jpn. J. Pharmacol. 1988, 46, 117–125. [Google Scholar] [CrossRef]
- Parks, R.J.; Howlett, S.E. Sex differences in mechanisms of cardiac excitation–contraction coupling. Pflüg. Arch.-Eur. J. Physiol. 2013, 465, 747–763. [Google Scholar] [CrossRef]
- Farrell, S.R.; Ross, J.L.; Howlett, S.E. Sex differences in mechanisms of cardiac excitation-contraction coupling in rat ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H36–H45. [Google Scholar] [CrossRef]
- Johnson, B.D.; Zheng, W.; Korach, K.; Scheuer, T.; Catterall, W.A.; Rubanyi, G.M. Increased Expression of the Cardiac L-type Calcium Channel in Estrogen Receptor–deficient Mice. J. Gen. Physiol. 1997, 110, 135–140. [Google Scholar] [CrossRef]
- Nakajima, T.; Iwasawa, K.; Oonuma, H.; Morita, T.; Goto, A.; Wang, Y.; Hazama, H. Antiarrhythmic effect and its underlying ionic mechanism of 17beta-estradiol in cardiac myocytes. Br. J. Pharmacol. 1999, 127, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Yeap, B.B.; Hyde, Z.; Almeida, O.P.; Norman, P.E.; Chubb, S.A.P.; Jamrozik, K.; Flicker, L.; Hankey, G.J. Lower Testosterone Levels Predict Incident Stroke and Transient Ischemic Attack in Older Men. J. Clin. Endocrinol. Metab. 2009, 94, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, L.L.; Jørgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Olsen, T.S.; Winther, K. Decreased Serum Testosterone in Men With Acute Ischemic Stroke. Arter. Thromb. Vasc. Biol. 1996, 16, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Basaria, S.; Coviello, A.D.; Travison, T.G.; Storer, T.W.; Farwell, W.R.; Jette, A.; Eder, R.; Tennstedt, S.; Ulloor, J.; Zhang, A.; et al. Adverse Events Associated with Testosterone Administration. N. Engl. J. Med. 2010, 363, 109–122. [Google Scholar] [CrossRef]
- Gonzales, R.J. Androgens and the cerebrovasculature: Modulation of vascular function during normal and pathophysiological conditions. Pflügers Arch.-Eur. J. Physiol. 2013, 465, 627–642. [Google Scholar] [CrossRef]
- Gonzales, R.J.; Krause, D.N.; Duckles, S.P. Testosterone suppresses endothelium-dependent dilation of rat middle cerebral arteries. Am. J. Physiol. Circ. Physiol. 2004, 286, H552–H560. [Google Scholar] [CrossRef]
- Gonzales, R.J.; Ghaffari, A.A.; Duckles, S.P.; Krause, D.N. Testosterone treatment increases thromboxane function in rat cerebral arteries. Am. J. Physiol. Circ. Physiol. 2005, 289, H578–H585. [Google Scholar] [CrossRef]
- Wabitsch, M.; Blum, W.F.; Muche, R.; Braun, M.; Hube, F.; Rascher, W.; Heinze, E.; Teller, W.; Hauner, H. Contribution of androgens to the gender difference in leptin production in obese children and adolescents. J. Clin. Investig. 1997, 100, 808–813. [Google Scholar] [CrossRef]
- Deng, Z.-H.; Liao, J.; Zhang, J.-Y.; Liang, C.; Song, C.-H.; Han, M.; Wang, L.-H.; Xue, H.; Zhang, K.; Zabeau, L.; et al. Inhibition of the Connexin 43 Elevation May be Involved in the Neuroprotective Activity of Leptin Against Brain Ischemic Injury. Cell. Mol. Neurobiol. 2014, 34, 871–879. [Google Scholar] [CrossRef]
- Sarfo, F.S.; Akassi, J.; Adamu, S.; Obese, V.; Ovbiagele, B. Burden and Predictors of Poststroke Cognitive Impairment in a Sample of Ghanaian Stroke Survivors. J. Stroke Cerebrovasc. Dis. 2017, 26, 2553–2562. [Google Scholar] [CrossRef]
- Nead, K.T.; Gaskin, G.; Chester, C.; Swisher-McClure, S.; Leeper, N.J.; Shah, N.H. Association Between Androgen Deprivation Therapy and Risk of Dementia. JAMA Oncol. 2017, 3, 49–55. [Google Scholar] [CrossRef]
- Fukai, S.; Akishita, M.; Yamada, S.; Toba, K.; Ouchi, Y. EFFECTS OF TESTOSTERONE IN OLDER MEN WITH MILD-TO-MODERATE COGNITIVE IMPAIRMENT. J. Am. Geriatr. Soc. 2010, 58, 1419–1421. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tong, Y.; Chen, P.F.; Miao, S.; Zhou, R.Y. Neuroprotection of dihydrotestosterone via suppression of the toll-like receptor 4/nuclear factor-kappa B signaling pathway in high glucose-induced BV-2 microglia inflammatory responses. Neuroreport 2020, 31, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Pike, C.J.; Nguyen, T.-V.V.; Ramsden, M.; Yao, M.; Murphy, M.P.; Rosario, E.R. Androgen cell signaling pathways involved in neuroprotective actions. Horm. Behav. 2008, 53, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Li, H. An Updated Review: Androgens and Cognitive Impairment in Older Men. Front. Endocrinol. 2020, 11, 586909. [Google Scholar] [CrossRef]
- Koss, W.A.; Frick, K.M. Activation of androgen receptors protects intact male mice from memory impairments caused by aromatase inhibition. Horm. Behav. 2019, 111, 96–104. [Google Scholar] [CrossRef]
- Yaffe, K.; Edwards, E.R.; Lui, L.-Y.; Zmuda, J.M.; E Ferrell, R.; A Cauley, J. Androgen receptor CAG repeat polymorphism is associated with cognitive function in older men. Biol. Psychiatry 2003, 54, 943–946. [Google Scholar] [CrossRef]
- Carswell, H.V.O.; Dominiczak, A.F.; Macrae, I.M. Estrogen status affects sensitivity to focal cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am. J. Physiol. Circ. Physiol. 2000, 278, H290–H294. [Google Scholar] [CrossRef]
- Alkayed, N.J.; Harukuni, I.; Kimes, A.S.; London, E.D.; Traystman, R.J.; Hurn, P.D. Gender-Linked Brain Injury in Experimental Stroke. Stroke 1998, 29, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yuan, R.; Benashski, S.E.; McCullough, L.D. Changes in Experimental Stroke Outcome across the Life Span. Br. J. Pharmacol. 2009, 29, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Hawk, T.; Zhang, Y.-Q.; Rajakumar, G.; Day, A.L.; Simpkins, J.W. Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res. 1998, 796, 296–298. [Google Scholar] [CrossRef]
- Yang, S.-H.; Perez, E.; Cutright, J.; Liu, R.; He, Z.; Day, A.L.; Simpkins, J.W. Testosterone increases neurotoxicity of glutamate in vitro and ischemia-reperfusion injury in an animal model. J. Appl. Physiol. 2002, 92, 195–201. [Google Scholar] [CrossRef]
- Alkayed, N.J.; Murphy, S.J.; Traystman, R.J.; Hurn, P.D.; Miller, V.M. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke 2000, 31, 161–168. [Google Scholar] [CrossRef]
- Cai, M.; Ma, Y.; Qin, P.; Li, Y.; Zhang, L.-X.; Nie, H.; Peng, Z.; Dong, H.; Dong, H.-L.; Hou, W.-G.; et al. The loss of estrogen efficacy against cerebral ischemia in aged postmenopausal female mice. Neurosci. Lett. 2014, 558, 115–119. [Google Scholar] [CrossRef]
- Ma, Y.; Qin, P.; Li, Y.; Shen, L.; Wang, S.-Q.; Dong, H.-L.; Hou, W.-G.; Xiong, L.-Z. The effects of different doses of estradiol (E2) on cerebral ischemia in an in vitro model of oxygen and glucose deprivation and reperfusion and in a rat model of middle carotid artery occlusion. BMC Neurosci. 2013, 14, 118. [Google Scholar] [CrossRef]
- Strom, J.O.; Theodorsson, A.; Theodorsson, E. Mechanisms of estrogens’ dose-dependent neuroprotective and neurodamaging effects in experimental models of cerebral ischemia. Int. J. Mol. Sci 2011, 12, 1533–1562. [Google Scholar] [CrossRef] [PubMed]
- Ström, J.; Theodorsson, A.; Theodorsson, E. Dose-Related Neuroprotective versus Neurodamaging Effects of Estrogens in Rat Cerebral Ischemia: A Systematic Analysis. Br. J. Pharmacol. 2009, 29, 1359–1372. [Google Scholar] [CrossRef]
- Leon, R.L.; Li, X.; Huber, J.D.; Rosen, C.L. Worsened Outcome from Middle Cerebral Artery Occlusion in Aged Rats Receiving 17β-Estradiol. Endocrinology 2012, 153, 3386–3393. [Google Scholar] [CrossRef] [PubMed]
- Batnasan, E.; Wang, R.; Wen, J.; Ke, Y.; Li, X.; Bohio, A.A.; Zeng, X.; Huo, H.; Han, L.; Boldogh, I.; et al. 17-beta estradiol inhibits oxidative stress-induced accumulation of AIF into nucleolus and PARP1-dependent cell death via estrogen receptor alpha. Toxicol. Lett. 2015, 232, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.; Zendedel, A.; Lammerding, L.; Beyer, C.; Slowik, A. Impact of 17beta-estradiol and progesterone on inflammatory and apoptotic microRNA expression after ischemia in a rat model. J. Steroid Biochem. Mol. Biol. 2017, 167, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, J.; Sun, S.; Zhao, J.; Dong, X.; Wang, J. Effects of Estradiol on Autophagy and Nrf-2/ARE Signals after Cerebral Ischemia. Cell. Physiol. Biochem. 2017, 41, 2027–2036. [Google Scholar] [CrossRef]
- Zhang, Z.-L.; Qin, P.; Liu, Y.; Zhang, L.-X.; Guo, H.; Deng, Y.-L.; Liu, Y.-; Hou, Y.-S.; Wang, L.-Y.; Miao, Y.; et al. Alleviation of ischaemia-reperfusion injury by endogenous estrogen involves maintaining Bcl-2 expression via the ERα signalling pathway. Brain Res. 2017, 1661, 15–23. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, P.; Li, X.; Lei, S.; Li, W.; He, X.; Zhang, J.; Wang, N.; Qi, C.; Chen, X.; et al. Post-stroke estradiol treatment enhances neurogenesis in the subventricular zone of rats after permanent focal cerebral ischemia. Neuroscience 2012, 231, 82–90. [Google Scholar] [CrossRef]
- Deroo, B.J.; Korach, K.S. Estrogen receptors and human disease. J. Clin. Investig. 2006, 116, 561–570. [Google Scholar] [CrossRef]
- Yaffe, K.; Lui, L.Y.; Grady, D.; Stone, K.; Morin, P. Estrogen receptor 1 polymorphisms and risk of cognitive impairment in older women. Biol. Psychiatry 2002, 51, 677–682. [Google Scholar] [CrossRef]
- Dresner-Pollak, R.; Kinnar, T.; Friedlander, Y.; Sharon, N.; Rosenmann, H.; Pollak, A. Estrogen Receptor Beta Gene Variant Is Associated with Vascular Dementia in Elderly Women. Genet. Test. Mol. Biomarkers 2009, 13, 339–342. [Google Scholar] [CrossRef]
- Ardelt, A.A.; McCullough, L.D.; Korach, K.S.; Wang, M.M.; Munzenmaier, D.H.; Hurn, P.D. Estradiol regulates angiopoietin-1 mRNA expression through estrogen receptor-alpha in a rodent experimental stroke model. Stroke 2005, 36, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Bretler, D.M.; Hansen, P.R.; Lindhardsen, J.; Ahlehoff, O.; Andersson, C.; Jensen, T.B.; Raunsø, J.; Torp-Pedersen, C.; Gislason, G.H. Hormone replacement therapy and risk of new-onset atrial fibrillation after myocardial infarction—A nationwide cohort study. PLoS ONE 2012, 7, e51580. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Haung, Y.-B.; Kuo, H.-F.; Tang, W.-H.; Hsu, P.-C.; Su, H.-M.; Lin, T.-H.; Chu, C.-S.; Jhuo, S.-J.; Lee, K.-T.; et al. Hormone replacement therapy and risk of atrial fibrillation in Taiwanese menopause women: A nationwide cohort study. Sci. Rep. 2016, 6, 24132. [Google Scholar] [CrossRef]
- Wong, J.A.; Rexrode, K.; Sandhu, R.K.; Moorthy, M.V.; Conen, D.; Albert, C. Menopausal age, postmenopausal hormone therapy and incident atrial fibrillation. Heart 2017, 103, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.; Leonardo, F.; Dicandia, C.; Sheiban, I.; Pagnotta, P.; Pappone, C.; Chierchia, S.L. Acute electrophysiologic effect of estradiol 17beta in menopausal women. Am. J. Cardiol. 2000, 86, 1385–1387. [Google Scholar] [CrossRef]
- Saba, S.; Zhu, W.; Aronovitz, M.J.; Estes, N.M.; Wang, P.J.; Mendelsohn, M.E.; Karas, R.H. Effects of estrogen on cardiac electrophysiology in female mice. J. Cardiovasc. Electrophysiol. 2002, 13, 276–280. [Google Scholar] [CrossRef]
- Sharma, R.; Oni, O.A.; Gupta, K.; Sharma, M.; Sharma, R.; Singh, V.; Parashara, D.; Kamalakar, S.; Dawn, B.; Chen, G.; et al. Normalization of Testosterone Levels After Testosterone Replacement Therapy Is Associated With Decreased Incidence of Atrial Fibrillation. J. Am. Heart Assoc. 2017, 6, e004880. [Google Scholar] [CrossRef]
- Tsuneda, T.; Yamashita, T.; Kato, T.; Sekiguchi, A.; Sagara, K.; Sawada, H.; Aizawa, T.; Fu, L.-T.; Fujiki, A.; Inoue, H. Deficiency of Testosterone Associates with the Substrate of Atrial Fibrillation in the Rat Model. J. Cardiovasc. Electrophysiol. 2009, 20, 1055–1060. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Lee, T.-I.; Chen, Y.-C.; Kao, Y.-H.; Lu, Y.-Y.; Lin, Y.-K.; Chen, S.-A.; Chen, Y.-J. Testosterone replacement increases aged pulmonary vein and left atrium arrhythmogenesis with enhanced adrenergic activity. Int. J. Cardiol. 2014, 176, 110–118. [Google Scholar] [CrossRef]
- Carrasquilla, G.D.; Frumento, P.; Berglund, A.; Borgfeldt, C.; Bottai, M.; Chiavenna, C.; Eliasson, M.; Engström, G.; Hallmans, G.; Jansson, J.-H.; et al. Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies. PLoS Med. 2017, 14, e1002445. [Google Scholar] [CrossRef]
- Miller, V.M.; Harman, S.M. An update on hormone therapy in postmenopausal women: Mini-review for the basic scientist. Am. J. Physiol. Circ. Physiol. 2017, 313, H1013–H1021. [Google Scholar] [CrossRef] [PubMed]
- van Londen, G.J.; Beckjord, E.B.; Dew, M.A.; Cooper, K.L.; Davidson, N.E.; Bovbjerg, D.H.; Donovan, H.S.; Thurston, R.C.; Morse, J.Q.; Nutt, S.; et al. Associations between adjuvant endocrine therapy and onset of physical and emotional concerns among breast cancer survivors. Support. Care Cancer 2013, 22, 937–945. [Google Scholar] [CrossRef]
- Luine, V.N. Estradiol and cognitive function: Past, present and future. Horm. Behav. 2014, 66, 602–618. [Google Scholar] [CrossRef]
- Brinton, R.D.; Yao, J.; Yin, F.; Mack, W.J.; Cadenas, E. Perimenopause as a neurological transition state. Nat. Rev. Endocrinol. 2015, 11, 393–405. [Google Scholar] [CrossRef]
- Finley, S.K.; Kritzer, M.K. Immunoreactivity for intracellular androgen receptors in identified subpopulations of neurons, astrocytes and oligodendrocytes in primate prefrontal cortex. J. Neurobiol. 1999, 40, 446–457. [Google Scholar] [CrossRef]
- Yoon, B.-K.; Chin, J.; Kim, J.-W.; Shin, M.-H.; Ahn, S.; Lee, D.-Y.; Seo, S.W.; Na, D.L. Menopausal hormone therapy and mild cognitive impairment: A randomized, placebo-controlled trial. Menopause 2018, 25, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, R.A.; Quesenberry, C.P.; Zhou, J.; Yaffe, K. Timing of hormone therapy and dementia: The critical window theory re-visited. Ann. Neurol. 2011, 69, 163–169. [Google Scholar] [CrossRef]
- Abdelkader, N.F.; Abd El-Latif, A.M.; Khattab, M.M. Telmisartan/17β-estradiol mitigated cognitive deficit in an ovariectomized rat model of Alzheimer’s disease: Modulation of ACE1/ACE2 and AT1/AT2 ratio. Life Sci. 2020, 245, 117388. [Google Scholar] [CrossRef]
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-T.; Chen, Y.-L.; Kang, H.-Y. Revealing the Influences of Sex Hormones and Sex Differences in Atrial Fibrillation and Vascular Cognitive Impairment. Int. J. Mol. Sci. 2021, 22, 8776. https://doi.org/10.3390/ijms22168776
Chang Y-T, Chen Y-L, Kang H-Y. Revealing the Influences of Sex Hormones and Sex Differences in Atrial Fibrillation and Vascular Cognitive Impairment. International Journal of Molecular Sciences. 2021; 22(16):8776. https://doi.org/10.3390/ijms22168776
Chicago/Turabian StyleChang, Ya-Ting, Yung-Lung Chen, and Hong-Yo Kang. 2021. "Revealing the Influences of Sex Hormones and Sex Differences in Atrial Fibrillation and Vascular Cognitive Impairment" International Journal of Molecular Sciences 22, no. 16: 8776. https://doi.org/10.3390/ijms22168776
APA StyleChang, Y.-T., Chen, Y.-L., & Kang, H.-Y. (2021). Revealing the Influences of Sex Hormones and Sex Differences in Atrial Fibrillation and Vascular Cognitive Impairment. International Journal of Molecular Sciences, 22(16), 8776. https://doi.org/10.3390/ijms22168776






