Biomaterial-Assisted Regenerative Medicine
Abstract
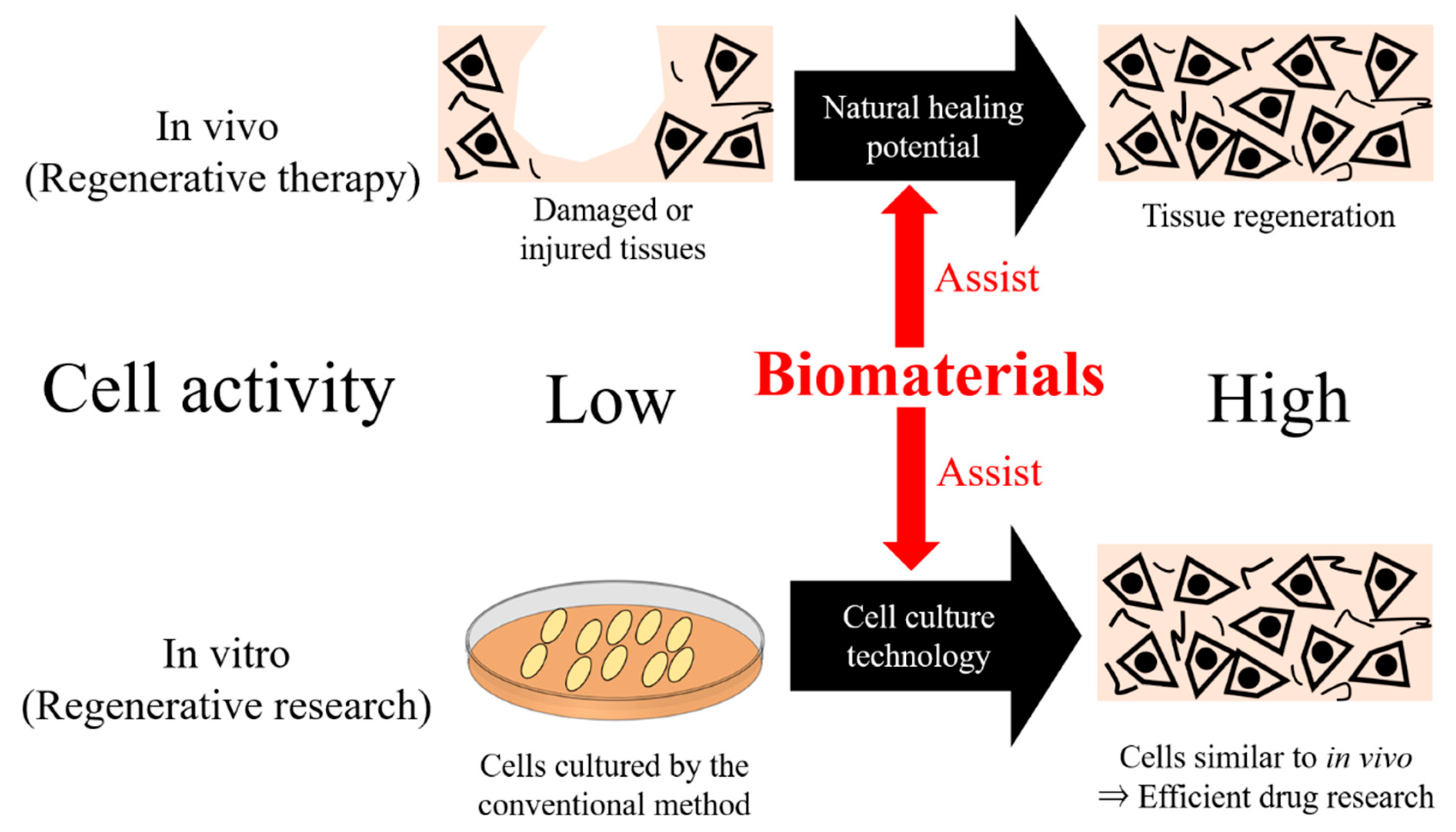
:1. Introduction
2. Regenerative Medicine Combined with Biomaterials
2.1. Collagen
2.2. Gelatin
2.3. Alginate
2.4. Chitosan
2.5. Silk Fibroin
2.6. Agarose
2.7. Matrigel
2.8. Poly(lactic acid)
2.9. Poly(lactic-co-glycolic acid)
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nii, T.; Makino, K.; Tabata, Y. Three-dimensional culture system of cancer cells combined with biomaterials for drug screening. Cancers 2020, 12, 2754. [Google Scholar] [CrossRef]
- Fukuda, J.; Sakai, Y.; Nakazawa, K. Novel hepatocyte culture system developed using microfabrication and collagen/polyethylene glycol microcontact printing. Biomaterials 2006, 27, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.; Jung, F.; Pietzsch, J. Human Endothelial Cell Models in Biomaterial Research. Trends Biotechnol. 2017, 35, 265–277. [Google Scholar] [CrossRef]
- Rodríguez-Enríquez, S.; Gallardo-Pérez, J.C.; Avilés-Salas, A.; Marín-Hernández, A.; Carreño-Fuentes, L.; Maldonado-Lagunas, V.; Moreno-Sánchez, R. Energy metabolism transition in multi-cellular human tumor spheroids. J. Cell. Physiol. 2008, 216, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Asada, N.; Kunisaki, Y.; Pierce, H.; Wang, Z.; Fernandez, N.F.; Birbrair, A.; Ma’ayan, A.; Frenette, P.S. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat. Cell Biol. 2017, 19, 214–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nii, T.; Makino, K.; Tabata, Y. A cancer invasion model of cancer-associated fibroblasts aggregates combined with TGF-β1 release system. Regen. Ther. 2020, 14, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef]
- Ramadan, Q.; Zourob, M. Organ-on-a-chip engineering: Toward bridging the gap between lab and industry. Biomicrofluidics 2020, 14, 041501. [Google Scholar] [CrossRef] [PubMed]
- Brighi, C.; Cordella, F.; Chiriatti, L.; Soloperto, A.; Di Angelantonio, S. Retinal and Brain Organoids: Bridging the Gap Between in vivo Physiology and in vitro Micro-Physiology for the Study of Alzheimer’s Diseases. Front. Neurosci. 2020, 14, 655. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Hinderer, S.; Layland, S.L.; Schenke-Layland, K. ECM and ECM-like materials—Biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 2016, 97, 260–269. [Google Scholar] [CrossRef]
- Lee, Y.B.; Kim, E.M.; Byun, H.; Chang, H.k.; Jeong, K.; Aman, Z.M.; Choi, Y.S.; Park, J.; Shin, H. Engineering spheroids potentiating cell-cell and cell-ECM interactions by self-assembly of stem cell microlayer. Biomaterials 2018, 165, 105–120. [Google Scholar] [CrossRef]
- Sainio, A.; Järveläinen, H. Extracellular matrix-cell interactions: Focus on therapeutic applications. Cell. Signal. 2020, 66, 109487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kawazoe, N.; Chen, G. Preparation of dexamethasone-loaded biphasic calcium phosphate nanoparticles/collagen porous composite scaffolds for bone tissue engineering. Acta Biomater. 2018, 67, 341–353. [Google Scholar] [CrossRef]
- Heo, D.N.; Hospodiuk, M.; Ozbolat, I.T. Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering. Acta Biomater. 2019, 95, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, R.; Jia, Z.; Wang, C.; Xu, Y.; Li, C.; Xia, H.; Meng, D. Porous fish collagen for cartilage tissue engineering. Am. J. Transl. Res. 2020, 12, 6107–6121. [Google Scholar]
- Chen, S.; Kawazoe, N.; Chen, G. Biomimetic Assembly of Vascular Endothelial Cells and Muscle Cells in Microgrooved Collagen Porous Scaffolds. Tissue Eng.-Part C Methods 2017, 23, 367–376. [Google Scholar] [CrossRef]
- Campbell, J.J.; Husmann, A.; Hume, R.D.; Watson, C.J.; Cameron, R.E. Development of three-dimensional collagen scaffolds with controlled architecture for cell migration studies using breast cancer cell lines. Biomaterials 2017, 114, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Saini, H.; Rahmani Eliato, K.; Silva, C.; Allam, M.; Mouneimne, G.; Ros, R.; Nikkhah, M. The Role of Desmoplasia and Stromal Fibroblasts on Anti-cancer Drug Resistance in a Microengineered Tumor Model. Cell. Mol. Bioeng. 2018, 11, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Oyanagi, J.; Hoshino, D.; Togo, S.; Kumagai, H.; Miyagi, Y. Cancer cell migration on elongate protrusions of fibroblasts in collagen matrix. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapudom, J.; Kalbitzer, L.; Wu, X.; Martin, S.; Kroy, K.; Pompe, T. Fibril bending stiffness of 3D collagen matrices instructs spreading and clustering of invasive and non-invasive breast cancer cells. Biomaterials 2019, 193, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Masumoto, H.; Tajima, S.; Ikuno, T.; Katayama, S.; Minakata, K.; Ikeda, T.; Yamamizu, K.; Tabata, Y.; Sakata, R.; et al. Efficient long-term survival of cell grafts after myocardial infarction with thick viable cardiac tissue entirely from pluripotent stem cells. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Masumoto, H.; Jo, J.I.; Yamazaki, K.; Ikeda, T.; Tabata, Y.; Minatoya, K. Sustained release of basic fibroblast growth factor using gelatin hydrogel improved left ventricular function through the alteration of collagen subtype in a rat chronic myocardial infarction model. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Tajima, S.; Tabata, Y. Preparation of EpH4 and 3T3L1 cells aggregates incorporating gelatin hydrogel microspheres for a cell condition improvement. Regen. Ther. 2017, 6, 90–99. [Google Scholar] [CrossRef]
- Tajima, S.; Tabata, Y. Preparation of epithelial cell aggregates incorporating matrigel microspheres to enhance proliferation and differentiation of epithelial cells. Regen. Ther. 2017, 7, 34–44. [Google Scholar] [CrossRef]
- Inoo, K.; Bando, H.; Tabata, Y. Enhanced survival and insulin secretion of insulinoma cell aggregates by incorporating gelatin hydrogel microspheres. Regen. Ther. 2018, 8, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Nakamura, H.; Tabata, Y.; Fujimori, Y.; Kumasawa, K.; Kimura, T. Effect of sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogels on frozen-thawed human ovarian tissue in a xenograft model. J. Obstet. Gynaecol. Res. 2018, 44, 1947–1955. [Google Scholar] [CrossRef]
- Notodihardjo, S.C.; Morimoto, N.; Kakudo, N.; Mitsui, T.; Le, T.M.; Tabata, Y.; Kusumoto, K. Efficacy of Gelatin Hydrogel Impregnated with Concentrated Platelet Lysate in Murine Wound Healing. J. Surg. Res. 2019, 234, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Nii, T.; Makino, K.; Tabata, Y. A Cancer Invasion Model Combined with Cancer-Associated Fibroblasts Aggregates Incorporating Gelatin Hydrogel Microspheres Containing a p53 Inhibitor. Tissue Eng.-Part C Methods 2019, 25, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Nii, T.; Kuwahara, T.; Makino, K.; Tabata, Y. A co-culture system of three-dimensional tumor-associated macrophages and three-dimensional cancer-associated fibroblasts combined with biomolecule release for cancer cell migration. Tissue Eng.-Part A 2020, 26, 1272–1282. [Google Scholar] [CrossRef]
- Mansouri, V.; Salehi, M.; Omrani, M.d.; Niknam, Z.; Ardeshirylajimi, A. Collagen-alginate microspheres as a 3D culture system for mouse embryonic stem cells differentiation to primordial germ cells. Biologicals 2017, 48, 114–120. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Liu, W.; Zhang, Y.; Pang, B.; Liu, H.; Zhang, Y.; Zhang, H.; Zhang, L.; Liao, H.; Ren, C.; et al. Continuous microfluidic encapsulation of single mesenchymal stem cells using alginate microgels as injectable fillers for bone regeneration. Acta Biomater. 2020, 111, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Anamizu, M.; Tabata, Y. Design of injectable hydrogels of gelatin and alginate with ferric ions for cell transplantation. Acta Biomater. 2019, 100, 184–190. [Google Scholar] [CrossRef]
- Sarker, B.; Zehnder, T.; Rath, S.N.; Horch, R.E.; Kneser, U.; Detsch, R.; Boccaccini, A.R. Oxidized Alginate-Gelatin Hydrogel: A Favorable Matrix for Growth and Osteogenic Differentiation of Adipose-Derived Stem Cells in 3D. ACS Biomater. Sci. Eng. 2017, 3, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Etter, J.N.; Karasinski, M.; Ware, J.; Oldinski, R.A. Dual-crosslinked homogeneous alginate microspheres for mesenchymal stem cell encapsulation. J. Mater. Sci. Mater. Med. 2018, 29, 1–10. [Google Scholar] [CrossRef]
- Acarregui, A.; Ciriza, J.; Saenz del Burgo, L.; Gurruchaga Iribar, H.; Yeste, J.; Illa, X.; Orive, G.; Hernández, R.M.; Villa, R.; Pedraz, J.L. Characterization of an encapsulated insulin secreting human pancreatic beta cell line in a modular microfluidic device. J. Drug Target. 2018, 26, 36–44. [Google Scholar] [CrossRef]
- Somo, S.I.; Langert, K.; Yang, C.Y.; Vaicik, M.K.; Ibarra, V.; Appel, A.A.; Akar, B.; Cheng, M.H.; Brey, E.M. Synthesis and evaluation of dual crosslinked alginate microbeads. Acta Biomater. 2018, 65, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Estrada, M.F.; Rebelo, S.P.; Davies, E.J.; Pinto, M.T.; Pereira, H.; Santo, V.E.; Smalley, M.J.; Barry, S.T.; Gualda, E.J.; Alves, P.M.; et al. Modelling the tumour microenvironment in long-term microencapsulated 3D co-cultures recapitulates phenotypic features of disease progression. Biomaterials 2016, 78, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Liu, Y.; Xu, X.X.; Guo, X.; Sun, G.W.; Ma, X.J. Mesenchymal stem cells enhance the metastasis of 3D-cultured hepatocellular carcinoma cells. BMC Cancer 2016, 16, 566. [Google Scholar] [CrossRef] [Green Version]
- Badhe, R.V.; Bijukumar, D.; Chejara, D.R.; Mabrouk, M.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Pillay, V. A composite chitosan-gelatin bi-layered, biomimetic macroporous scaffold for blood vessel tissue engineering. Carbohydr. Polym. 2017, 157, 1215–1225. [Google Scholar] [CrossRef]
- Fiqrianti, I.A.; Widiyanti, P.; Manaf, M.A.; Savira, C.Y.; Cahyani, N.R.; Bella, F.R. Poly-L-Lactic acid (PLLA)-chitosan-collagen electrospun tube for vascular graft application. J. Funct. Biomater. 2018, 9, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wang, D.; Jiang, X.; He, L.; Fu, L.; Zhao, Y.; Wang, Y.; Mo, H.; Shen, J. Multistructured vascular patches constructed via layer-by-layer self-assembly of heparin and chitosan for vascular tissue engineering applications. Chem. Eng. J. 2019, 370, 1057–1067. [Google Scholar] [CrossRef]
- Rodrigues, M.N.; Oliveira, M.B.; Costa, R.R.; Mano, J.F. Chitosan/Chondroitin Sulfate Membranes Produced by Polyelectrolyte Complexation for Cartilage Engineering. Biomacromolecules 2016, 17, 2178–2188. [Google Scholar] [CrossRef] [PubMed]
- Mirmusavi, M.H.; Zadehnajar, P.; Semnani, D.; Karbasi, S.; Fekrat, F.; Heidari, F. Evaluation of physical, mechanical and biological properties of poly 3-hydroxybutyrate-chitosan-multiwalled carbon nanotube/silk nano-micro composite scaffold for cartilage tissue engineering applications. Int. J. Biol. Macromol. 2019, 132, 822–835. [Google Scholar] [CrossRef]
- Shamekhi, M.A.; Mirzadeh, H.; Mahdavi, H.; Rabiee, A.; Mohebbi-Kalhori, D.; Baghaban Eslaminejad, M. Graphene oxide containing chitosan scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2019, 127, 396–405. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, F.; Wang, Q.; Wu, X. Fabrication of chitosan/graphene oxide polymer nanofiber and its biocompatibility for cartilage tissue engineering. Mater. Sci. Eng. C 2017, 79, 697–701. [Google Scholar] [CrossRef]
- Kar, S.; Kaur, T.; Thirugnanam, A. Microwave-assisted synthesis of porous chitosan-modified montmorillonite-hydroxyapatite composite scaffolds. Int. J. Biol. Macromol. 2016, 82, 628–636. [Google Scholar] [CrossRef]
- Lei, Y.; Xu, Z.; Ke, Q.; Yin, W.; Chen, Y.; Zhang, C.; Guo, Y. Strontium hydroxyapatite/chitosan nanohybrid scaffolds with enhanced osteoinductivity for bone tissue engineering. Mater. Sci. Eng. C 2017, 72, 134–142. [Google Scholar] [CrossRef]
- Ghorbani, M.; Ai, J.; Nourani, M.R.; Azami, M.; Hashemi Beni, B.; Asadpour, S.; Bordbar, S. Injectable natural polymer compound for tissue engineering of intervertebral disc: In vitro study. Mater. Sci. Eng. C 2017, 80, 502–508. [Google Scholar] [CrossRef]
- Alinejad, Y.; Adoungotchodo, A.; Grant, M.P.; Epure, L.M.; Antoniou, J.; Mwale, F.; Lerouge, S. Injectable Chitosan Hydrogels with Enhanced Mechanical Properties for Nucleus Pulposus Regeneration. Tissue Eng.-Part A 2019, 25, 303–313. [Google Scholar] [CrossRef]
- Yuan, D.; Chen, Z.; Xiang, X.; Deng, S.; Liu, K.; Xiao, D.; Deng, L.; Feng, G. The establishment and biological assessment of a whole tissue-engineered intervertebral disc with PBST fibers and a chitosan hydrogel in vitro and in vivo. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2019, 107, 2305–2316. [Google Scholar] [CrossRef]
- Trinca, R.B.; Westin, C.B.; da Silva, J.A.F.; Moraes, Â.M. Electrospun multilayer chitosan scaffolds as potential wound dressings for skin lesions. Eur. Polym. J. 2017, 88, 161–170. [Google Scholar] [CrossRef]
- Madni, A.; Khan, R.; Ikram, M.; Naz, S.S.; Khan, T.; Wahid, F. Fabrication and characterization of chitosan–Vitamin C–lactic acid composite membrane for potential skin tissue engineering. Int. J. Polym. Sci. 2019, 2019. [Google Scholar] [CrossRef]
- Luetchford, K.A.; Chaudhuri, J.B.; De Bank, P.A. Silk fibroin/gelatin microcarriers as scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2020, 106. [Google Scholar] [CrossRef]
- Gambari, L.; Amore, E.; Raggio, R.; Bonani, W.; Barone, M.; Lisignoli, G.; Grigolo, B.; Motta, A.; Grassi, F. Hydrogen sulfide-releasing silk fibroin scaffold for bone tissue engineering. Mater. Sci. Eng. C 2019, 102, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Singh, Y.P.; Devi, D.; Kandimalla, R.; Kotoky, J.; Mandal, B.B. Potential of silk fibroin/chondrocyte constructs of muga silkworm Antheraea assamensis for cartilage tissue engineering. J. Mater. Chem. B 2016, 4, 3670–3684. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H.; et al. Structurally and Functionally Optimized Silk-Fibroin–Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In Vitro and In Vivo. Adv. Mater. 2017, 29, 1–7. [Google Scholar] [CrossRef]
- Singh, Y.P.; Bhardwaj, N.; Mandal, B.B. Potential of Agarose/Silk Fibroin Blended Hydrogel for in Vitro Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 21236–21249. [Google Scholar] [CrossRef] [PubMed]
- Allardyce, B.J.; Rajkhowa, R.; Dilley, R.J.; Xie, Z.; Campbell, L.; Keating, A.; Atlas, M.D.; von Unge, M.; Wang, X. Comparative acoustic performance and mechanical properties of silk membranes for the repair of chronic tympanic membrane perforations. J. Mech. Behav. Biomed. Mater. 2016, 64, 65–74. [Google Scholar] [CrossRef]
- Su, T.; Zhang, M.; Zeng, Q.; Pan, W.; Huang, Y.; Qian, Y.; Dong, W.; Qi, X.; Shen, J. Mussel-inspired agarose hydrogel scaffolds for skin tissue engineering. Bioact. Mater. 2021, 6, 579–588. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, L.; Campos, F.; Godoy-Guzmán, C.; del Carmen Sánchez-Quevedo, M.; Garzón, I.; Alaminos, M.; Campos, A.; Carriel, V. Encapsulation of human elastic cartilage-derived chondrocytes in nanostructured fibrin-agarose hydrogels. Histochem. Cell Biol. 2017, 147, 83–95. [Google Scholar] [CrossRef]
- Bagheri, B.; Zarrintaj, P.; Surwase, S.S.; Baheiraei, N.; Saeb, M.R.; Mozafari, M.; Kim, Y.C.; Park, O.O. Self-gelling electroactive hydrogels based on chitosan–aniline oligomers/agarose for neural tissue engineering with on-demand drug release. Colloids Surf. B Biointerfaces 2019, 184. [Google Scholar] [CrossRef]
- Carriel, V.; Garzón, I.; Campos, A.; Cornelissen, M.; Alaminos, M. Differential expression of GAP-43 and neurofilament during peripheral nerve regeneration through bio-artificial conduits. J. Tissue Eng. Regen. Med. 2017, 11, 553–563. [Google Scholar] [CrossRef]
- Carriel, V.; Scionti, G.; Campos, F.; Roda, O.; Castro, B.; Cornelissen, M.; Garzón, I.; Alaminos, M. In vitro characterization of a nanostructured fibrin agarose bio-artificial nerve substitute. J. Tissue Eng. Regen. Med. 2017, 11, 1412–1426. [Google Scholar] [CrossRef] [Green Version]
- Cavo, M.; Caria, M.; Pulsoni, I.; Beltrame, F.; Fato, M.; Scaglione, S. A new cell-laden 3D Alginate-Matrigel hydrogel resembles human breast cancer cell malignant morphology, spread and invasion capability observed “in vivo”. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anguiano, M.; Castilla, C.; Maška, M.; Ederra, C.; Peláez, R.; Morales, X.; Muñoz-Arrieta, G.; Mujika, M.; Kozubek, M.; Muñoz-Barrutia, A.; et al. Characterization of three-dimensional cancer cell migration in mixed collagen-Matrigel scaffolds using microfluidics and image analysis. PLoS ONE 2017, 12, e0171417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, J.; Lin, J.; Su, C.; Yu, S.; Cui, Z.; Wang, Q.; Chen, W.; Turng, L.S. Ultrasonication-Induced Modification of Hydroxyapatite Nanoparticles onto a 3D Porous Poly(lactic acid) Scaffold with Improved Mechanical Properties and Biocompatibility. Macromol. Mater. Eng. 2019, 304, 1900081. [Google Scholar] [CrossRef]
- Zimina, A.; Senatov, F.; Choudhary, R.; Kolesnikov, E.; Anisimova, N.; Kiselevskiy, M.; Orlova, P.; Strukova, N.; Generalova, M.; Manskikh, V.; et al. Biocompatibility and physico-chemical properties of highly porous PLA/HA scaffolds for bone reconstruction. Polymers 2020, 12, 2938. [Google Scholar] [CrossRef] [PubMed]
- Oladapo, B.I.; Daniyan, I.A.; Ikumapayi, O.M.; Malachi, O.B.; Malachi, I.O. Microanalysis of hybrid characterization of PLA/cHA polymer scaffolds for bone regeneration. Polym. Test. 2020, 83, 106341. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, G.; Junka, R.; Chang, N.; Anwar, A.; Wang, H.; Yu, X. Fabrication of polylactic acid (PLA)-based porous scaffold through the combination of traditional bio-fabrication and 3D printing technology for bone regeneration. Colloids Surf. B Biointerfaces 2021, 197. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, X. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Eslami, H.; Azimi Lisar, H.; Jafarzadeh Kashi, T.S.; Tahriri, M.; Ansari, M.; Rafiei, T.; Bastami, F.; Shahin-Shamsabadi, A.; Mashhadi Abbas, F.; Tayebi, L. Poly (lactic-co-glycolic acid)(PLGA)/TiO2 nanotube bioactive composite as a novel scaffold for bone tissue engineering: In vitro and in vivo studies. Biologicals 2018, 53, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Babilotte, J.; Martin, B.; Guduric, V.; Bareille, R.; Agniel, R.; Roques, S.; Héroguez, V.; Dussauze, M.; Gaudon, M.; Le Nihouannen, D.; et al. Development and characterization of a PLGA-HA composite material to fabricate 3D-printed scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2021, 118, 111334. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, M.A.; Shin, J.Y.; Jeon, J.H.; Lee, S.J.; Yoon, M.Y.; Kim, H.J.; Choi, E.J.; Do, S.H.; Yang, V.C.; et al. Intra-articular delivery of synovium-resident mesenchymal stem cells via BMP-7-loaded fibrous PLGA scaffolds for cartilage repair. J. Control. Release 2019, 302, 169–180. [Google Scholar] [CrossRef]
- Wei, P.; Xu, Y.; Gu, Y.; Yao, Q.; Li, J.; Wang, L. IGF-1-releasing PLGA nanoparticles modified 3D printed PCL scaffolds for cartilage tissue engineering. Drug Deliv. 2020, 27, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Moradian, H.; Keshvari, H.; Fasehee, H.; Dinarvand, R.; Faghihi, S. Combining NT3-overexpressing MSCs and PLGA microcarriers for brain tissue engineering: A potential tool for treatment of Parkinson’s disease. Mater. Sci. Eng. C 2017, 76, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Tu, J.; Fang, G.; Deng, L.; Gao, X.; Guo, K.; Kong, J.; Lv, J.; Guan, W.; Yang, C. Combining PLGA scaffold and MSCs for brain tissue engineering: A potential tool for treatment of brain injury. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens-Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawelec, K.M.; Best, S.M.; Cameron, R.E. Collagen: A network for regenerative medicine. J. Mater. Chem. B 2016, 4, 6484–6496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maller, O.; Drain, A.P.; Barrett, A.S.; Borgquist, S.; Ruffell, B.; Zakharevich, I.; Pham, T.T.; Gruosso, T.; Kuasne, H.; Lakins, J.N.; et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat. Mater. 2021, 20, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Barcellos-de-Souza, P.; Gori, V.; Bambi, F.; Chiarugi, P. Tumor microenvironment: Bone marrow-mesenchymal stem cells as key players. Biochim. Biophys. Acta-Rev. Cancer 2013, 1836, 321–335. [Google Scholar] [CrossRef]
- Law, J.X.; Liau, L.L.; Saim, A.; Yang, Y.; Idrus, R. Electrospun Collagen Nanofibers and Their Applications in Skin Tissue Engineering. Tissue Eng. Regen. Med. 2017, 14, 699–718. [Google Scholar] [CrossRef]
- Mizuno, M.; Fujisawa, R.; Kuboki, Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-α2β1 integrin interaction. J. Cell. Physiol. 2000, 184, 207–213. [Google Scholar] [CrossRef]
- Irawan, V.; Sung, T.C.; Higuchi, A.; Ikoma, T. Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development. Tissue Eng. Regen. Med. 2018, 15, 673–697. [Google Scholar] [CrossRef]
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-based tissue engineering strategies for vascular medicine. Front. Bioeng. Biotechnol. 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen-Ngoc, K.V.; Cheung, K.J.; Brenot, A.; Shamir, E.R.; Gray, R.S.; Hines, W.C.; Yaswen, P.; Werb, Z.; Ewald, A.J. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc. Natl. Acad. Sci. USA 2012, 109, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuczek, D.E.; Larsen, A.M.H.; Carretta, M.; Kalvisa, A.; Siersbæk, M.S.; Simões, A.M.C.; Roslind, A.; Engelholm, L.H.; Donia, M.; Svane, I.M.; et al. Collagen density regulates the activity of tumor-infiltrating T cells. J. Immunother. Cancer 2018, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Tabata, Y.; Ikada, Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998, 31, 287–301. [Google Scholar] [CrossRef]
- Nii, T.; Makino, K.; Tabata, Y. Influence of shaking culture on the biological functions of cell aggregates incorporating gelatin hydrogel microspheres. J. Biosci. Bioeng. 2019, 128, 606–612. [Google Scholar] [CrossRef]
- Hayashi, K.; Tabata, Y. Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater. 2011, 7, 2797–2803. [Google Scholar] [CrossRef] [Green Version]
- Kellner, K.; Liebsch, G.; Klimant, I.; Wolfbeis, O.S.; Blunk, T.; Schulz, M.B.; Göpferich, A. Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnol. Bioeng. 2002, 80, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Suzuki, S.; Tabata, Y.; Ikada, Y.; Nishimura, Y. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials 2000, 21, 489–499. [Google Scholar] [CrossRef]
- Tabata, Y.; Ikada, Y. Vascularization effect of basic fibroblast growth factor released from gelatin hydrogels with different biodegradabilities. Biomaterials 1999, 20, 2169–2175. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA: Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-associated fibroblasts: Their characteristics and their roles in tumor growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef]
- Matsuno, K.; Saotome, T.; Shimada, N.; Nakamura, K.; Tabata, Y. Effect of cell seeding methods on the distribution of cells into the gelatin hydrogel nonwoven fabric. Regen. Ther. 2020, 14, 160–164. [Google Scholar] [CrossRef]
- Nakamura, K.; Saotome, T.; Shimada, N.; Matsuno, K.; Tabata, Y. A Gelatin Hydrogel Nonwoven Fabric Facilitates Metabolic Activity of Multilayered Cell Sheets. Tissue Eng.-Part C Methods 2019, 25, 344–352. [Google Scholar] [CrossRef]
- Nakamura, K.; Nobutani, K.; Shimada, N.; Tabata, Y. Gelatin hydrogel-fragmented fibers suppress shrinkage of cell sheet. Tissue Eng.-Part C Methods 2020, 26, 216–224. [Google Scholar] [CrossRef]
- Murata, Y.; Jo, J.I.; Tabata, Y. Intracellular controlled release of molecular beacon prolongs the time period of mRNA visualization. Tissue Eng.-Part A 2019, 25, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Jo, J.I.; Tabata, Y. Visualization of Apoptosis in Three-Dimensional Cell Aggregates Based on Molecular Beacon Imaging. Tissue Eng.-Part C Methods 2021, 27, 264–275. [Google Scholar] [CrossRef]
- Yoshimoto, Y.; Jo, J.I.; Tabata, Y. Preparation of antibody-immobilized gelatin nanospheres incorporating a molecular beacon to visualize the biological function of macrophages. Regen. Ther. 2020, 14, 11–18. [Google Scholar] [CrossRef]
- Murata, Y.; Jo, J.-I.; Tabata, Y. Molecular Beacon Imaging to Visualize Ki67 mRNA for Cell Proliferation Ability. Tissue Eng. Part A 2021, 27, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.E. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992, 6, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sanchez, P.; Fredriksson, N.; Larsson, A.; Altskär, A.; Ström, A. High sugar content impacts microstructure, mechanics and release of calcium-alginate gels. Food Hydrocoll. 2018, 84, 26–33. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-induced gelation of alginate: Mechanisms and applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Kanafi, M.M.; Ramesh, A.; Gupta, P.K.; Bhonde, R.R. Dental pulp stem cells immobilized in alginate microspheres for applications in bone tissue engineering. Int. Endod. J. 2014, 47, 687–697. [Google Scholar] [CrossRef]
- Utech, S.; Prodanovic, R.; Mao, A.S.; Ostafe, R.; Mooney, D.J.; Weitz, D.A. Microfluidic Generation of Monodisperse, Structurally Homogeneous Alginate Microgels for Cell Encapsulation and 3D Cell Culture. Adv. Healthc. Mater. 2015, 4, 1628–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, R.P.H.; Mahou, R.; Morel, P.; Meyer, J.; Montanari, E.; Muller, Y.D.; Christofilopoulos, P.; Wandrey, C.; Gonelle-Gispert, C.; Bühler, L.H. Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J. Hepatol. 2015, 62, 634–641. [Google Scholar] [CrossRef]
- Qi, M.; Mørch, Y.; Lacík, I.; Formo, K.; Marchese, E.; Wang, Y.; Danielson, K.K.; Kinzer, K.; Wang, S.; Barbaro, B.; et al. Survival of human islets in microbeads containing high guluronic acid alginate crosslinked with Ca2+ and Ba2+. Xenotransplantation 2012, 19, 355–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Xie, H.X.; Huang, C.; Li, L.; Yu, X. Preparation of chitosan/silk fibroin blending membrane fixed with alginate dialdehyde for wound dressing. Int. J. Biol. Macromol. 2013, 58, 121–126. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Comparative study on antimicrobial activity and biocompatibility of N-selective chitosan derivatives. React. Funct. Polym. 2018, 124, 149–155. [Google Scholar] [CrossRef]
- Kumar, R.; Oves, M.; Ameelbi, T.; Al-Makishah, N.H.; Barakat, M.A. Hybrid chitosan/polyaniline-polypyrrole biomaterial for enhanced adsorption and antimicrobial activity. J. Colloid Interface Sci. 2017, 490, 488–496. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as A Preservative for Fruits and Vegetables: A Review on Chemistry and Antimicrobial Properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Rocha, M.A.M.; Coimbra, M.A.; Nunes, C. Applications of chitosan and their derivatives in beverages: A critical review. Curr. Opin. Food Sci. 2017, 15, 61–69. [Google Scholar] [CrossRef]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Tzaneva, D.; Simitchiev, A.; Petkova, N.; Nenov, V.; Stoyanova, A.; Denev, P. Synthesis of carboxymethyl chitosan and its rheological behaviour in pharmaceutical and cosmetic emulsions. J. Appl. Pharm. Sci. 2017, 7, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Neves, S.C.; Moreira Teixeira, L.S.; Moroni, L.; Reis, R.L.; Van Blitterswijk, C.A.; Alves, N.M.; Karperien, M.; Mano, J.F. Chitosan/Poly (ε-caprolactone) blend scaffolds for cartilage repair. Biomaterials 2011, 32, 1068–1079. [Google Scholar] [CrossRef] [Green Version]
- Kashi, M.; Baghbani, F.; Moztarzadeh, F.; Mobasheri, H.; Kowsari, E. Green synthesis of degradable conductive thermosensitive oligopyrrole/chitosan hydrogel intended for cartilage tissue engineering. Int. J. Biol. Macromol. 2018, 107, 1567–1575. [Google Scholar] [CrossRef]
- Aranaz, I.; Martínez-Campos, E.; Moreno-Vicente, C.; Civantos, A.; García-Arguelles, S.; del Monte, F. Macroporous calcium phosphate/chitosan composites prepared via unidirectional ice segregation and subsequent freeze-drying. Materials 2017, 10, 516. [Google Scholar] [CrossRef] [Green Version]
- Balagangadharan, K.; Dhivya, S.; Selvamurugan, N. Chitosan based nanofibers in bone tissue engineering. Int. J. Biol. Macromol. 2017, 104, 1372–1382. [Google Scholar] [CrossRef]
- Logithkumar, R.; Keshavnarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Doench, I.; Torres-Ramos, M.E.W.; Montembault, A.; de Oliveira, P.N.; Halimi, C.; Viguier, E.; Heux, L.; Siadous, R.; Thiré, R.M.S.M.; Osorio-Madrazo, A. Injectable and gellable chitosan formulations filled with cellulose nanofibers for intervertebral disc tissue engineering. Polymers 2018, 10, 1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doench, I.; Tran, T.; David, L.; Montembault, A.; Viguier, E.; Gorzelanny, C.; Sudre, G.; Cachon, T.; Louback-Mohamed, M.; Horbelt, N.; et al. Cellulose Nanofiber-Reinforced Chitosan Hydrogel Composites for Intervertebral Disc Tissue Repair. Biomimetics 2019, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Tchemtchoua, V.T.; Atanasova, G.; Aqil, A.; Filée, P.; Garbacki, N.; Vanhooteghem, O.; Deroanne, C.; Noël, A.; Jérome, C.; Nusgens, B.; et al. Development of a Chitosan Nanofibrillar Scaffold for Skin Repair and Regeneration. Biomacromolecules 2011, 12, 3194–3204. [Google Scholar] [CrossRef] [PubMed]
- Keong, L.C.; Halim, A.S. In Vitro models in biocompatibility assessment for biomedical-grade chitosan derivatives in wound management. Int. J. Mol. Sci. 2009, 10, 1300–1313. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Song, Y.W.; Jin, L.; Wang, Z.J.; Pu, D.Y.; Lin, S.Q.; Zhou, C.; You, H.J.; Ma, Y.; Li, J.M.; et al. Silk structure and degradation. Colloids Surf. B Biointerfaces 2015, 131, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Malay, A.D.; Sato, R.; Yazawa, K.; Watanabe, H.; Ifuku, N.; Masunaga, H.; Hikima, T.; Guan, J.; Mandal, B.B.; Damrongsakkul, S.; et al. Relationships between physical properties and sequence in silkworm silks. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.-H.; Le, T.-H.; Huynh, V.Q.N.; Vo, D.-V.N.; Trinh, Q.T.; Kim, S.Y.; Le, Q.V. Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Z.; Song, Y.; Jin, Y.; Zhang, C.; Peng, D.; Chen, Z.; Chang, P.; Kundu, S.C.; Wang, G.; Wang, Z.; et al. In Vivo Characterizations of the Immune Properties of Sericin: An Ancient Material with Emerging Value in Biomedical Applications. Macromol. Biosci. 2017, 17, 1–6. [Google Scholar] [CrossRef]
- Panilaitis, B.; Altman, G.H.; Chen, J.; Jin, H.J.; Karageorgiou, V.; Kaplan, D.L. Macrophage responses to silk. Biomaterials 2003, 24, 3079–3085. [Google Scholar] [CrossRef]
- Sahu, N.; Pal, S.; Sapru, S.; Kundu, J.; Talukdar, S.; Singh, N.I.; Yao, J.; Kundu, S.C. Non-Mulberry and Mulberry Silk Protein Sericins as Potential Media Supplement for Animal Cell Culture. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. Silk fibroin as a functional biomaterial for drug and gene delivery. Pharmaceutics 2019, 11, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Wang, B. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef] [Green Version]
- Meinel, L.; Hofmann, S.; Betz, O.; Fajardo, R.; Merkle, H.P.; Langer, R.; Evans, C.H.; Vunjak-Novakovic, G.; Kaplan, D.L. Osteogenesis by human mesenchymal stem cells cultured on silk biomaterials: Comparison of adenovirus mediated gene transfer and protein delivery of BMP-2. Biomaterials 2006, 27, 4993–5002. [Google Scholar] [CrossRef]
- Uebersax, L.; Merkle, H.P.; Meinel, L. Insulin-like growth factor I releasing silk fibroin scaffolds induce chondrogenic differentiation of human mesenchymal stem cells. J. Control. Release 2008, 127, 12–21. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.; Wang, Y.; Toh, S.L.; Goh, J.C.H. The interaction between a combined knitted silk scaffold and microporous silk sponge with human mesenchymal stem cells for ligament tissue engineering. Biomaterials 2008, 29, 662–674. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Chen, J.; Wang, L.; Gui, X.; Ran, J.; Xu, G.; Zhao, H.; Zeng, M.; Ji, J.; et al. Silk Fibroin Biomaterial Shows Safe and Effective Wound Healing in Animal Models and a Randomized Controlled Clinical Trial. Adv. Healthc. Mater. 2017, 6, 1–16. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of Silk Fibroin Use in Wound Dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Wang, S.; Zhao, J.; Xu, L.; Zhu, C.; Zeng, D.; Chen, J.; Zhang, Z.; Kaplan, D.L.; et al. The use of injectable sonication-induced silk hydrogel for VEGF 165 and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials 2011, 32, 9415–9424. [Google Scholar] [CrossRef] [Green Version]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Bakhshandeh, B.; Rezaeian, I.; Heshmatian, B.; Ganjali, M.R. A Novel Electroactive Agarose-Aniline Pentamer Platform as a Potential Candidate for Neural Tissue Engineering. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Jeong, D.; Kim, S.; Kim, Y.; Jung, S. Cyclodextrin functionalized agarose gel with low gelling temperature for controlled drug delivery systems. Carbohydr. Polym. 2019, 222, 115011. [Google Scholar] [CrossRef] [PubMed]
- Rahfoth, B.; Weisser, J.; Sternkopf, F.; Aigner, T.; Von Der Mark, K.; Bräuer, R. Transplantation of allograft chondrocytes embedded in agarose gel into cartilage defects of rabbits. Osteoarthr. Cartil. 1998, 6, 50–65. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, A.M.; Cardona, J.; Ghinea, R.; Garzón Bello, I.; González-Andrades, M.; Alaminos, M.; Pérez, M.; del Mar Pérez, M. Optical properties of an anterior lamellar human cornea model based on fibrin-agarose. In Third International Conference on Applications of Optics and Photonics; International Society for Optics and Photonics: Bellingham, WA, USA, 2017; Volume 10453, p. 104532J. [Google Scholar] [CrossRef]
- Miller, R.T. Mechanical properties of basement membrane in health and disease. Matrix Biol. 2017, 57–58, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Glentis, A.; Oertle, P.; Mariani, P.; Chikina, A.; El Marjou, F.; Attieh, Y.; Zaccarini, F.; Lae, M.; Loew, D.; Dingli, F.; et al. Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Chang, J.; Chaudhuri, O. Beyond proteases: Basement membrane mechanics and cancer invasion. J. Cell Biol. 2019, 218, 2456–2469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Serratì, S.; Porcelli, L.; Guida, S.; Ferretta, A.; Iacobazzi, R.M.; Cocco, T.; Maida, I.; Tamasi, G.; Rossi, C.; Manganelli, M.; et al. Tomatine displays antitumor potential in in vitro models of metastatic melanoma. Int. J. Mol. Sci. 2020, 21, 5243. [Google Scholar] [CrossRef]
- Kenny, P.A.; Lee, G.Y.; Myers, C.A.; Neve, R.M.; Semeiks, J.R.; Spellman, P.T.; Lorenz, K.; Lee, E.H.; Barcellos-Hoff, M.H.; Petersen, O.W.; et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol. Oncol. 2007, 1, 84–96. [Google Scholar] [CrossRef]
- Buzarovska, A.; Dinescu, S.; Chitoiu, L.; Costache, M. Porous poly(l-lactic acid) nanocomposite scaffolds with functionalized TiO2 nanoparticles: Properties, cytocompatibility and drug release capability. J. Mater. Sci. 2018, 53, 11151–11166. [Google Scholar] [CrossRef]
- Grémare, A.; Guduric, V.; Bareille, R.; Heroguez, V.; Latour, S.; L’heureux, N.; Fricain, J.C.; Catros, S.; Le Nihouannen, D. Characterization of printed PLA scaffolds for bone tissue engineering. J. Biomed. Mater. Res.-Part A 2018, 106, 887–894. [Google Scholar] [CrossRef]
- Chen, J.; Yu, M.; Guo, B.; Ma, P.X.; Yin, Z. Conductive nanofibrous composite scaffolds based on in-situ formed polyaniline nanoparticle and polylactide for bone regeneration. J. Colloid Interface Sci. 2018, 514, 517–527. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly (lactic acid) composites in tissue engineering and drug delivery. Compos. Part B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Skidmore, S.; Hadar, J.; Garner, J.; Park, H.; Park, K.; Wang, Y.; Jiang, X. (Jeff) Complex sameness: Separation of mixed poly (lactide-co-glycolide) s based on the lactide:glycolide ratio. J. Control. Release 2019, 300, 174–184. [Google Scholar] [CrossRef]
- Nii, T.; Takeuchi, I.; Kimura, Y.; Makino, K. Effects of the conformation of PLGA molecules in the organic solvent on the aerodynamic diameter of spray dried microparticles. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 347–353. [Google Scholar] [CrossRef]
- Enayati, M.; Mobedi, H.; Hojjati-Emami, S.; Mirzadeh, H.; Jafari-Nodoushan, M. In situ forming PLGA implant for 90 days controlled release of leuprolide acetate for treatment of prostate cancer. Polym. Adv. Technol. 2017, 28, 867–875. [Google Scholar] [CrossRef]
- Sulong, A.F.; Fiassan, N.H.; Hwei, N.M.; Lokanathan, Y.; Naicker, A.S.; Abdullah, S.; Yusof, M.R.; Htwe, O.; Idrus, R.B.H.; Haflah, N.H.M. Collagen-Coated Polylactic-Glycolic Acid (PLGA) seeded with neural-differentiated human mesenchymal stem cells as a potential nerve conduit. Adv. Clin. Exp. Med. 2014, 23, 353–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadlock, T.; Sundback, C.; Hunter, D.; Cheney, M.; Vacanti, J.P. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000, 6, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lv, P.; Zhu, Y.; Wu, H.; Zhang, K.; Xu, F.; Zheng, L.; Zhao, J. Salidroside promotes peripheral nerve regeneration based on tissue engineering strategy using Schwann cells and PLGA: In vitro and in vivo. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Momotori, N.; Jo, J.i.; Tabata, Y. Preparation of polymer microspheres capable for pioglitazone release to modify macrophages function. Regen. Ther. 2019, 11, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B. Hide and seek: Nanomaterial interactions with the immune system. Front. Immunol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Keshavan, S.; Calligari, P.; Stella, L.; Fusco, L.; Delogu, L.G.; Fadeel, B. Nano-bio interactions: A neutrophil-centric view. Cell Death Dis. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
| Biomaterials | Ref. | Date | Tissue Targeted | In Vitro (Cell Type)/In Vivo (Animal Type) Testing | Results Featured |
|---|---|---|---|---|---|
| Collagen | [16] | 2018 | Bone | In vitro (human mesenchymal stem cells (MSC))/In vivo (mouse) | The scaffold of collagen and biphasic calcium phosphate nanoparticles with a controlled release of dexamethasone enabled the enhancement of osteogenesis from human MSC. In addition, bone regeneration was observed in nude mice. |
| [17] | 2019 | Bone | In vitro (human MSC and human umbilical vein endothelial cells) | MSC and umbilical vein endothelial cells multicellular spheroids encapsulated in collagen/fibrin hydrogel showed efficient osteogenic differentiation. | |
| [18] | 2020 | Cartilage | In vitro (rabbit chondrocytes)/In vivo (mouse) | Porous fish collagen scaffolds promoted cartilage formation in vitro and in vivo. | |
| [19] | 2017 | Muscle | In vitro (rat skeletal myoblasts) | The 3D microgroove collagen scaffolds triggered cell assembly into anisotropic muscle bundles. | |
| [20] | 2017 | Cancer | In vitro (human breast cancer cells) | Anisotropic scaffolds supported the migration of invasive cancer cells. | |
| [21] | 2018 | Cancer | In vitro (human breast cancer cells and fibroblasts) | Tool of cancer cells and collagen gels containing fibroblasts combination system enabled the evaluation of desmoplasia, cancer proliferation, or invasion. | |
| [22] | 2019 | Cancer | In vitro (human pancreatic cancer cells, human lung cancer cells, and fibroblasts) | Cancer cells attached and migrated on the collagen matrix containing fibroblasts. | |
| [23] | 2019 | Cancer | In vitro (human breast cancer cells) | Collagen matrices with fibril bending stiffness indicated the spreading and clustering of invasive cancer cells. | |
| Gelatin | [24] | 2015 | Cardiac | In vitro (human cardiovascular cell derived from iPS cells)/In vivo (mouse) | Multilayered thick cell sheets were viable by stacked with gelatin gels between each cell sheet. |
| [25] | 2018 | Cardiac | In vivo (rat) | Basic fibroblast growth factor release from gelatin gels enabled the cell sheets to improve cardiac contractile function. | |
| [26] | 2017 | Epithelial | In vitro (mouse mammary epithelial cells and mouse preadipocyte cells) | Epithelial and preadipocyte spheroids incorporating gelatin gels promoted the expression level of laminin. | |
| [27] | 2017 | Epithelial | In vitro (mouse mammary epithelial cells) | β-casein expression was high for epithelial spheroids incorporating gelatin gels. | |
| [28] | 2018 | Pancreas | In vitro (rat insulinoma cells) | The incorporation of gelatin gels into insulinoma spheroids enabled insulin secretion. | |
| [29] | 2018 | Ovarian | In vivo (mouse) | The transplantation of gelatin sheets capable of basic fibroblast growth factor with ovarian tissues significantly increased the proliferation of stromal and endothelial cells. | |
| [30] | 2019 | Wound healing | In vivo (mouse) | Gelatin sheets impregnated platelet-rich plasma accelerated the capillary and tissue formation. | |
| [31] | 2019 | Cancer | In vitro (human lung cancer cells and fibroblasts) | A co-culture tool of cancer cells and fibroblast spheroids incorporating gelatin gels containing a p53 inhibitor can evaluate the invasion level of cancer cells. | |
| [6] | 2020 | Cancer | In vitro (human lung cancer cells and fibroblasts) | The fibroblasts spheroids incorporating gelatin gels capable of transforming growth factor-β1 increased the invasion rate of cancer cells similar to in vivo. | |
| [32] | 2020 | Cancer | In vitro (human lung, breast, and hepatic cancer cells, fibroblasts, and macrophages) | The gelatin gel-based drug release system was able to mimic the invasion ability of cancer cells, responding to the tissue region. | |
| Alginate | [33] | 2017 | Germ cells | In vitro (mouse embryonic stem cells) | Alginate-collagen gels enhance primordial germ cell differentiation of embryonic stem cells. |
| [34] | 2020 | Bone | In vitro (rat MSC)/In vivo (rat) | The osteogenesis and mineralization were observed when MSC were encapsulated into alginate gels. | |
| [35] | 2019 | Bone | In vitro (murine bone calvaria pre-osteoblast)/in vivo (mouse) | The osteoblast differentiation of pre-osteoblast was high in vitro and in vivo by encapsulating into alginate-gelatin injectable gels. | |
| [36] | 2017 | Bone | In vitro (human adipose-derived MSC) | The crosslinked oxidized alginate-gelatin hydrogel was prepared by changing the mixing ratio of alginate/gelatin. The ratio influenced osteogenic differentiation. | |
| [37] | 2018 | None | In vitro (human bone marrow-derived MSC) | Preparation of dual crosslinking homogeneous alginate microspheres combined with a microfluidics system to encapsulate MSC. | |
| [38] | 2018 | Pancreas | In vitro (human pancreatic islets) | The first trial to encapsulate human pancreatic islets in a dynamic condition, such as an organ-on-chip. | |
| [39] | 2018 | Pancreas | In vitro (mouse pancreatic β cells)/In vivo (rat) | Dual cross-linked alginate microbeads were stable under the inflammation condition in vitro and in vivo. | |
| [40] | 2016 | Cancer | In vitro (human breast cancer cells and human fibroblasts) | Alginate gels encapsulating human breast cancer cells and fibroblasts replicated phenotypic functions of cancer disease progression in vitro. | |
| [41] | 2016 | Cancer | In vitro (human umbilical cord-derived MSC and human hepatocellular carcinoma) | EMT induction or metastasis was observed when the alginate gels encapsulating hepatocellular carcinoma were co-cultured with MSC. | |
| Chitosan | [42] | 2017 | Blood vessel | In vitro (human dermal fibroblast cells) | Chitosan-gelatin-based bi-layer was an appropriate scaffold to mimic the biological blood vessel, such as morphology and mechanism. |
| [43] | 2018 | Blood vessel | In vitro (human lymphocyte cell T) | The properties of the tube showed the range value of native blood vessels (tensile strength: 2.13 MPa and burst pressure: 2593 mmHg). In addition, the tube was of high hemocompatibility and low cytotoxicity. | |
| [44] | 2019 | Blood vessel | In vitro (endothelial progenitor cells, red blood cells, or platelet-rich plasma)/In vivo (pig) | A heparin–chitosan multilayered vascular patch was biocompatible, such as a low hemolysis rate. | |
| [45] | 2016 | Cartilage | In vitro (mouse pre-chondrocytes) | The membrane of chitosan and chondroitin sulfate improved cell adhesion and enhance the expression of cartilage markers. | |
| [46] | 2019 | Cartilage | In vitro (rabbit chondrocytes) | They evaluated the mechanical and biological properties of the poly 3-hydroxybutyrate-chitosan/silk scaffold for chondrocyte viability. | |
| [47] | 2019 | Cartilage | In vitro (human cartilage) | When the graphene oxide concentration in the chitosan scaffold was high, physical and mechanical properties were improved, resulting in enhanced proliferation of chondrocytes. | |
| [48] | 2017 | Cartilage | In vitro (mouse pre-chondrocytes) | Preparation of chitosan/poly(vinyl alcohol)/graphene oxide nanofiber for cartilage tissue engineering. | |
| [49] | 2016 | Bone | In vitro (human bone osteosarcoma cells) | Chitosan-montmorillonite-hydroxyapatite composite scaffolds were non-cytotoxic, and the properties, such as bioactivity or protein absorption, were improved compared with chitosan or chitosan-montmorillonite scaffolds. | |
| [50] | 2017 | Bone | In vitro (human bone marrow-derived MSC) | Chitosan nanohybrid combined with strontium hydroxyapatite enhanced osteoconductivity. | |
| [51] | 2017 | Intervertebral disc | In vitro (rabbit nucleus pulposus cells from lumbar disc) | Chitosan-based injectable gels indicated constant storage modulus similar to the intervertebral disc ECM. | |
| [52] | 2019 | Intervertebral disc | In vitro (bovine nucleus pulposus cells from coccygeal intervertebral disc) | Thermosensitive chitosan hydrogels with high strength and rheological properties were prepared. | |
| [53] | 2019 | Intervertebral disc | In vitro (rabbit nucleus pulposus cells and annulus fibrosus cells)/In vivo (rabbit) | Preparation of chitosan hydrogel/poly (butylene succinate-co-terephthalate) copolyester electrospun fibers for intervertebral disc therapy. | |
| [54] | 2017 | Skin | In vitro (mouse fibroblast cells) | Electrospun multilayer chitosan scaffolds with low cytotoxicity were prepared. The scaffolds have high porosity, and the mechanical properties of the scaffolds matched those of the human skin. | |
| [55] | 2019 | Skin | In vitro (mouse fibroblast cells) | The chitosan-vitamin C scaffolds with glycerol and polyethylene glycol enhanced the activity of skin cells. | |
| Silk fibroin | [56] | 2020 | Bone | In vitro (rat bone marrow-derived MSC) | They evaluated the appropriate mixing ratio of silk fibroin/gelatin as a microcarrier for efficient osteogenic differentiation. |
| [57] | 2019 | Bone | In vitro (human bone marrow-derived MSC) | Hydrogen sulfide-releasing silk fibroin scaffolds induced osteogenesis. | |
| [58] | 2016 | Cartilage | In vitro (porcine chondrocytes)/In vivo (rat) | When the chondrocytes were cultured on the silk fibroin scaffolds of Antheraea assamensis, sulfated glycosaminoglycans and type Ⅱ collagen production increased. | |
| [59] | 2017 | Cartilage | In vitro (rat bone marrow-derived MSC)/In vivo (rabbit) | They optimized the mixing ratio of silk fibroin to gelatin as scaffolds prepared using 3D printing for cartilage repair. | |
| [60] | 2016 | Cartilage | In vitro (pig auricular chondrocytes) | The combination of agarose and silk fibroin enhanced the polymeric network, leading to the up-regulation of cartilage-specific genes. | |
| [61] | 2016 | Tympanic membrane | In vitro (pig cartilage) | The first report on the effect of silk fibroin membranes on the acoustic energy transfer and tensile strength to cartilage. | |
| Agarose | [62] | 2021 | Skin | In vitro (human normal embryonic lung fibroblast cells)/In vivo (mouse) | Agarose-polydopamine hydrogels were biocompatible scaffolds capable of promoting collagen deposition and angiogenesis, finally skin defect healing. |
| [63] | 2017 | Cartilage | In vitro (human elastic cartilage-derived chondrocytes) | Nanostructured fibrin–agarose hydrogel enabled chondrocytes encapsulation and support of culture. | |
| [64] | 2019 | Nerve | In vitro (rat neuronal cells) | Electrical stimulation facilitated dexamethasone release from hydrogels. | |
| [65] | 2017 | Nerve | In vitro (rat adipose-derived MSC)/In vivo (rat) | Collagen conduits filled with fibrin–agarose hydrogels containing stem cells were prepared for nerve regeneration. | |
| [66] | 2017 | Nerve | In vitro (human adipose-derived MSC) | A nanostructured fibrin-agarose bioartificial nerve substitute enabled stem cells to proliferate. | |
| Matrigel | [67] | 2018 | Cancer | In vitro (human breast cancer cells) | The cancer cell-laden gels composed of the appropriate mixing ratio of Matrigel and alginate replicate the behavior of cancer cells. |
| [68] | 2017 | Cancer | In vitro (human non-small cell lung carcinoma) | Matrigel and collagen-based microfluidics systems can control the migration of cancer cells by changing the Matrigel concentration. | |
| Poly(lactic acid) (PLA) | [69] | 2019 | Bone | In vitro (mouse embryonic osteoblast cells) | The attachment and proliferation of cells on poly(lactic acid)-hydroxyapatite (HA) hybrid scaffolds increased. The result is mainly because of the interaction between cells and scaffolds via HA. |
| [70] | 2020 | Bone | In vitro (cat bone marrow-derived MSC)/In vivo (mouse) | PLA-HA improved the adhesion of cells, and widespread ingrowth of tissues into the implant pores was observed. | |
| [71] | 2020 | Bone | None | Microanalysis of PLA-HA scaffolds was performed. | |
| [72] | 2021 | Bone | In vitro (human fetal osteoblast cells) | PLA-based scaffolds provided porous networks and gave cells good biological functions, such as osteogenesis. | |
| [73] | 2021 | Bone | In vitro (rabbit MSC)/In vivo (rabbit) | PLA scaffolds incorporating a high concentration of HA showed efficient bone regeneration. | |
| Poly(lactic-co-glycolic acid) (PLGA) | [74] | 2018 | Bone | In vitro (human osteosarcoma cells)/In vivo (rabbit) | The amount of bone formation for TiO2 nanotube/PLGA scaffolds was much higher than for PLGA scaffolds. |
| [75] | 2021 | Bone | In vitro (human adipose or bone marrow-derived MSC)/In vivo (rat) | PLGA-hydroxyapatite (HA) nanoparticles promoted osteodifferentiation compared to the PLGA scaffold. | |
| [76] | 2019 | Cartilage | In vitro (rabbit synovium-resident MSC)/In vivo (rabbit) | Bone morphogenetic proteins-7 loaded fibrous PLGA scaffolds combined with MSC showed a cartilage formation. | |
| [77] | 2020 | Cartilage | In vitro (rabbit bone marrow-derived MSC and rabbit chondrocytes) | When cells were cultured on insulin-like growth factor-1 laden PLGA/polydopamine/poly-ε-caprolactone scaffolds, glycosaminoglycan content, chondrogenic protein, and gene expression increased. | |
| [78] | 2017 | Nerve | In vitro (rat bone marrow-derived MSC) | PLGA microcarriers were promising scaffolds to support the culture of neurotrophin-3-overexpressing stem cells. | |
| [79] | 2018 | Nerve | In vitro (rat bone marrow-derived MSC and rat cortical neurons) | Stem cells and neurons could grow and migrate in the PLGA scaffolds. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nii, T.; Katayama, Y. Biomaterial-Assisted Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8657. https://doi.org/10.3390/ijms22168657
Nii T, Katayama Y. Biomaterial-Assisted Regenerative Medicine. International Journal of Molecular Sciences. 2021; 22(16):8657. https://doi.org/10.3390/ijms22168657
Chicago/Turabian StyleNii, Teruki, and Yoshiki Katayama. 2021. "Biomaterial-Assisted Regenerative Medicine" International Journal of Molecular Sciences 22, no. 16: 8657. https://doi.org/10.3390/ijms22168657
APA StyleNii, T., & Katayama, Y. (2021). Biomaterial-Assisted Regenerative Medicine. International Journal of Molecular Sciences, 22(16), 8657. https://doi.org/10.3390/ijms22168657






