Platelet-Derived Extracellular Vesicles for Regenerative Medicine
Abstract
1. Introduction
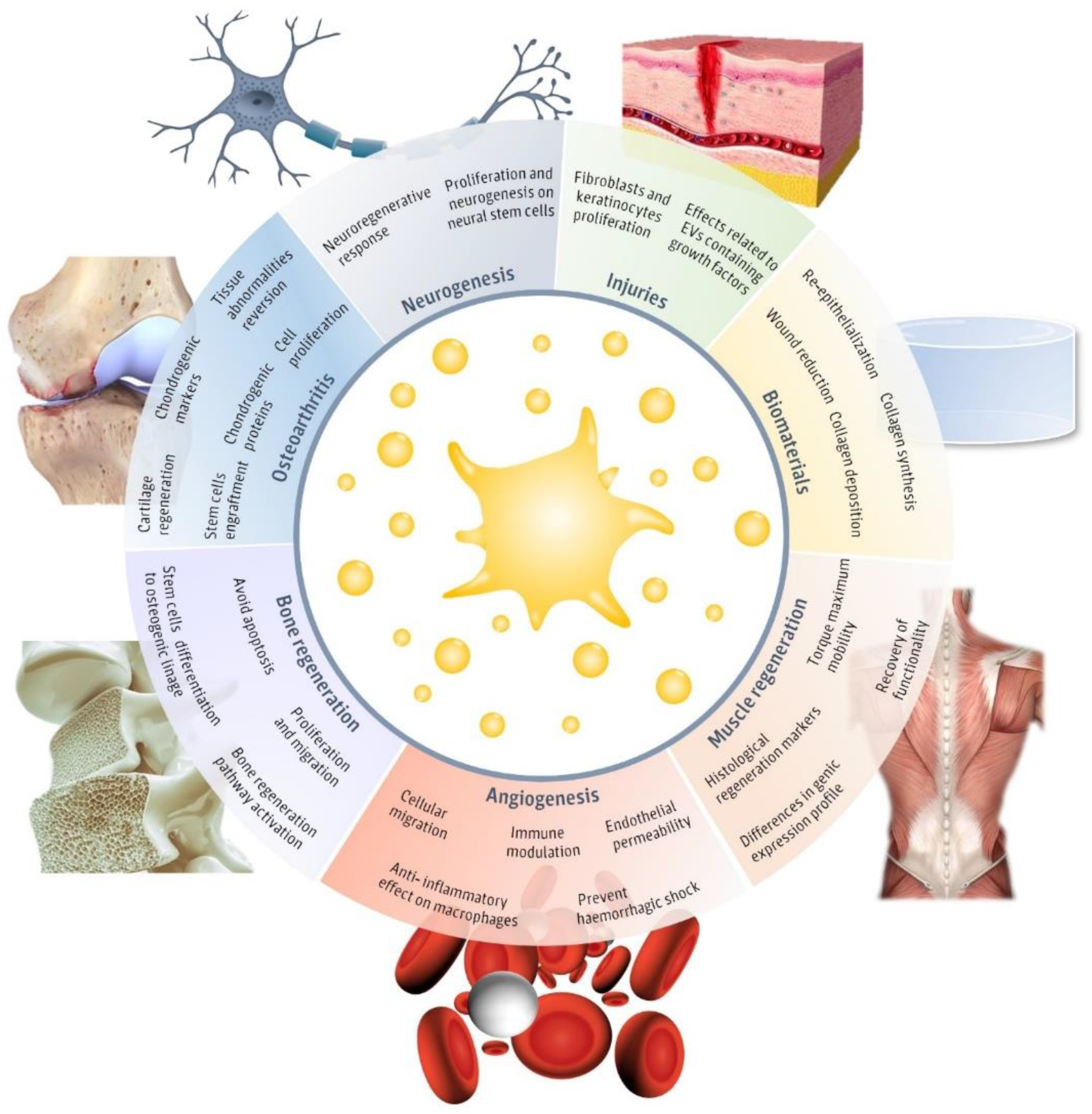
2. Regenerative Effects of pEVs
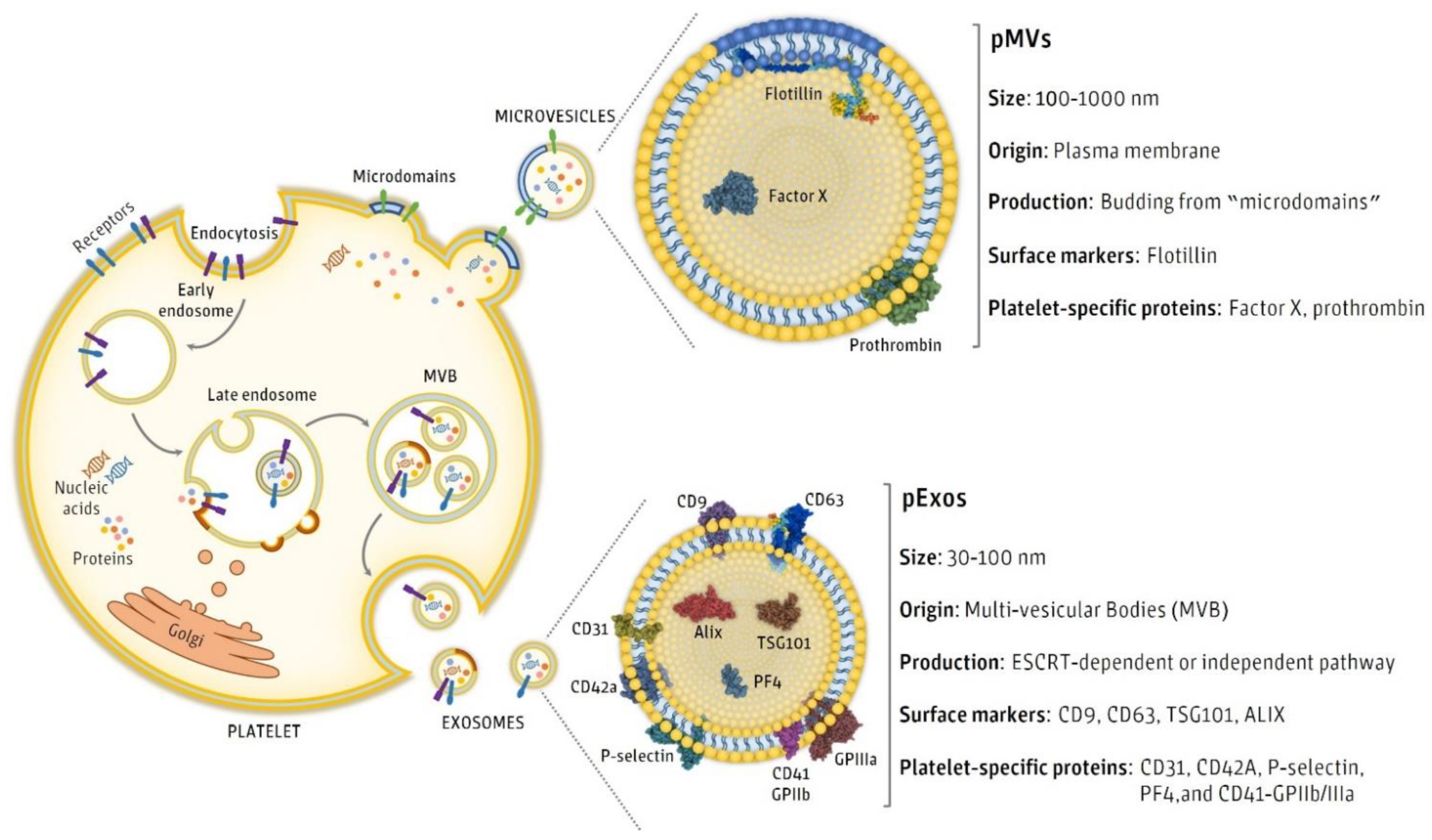
3. Isolation and Characterization of pEVs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Veerman, R.E.; Akpinar, G.G.; Eldh, M.; Gabrielsson, S. Immune Cell-Derived Extracellular Vesicles—Functions and Therapeutic Applications. Trends Mol. Med. 2019, 25, 382–394. [Google Scholar] [CrossRef]
- Johnson, J.; Wu, Y.-W.; Blyth, C.; Lichtfuss, G.; Goubran, H.; Burnouf, T. Prospective Therapeutic Applications of Platelet Extracellular Vesicles. Trends Biotechnol. 2021, 39, 598–612. [Google Scholar] [CrossRef]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Samadi, P.; Sheykhhasan, M.; Khoshinani, H.M. The Use of Platelet-Rich Plasma in Aesthetic and Regenerative Medicine: A Comprehensive Review. Aesthetic Plast. Surg. 2019, 43, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.I.-K.; Diaz, R.; Borg-Stein, J. Platelet-Rich Plasma. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 825–853. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.S.; Schwarz, E.M.; Maloney, M.D. Platelet-rich plasma therapy—Future or trend? Arthritis Res. Ther. 2012, 14, 219. [Google Scholar] [CrossRef]
- Etulain, J. Platelets in wound healing and regenerative medicine. Platelets 2018, 29, 556–568. [Google Scholar] [CrossRef]
- Tao, S.-C.; Guo, S.-C.; Zhang, C.-Q. Platelet-derived Extracellular Vesicles: An Emerging Therapeutic Approach. Int. J. Biol. Sci. 2017, 13, 828–834. [Google Scholar] [CrossRef]
- Puhm, F.; Boilard, E.; Machlus, K.R. Platelet Extracellular Vesicles. Arter. Thromb. Vasc. Biol. 2020, 2020, 87–96. [Google Scholar] [CrossRef]
- Wolf, P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Crawford, N. The Presence of Contractile Proteins in Platelet Microparticles Isolated from Human and Animal Platelet-free Plasma. Br. J. Haematol. 1971, 21, 53–69. [Google Scholar] [CrossRef]
- Fadadu, P.P.; Mazzola, A.J.; Hunter, C.W.; Davis, T.T. Review of concentration yields in commercially available platelet-rich plasma (PRP) systems: A call for PRP standardization. Reg. Anesthesia Pain Med. 2019, 44, 652–659. [Google Scholar] [CrossRef]
- Fioravanti, C.; Frustaci, I.; Armellin, E.; Condò, R.; Arcuri, C.; Cerroni, L. Autologous blood preparations rich in platelets, fibrin and growth factors. Oral Implant. 2016, 8, 96–113. [Google Scholar] [CrossRef]
- Lippross, S.; Alini, M. Platelet-rich plasma for bone healing—To use or not to use? AO Dialogue 2007, 25–29. [Google Scholar]
- Marques, L.F.; Stessuk, T.; Camargo, I.C.C.; Junior, N.S.; Dos Santos, L.; Ribeiro-Paes, J.T. Platelet-rich plasma (PRP): Methodological aspects and clinical applications. Platelets 2014, 26, 101–113. [Google Scholar] [CrossRef]
- Harmon, K.; Hanson, R.; Bowen, J.; Greenberg, S.; Magaziner, E.; Vandenbosch, J.; Harshfield, D.; Shiple, B.; Audley, D. Guidelines for the use of Platelet Rich Plasma—Draft. Int. Cell Med. Soc. 2010, 1–11. Available online: http://www.cellmedicinesociety.org/attachments/206_ICMS%20-%20Guidelines%20for%20the%20use%20of%20Platelet%20Rich%20Plasma%20-%20Draft.pdf (accessed on 31 December 2011).
- Antich-Rosselló, M.; Forteza-Genestra, M.A.; Calvo, J.; Gayà, A.; Monjo, M.; Ramis, J.M. Platelet-derived extracellular vesicles promote osteoinduction of mesenchymal stromal cells. Bone Jt. Res. 2020, 9, 667–674. [Google Scholar] [CrossRef]
- Torreggiani, E.; Perut, F.; Roncuzzi, L.; Zini, N.; Baglio, S.R.; Baldini, N. Exosomes: Novel effectors of human platelet lysate activity. Eur. Cells Mater. 2014, 28, 137–151. [Google Scholar] [CrossRef]
- Sinauridze, E.I.; Kireev, D.A.; Popenko, N.Y.; Pichugin, A.V.; Panteleev, M.A.; Krymskaya, O.V.; Ataullakhanov, F.I. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb. Haemost. 2007, 97, 425–434. [Google Scholar] [CrossRef]
- Melki, I.; Tessandier, N.; Zufferey, A.; Boilard, E. Platelet microvesicles in health and disease. Platelets 2017, 28, 214–221. [Google Scholar] [CrossRef]
- Kerris, E.W.J.; Hoptay, C.; Calderon, T.; Freishtat, R. Platelets and platelet extracellular vesicles in hemostasis and sepsis. J. Investig. Med. 2019, 68, 813–820. [Google Scholar] [CrossRef]
- Guo, S.-C.; Tao, S.-C.; Yin, W.-J.; Qi, X.; Yuan, T.; Zhang, C.-Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 2017, 7, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Wang, L.; Guan, J.; Tang, C.; He, N.; Zhang, W.; Fu, S. Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int. J. Biol. Macromol. 2018, 117, 102–107. [Google Scholar] [CrossRef]
- Lovisolo, F.; Carton, F.; Gino, S.; Migliario, M.; Renò, F. Platelet rich plasma-derived microvesicles increased in vitro wound healing. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9658–9664. [Google Scholar] [PubMed]
- Lopez, E.; Srivastava, A.; Burchfield, J.; Wang, Y.-W.; Cardenas, J.C.; Togarrati, P.P.; Miyazawa, B.; Gonzalez, E.; Holcomb, J.B.; Pati, S.; et al. Platelet-derived-Extracellular Vesicles Promote Hemostasis and Prevent the Development of Hemorrhagic Shock. Sci. Rep. 2019, 9, 17676. [Google Scholar] [CrossRef]
- Miyazawa, B.; Trivedi, A.; Togarrati, P.P.; Potter, D.; Baimukanova, G.; Vivona, L.; Lin, M.; Lopez, E.; Callcut, R.; Srivastava, A.; et al. Regulation of endothelial cell permeability by platelet-derived extracellular vesicles. J. Trauma Acute Care Surg. 2019, 86, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jung, H.; Song, J.; Choi, E.S.; You, G.; Mok, H. Activated Platelet-Derived Vesicles for Efficient Hemostatic Activity. Macromol. Biosci. 2020, 20, e1900338. [Google Scholar] [CrossRef]
- Sadallah, S.; Eken, C.; Martin, P.; Schifferli, J.A. Microparticles (Ectosomes) Shed by Stored Human Platelets Downregulate Macrophages and Modify the Development of Dendritic Cells. J. Immunol. 2011, 186, 6543–6552. [Google Scholar] [CrossRef] [PubMed]
- Hayon, Y.; Dashevsky, O.; Shai, E.; Varon, D.; Leker, R.R. Platelet Microparticles Promote Neural Stem Cell Proliferation, Survival and Differentiation. J. Mol. Neurosci. 2012, 47, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Hayon, Y.; Dashevsky, O.; Shai, E.; Brill, A.; Varon, D.; Leker, R.R. Platelet Microparticles Induce Angiogenesis and Neurogenesis after Cerebral Ischemia. Curr. Neurovasc. Res. 2012, 9, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.R.; Scheiber, A.L.; Yarowsky, P.; Henn, I.R.F.; Otsuru, S.; Lovering, R.M. Exosomes Isolated From Platelet-Rich Plasma and Mesenchymal Stem Cells Promote Recovery of Function After Muscle Injury. Am. J. Sports Med. 2020, 48, 2277–2286. [Google Scholar] [CrossRef]
- Mause, S.F.; Ritzel, E.; Liehn, E.A.; Hristov, M.; Bidzhekov, K.; Müller-Newen, G.; Soehnlein, O.; Weber, C. Platelet Microparticles Enhance the Vasoregenerative Potential of Angiogenic Early Outgrowth Cells After Vascular Injury. Circulation 2010, 122, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Song, K.S.; Chung, J.-H.; Lee, K.R.; Lee, S.-N. Platelet microparticles induce angiogenesisin vitro. Br. J. Haematol. 2004, 124, 376–384. [Google Scholar] [CrossRef]
- Brill, A.; Dashevsky, O.; Rivo, J.; Gozal, Y.; Varon, D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc. Res. 2005, 67, 30–38. [Google Scholar] [CrossRef]
- Ferreira, M.R.; Zambuzzi, W.F. Platelet microparticles load a repertory of miRNAs programmed to drive osteogenic phenotype. J. Biomed. Mater. Res. Part A 2021, 109, 1502–1511. [Google Scholar] [CrossRef]
- Tao, S.-C.; Yuan, T.; Rui, B.-Y.; Zhu, Z.-Z.; Guo, S.-C.; Zhang, C.-Q. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics 2017, 7, 733–750. [Google Scholar] [CrossRef]
- Moest, T.; Koehler, F.; Prechtl, C.; Schmitt, C.; Watzek, G.; Schlegel, K.A. Bone formation in peri-implant defects grafted with microparticles: A pilot animal experimental study. J. Clin. Periodontol. 2014, 41, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Otahal, A.; Kuten-Pella, O.; Kramer, K.; Neubauer, M.; Lacza, Z.; Nehrer, S.; De Luna, A. Functional repertoire of EV-associated miRNA profiles after lipoprotein depletion via ultracentrifugation and size exclusion chromatography from autologous blood products. Sci. Rep. 2021, 11, 5823. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, L.; Ma, C.; Wang, G.; Zhang, Y.; Sun, S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J. Orthop. Surg. Res. 2019, 14, 470. [Google Scholar] [CrossRef]
- Otahal, A.; Kramer, K.; Kuten-Pella, O.; Weiss, R.; Stotter, C.; Lacza, Z.; Weber, V.; Nehrer, S.; De Luna, A. Characterization and Chondroprotective Effects of Extracellular Vesicles from Plasma- and Serum-Based Autologous Blood-Derived Products for Osteoarthritis Therapy. Front. Bioeng. Biotechnol. 2020, 8, 584050. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Huang, J.; Luo, P.; Wang, Z.; He, J.; Wu, S.; Peng, C.; Cao, X. Platelet-Derived Microparticles Mediate the Intra-Articular Homing of Mesenchymal Stem Cells in Early-Stage Cartilage Lesions. Stem Cells Dev. 2020, 29, 414–424. [Google Scholar] [CrossRef]
- Hess, J.R.; Lelkens, C.C.; Holcomb, J.B.; Scalea, T.M. Advances in military, field, and austere transfusion medicine in the last decade. Transfus. Apher. Sci. 2013, 49, 380–386. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—an ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Milioli, M.; Ibáñez-Vea, M.; Sidoli, S.; Palmisano, G.; Careri, M.; Larsen, M.R. Quantitative proteomics analysis of platelet-derived microparticles reveals distinct protein signatures when stimulated by different physiological agonists. J. Proteom. 2015, 121, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Gudbergsson, J.M.; Andresen, T.L.; Simonsen, J.B. What is the blood concentration of extracellular vesicles? Implications for the use of extracellular vesicles as blood-borne biomarkers of cancer. Biochim. Biophys. Acta (BBA) Bioenergy 2019, 1871, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Greening, D.; Zhu, H.-J.; Takahashi, N.; Simpson, R.J. Extracellular vesicle isolation and characterization: Toward clinical application. J. Clin. Investig. 2016, 126, 1152–1162. [Google Scholar] [CrossRef]
- Gandham, S.; Su, X.; Wood, J.; Nocera, A.L.; Alli, S.C.; Milane, L.; Zimmerman, A.; Amiji, M.; Ivanov, A.R. Technologies and Standardization in Research on Extracellular Vesicles. Trends Biotechnol. 2020, 38, 1066–1098. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, Y.; Ishikawa, Y. Remnant lipoproteins as strong key particles to atherogenesis. J. Atheroscler. Thromb. 2009, 16, 145–154. [Google Scholar] [CrossRef]
- Antwi-Baffour, S.; Adjei, J.; Aryeh, C.; Kyeremeh, R.; Kyei, F.; Seidu, M.A. Understanding the biosynthesis of platelets-derived extracellular vesicles. Immun. Inflamm. Dis. 2015, 3, 133–140. [Google Scholar] [CrossRef]
- Posma, J.J.N.; Posthuma, J.J.; Spronk, H.M.H. Coagulation and non-coagulation effects of thrombin. J. Thromb. Haemost. 2016, 14, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Hougie, C. The Activation of Platelets by Plasma. Br. J. Haematol. 1955, 1, 213–222. [Google Scholar] [CrossRef]
- Tissot, J.-D.; Canellini, G.; Rubin, O.; Angelillo-Scherrer, A.; Delobel, J.; Prudent, M.; Lion, N. Blood microvesicles: From proteomics to physiology. Transl. Proteom. 2013, 1, 38–52. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, Q.; Hu, X.; Mi, T.; Yu, H.; Liu, S.; Zhang, B.; Tang, M.; Huang, J.; Xiong, K. How does temperature play a role in the storage of extracellular vesicles? J. Cell. Physiol. 2020, 235, 7663–7680. [Google Scholar] [CrossRef] [PubMed]
- Maroto, R.; Zhao, Y.; Jamaluddin, M.; Popov, V.L.; Wang, H.; Kalubowilage, M.; Zhang, Y.; Luisi, J.; Sun, H.; Culbertson, C.T.; et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J. Extracell. Vesicles 2017, 6, 1359478. [Google Scholar] [CrossRef] [PubMed]
- Lőrincz, Á.; Timar, C.; Marosvári, K.A.; Veres, D.S.; Otrokocsi, L.; Kittel, Á.; Ligeti, E. Effect of storage on physical and functional properties of extracellular vesicles derived from neutrophilic granulocytes. J. Extracell. Vesicles 2014, 3, 25465. [Google Scholar] [CrossRef]
- Royo, F.; Théry, C.; Falcón-Pérez, J.M.; Nieuwland, R.; Witwer, K.W. Methods for Separation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey Performed by the ISEV Rigor and Standardization Subcommittee. Cells 2020, 9, 1955. [Google Scholar] [CrossRef] [PubMed]
- De Luna, A.; Otahal, A.; Nehrer, S. Mesenchymal Stromal Cell-Derived Extracellular Vesicles—Silver Linings for Cartilage Regeneration? Front. Cell Dev. Biol. 2020, 8, 1548. [Google Scholar] [CrossRef] [PubMed]
- French, S.L.; Butov, K.R.; Allaeys, I.; Canas, J.; Morad, G.; Davenport, P.; Laroche, A.; Trubina, N.M.; Italiano, J.E.; Moses, M.A.; et al. Platelet-derived extracellular vesicles infiltrate and modify the bone marrow during inflammation. Blood Adv. 2020, 4, 3011–3023. [Google Scholar] [CrossRef]
- Penolazzi, L.; Vecchiatini, R.; Bignardi, S.; Lambertini, E.; Torreggiani, E.; Canella, A.; Franceschetti, T.; Calura, G.; Vesce, F.; Piva, R. Influence of obstetric factors on osteogenic potential of umbilical cord-derived mesenchymal stem cells. Reprod. Biol. Endocrinol. 2009, 7, 106. [Google Scholar] [CrossRef]
- Soleymani, S.; Yari, F.; Bolhassani, A.; Bakhshandeh, H. Platelet microparticles: An effective delivery system for anti-viral drugs. J. Drug Deliv. Sci. Technol. 2019, 51, 290–296. [Google Scholar] [CrossRef]
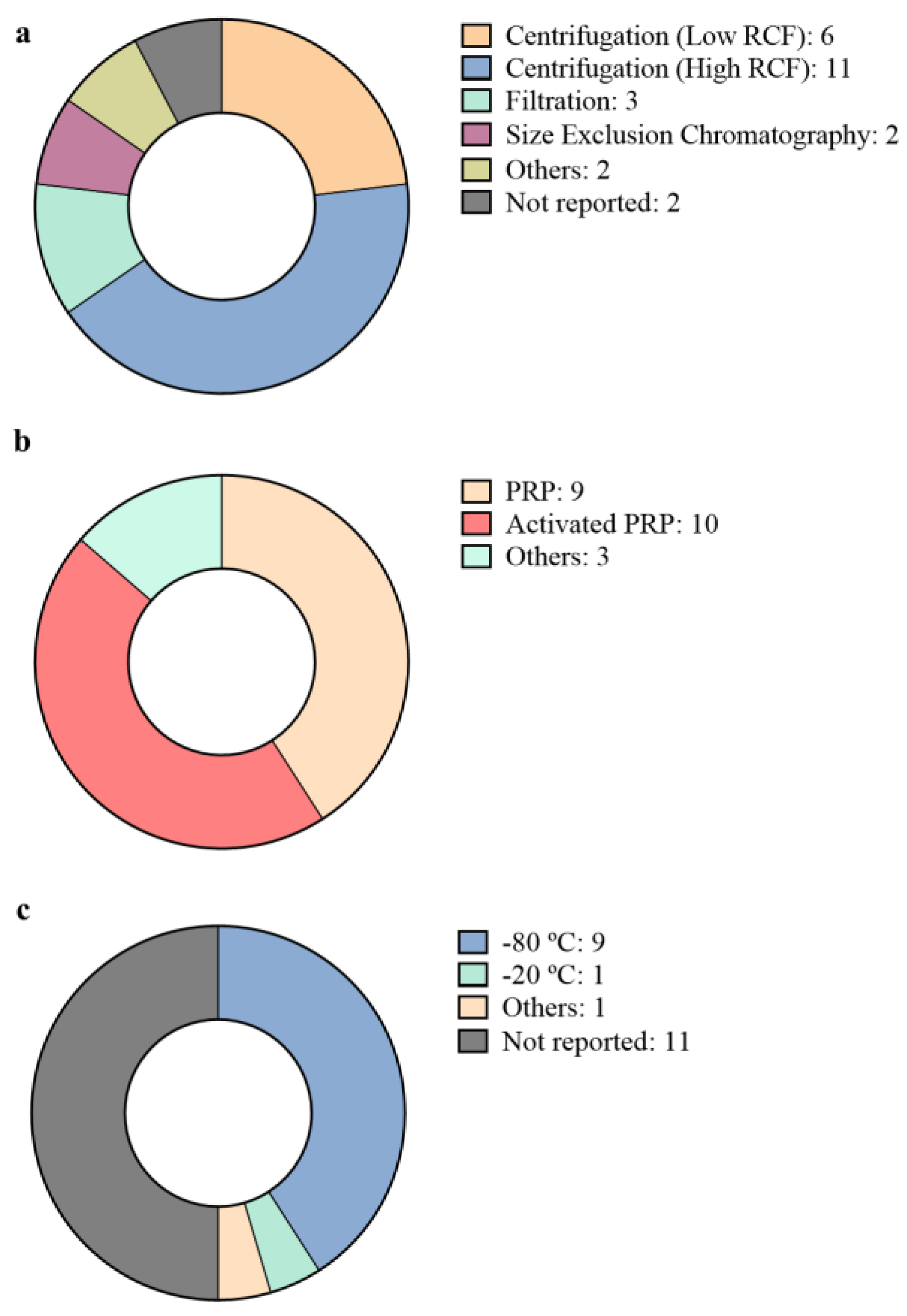

| Regenerative Medicine Field | Platelet Source | Isolation Method | pEVs Storage Conditions | Characterization | Study Model | Reference |
|---|---|---|---|---|---|---|
| Injuries and wounds Biomaterials Angiogenesis | PRP | High RCF centrifugation | Frozen at −80 °C | Physical characterization and pEV marker detection | In vitro cell culture In vivo diabetic rat model | [30] |
| PRP | Not specified | Not specified | Not specified | In vivo diabetic rat model | [31] | |
| Injuries and wounds | Activated PRP | Low RCF centrifugation | Not specified | Physical characterization | In vitro cell culture | [32] |
| PRP | Filtration | Frozen at −20 °C | Physical characterization and pEV marker detection | In vitro blood samples In vivo bleeding rat model | [33] | |
| Activated PRP | High RCF centrifugation | Frozen at −80 °C | Physical characterization and pEV marker detection | In vitro cell culture In vitro blood samples In vivo mice model | [34] | |
| 3 days stored activated platelets | Sonication | Not specified | Physical characterization | In vitro cell culture In vivo mice model. | [35] | |
| 5 days stored PRP | High RCF centrifugation | Stored at −80 °C until final centrifugation. | Physical characterization and pEV marker detection | In vitro cell culture | [36] | |
| Angiogenesis | Activated platelets | Low RCF centrifugation | Not specified | Not specified | In vitro cell culture | [40] |
| Activated PRP | Low RCF centrifugation | Not specified | Not specified | In vitro cell culture | [41] | |
| Activated PRP | High RCF centrifugation | Not specified | Physical characterization and pEV marker detection | In vitro cell culture In vivo ischemic heart rat model | [42] | |
| Angiogenesis Neural regeneration | Activated PRP | High RCF centrifugation | Not specified | Physical characterization and pEV marker detection | In vitro cell culture | [37] |
| Activated PRP | High RCF centrifugation | Not specified | pEV marker detection | In vivo focal ischemia rat model | [38] | |
| Osteoarthritis | PRP | High RCF centrifugation Filtration Size exclusion chromatography A combination of different techniques | Frozen at −80 °C | Physical characterization and pEV marker detection | miRNA profiling | [46] |
| PRP | Spin column based commercial kit | Frozen at −80 °C | Physical characterization and pEV marker detection | In vitro cell culture In vivo osteoarthritic rabbit model | [47] | |
| PRP | High RCF centrifugation | Frozen at −80 °C | Physical characterization and pEV marker detection | In vitro cell culture | [48] | |
| Activated PRP | Low RCF centrifugation | Not specified | Not specified | In vitro cell culture In vivo rat model | [49] | |
| Musculoskeletal regeneration | Not appliable | Not appliable | Not appliable | Not appliable | In silico miRNA profiling | [43] |
| PL | High RCF centrifugation Size Exclusion Chromatography | Frozen at −80 °C | Physical characterization and pEV marker detection | In vitro cell culture | [25] | |
| PL | High RCF centrifugation | Frozen at −80 °C | Physical characterization and pEV marker detection | In vitro cell culture | [26] | |
| PRP | High RCF centrifugation | Frozen at −80 °C | Physical characterization and pEV marker detection | In vitro cell culture In vivo rat model | [44] | |
| PRP | Sonication | Not specified | Not specified | In vivo pig model | [45] | |
| Activated PRP | High RCF centrifugation | Frozen at −80 °C | Physical characterization and pEV marker detection | In vivo rat model | [39] |
| Kind of Proteins Commonly Reported | pEV Markers | References |
|---|---|---|
| EV membrane markers | CD9 | [25,30,33,34,39,44,46,47,48] |
| CD61 | [33,36] | |
| CD63 | [25,26,30,33,34,39,44,47,48,66] | |
| D81 | [30,33,34,39,44,47] | |
| Platelet source markers | CD31 | [34] |
| CD41 | [33,34,37,38,42,44,48,67,68,69] | |
| CD42 | [40] | |
| EV cytosolic markers | ALIX | [46,48] |
| HSP90 | [33] | |
| HPS101 | [47] | |
| TSG101 | [44] | |
| Non-EVs structures | APOA1 | [46,48] |
| APOB100 | [46,48] | |
| Calnexin | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antich-Rosselló, M.; Forteza-Genestra, M.A.; Monjo, M.; Ramis, J.M. Platelet-Derived Extracellular Vesicles for Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8580. https://doi.org/10.3390/ijms22168580
Antich-Rosselló M, Forteza-Genestra MA, Monjo M, Ramis JM. Platelet-Derived Extracellular Vesicles for Regenerative Medicine. International Journal of Molecular Sciences. 2021; 22(16):8580. https://doi.org/10.3390/ijms22168580
Chicago/Turabian StyleAntich-Rosselló, Miquel, Maria Antònia Forteza-Genestra, Marta Monjo, and Joana M. Ramis. 2021. "Platelet-Derived Extracellular Vesicles for Regenerative Medicine" International Journal of Molecular Sciences 22, no. 16: 8580. https://doi.org/10.3390/ijms22168580
APA StyleAntich-Rosselló, M., Forteza-Genestra, M. A., Monjo, M., & Ramis, J. M. (2021). Platelet-Derived Extracellular Vesicles for Regenerative Medicine. International Journal of Molecular Sciences, 22(16), 8580. https://doi.org/10.3390/ijms22168580







