Investigation of LINC00493/SMIM26 Gene Suggests Its Dual Functioning at mRNA and Protein Level
Abstract
1. Introduction
2. Results
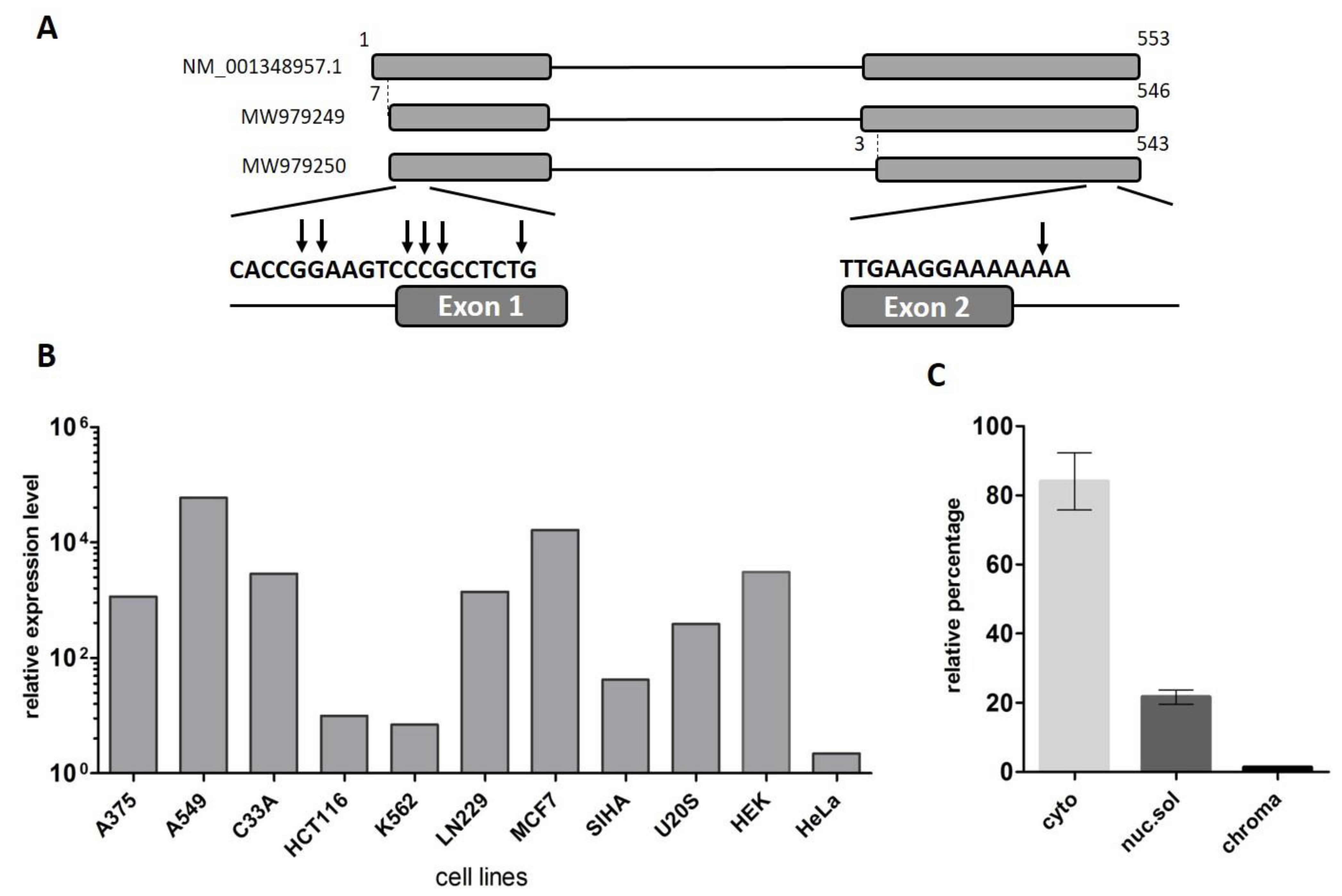
2.1. LINC00493 Transcript Structure
2.2. LINC00493 Is Widely Expressed in Human Tissues and Cell Lines
2.3. Cytoplasmic Localization of LINC00493
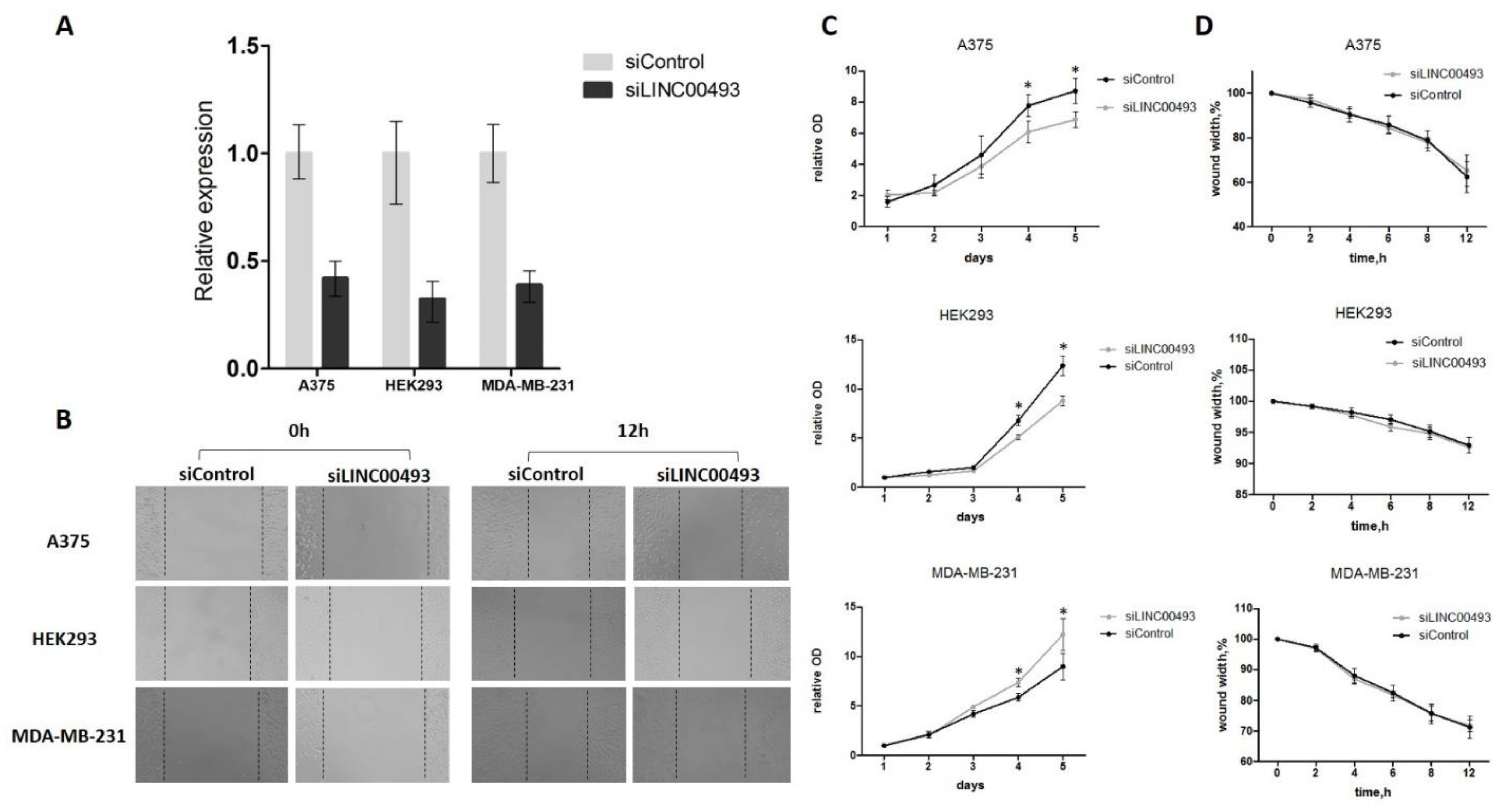
2.4. Knockdown of LINC00493 Affects Cell Growth in a Cell-Type-Specific Manner
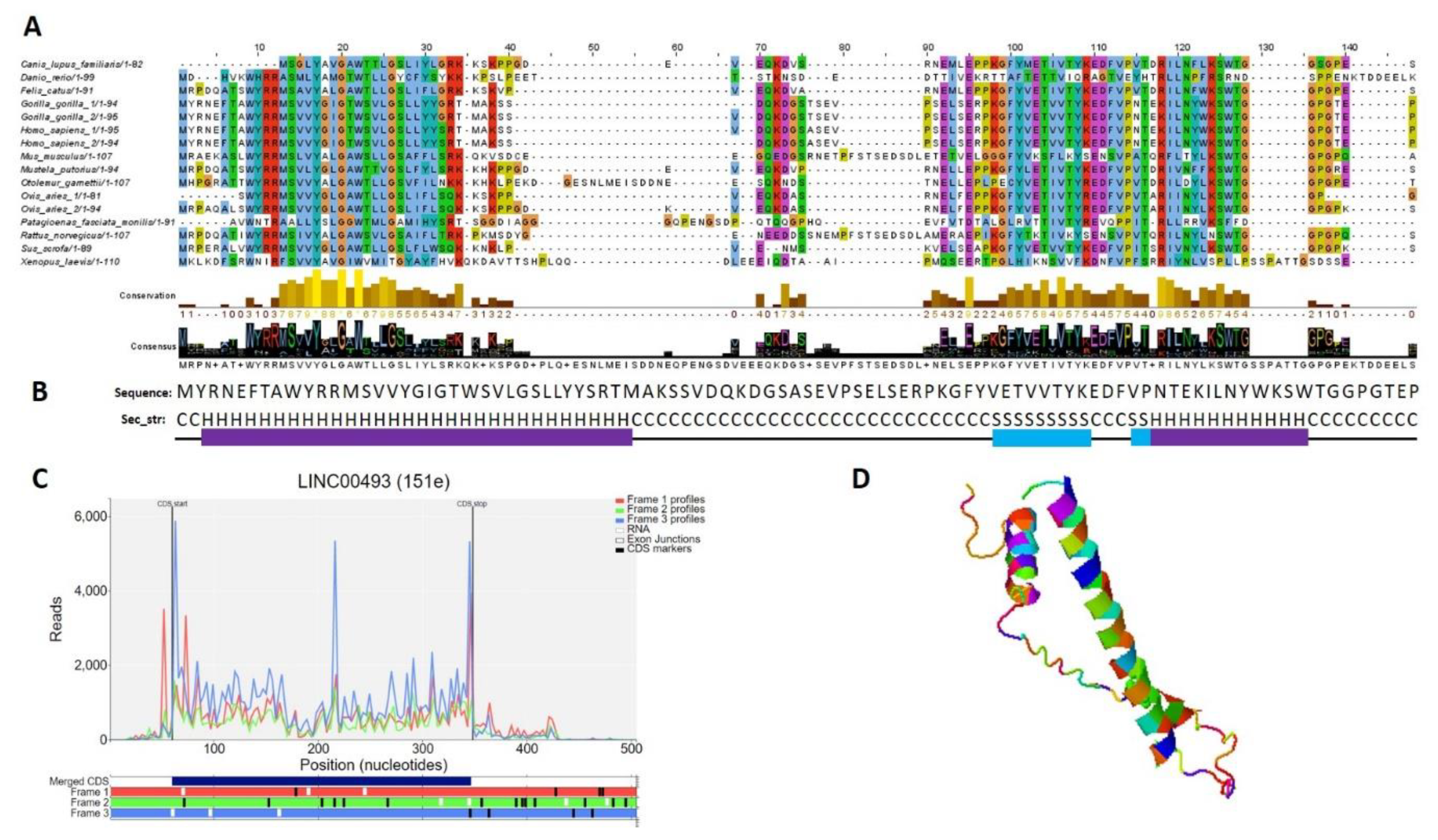
2.5. Small Protein Is Translated from LINC00493 RNA
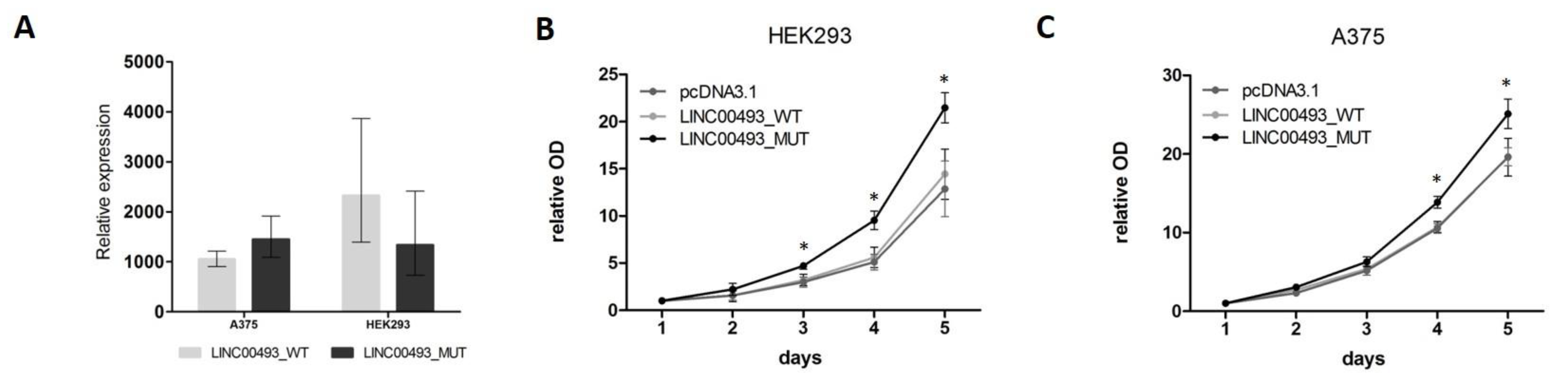
2.6. SMIM26 Protein Affects Cell Viability
3. Discussion
4. Materials and Methods
4.1. Bioinformatics Tools
4.2. Cell Culture
4.3. RNA Extraction and Reverse Transcription-Quantitative PCR (RT-qPCR)
4.4. Rapid Amplification of cDNA Ends (RACE)
4.5. Subcellular RNA Localization
4.6. LINC00493 Knockdown
4.7. LINC00493 Overexpression Experiments
4.8. Cell Proliferation and Migration Assays
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frankish, A.; Diekhans, M.; Ferreira, A.M.; Johnson, R.; Jungreis, I.; Loveland, J.; Mudge, J.M.; Sisu, C.; Wright, J.; Armstrong, J.; et al. Gencode reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019, 47, D766–D773. [Google Scholar] [CrossRef]
- Hon, C.C.; Ramilowski, J.A.; Harshbarger, J.; Bertin, N.; Rackham, O.J.; Gough, J.; Denisenko, E.; Schmeier, S.; Poulsen, T.M.; Severin, J.; et al. An atlas of human long non-coding RNAs with accurate 5’ ends. Nature 2017, 543, 199–204. [Google Scholar] [CrossRef]
- Dykes, I.M.; Emanueli, C. Transcriptional and Post-transcriptional Gene Regulation by Long Non-coding RNA. Genom. Proteom. Bioinform. 2017, 15, 177–186. [Google Scholar] [CrossRef]
- St Laurent, G.; Wahlestedt, C.; Kapranov, P. The Landscape of long noncoding RNA classification. Trends Genet. TIG 2015, 31, 239–251. [Google Scholar] [CrossRef]
- Panni, S.; Lovering, R.C.; Porras, P.; Orchard, S. Non-coding RNA regulatory networks. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194417. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Cullen, B.R. The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA 2007, 13, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.R.; Mondal, T.; Mohammad, F.; Enroth, S.; Redrup, L.; Komorowski, J.; Nagano, T.; Mancini-Dinardo, D.; Kanduri, C. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol. Cell 2008, 32, 232–246. [Google Scholar] [CrossRef]
- Orom, U.A.; Derrien, T.; Beringer, M.; Gumireddy, K.; Gardini, A.; Bussotti, G.; Lai, F.; Zytnicki, M.; Notredame, C.; Huang, Q.; et al. Long noncoding RNAs with enhancer-like function in human cells. Cell 2010, 143, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Orom, U.A.; Derrien, T.; Guigo, R.; Shiekhattar, R. Long noncoding RNAs as enhancers of gene expression. Cold Spring Harb. Symp. Quant. Biol. 2010, 75, 325–331. [Google Scholar] [CrossRef]
- Gong, C.; Maquat, L.E. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3’ UTRs via Alu elements. Nature 2011, 470, 284–288. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Jiang, J.; Xu, C.; Kang, J.; Xiao, L.; Wu, M.; Xiong, J.; Guo, X.; Liu, H. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev. Cell 2013, 25, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Keniry, A.; Oxley, D.; Monnier, P.; Kyba, M.; Dandolo, L.; Smits, G.; Reik, W. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat. Cell Biol. 2012, 14, 659–665. [Google Scholar] [CrossRef]
- Liu, S.J.; Horlbeck, M.A.; Cho, S.W.; Birk, H.S.; Malatesta, M.; He, D.; Attenello, F.J.; Villalta, J.E.; Cho, M.Y.; Chen, Y.; et al. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science 2017, 355. [Google Scholar] [CrossRef]
- Sparber, P.; Filatova, A.; Khantemirova, M.; Skoblov, M. The role of long non-coding RNAs in the pathogenesis of hereditary diseases. BMC Med. Genom. 2019, 12, 42. [Google Scholar] [CrossRef]
- Bao, Z.; Yang, Z.; Huang, Z.; Zhou, Y.; Cui, Q.; Dong, D. LncRNADisease 2.0: An updated database of long non-coding RNA-associated diseases. Nucleic Acids Res. 2019, 47, D1034–D1037. [Google Scholar] [CrossRef]
- Lorenzen, J.M.; Thum, T. Long noncoding RNAs in kidney and cardiovascular diseases. Nat. Rev. Nephrol. 2016, 12, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, J. LncRNAs: Macromolecules with big roles in neurobiology and neurological diseases. Metab. Brain Dis. 2017, 32, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Wang, D.; Wang, J.; Yu, W.; Yang, J. Long Non-Coding RNA in the Pathogenesis of Cancers. Cells 2019, 8, 1015. [Google Scholar] [CrossRef]
- St Laurent, G.; Vyatkin, Y.; Kapranov, P. Dark matter RNA illuminates the puzzle of genome-wide association studies. BMC Med. 2014, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Hindorff, L.A.; Sethupathy, P.; Junkins, H.A.; Ramos, E.M.; Mehta, J.P.; Collins, F.S.; Manolio, T.A. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc. Natl. Acad. Sci. USA 2009, 106, 9362–9367. [Google Scholar] [CrossRef]
- Chew, G.L.; Pauli, A.; Rinn, J.L.; Regev, A.; Schier, A.F.; Valen, E. Ribosome profiling reveals resemblance between long non-coding RNAs and 5’ leaders of coding RNAs. Development 2013, 140, 2828–2834. [Google Scholar] [CrossRef]
- Aspden, J.L.; Eyre-Walker, Y.C.; Phillips, R.J.; Amin, U.; Mumtaz, M.A.; Brocard, M.; Couso, J.P. Extensive translation of small Open Reading Frames revealed by Poly-Ribo-Seq. eLife 2014, 3, e03528. [Google Scholar] [CrossRef]
- Bazzini, A.A.; Johnstone, T.G.; Christiano, R.; Mackowiak, S.D.; Obermayer, B.; Fleming, E.S.; Vejnar, C.E.; Lee, M.T.; Rajewsky, N.; Walther, T.C.; et al. Identification of small ORFs in vertebrates using ribosome footprinting and evolutionary conservation. EMBO J. 2014, 33, 981–993. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, H.W.; Nam, J.W. The small peptide world in long noncoding RNAs. Brief. Bioinform. 2019, 20, 1853–1864. [Google Scholar] [CrossRef]
- Stein, C.S.; Jadiya, P.; Zhang, X.; McLendon, J.M.; Abouassaly, G.M.; Witmer, N.H.; Anderson, E.J.; Elrod, J.W.; Boudreau, R.L. Mitoregulin: A lncRNA-Encoded Microprotein that Supports Mitochondrial Supercomplexes and Respiratory Efficiency. Cell Rep. 2018, 23, 3710–3720.e3718. [Google Scholar] [CrossRef]
- Chugunova, A.; Loseva, E.; Mazin, P.; Mitina, A.; Navalayeu, T.; Bilan, D.; Vishnyakova, P.; Marey, M.; Golovina, A.; Serebryakova, M.; et al. LINC00116 codes for a mitochondrial peptide linking respiration and lipid metabolism. Proc. Natl. Acad. Sci. USA 2019, 116, 4940–4945. [Google Scholar] [CrossRef]
- Konina, D.O.; Filatova, A.Y.; Skoblov, M.Y. LINC01420 RNA structure and influence on cell physiology. BMC Genom. 2019, 20, 298. [Google Scholar] [CrossRef] [PubMed]
- Haeussler, M.; Zweig, A.S.; Tyner, C.; Speir, M.L.; Rosenbloom, K.R.; Raney, B.J.; Lee, C.M.; Lee, B.T.; Hinrichs, A.S.; Gonzalez, J.N.; et al. The UCSC Genome Browser database: 2019 update. Nucleic Acids Res. 2019, 47, D853–D858. [Google Scholar] [CrossRef] [PubMed]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef]
- FANTOM Consortium; The RIKEN PMI; CLST (DGT); Forrest, A.R.; Kawaji, H.; Rehli, M.; Baillie, J.K.; de Hoon, M.J.; Haberle, V.; Lassmann, T.; et al. A promoter-level mammalian expression atlas. Nature 2014, 507, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Michel, A.M.; Kiniry, S.J.; O’Connor, P.B.F.; Mullan, J.P.; Baranov, P.V. GWIPS-viz: 2018 update. Nucleic Acids Res. 2018, 46, D823–D830. [Google Scholar] [CrossRef]
- Kiniry, S.J.; O’Connor, P.B.F.; Michel, A.M.; Baranov, P.V. Trips-Viz: A transcriptome browser for exploring Ribo-Seq data. Nucleic Acids Res. 2019, 47, D847–D852. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Anishchenko, I.; Park, H.; Peng, Z.; Ovchinnikov, S.; Baker, D. Improved protein structure prediction using predicted interresidue orientations. Proc. Natl. Acad. Sci. USA 2020, 117, 1496–1503. [Google Scholar] [CrossRef]
- Ramilowski, J.A.; Yip, C.W.; Agrawal, S.; Chang, J.C.; Ciani, Y.; Kulakovskiy, I.V.; Mendez, M.; Ooi, J.L.C.; Ouyang, J.F.; Parkinson, N.; et al. Functional annotation of human long noncoding RNAs via molecular phenotyping. Genome Res. 2020, 30, 1060–1072. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, B.; Lu, J.; Song, Y.; Wang, W.; Zhang, W.; Shao, F.; Gong, M.; Wang, M.; Liang, X.; et al. Long noncoding RNA PM maintains cerebellar synaptic integrity and Cbln1 activation via Pax6/Mll1-mediated H3K4me3. PLoS Biol. 2021, 19, e3001297. [Google Scholar] [CrossRef]
- Mukherjee, N.; Calviello, L.; Hirsekorn, A.; de Pretis, S.; Pelizzola, M.; Ohler, U. Integrative classification of human coding and noncoding genes through RNA metabolism profiles. Nat. Struct. Mol. Biol. 2017, 24, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Yeasmin, F.; Imamachi, N.; Tanu, T.; Taniue, K.; Kawamura, T.; Yada, T.; Akimitsu, N. Identification and analysis of short open reading frames (sORFs) in the initially annotated noncoding RNA LINC00493 from human cells. J. Biochem. 2021, 169, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Reljić, B.; Liang, C.; Kerouanton, B.; Francisco, J.C.; Peh, J.H.; Mary, C.; Jagannathan, N.S.; Olexiouk, V.; Tang, C.; et al. Mitochondrial peptide BRAWNIN is essential for vertebrate respiratory complex III assembly. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alfoldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Nam, J.W.; Choi, S.W.; You, B.H. Incredible RNA: Dual Functions of Coding and Noncoding. Mol. Cells 2016, 39, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Rubtsova, M.; Naraykina, Y.; Vasilkova, D.; Meerson, M.; Zvereva, M.; Prassolov, V.; Lazarev, V.; Manuvera, V.; Kovalchuk, S.; Anikanov, N.; et al. Protein encoded in human telomerase RNA is involved in cell protective pathways. Nucleic Acids Res. 2018, 46, 8966–8977. [Google Scholar] [CrossRef]
- Spencer, H.L.; Sanders, R.; Boulberdaa, M.; Meloni, M.; Cochrane, A.; Spiroski, A.M.; Mountford, J.; Emanueli, C.; Caporali, A.; Brittan, M.; et al. The LINC00961 transcript and its encoded micropeptide, small regulatory polypeptide of amino acid response, regulate endothelial cell function. Cardiovasc. Res. 2020, 116, 1981–1994. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.G.; Brown, C.M. In silico estimation of translation efficiency in human cell lines: Potential evidence for widespread translational control. PLoS ONE 2013, 8, e57625. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, S.; Zhou, H.; Qu, L.H.; Yang, J.H. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014, 42, D92–D97. [Google Scholar] [CrossRef]
- Lietman, S.A.; Yin, L.; Levine, M.A. SH3BP2 is an activator of NFAT activity and osteoclastogenesis. Biochem. Biophys. Res. Commun. 2008, 371, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.; Munch, A.; Neumann, S.; Kremmer, E.; Tatzelt, J.; Lichtenthaler, S.F. The novel membrane protein TMEM59 modulates complex glycosylation, cell surface expression, and secretion of the amyloid precursor protein. J. Biol. Chem. 2010, 285, 20664–20674. [Google Scholar] [CrossRef]
- Haase, M.; Fitze, G. HSP90AB1: Helping the good and the bad. Gene 2016, 575, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Bereshchenko, O.; Migliorati, G.; Bruscoli, S.; Riccardi, C. Glucocorticoid-Induced Leucine Zipper: A Novel Anti-inflammatory Molecule. Front. Pharmacol. 2019, 10, 308. [Google Scholar] [CrossRef]
- Mascarenhas, R.; Dougherty, J.A.; Schoenberg, D.R. SMG6 cleavage generates metastable decay intermediates from nonsense-containing beta-globin mRNA. PLoS ONE 2013, 8, e74791. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, P.; Josi, C.; Kurosawa, H.; Yamashita, A.; Muhlemann, O. A novel phosphorylation-independent interaction between SMG6 and UPF1 is essential for human NMD. Nucleic Acids Res. 2014, 42, 9217–9235. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhu, Y.; Liu, Z.J.; Ouyang, S. The emerging roles of the DDX41 protein in immunity and diseases. Protein Cell 2017, 8, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhang, M.; Ao, J.; Zhen, Z.; Gao, X.; Li, M. NUCKS1 is a novel regulator of milk synthesis in and proliferation of mammary epithelial cells via the mTOR signaling pathway. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Shao, D.; Xu, K.; Lu, Z.; Lu, Z.J.; Yang, Y.T.; Zhang, Q.C. RISE: A database of RNA interactome from sequencing experiments. Nucleic Acids Res. 2018, 46, D194–D201. [Google Scholar] [CrossRef]
- Paraskevopoulou, M.D.; Hatzigeorgiou, A.G. Analyzing MiRNA-LncRNA Interactions. Methods Mol. Biol. 2016, 1402, 271–286. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, B.; Liu, M.; Liu, Y.; Gao, R. miR-126-5p Restoration Promotes Cell Apoptosis in Cervical Cancer by Targeting Bcl2l2. Oncol. Res. 2017, 25, 463–470. [Google Scholar] [CrossRef]
- Villain, G.; Poissonnier, L.; Noueihed, B.; Bonfils, G.; Rivera, J.C.; Chemtob, S.; Soncin, F.; Mattot, V. miR-126-5p promotes retinal endothelial cell survival through SetD5 regulation in neurons. Development 2018. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Zhao, W.; Cui, S.; Song, Y. miR-153-3p regulates progression of ovarian carcinoma in vitro and in vivo by targeting MCL1 gene. J. Cell. Biochem. 2019, 120, 19147–19158. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhou, H.G.; Chen, L.H.; Qu, D.C.; Wang, C.J.; Xia, Z.Y.; Zheng, J.H. MiR-32-5p regulates the proliferation and metastasis of cervical cancer cells by targeting HOXB8. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 87–95. [Google Scholar] [CrossRef]
- Ye, Z.; Shi, J.; Ning, Z.; Hou, L.; Hu, C.Y.; Wang, C. MiR-92b-3p inhibits proliferation and migration of C2C12 cells. Cell Cycle 2020, 19, 2906–2917. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Zhou, X.; Guo, R.; Yin, J.; Li, Y.; Ma, G. miR-137: A Novel Therapeutic Target for Human Glioma. Mol. Ther. Nucleic Acids 2020, 21, 614–622. [Google Scholar] [CrossRef]
- Wei, H.; Yu, K.; Liu, Y.; Li, L.; Wang, G. Tumor expression of miR-448 is a prognostic marker in oral squamous cell carcinoma. BMC Cancer 2020, 20, 756. [Google Scholar] [CrossRef] [PubMed]
- Lou, Q.; Liu, R.; Yang, X.; Li, W.; Huang, L.; Wei, L.; Tan, H.; Xiang, N.; Chan, K.; Chen, J.; et al. miR-448 targets IDO1 and regulates CD8(+) T cell response in human colon cancer. J. Immunother. Cancer 2019, 7, 210. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Sun, H.; Wu, J.; Kuang, Y. MiR-92b-3p regulates oxygen and glucose deprivation-reperfusion-mediated apoptosis and inflammation by targeting TRAF3 in PC12 cells. Exp. Physiol. 2020, 105, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.; Achuthan, P.; Akanni, W.; Allen, J.; Amode, M.R.; Armean, I.M.; Bennett, R.; Bhai, J.; Billis, K.; Boddu, S.; et al. Ensembl 2019. Nucleic Acids Res. 2019, 47, D745–D751. [Google Scholar] [CrossRef]
- Noguchi, S.; Arakawa, T.; Fukuda, S.; Furuno, M.; Hasegawa, A.; Hori, F.; Ishikawa-Kato, S.; Kaida, K.; Kaiho, A.; Kanamori-Katayama, M.; et al. FANTOM5 CAGE profiles of human and mouse samples. Sci. Data 2017, 4, 170112. [Google Scholar] [CrossRef] [PubMed]
- Consortium, G.T. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef]
- Kall, L.; Krogh, A.; Sonnhammer, E.L. Advantages of combined transmembrane topology and signal peptide prediction—the Phobius web server. Nucleic Acids Res. 2007, 35, W429–W432. [Google Scholar] [CrossRef]
- Viklund, H.; Bernsel, A.; Skwark, M.; Elofsson, A. SPOCTOPUS: A combined predictor of signal peptides and membrane protein topology. Bioinformatics 2008, 24, 2928–2929. [Google Scholar] [CrossRef] [PubMed]
- Nugent, T.; Ward, S.; Jones, D.T. The MEMPACK alpha-helical transmembrane protein structure prediction server. Bioinformatics 2011, 27, 1438–1439. [Google Scholar] [CrossRef] [PubMed]
- Ulintz, P.J.; Wu, W.; Gates, C.M. Bioinformatics Analysis of Whole Exome Sequencing Data. Methods Mol. Biol. 2019, 1881, 277–318. [Google Scholar] [CrossRef]
- Cabianca, D.S.; Casa, V.; Bodega, B.; Xynos, A.; Ginelli, E.; Tanaka, Y.; Gabellini, D. A long ncRNA links copy number variation to a polycomb/trithorax epigenetic switch in FSHD muscular dystrophy. Cell 2012, 149, 819–831. [Google Scholar] [CrossRef]
- Vyakhireva, J.V.; Filatova, A.Y.; Krivosheeva, I.A.; Skoblov, M.Y. siRNA-mediated gene silencing. Bull. RSMU 2017. [Google Scholar] [CrossRef][Green Version]
- Huang, Y.; Zhang, L. An In Vitro Single-Primer Site-Directed Mutagenesis Method for Use in Biotechnology. Methods Mol. Biol. 2017, 1498, 375–383. [Google Scholar] [CrossRef] [PubMed]
| Title | Sequence |
|---|---|
| Ex1f2 | 5′-TGGCGTACCCATGTATCGAA-3′ |
| Ex1r1 | 5′-AAAGCAGTGAGCCCAACACA-3′ |
| Prom-f1 | 5′-GACGCCCTCACCGGAAGT-3′ |
| Prom-f2 | 5′-GCGGCAGGGACCGCAGC-3′ |
| Ex2f1 | 5′-TACAGAAAAGATCCTCAACTAT-3′ |
| Ex2r1sh | 5′-TAAATGTTGAACCAAGTCCTG-3′ |
| Ex2r2l | 5′-TTGCATATTATTAGTGATTATGTT-3′ |
| Add-f1 | 5′-CGAGGCTGGTCTCAAACAC-3′ |
| Add-r1 | 5′-CTCCAACCCCAATAATGAAGG-3′ |
| Ex1f1 | 5′-CCCGCCTCTGCCGTGGG-3′ |
| F3 | 5′-ATAGCCGGACAATGGCGAAG-3′ |
| R3 | 5′-TGGGCGTTCAGAGAGTTCAC-3′ |
| Clon-F-HindIII | 5′-AAAAAAGCTTCGTGGGCCTGCGAATCGAG-3′ |
| Clon-R-XholI | 5′-AAAACTCGAGAACGCAGATAGTTTCCTTCAAG-3′ |
| LINC-ATGmut | 5′-CGAGGCACTCGCTGGCGTACCTTTGTATCGAAATGAGTTCACGG-3′ |
| HPRTf | 5′-TGTAATGACCAGTCAACAGGG-3′ |
| HPRTr | 5′-TGCGACCTTGACCATCTTTG-3′ |
| B2Mf | 5′-TCTTTCAGCAAGGACTGGTC-3′ |
| B2Mr | 5′-GGCATCTTCAAACCTCCATG-3′ |
| TBPf | 5′-CGGAGAGTTCTGGGATTGTAC-3′ |
| TBPr | 5′-GTGGTTCGTGGCTCTCTTAT-3′ |
| TFRCf | 5′-TCCTTGCATATTCTGGAATCCC-3′ |
| TFRCr | 5′-ATCACGAACTGACCAGCG-3′ |
| siLINC00493 | 5′-GGCGUACCCAUGUAUCGAAAUdTdT-3′ 3’-dTdTCCGCAUGGGUACAUAGCUUUA-5′ |
| siControl | 5′-AGGUAGUGUAAUCGCCUUGdTdT-3′ 3′-dTdTUCCAUCACAUUAGCGGAAC-5′ |
| FAM-control | 5′-FAM-AGGUCGAACUACGGGUCAAdTdC-3′ 3′-dGdAUCCAGCUUGAUGCCCAGUU-FAM-5′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konina, D.; Sparber, P.; Viakhireva, I.; Filatova, A.; Skoblov, M. Investigation of LINC00493/SMIM26 Gene Suggests Its Dual Functioning at mRNA and Protein Level. Int. J. Mol. Sci. 2021, 22, 8477. https://doi.org/10.3390/ijms22168477
Konina D, Sparber P, Viakhireva I, Filatova A, Skoblov M. Investigation of LINC00493/SMIM26 Gene Suggests Its Dual Functioning at mRNA and Protein Level. International Journal of Molecular Sciences. 2021; 22(16):8477. https://doi.org/10.3390/ijms22168477
Chicago/Turabian StyleKonina, Daria, Peter Sparber, Iuliia Viakhireva, Alexandra Filatova, and Mikhail Skoblov. 2021. "Investigation of LINC00493/SMIM26 Gene Suggests Its Dual Functioning at mRNA and Protein Level" International Journal of Molecular Sciences 22, no. 16: 8477. https://doi.org/10.3390/ijms22168477
APA StyleKonina, D., Sparber, P., Viakhireva, I., Filatova, A., & Skoblov, M. (2021). Investigation of LINC00493/SMIM26 Gene Suggests Its Dual Functioning at mRNA and Protein Level. International Journal of Molecular Sciences, 22(16), 8477. https://doi.org/10.3390/ijms22168477






