Improving Human Induced Pluripotent Stem Cell-Derived Megakaryocyte Differentiation and Platelet Production
Abstract
:1. Introduction
2. Results
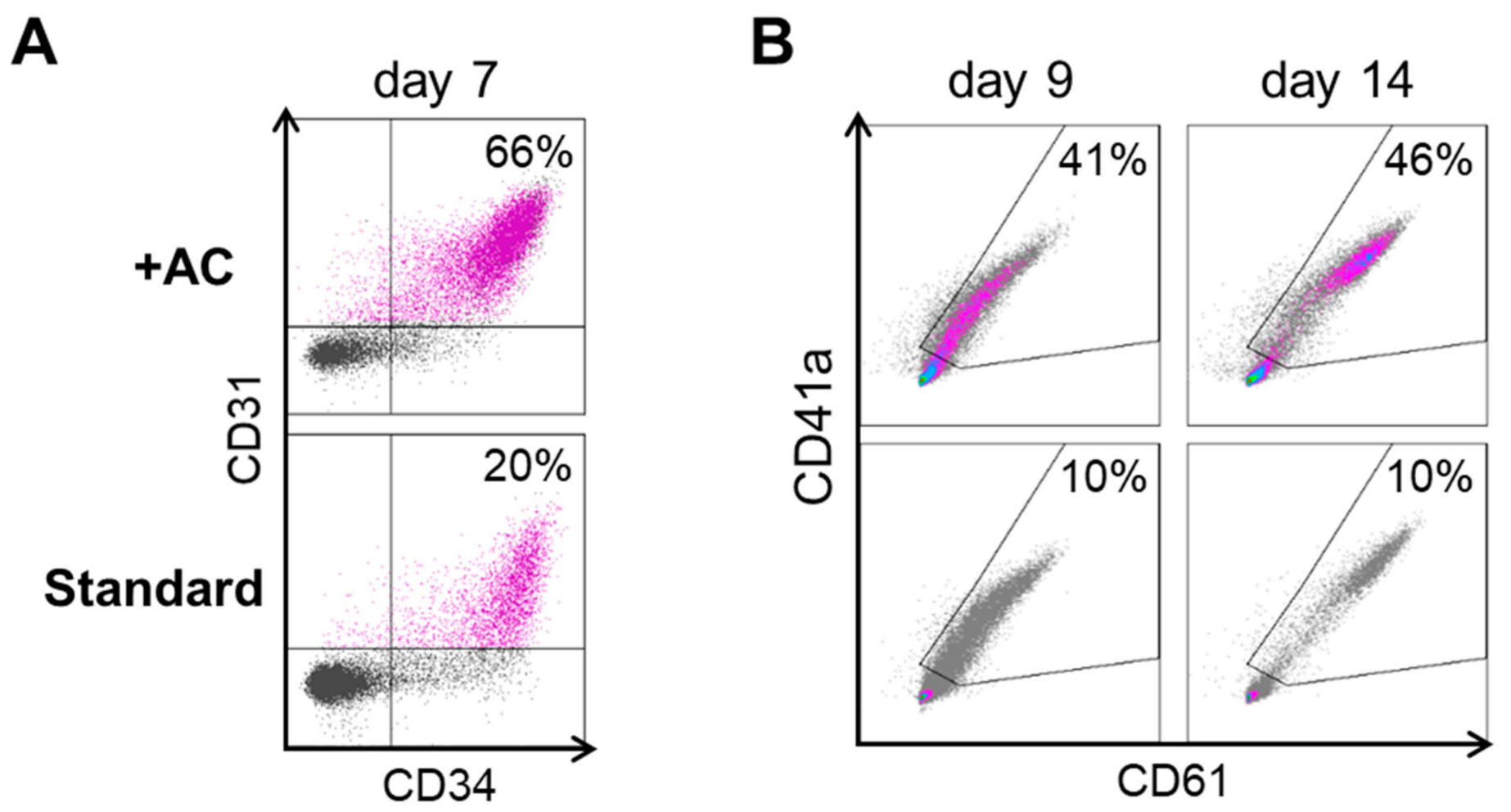
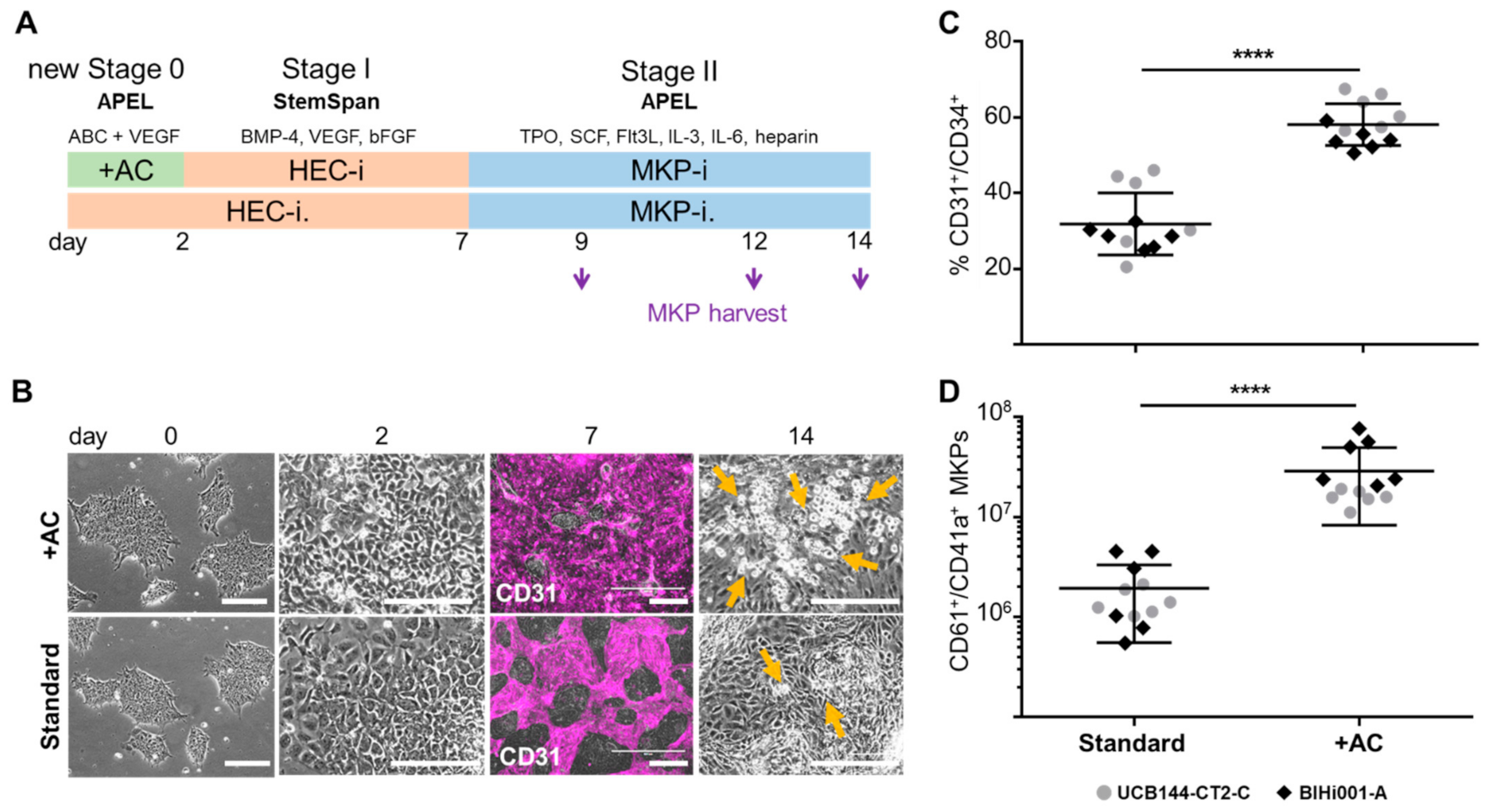
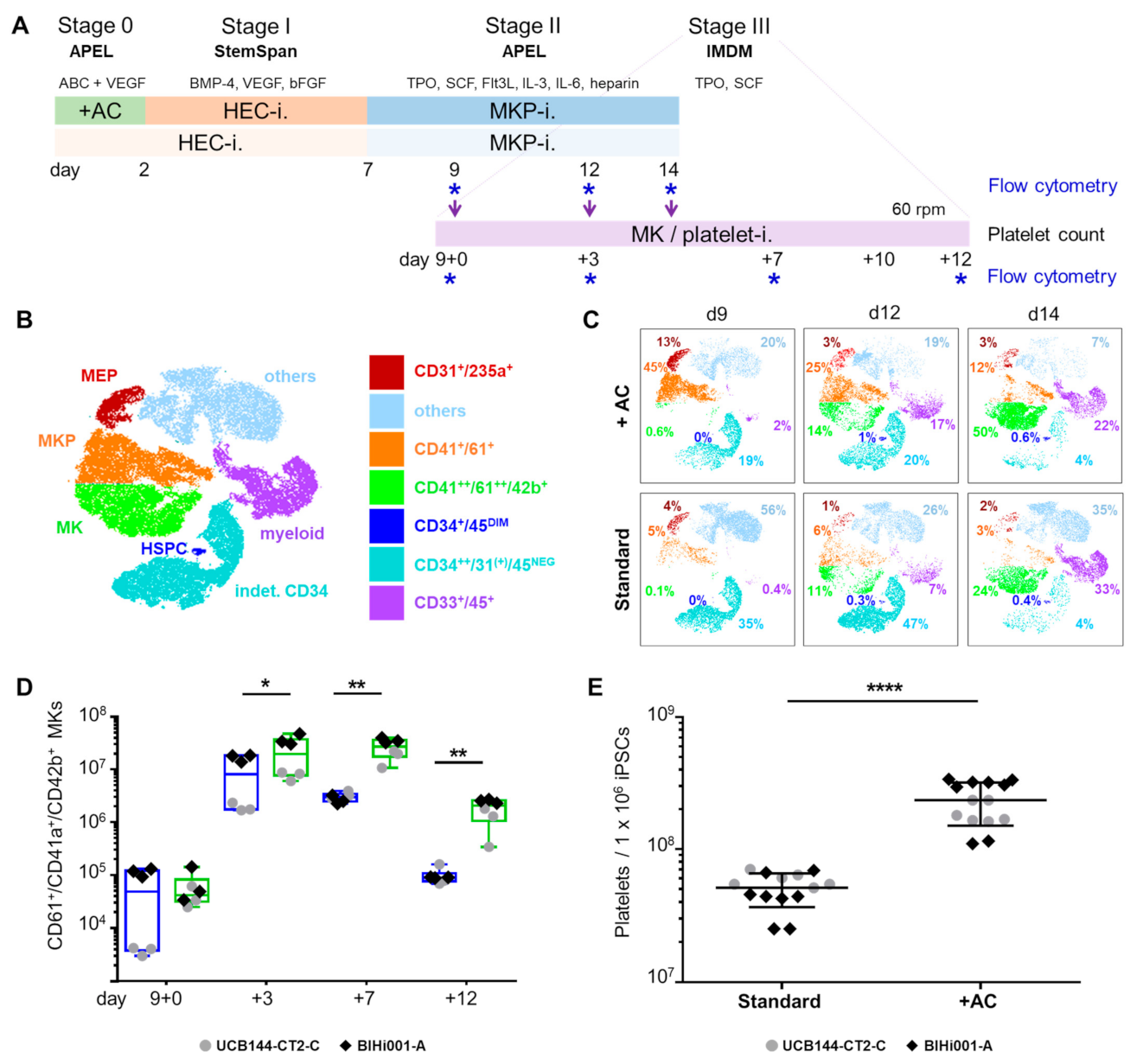
2.1. Improving HEC Differentiation and MK Progenitor (MKP) Production from hiPSCs
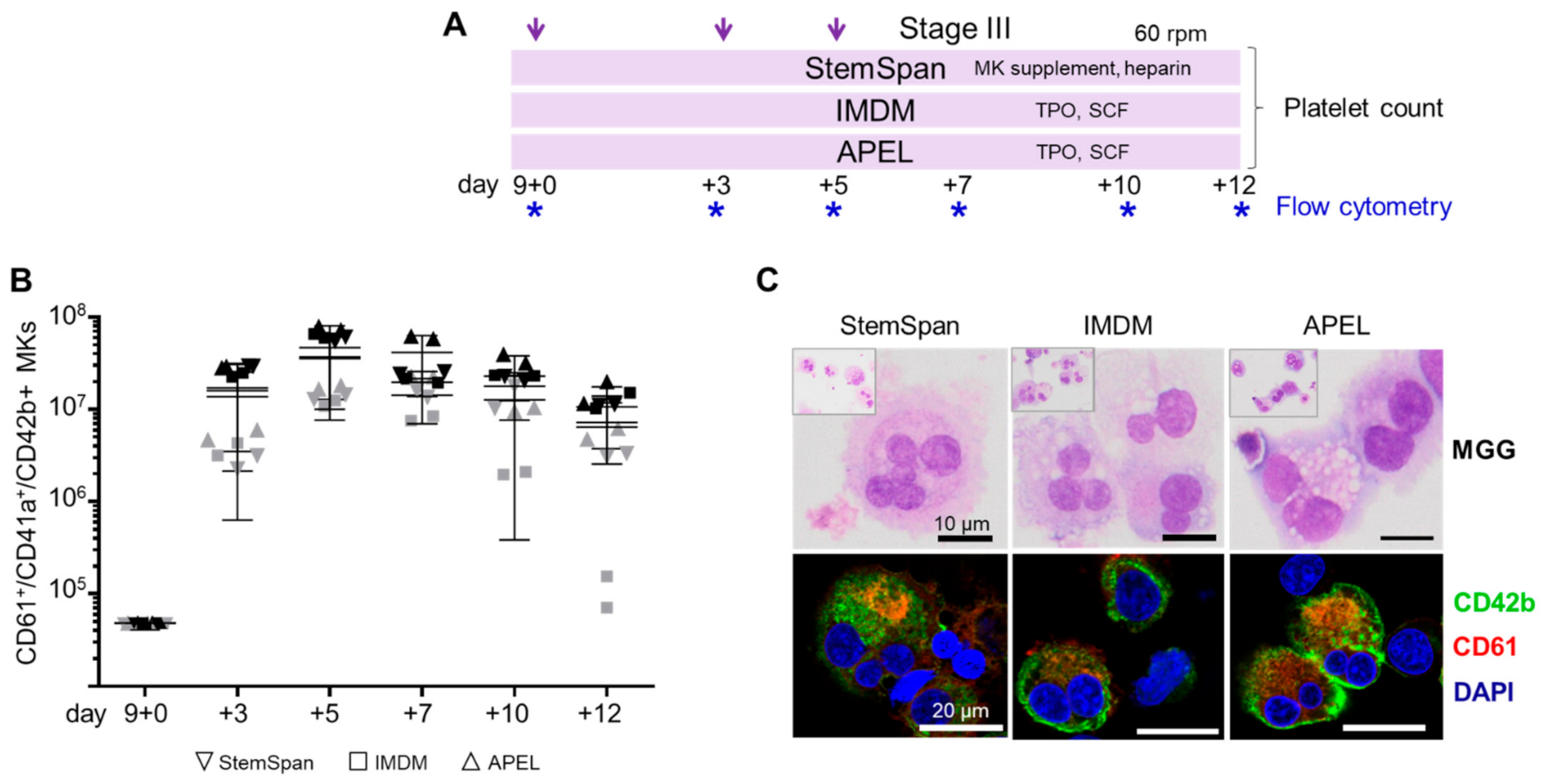
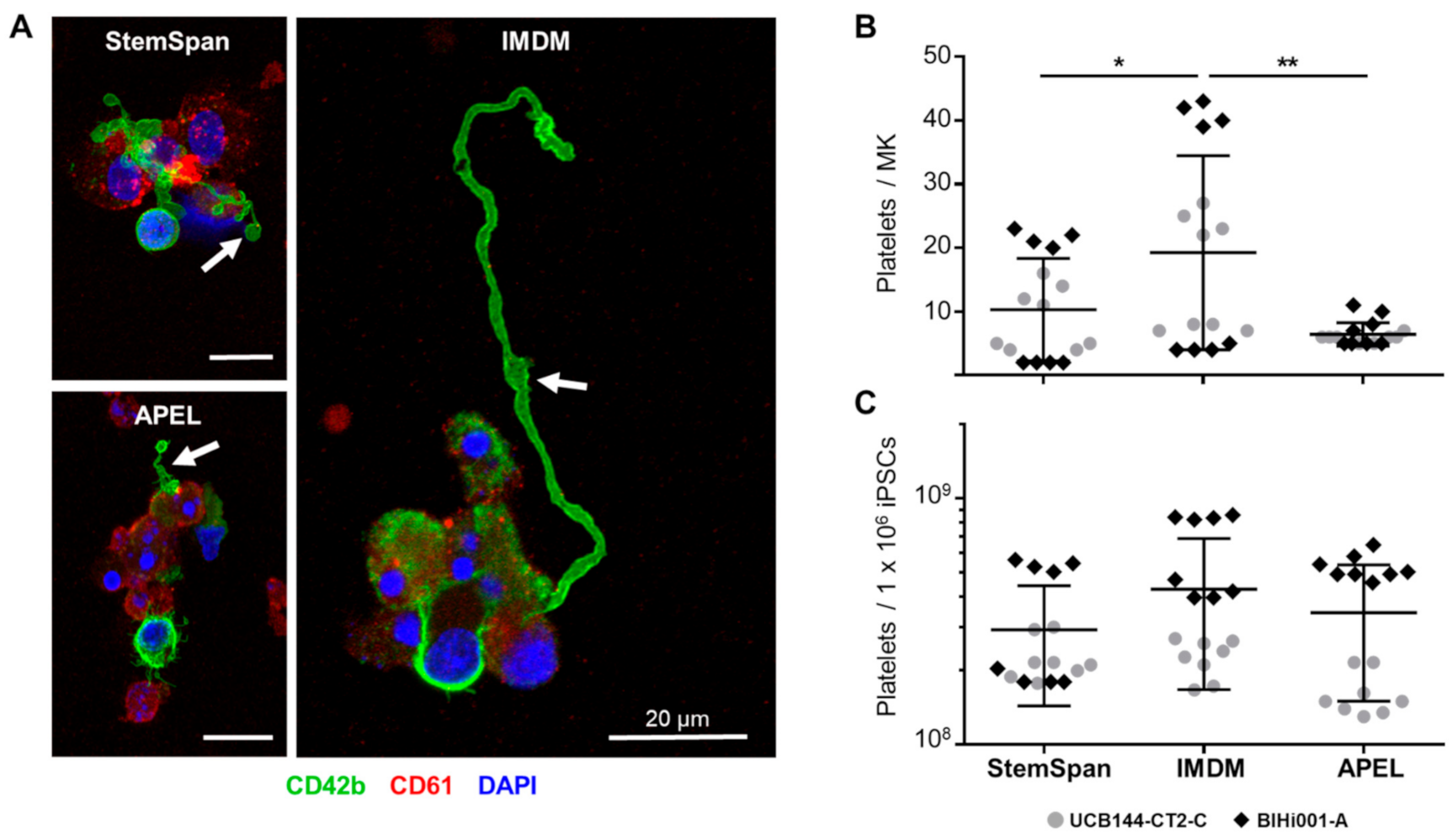
2.2. Expansion, Maturation and Platelet Production of hiPSC-MKs in Different Media Conditions
2.3. Validation That the Modified Protocol Improves Platelet Production from hiPSCs
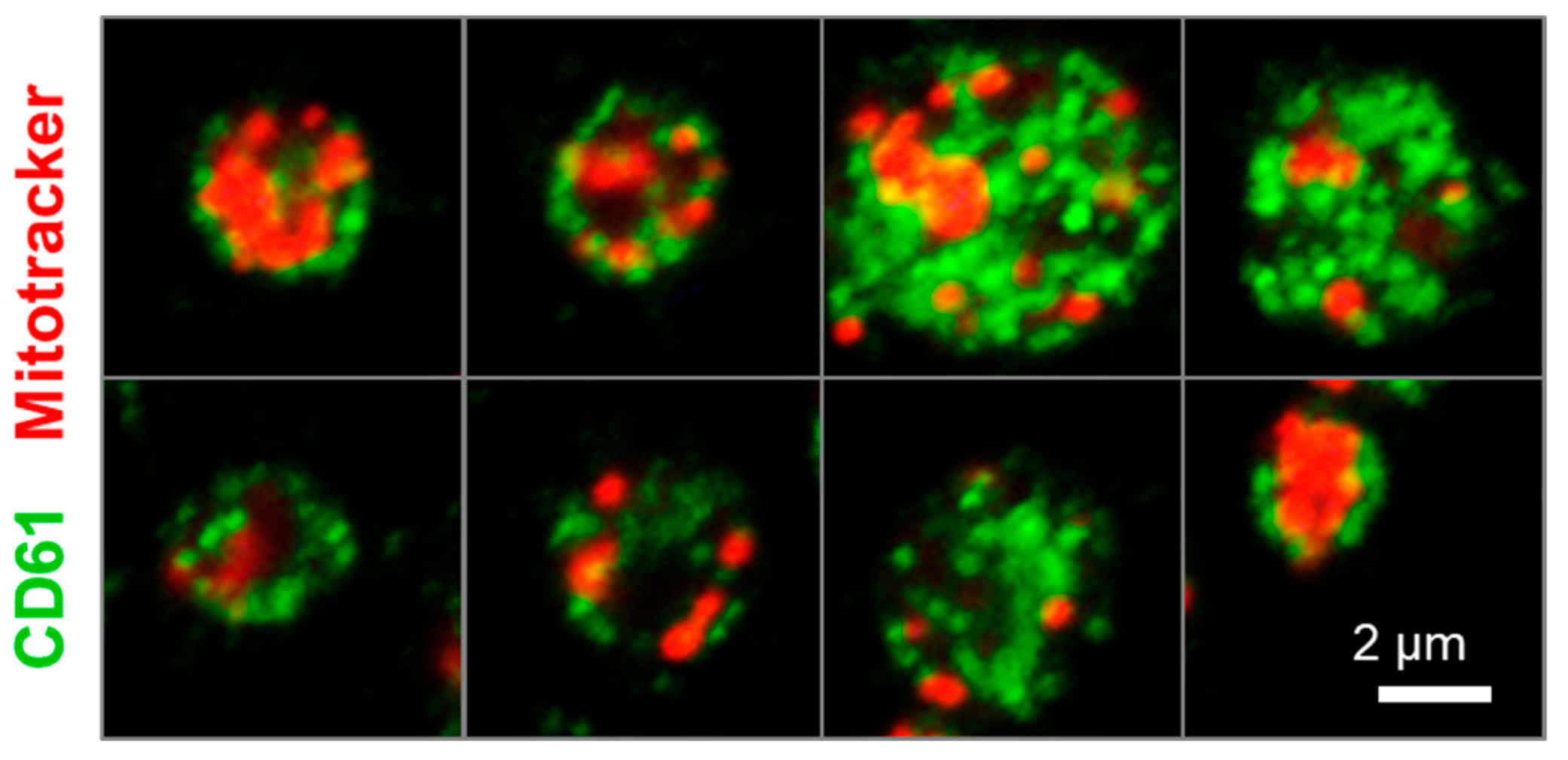
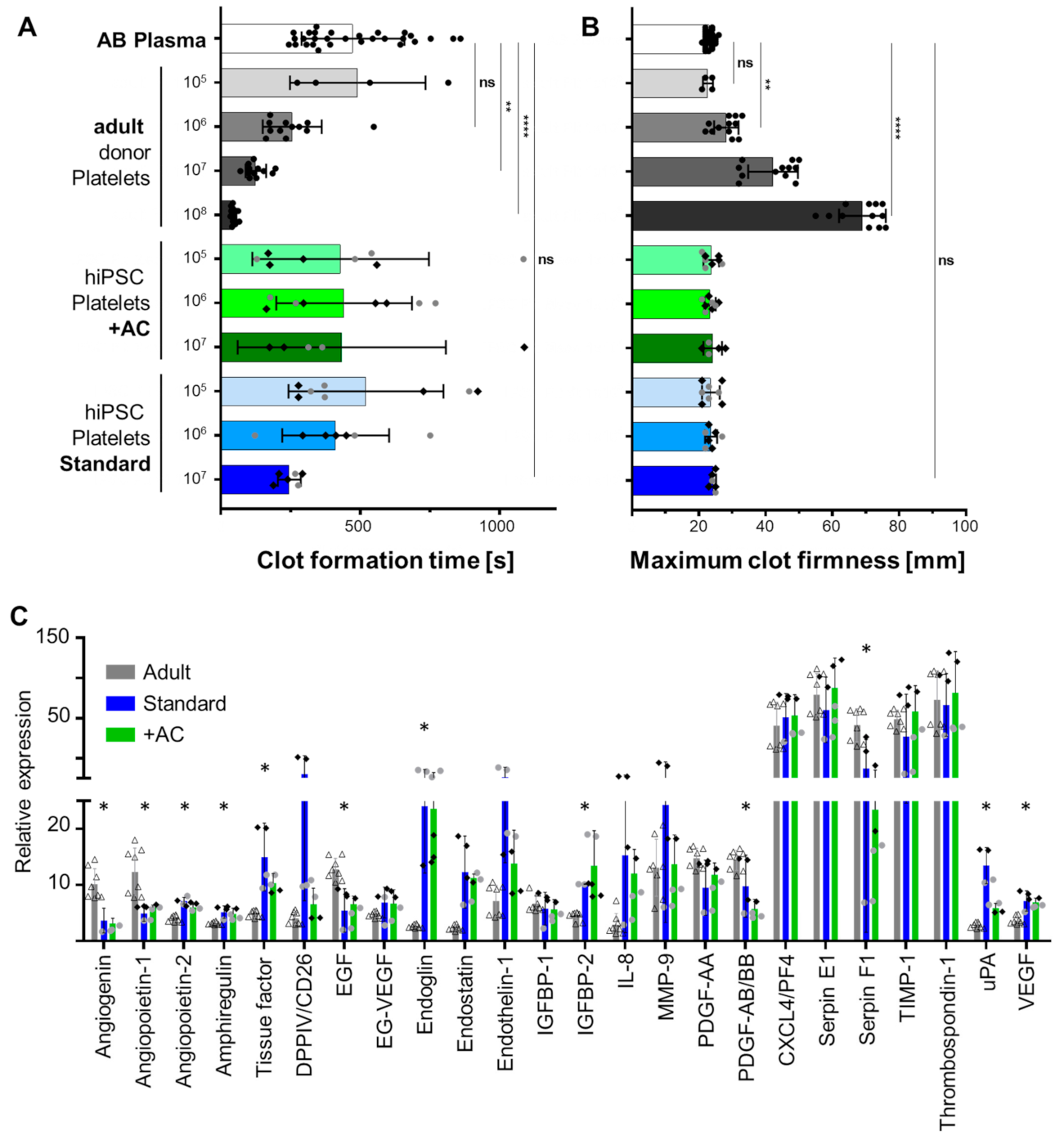
2.4. Human iPSC-Derived Platelets Are Functional and Contain Angiogenesis-Related Proteins
3. Discussion
4. Materials and Methods
4.1. Maintenance and Expansion of hiPSC Lines
4.2. Differentiation of hiPSCs into MKs and Platelets
4.3. Flow Cytometry
4.4. Harvesting and Quantification of hiPSC-derived MKPs, MKs and Platelets
4.5. Immunocytochemistry and May-Grünwald-Giemsa Staining
4.6. Image Acquisition
4.7. Proteome Profiler Array
4.8. Rotational Thromboelastometry
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Semple, J.W.; Italiano, J.E., Jr.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Nachman, R.L.; Rafii, S. Platelets, petechiae, and preservation of the vascular wall. N. Engl. J. Med. 2008, 359, 1261–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blood Safety and Availability. Available online: https://www.who.int/news-room/fact-sheets/detail/blood-safety-and-availability (accessed on 30 June 2021).
- Machlus, K.R.; Italiano, J.E., Jr. The incredible journey: From megakaryocyte development to platelet formation. J. Cell Biol. 2013, 201, 785–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greinacher, A.; Weitmann, K.; Schonborn, L.; Alpen, U.; Gloger, D.; Stangenberg, W.; Stupmann, K.; Greger, N.; Kiefel, V.; Hoffmann, W. A population-based longitudinal study on the implication of demographic changes on blood donation and transfusion demand. Blood Adv. 2017, 1, 867–874. [Google Scholar] [CrossRef] [Green Version]
- Sim, X.; Poncz, M.; Gadue, P.; French, D.L. Understanding platelet generation from megakaryocytes: Implications for in vitro-derived platelets. Blood 2016, 127, 1227–1233. [Google Scholar] [CrossRef] [Green Version]
- Harker, L.A. The kinetics of platelet production and destruction in man. Clin. Haematol. 1977, 6, 671–693. [Google Scholar] [CrossRef]
- Kaushansky, K. Determinants of platelet number and regulation of thrombopoiesis. Hematol. Am. Soc. Hematol. Educ. Program. 2009, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Strassel, C.; Gachet, C.; Lanza, F. On the Way to in vitro Platelet Production. Front. Med. 2018, 5, 239. [Google Scholar] [CrossRef] [Green Version]
- Takayama, N.; Eto, K. Pluripotent stem cells reveal the developmental biology of human megakaryocytes and provide a source of platelets for clinical application. Cell Mol. Life Sci. 2012, 69, 3419–3428. [Google Scholar] [CrossRef] [Green Version]
- Pang, L.; Weiss, M.J.; Poncz, M. Megakaryocyte biology and related disorders. J. Clin. Investig. 2005, 115, 3332–3338. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Shabrani, N.; Thon, J.N.; Huo, H.; Thiel, A.; Machlus, K.R.; Kim, K.; Brooks, J.; Li, F.; Luo, C.; et al. Scalable generation of universal platelets from human induced pluripotent stem cells. Stem Cell Rep. 2014, 3, 817–831. [Google Scholar] [CrossRef] [Green Version]
- Thon, J.N.; Mazutis, L.; Wu, S.; Sylman, J.L.; Ehrlicher, A.; Machlus, K.R.; Feng, Q.; Lu, S.; Lanza, R.; Neeves, K.B.; et al. Platelet bioreactor-on-a-chip. Blood 2014, 124, 1857–1867. [Google Scholar] [CrossRef] [Green Version]
- Borger, A.K.; Eicke, D.; Wolf, C.; Gras, C.; Aufderbeck, S.; Schulze, K.; Engels, L.; Eiz-Vesper, B.; Schambach, A.; Guzman, C.A.; et al. Generation of HLA-Universal iPSC-Derived Megakaryocytes and Platelets for Survival Under Refractoriness Conditions. Mol. Med. 2016, 22, 274–285. [Google Scholar] [CrossRef] [Green Version]
- Evans, A.L.; Dalby, A.; Foster, H.R.; Howard, D.; Waller, A.K.; Taimoor, M.; Lawrence, M.; Mookerjee, S.; Lehmann, M.; Burton, A.; et al. Transfer to the clinic: Refining forward programming of hPSCs to megakaryocytes for platelet production in bioreactors. Blood Adv. 2021, 5, 1977–1990. [Google Scholar] [CrossRef]
- Galat, Y.; Elcheva, I.; Dambaeva, S.; Katukurundage, D.; Beaman, K.; Iannaccone, P.M.; Galat, V. Application of small molecule CHIR99021 leads to the loss of hemangioblast progenitor and increased hematopoiesis of human pluripotent stem cells. Exp. Hematol. 2018, 65, 38–48. [Google Scholar] [CrossRef]
- Sturgeon, C.M.; Ditadi, A.; Awong, G.; Kennedy, M.; Keller, G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 554–561. [Google Scholar] [CrossRef] [Green Version]
- Creamer, J.P.; Dege, C.; Ren, Q.; Ho, J.T.K.; Valentine, M.C.; Druley, T.E.; Sturgeon, C.M. Human definitive hematopoietic specification from pluripotent stem cells is regulated by mesodermal expression of CDX4. Blood 2017, 129, 2988–2992. [Google Scholar] [CrossRef]
- Eicke, D.; Baigger, A.; Schulze, K.; Latham, S.L.; Halloin, C.; Zweigerdt, R.; Guzman, C.A.; Blasczyk, R.; Figueiredo, C. Large-scale production of megakaryocytes in microcarrier-supported stirred suspension bioreactors. Sci. Rep. 2018, 8, 10146. [Google Scholar] [CrossRef] [Green Version]
- Moreau, T.; Evans, A.L.; Vasquez, L.; Tijssen, M.R.; Yan, Y.; Trotter, M.W.; Howard, D.; Colzani, M.; Arumugam, M.; Wu, W.H.; et al. Large-scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming. Nat. Commun. 2016, 7, 11208. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Howard, D.; Waller, A.K.; Foster, H.R.; Mueller, A.; Moreau, T.; Evans, A.L.; Arumugam, M.; Bouet Chalon, G.; Vriend, E.; et al. Structurally graduated collagen scaffolds applied to the ex vivo generation of platelets from human pluripotent stem cell-derived megakaryocytes: Enhancing production and purity. Biomaterials 2018, 182, 135–144. [Google Scholar] [CrossRef]
- Stavish, D.; Boiers, C.; Price, C.; Frith, T.J.R.; Halliwell, J.; Saldana-Guerrero, I.; Wray, J.; Brown, J.; Carr, J.; James, C.; et al. Generation and trapping of a mesoderm biased state of human pluripotency. Nat. Commun. 2020, 11, 4989. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Nakamura, S.; Sugimoto, N.; Shigemori, T.; Kato, Y.; Ohno, M.; Sakuma, S.; Ito, K.; Kumon, H.; Hirose, H.; et al. Turbulence Activates Platelet Biogenesis to Enable Clinical Scale Ex Vivo Production. Cell 2018, 174, 636–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, N.; Eto, K. Platelet production from induced pluripotent stem cells. J. Thromb. Haemost. 2017, 15, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, N.; Eto, K. Generation and manipulation of human iPSC-derived platelets. Cell Mol. Life Sci 2021, 78, 3385–3401. [Google Scholar] [CrossRef]
- Burnouf, T.; Strunk, D.; Koh, M.B.; Schallmoser, K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef]
- Scharler, S.; Poupardin, R.; Peking, P.; Wolf, M.; Brachtl, G.; Daheron, L.; Schallmoser, K.; Jürchott, K.; Stachelscheid, H.; Volk, H.D.; et al. Extra-hematopoietic immunomodulatory role of the SCID-susceptibility gene DOCK-2 identified by stepwise maturation of human iPSCs into clonogenic mesodermal stromal progenitors. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ebner-Peking, P.; Krisch, L.; Wolf, M.; Hoog, A.; Vári, B.; Muigg, K.; Poupardin, R.; Scharler, C.; Russe, E.; Stachelscheid, H.; et al. Self-assembly of progenitor cells under the aegis of platelet factors facilitates human skin organoid formation and vascularized wound healing. Theranostics 2021, 11, 8430–8447. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Tan, Z.; Wang, C.; Liu, T.; Chen, L.; Yong, J.; Jiang, W.; Sun, X.; Du, L.; et al. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood 2008, 111, 1933–1941. [Google Scholar] [CrossRef] [Green Version]
- Navarro, F.; Gutman, D.; Meire, E.; Caceres, M.; Rigoutsos, I.; Bentwich, Z.; Lieberman, J. miR-34a contributes to megakaryocytic differentiation of K562 cells independently of p53. Blood 2009, 114, 2181–2192. [Google Scholar] [CrossRef] [Green Version]
- Benbarche, S.; Strassel, C.; Angenieux, C.; Mallo, L.; Freund, M.; Gachet, C.; Lanza, F.; de la Salle, H. Dual role of IL-21 in megakaryopoiesis and platelet homeostasis. Haematologica 2017, 102, 637–646. [Google Scholar] [CrossRef] [Green Version]
- Mookerjee, S.; Foster, H.R.; Waller, A.K.; Ghevaert, C.J. In vitro-derived platelets: The challenges we will have to face to assess quality and safety. Platelets 2020, 31, 724–730. [Google Scholar] [CrossRef]
- Oeller, M.; Laner-Plamberger, S.; Hochmann, S.; Ketterl, N.; Feichtner, M.; Brachtl, G.; Hochreiter, A.; Scharler, C.; Bieler, L.; Romanelli, P.; et al. Selection of Tissue Factor-Deficient Cell Transplants as a Novel Strategy for Improving Hemocompatibility of Human Bone Marrow Stromal Cells. Theranostics 2018, 8, 1421–1434. [Google Scholar] [CrossRef] [Green Version]
- Andrade, A.C.; Wolf, M.; Binder, H.M.; Gomes, F.G.; Manstein, F.; Ebner-Peking, P.; Poupardin, R.; Zweigerdt, R.; Schallmoser, K.; Strunk, D. Hypoxic Conditions Promote the Angiogenic Potential of Human Induced Pluripotent Stem Cell-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 3890. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, X.; Zi, G.; He, L.; Wang, S.; Chen, L.; Fan, Z.; Nan, X.; Xi, J.; Yue, W.; et al. Large-scale generation of megakaryocytes from human embryonic stem cells using transgene-free and stepwise defined suspension culture conditions. Cell Prolif. 2021, 54, e13002. [Google Scholar] [CrossRef]
- Thon, J.N.; Dykstra, B.J.; Beaulieu, L.M. Platelet bioreactor: Accelerated evolution of design and manufacture. Platelets 2017, 28, 472–477. [Google Scholar] [CrossRef]
- Pallotta, I.; Lovett, M.; Kaplan, D.L.; Balduini, A. Three-dimensional system for the in vitro study of megakaryocytes and functional platelet production using silk-based vascular tubes. Tissue Eng. Part. C Methods 2011, 17, 1223–1232. [Google Scholar] [CrossRef] [Green Version]
- Di Buduo, C.A.; Wray, L.S.; Tozzi, L.; Malara, A.; Chen, Y.; Ghezzi, C.E.; Smoot, D.; Sfara, C.; Antonelli, A.; Spedden, E.; et al. Programmable 3D silk bone marrow niche for platelet generation ex vivo and modeling of megakaryopoiesis pathologies. Blood 2015, 125, 2254–2264. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.V.T.; Witwer, K.W.; Verhaar, M.C.; Strunk, D.; van Balkom, B.W.M. Functional assays to assess the therapeutic potential of extracellular vesicles. J. Extracell Vesicles 2020, 10, e12033. [Google Scholar] [CrossRef]
- Guan, X.; Wang, L.; Wang, H.; Wang, H.; Dai, W.; Jiang, Y. Good Manufacturing Practice-Grade of Megakaryocytes Produced by a Novel Ex Vivo Culturing Platform. Clin. Transl. Sci. 2020, 13, 1115–1126. [Google Scholar] [CrossRef]
- Manstein, F.; Ullmann, K.; Kropp, C.; Halloin, C.; Triebert, W.; Franke, A.; Farr, C.M.; Sahabian, A.; Haase, A.; Breitkreuz, Y.; et al. High density bioprocessing of human pluripotent stem cells by metabolic control and in silico modeling. Stem Cells Transl. Med. 2021. [Google Scholar] [CrossRef]
- Whiting, D.; DiNardo, J.A. TEG and ROTEM: Technology and clinical applications. Am. J. Hematol. 2014, 89, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Margraf, A.; Nussbaum, C.; Sperandio, M. Ontogeny of platelet function. Blood Adv. 2019, 3, 692–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkelhorst, D.; Murphy, M.F.; Greinacher, A.; Shehata, N.; Bakchoul, T.; Massey, E.; Baker, J.; Lieberman, L.; Tanael, S.; Hume, H.; et al. Antenatal management in fetal and neonatal alloimmune thrombocytopenia: A systematic review. Blood 2017, 129, 1538–1547. [Google Scholar] [CrossRef] [Green Version]
- Sparger, K.A.; Assmann, S.F.; Granger, S.; Winston, A.; Christensen, R.D.; Widness, J.A.; Josephson, C.; Stowell, S.R.; Saxonhouse, M.; Sola-Visner, M. Platelet Transfusion Practices Among Very-Low-Birth-Weight Infants. JAMA Pediatr. 2016, 170, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Strunk, D.; Lozano, M.; Marks, D.C.; Loh, Y.S.; Gstraunthaler, G.; Schennach, H.; Rohde, E.; Laner-Plamberger, S.; Oller, M.; Nystedt, J.; et al. International Forum on GMP-grade human platelet lysate for cell propagation: Summary. Vox Sang. 2018, 113, 80–87. [Google Scholar] [CrossRef]
- Oeller, M.; Laner-Plamberger, S.; Krisch, L.; Rohde, E.; Strunk, D.; Schallmoser, K. Human Platelet Lysate for Good Manufacturing Practice-Compliant Cell Production. Int. J. Mol. Sci. 2021, 22, 5178. [Google Scholar] [CrossRef]
- Schallmoser, K.; Henschler, R.; Gabriel, C.; Koh, M.B.C.; Burnouf, T. Production and Quality Requirements of Human Platelet Lysate: A Position Statement from the Working Party on Cellular Therapies of the International Society of Blood Transfusion. Trends Biotechnol. 2020, 38, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Bieback, K.; Fernandez-Munoz, B.; Pati, S.; Schafer, R. Gaps in the knowledge of human platelet lysate as a cell culture supplement for cell therapy: A joint publication from the AABB and the International Society for Cell & Gene Therapy. Transfusion 2019, 59, 3448–3460. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, M.; Evans, A.; Moreau, T.; Bagnati, M.; Smart, M.; Hassan, E.; Hasan, J.; Pianella, M.; Kerby, J.; Ghevaert, C. Process analysis of pluripotent stem cell differentiation to megakaryocytes to make platelets applying European GMP. NPJ Regen Med. 2021, 6, 27. [Google Scholar] [CrossRef]
- Moradi, S.; Mahdizadeh, H.; Saric, T.; Kim, J.; Harati, J.; Shahsavarani, H.; Greber, B.; Moore, J.B.T. Research and therapy with induced pluripotent stem cells (iPSCs): Social, legal, and ethical considerations. Stem Cell Res. Ther. 2019, 10, 341. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, D.; Flahou, C.; Yoshikawa, N.; Stirblyte, I.; Hayashi, Y.; Sawaguchi, A.; Akasaka, M.; Nakamura, S.; Higashi, N.; Xu, H.; et al. iPSC-Derived Platelets Depleted of HLA Class I Are Inert to Anti-HLA Class I and Natural Killer Cell Immunity. Stem Cell Rep. 2020, 14, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, E.S.; Davis, R.; Stanley, E.G.; Elefanty, A.G. A protocol describing the use of a recombinant protein-based, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nat. Protoc. 2008, 3, 768–776. [Google Scholar] [CrossRef]
- Orlova, V.V.; van den Hil, F.E.; Petrus-Reurer, S.; Drabsch, Y.; Ten Dijke, P.; Mummery, C.L. Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells. Nat. Protoc. 2014, 9, 1514–1531. [Google Scholar] [CrossRef] [Green Version]
- Steevels, T.A.; Westerlaken, G.H.; Tijssen, M.R.; Coffer, P.J.; Lenting, P.J.; Akkerman, J.W.; Meyaard, L. Co-expression of the collagen receptors leukocyte-associated immunoglobulin-like receptor-1 and glycoprotein VI on a subset of megakaryoblasts. Haematologica 2010, 95, 2005–2012. [Google Scholar] [CrossRef]
| Stage/Differentiation | Basal Medium | Supplements | Reference |
|---|---|---|---|
| 0/incl. activin A + CHIR | StemDiff™ APEL2 | BMP-4 (30 ng/mL) | [54] |
| VEGF (50 ng/mL) | |||
| activin A (25 ng/mL) | |||
| CHIR99021 (1.5 µM) | |||
| I/HECs | StemSpan™ ACF | BMP-4 (30 ng/mL) | [12] |
| VEGF (50 ng/mL) | |||
| bFGF (50 ng/mL) | |||
| II/MKPs | StemDiff™ APEL2 | TPO (25 ng/mL) | [12] |
| SCF (25 ng/mL) | |||
| Flt3L (25 ng/mL) | |||
| IL-3 (10 ng/mL) | |||
| IL-6 (10 ng/mL) | |||
| heparin (5 U/mL) PFHM-II (5%) | |||
| III a/platelets | StemSpan™ ACF | StemSpan™ megakaryocyte expansion supplement (1x) | [12] |
| heparin (5 U/mL) | |||
| III b/platelets | IMDM | ITS+1 | [55] |
| 2-mercaptoethanol (50 µM) | |||
| SCF (50 ng/mL) | |||
| TPO (20–50 ng/mL) | |||
| III c/platelets | StemDiff™ APEL2 | PFHM-II (5%) | [14] |
| SCF (50 ng/mL) | |||
| TPO (50 ng/mL) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krisch, L.; Brachtl, G.; Hochmann, S.; Andrade, A.C.; Oeller, M.; Ebner-Peking, P.; Schallmoser, K.; Strunk, D. Improving Human Induced Pluripotent Stem Cell-Derived Megakaryocyte Differentiation and Platelet Production. Int. J. Mol. Sci. 2021, 22, 8224. https://doi.org/10.3390/ijms22158224
Krisch L, Brachtl G, Hochmann S, Andrade AC, Oeller M, Ebner-Peking P, Schallmoser K, Strunk D. Improving Human Induced Pluripotent Stem Cell-Derived Megakaryocyte Differentiation and Platelet Production. International Journal of Molecular Sciences. 2021; 22(15):8224. https://doi.org/10.3390/ijms22158224
Chicago/Turabian StyleKrisch, Linda, Gabriele Brachtl, Sarah Hochmann, André Cronemberger Andrade, Michaela Oeller, Patricia Ebner-Peking, Katharina Schallmoser, and Dirk Strunk. 2021. "Improving Human Induced Pluripotent Stem Cell-Derived Megakaryocyte Differentiation and Platelet Production" International Journal of Molecular Sciences 22, no. 15: 8224. https://doi.org/10.3390/ijms22158224
APA StyleKrisch, L., Brachtl, G., Hochmann, S., Andrade, A. C., Oeller, M., Ebner-Peking, P., Schallmoser, K., & Strunk, D. (2021). Improving Human Induced Pluripotent Stem Cell-Derived Megakaryocyte Differentiation and Platelet Production. International Journal of Molecular Sciences, 22(15), 8224. https://doi.org/10.3390/ijms22158224






