Caveolin-1 Expression in the Dorsal Striatum Drives Methamphetamine Addiction-Like Behavior
Abstract
1. Significance Statement
2. Introduction
3. Results
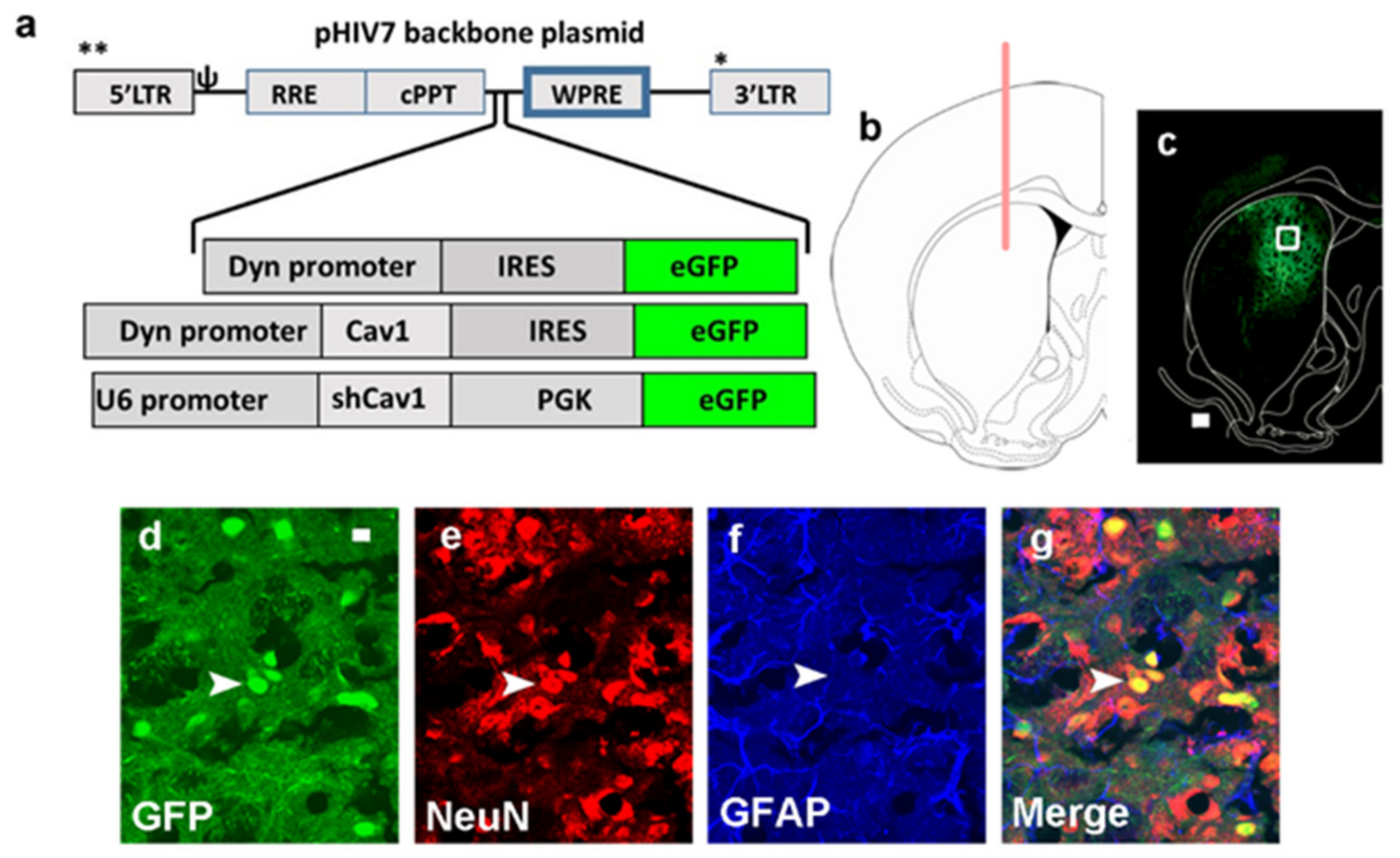
3.1. Green Fluorescent Protein (GFP) Expression Is Limited to Neurons and Is Not Observed in Astrocytes
3.2. Effect of LV-Cav1 and LV-shCav1 on Methamphetamine Self-Administration

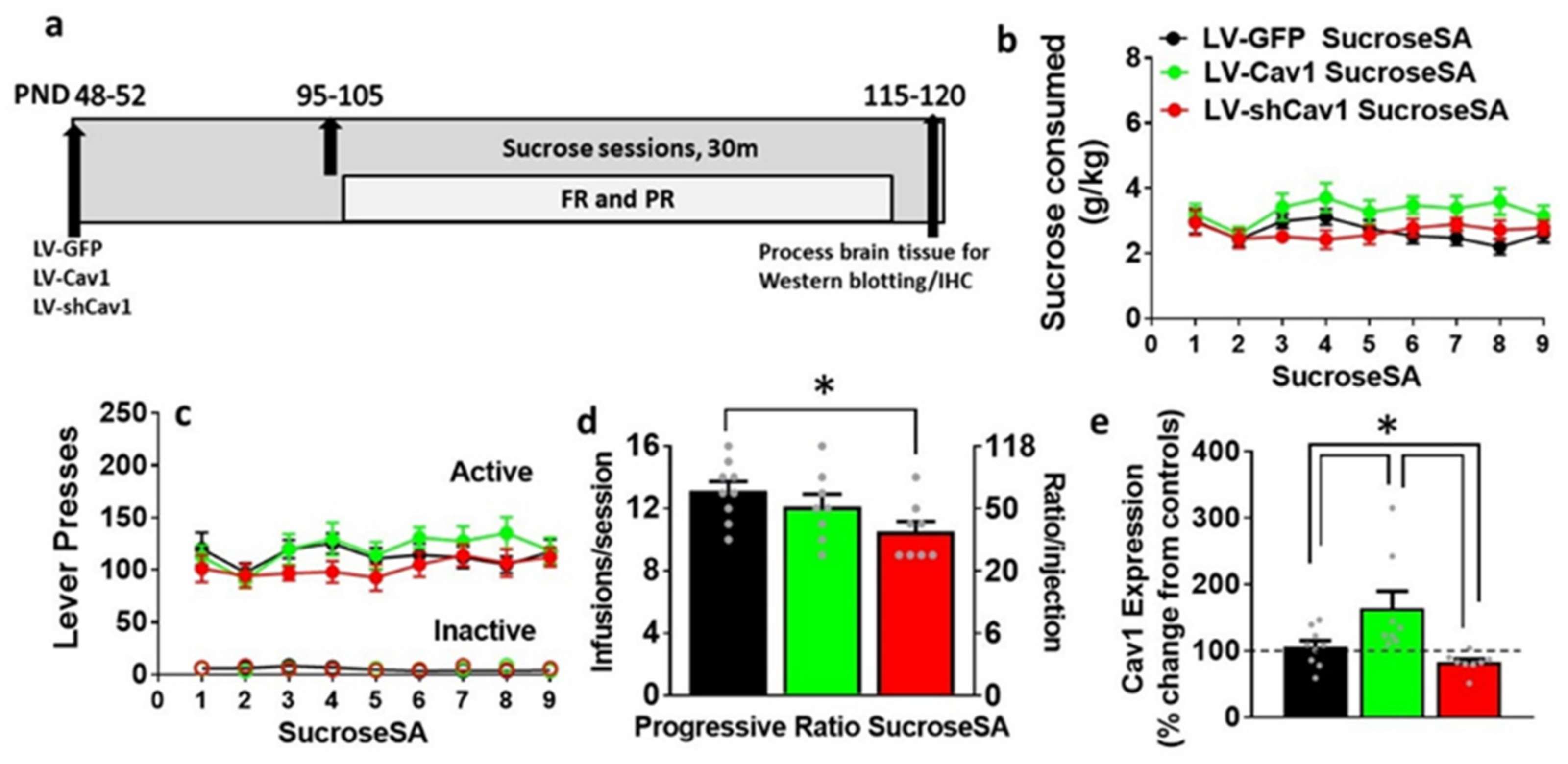
3.3. Effect of LV-Cav1 and LV-shCav1 on Sucrose Self-Administration
3.4. LV-Cav1 Enhances and LV-shCav1 Reduces Expression of Cav1 in Dorsal Striatum Tissue Homogenates
3.5. Basal Synaptic Transmission and Synaptic Plasticity Is Compromised after Acute Methamphetamine Treatment, but Restored in the Presence of SCH23390 and AM251 in Behavior, Virus and Drug Naive Rats
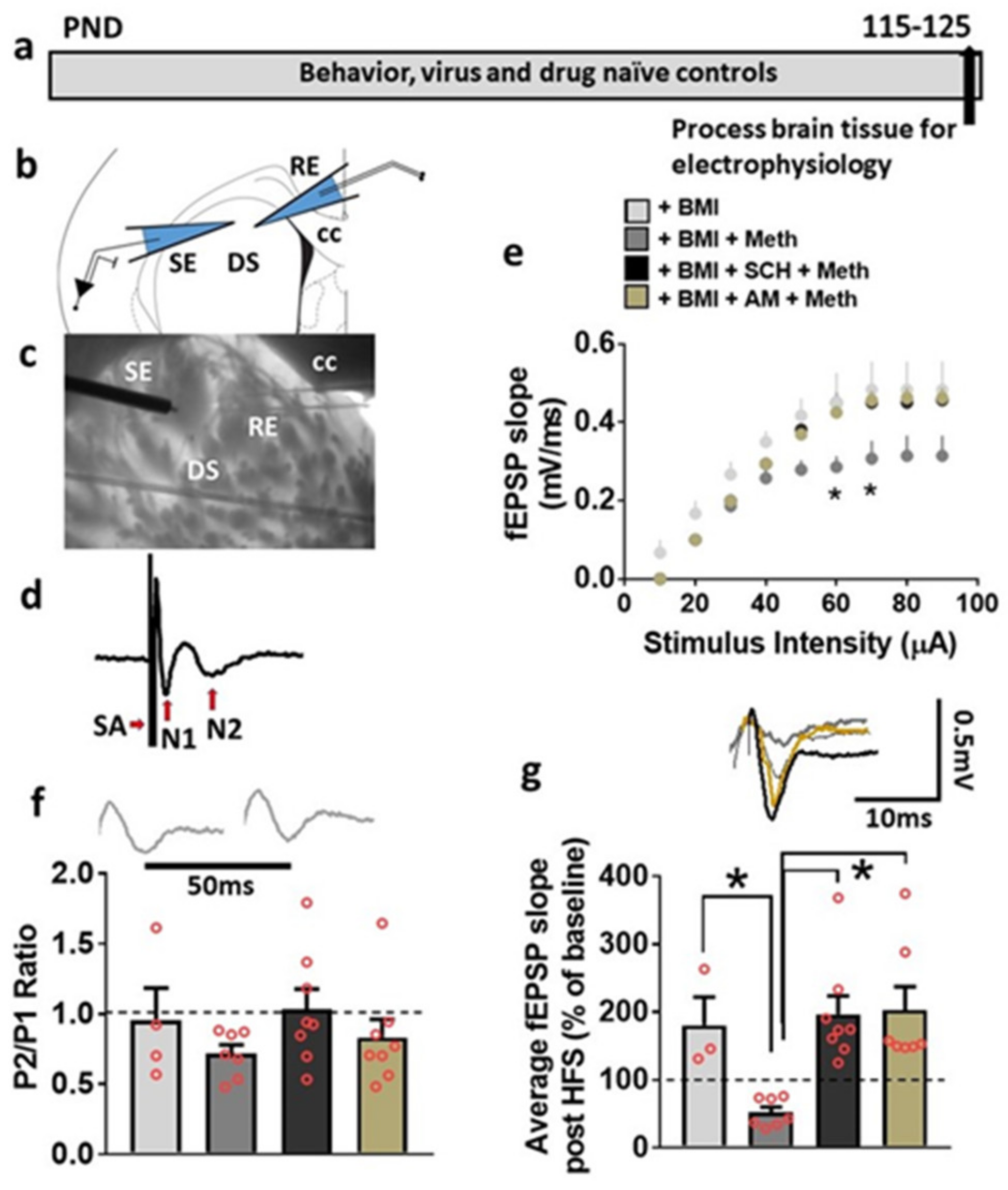
3.6. LV-GFP and LV-Cav1 Do Not Alter Synaptic Transmission and Plasticity; LV-shCav1 Produces In Vivo Deficits in Synaptic Plasticity without Altering Synaptic Transmission in Rats That Did Not Self-Administer Methamphetamine
3.7. HFS-Induced LTP Is Differentially Altered by Methamphetamine Self-Administration in LV-GFP vs. LV-Cav1 and LV-shCav1 Rats

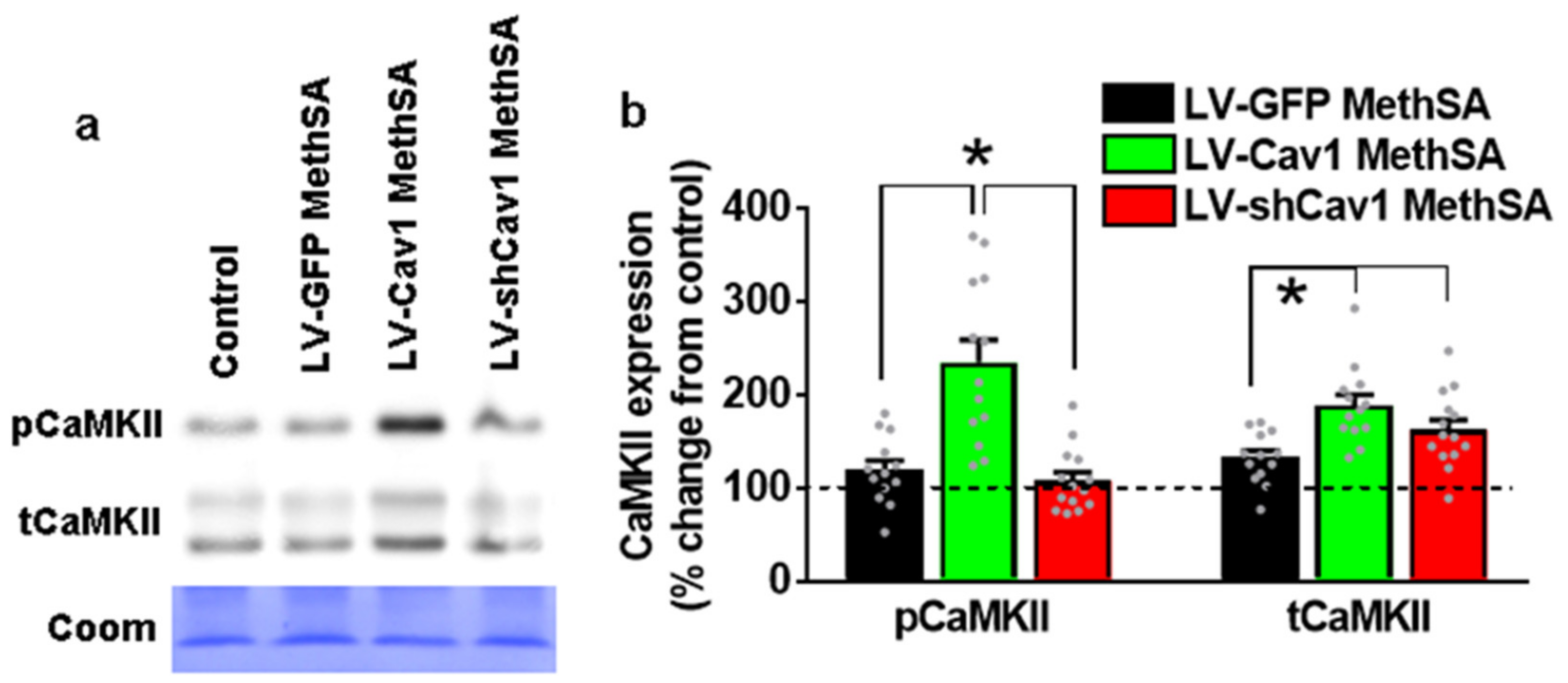
3.8. Methamphetamine Self-Administration in LV-Cav1 Enhances and in LV-shCav1 Does Not Alter Phosphorylation of CaMKII in Dorsal Striatum Tissue Homogenates Compared with LV-GFP Rats
4. Discussion
5. Methods
5.1. Animals
5.2. Viral Vector Construction, Surgery and Viral Gene Transfer
5.3. Intravenous Methamphetamine Self-Administration
5.4. Oral Sucrose Self-Administration
5.5. Brain Tissue Collection
5.6. Validation of Virus Infection
5.7. Western Blotting
5.8. Slice Preparation for Electrophysiology and Electrophysiological Analysis
5.9. Field Potential Recordings
5.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval
References
- Redish, A.D. Addiction as a computational process gone awry. Science 2004, 306, 1944–1947. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. Stress, corticotropin-releasing factor, and drug addiction. Ann. N. Y. Acad. Sci. 1999, 897, 27–45. [Google Scholar] [CrossRef]
- Koob, G.F. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 2009, 56 (Suppl. 1), 18–31. [Google Scholar] [CrossRef]
- Koob, G.F. Addiction is a Reward Deficit and Stress Surfeit Disorder. Front. Psychiatry 2013, 4, 72. [Google Scholar] [CrossRef]
- Vanderschuren, L.J.; Kalivas, P.W. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: A critical review of preclinical studies. Psychopharmacology 2000, 151, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Calabresi, P.; Maj, R.; Mercuri, N.B.; Bernardi, G. Coactivation of D1 and D2 dopamine receptors is required for long-term synaptic depression in the striatum. Neurosci. Lett. 1992, 142, 95–99. [Google Scholar] [CrossRef]
- Calabresi, P.; Maj, R.; Pisani, A.; Mercuri, N.B.; Bernardi, G. Long-term synaptic depression in the striatum: Physiological and pharmacological characterization. J. Neurosci. 1992, 12, 4224–4233. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Plotkin, J.; Shen, W. Dopamine and synaptic plasticity in dorsal striatal circuits controlling action selection. Curr. Opin. Neurobiol. 2009, 19, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Flajolet, M.; Greengard, P.; Surmeier, D.J. Dichotomous dopaminergic control of striatal synaptic plasticity. Science 2008, 321, 848–851. [Google Scholar] [CrossRef]
- Pawlak, V.; Kerr, J.N. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J. Neurosci. 2008, 28, 2435–2446. [Google Scholar] [CrossRef]
- Avchalumov, Y.; Mandyam, C.D. Synaptic Plasticity and its Modulation by Alcohol. Brain Plast. 2020, 6, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, S.; Nishi, M.; Sasaki, Y.; Takeshima, H.; Fukunaga, K. Aberrant behavioral sensitization by methamphetamine in junctophilin-deficient mice. Mol. Neurobiol. 2015, 51, 533–542. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Y.Y.; Shen, Y.; Cao, X.; Li, A.; Liu, Q.; Li, Z.; Zhang, L.B.; Dai, W.; Tan, T.; et al. Methamphetamine abuse impairs motor cortical plasticity and function. Mol. Psychiatry 2017, 22, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Avchalumov, Y.; Trenet, W.; Piña-Crespo, J.; Mandyam, C. SCH23390 Reduces Methamphetamine Self-Administration and Prevents Methamphetamine-Induced Striatal LTD. Int. J. Mol. Sci. 2020, 21, 6491. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.C. Metaplasticity: Tuning synapses and networks for plasticity. Nat. Rev. Neurosci. 2008, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Guerin, A.A.; Nestler, E.J.; Berk, M.; Lawrence, A.J.; Rossell, S.L.; Kim, J.H. Genetics of methamphetamine use disorder: A systematic review and meta-analyses of gene association studies. Neurosci. Biobehav. Rev. 2021, 120, 48–74. [Google Scholar] [CrossRef]
- Centonze, D.; Battista, N.; Rossi, S.; Mercuri, N.B.; Finazzi-Agrò, A.; Bernardi, G.; Calabresi, P.; Maccarrone, M. A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal gabaergic Transmission. Neuropsychopharmacology 2004, 29, 1488–1497. [Google Scholar] [CrossRef]
- Gerdeman, G.L.; Partridge, J.G.; Lupica, C.R.; Lovinger, D.M. It could be habit forming: Drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003, 26, 184–192. [Google Scholar] [CrossRef]
- Lipton, D.M.; Gonzales, B.J.; Citri, A. Dorsal Striatal Circuits for Habits, Compulsions and Addictions. Front. Syst. Neurosci. 2019, 13, 28. [Google Scholar] [CrossRef]
- Mathur, B.N.; Lovinger, D.M. Endocannabinoid-dopamine interactions in striatal synaptic plasticity. Front. Pharmacol. 2012, 3, 66. [Google Scholar] [CrossRef]
- Thomas, M.J.; Beurrier, C.; Bonci, A.; Malenka, R.C. Long-term depression in the nucleus accumbens: A neural correlate of behavioral sensitization to cocaine. Nat. Neurosci. 2001, 4, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Chiamulera, C.; Piva, A.; Abraham, W.C. Glutamate receptors and metaplasticity in addiction. Curr. Opin. Pharm. 2021, 56, 39–45. [Google Scholar] [CrossRef]
- Neuhofer, D.; Kalivas, P. Metaplasticity at the addicted tetrapartite synapse: A common denominator of drug induced adaptations and potential treatment target for addiction. Neurobiol. Learn. Mem. 2018, 154, 97–111. [Google Scholar] [CrossRef]
- Williams, T.M.; Lisanti, M.P. The Caveolin genes: From cell biology to medicine. Ann. Med. 2004, 36, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.A.; Halverson-Tamboli, R.A.; Rasenick, M.M. Lipid raft microdomains and neurotransmitter signalling. Nat. Rev. Neurosci. 2007, 8, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Stary, C.M.; Tsutsumi, Y.M.; Patel, P.M.; Head, B.P.; Patel, H.H.; Roth, D.M. Caveolins: Targeting pro-survival signaling in the heart and brain. Front. Physiol. 2012, 3, 393. [Google Scholar] [CrossRef] [PubMed]
- Egawa, J.; Zemljic-Harpf, A.; Mandyam, C.D.; Niesman, I.R.; Lysenko, L.V.; Kleschevnikov, A.M.; Roth, D.M.; Patel, H.H.; Patel, P.M.; Head, B.P. Neuron-Targeted Caveolin-1 Promotes Ultrastructural and Functional Hippocampal Synaptic Plasticity. Cereb. Cortex 2018, 28, 3255–3266. [Google Scholar] [CrossRef]
- Zhong, W.; Huang, Q.; Zeng, L.; Hu, Z.; Tang, X. Caveolin-1 and MLRs: A potential target for neuronal growth and neuroplasticity after ischemic stroke. Int. J. Med. Sci. 2019, 16, 1492–1503. [Google Scholar] [CrossRef] [PubMed]
- Mandyam, C.D.; Schilling, J.M.; Cui, W.; Egawa, J.; Niesman, I.R.; Kellerhals, S.E.; Staples, M.C.; Busija, A.R.; Risbrough, V.B.; Posadas, E.; et al. Neuron-Targeted Caveolin-1 Improves Molecular Signaling, Plasticity, and Behavior Dependent on the Hippocampus in Adult and Aged Mice. Biol. Psychiatry 2017, 81, 101–110. [Google Scholar] [CrossRef]
- Wang, S.; Leem, J.S.; Podvin, S.; Hook, V.; Kleschevnikov, N.; Savchenko, P.; Dhanani, M.; Zhou, K.; Kelly, I.C.; Zhang, T.; et al. Synapsin-caveolin-1 gene therapy preserves neuronal and synaptic morphology and prevents neurodegeneration in a mouse model of AD. Mol. Ther. Methods Clin. Dev. 2021, 21, 434–450. [Google Scholar] [CrossRef]
- Wang, S.; Wang, N.; Zheng, Y.; Zhang, J.; Zhang, F.; Wang, Z. Caveolin-1: An Oxidative Stress-Related Target for Cancer Prevention. Oxid. Med. Cell Longev. 2017, 2017, 7454031. [Google Scholar] [CrossRef] [PubMed]
- Jozic, I.; Sawaya, A.P.; Pastar, I.; Head, C.R.; Wong, L.L.; Glinos, G.D.; Wikramanayake, T.C.; Brem, H.; Kirsner, R.S.; Tomic-Canic, M. Pharmacological and Genetic Inhibition of Caveolin-1 Promotes Epithelialization and Wound Closure. Mol. Ther. 2019, 27, 1992–2004. [Google Scholar] [CrossRef] [PubMed]
- Kruglikov, I.L.; Scherer, P.E. Caveolin-1 as a target in prevention and treatment of hypertrophic scarring. NPJ Regen. Med. 2019, 4, 9. [Google Scholar] [CrossRef]
- Sáez, C.G.; Pereira-Flores, K.; Ebensperger, R.; Panes, O.; Massardo, T.; Hidalgo, P.; Mezzano, D.; Pereira, J. Atorvastatin reduces the proadhesive and prothrombotic endothelial cell phenotype induced by cocaine and plasma from cocaine consumers in vitro. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2439–2448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cui, W.; Ren, Y.; Wang, S.; Zeng, M.; Han, S.; Li, J.; Han, R. The role of caveolin-1 in morphine-induced structural plasticity in primary cultured mouse cerebral cortical neurons. Neurosci. Lett. 2018, 665, 38–42. [Google Scholar] [CrossRef]
- Ujcikova, H.; Hlouskova, M.; Cechova, K.; Stolarova, K.; Roubalova, L.; Svoboda, P. Determination of μ-, δ- and κ-opioid receptors in forebrain cortex of rats exposed to morphine for 10 days: Comparison with animals after 20 days of morphine withdrawal. PLoS ONE 2017, 12, e0186797. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Chang, A.; Liu, N.J.; Gintzler, A.R. Chronic opioid treatment augments caveolin-1 scaffolding: Relevance to stimulatory μ-opioid receptor adenylyl cyclase signaling. J. Neurochem. 2016, 139, 737–747. [Google Scholar] [CrossRef]
- Eisinger, K.R.T.; Chapp, A.D.; Swanson, S.P.; Tam, D.; Lopresti, N.M.; Larson, E.B.; Thomas, M.J.; Lanier, L.M.; Mermelstein, P.G. Caveolin-1 regulates medium spiny neuron structural and functional plasticity. Psychopharmacology 2020, 237, 2673–2684. [Google Scholar] [CrossRef] [PubMed]
- Somkuwar, S.S.; Fannon, M.J.; Head, B.P.; Mandyam, C.D. Methamphetamine reduces expression of caveolin-1 in the dorsal striatum: Implication for dysregulation of neuronal function. Neuroscience 2016, 328, 147–156. [Google Scholar] [CrossRef][Green Version]
- Kreisler, A.D.; Terranova, M.J.; Somkuwar, S.S.; Purohit, D.C.; Wang, S.; Head, B.P.; Mandyam, C.D. In vivo reduction of striatal D1R by RNA interference alters expression of D1R signaling-related proteins and enhances methamphetamine addiction in male rats. Brain Struct. Funct. 2020, 225, 1073–1088. [Google Scholar] [CrossRef]
- Kong, M.M.; Hasbi, A.; Mattocks, M.; Fan, T.; O’Dowd, B.F.; George, S.R. Regulation of D1 dopamine receptor trafficking and signaling by caveolin-1. Mol. Pharm. 2007, 72, 1157–1170. [Google Scholar] [CrossRef]
- Voulalas, P.J.; Schetz, J.; Undieh, A.S. Differential subcellular distribution of rat brain dopamine receptors and subtype-specific redistribution induced by cocaine. Mol. Cell Neurosci. 2011, 46, 645–654. [Google Scholar] [CrossRef]
- Bari, M.; Oddi, S.; De Simone, C.; Spagnolo, P.; Gasperi, V.; Battista, N.; Centonze, D.; Maccarrone, M. Type-1 cannabinoid receptors colocalize with caveolin-1 in neuronal cells. Neuropharmacology 2008, 54, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, O.; Wee, S.; Specio, S.E.; Koob, G.F.; Pulvirenti, L. Escalation of methamphetamine self-administration in rats: A dose-effect function. Psychopharmacology 2006, 186, 48–53. [Google Scholar] [CrossRef]
- Du, F.; Saitoh, F.; Tian, Q.B.; Miyazawa, S.; Endo, S.; Suzuki, T. Mechanisms for association of Ca2+/calmodulin-dependent protein kinase II with lipid rafts. Biochem. Biophys. Res. Commun. 2006, 347, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Du, F.; Tian, Q.B.; Zhang, J.; Endo, S. Ca2+/calmodulin-dependent protein kinase IIalpha clusters are associated with stable lipid rafts and their formation traps PSD-95. J. Neurochem. 2008, 104, 596–610. [Google Scholar] [PubMed]
- Swant, J.; Chirwa, S.; Stanwood, G.; Khoshbouei, H. Methamphetamine reduces LTP and increases baseline synaptic transmission in the CA1 region of mouse hippocampus. PLoS ONE 2010, 5, e11382. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wei, X.; Xu, X.; Yu, G.; Yong, Z.; Su, R.; Tao, L. Methamphetamine Inhibits Long-Term Memory Acquisition and Synaptic Plasticity by Evoking Endoplasmic Reticulum Stress. Front. Neurosci. 2020, 14, 630713. [Google Scholar] [CrossRef] [PubMed]
- Heysieattalab, S.; Naghdi, N.; Hosseinmardi, N.; Zarrindast, M.R.; Haghparast, A.; Khoshbouei, H. Methamphetamine-induced enhancement of hippocampal long-term potentiation is modulated by NMDA and GABA receptors in the shell-accumbens. Synapse 2016, 70, 325–335. [Google Scholar] [CrossRef]
- Ishikawa, A.; Kadota, T.; Kadota, K.; Matsumura, H.; Nakamura, S. Essential role of D1 but not D2 receptors in methamphetamine-induced impairment of long-term potentiation in hippocampal-prefrontal cortex pathway. Eur. J. Neurosci. 2005, 22, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Nishioku, T.; Shimazoe, T.; Yamamoto, Y.; Nakanishi, H.; Watanabe, S. Expression of long-term potentiation of the striatum in methamphetamine-sensitized rats. Neurosci. Lett. 1999, 268, 81–84. [Google Scholar] [CrossRef]
- Shahidi, S.; Komaki, A.; Sadeghian, R.; Asl, S.S. Different doses of methamphetamine alter long-term potentiation, level of BDNF and neuronal apoptosis in the hippocampus of reinstated rats. J. Physiol. Sci. 2019, 69, 409–419. [Google Scholar] [CrossRef]
- Jog, M.S.; Kubota, Y.; Connolly, C.I.; Hillegaart, V.; Graybiel, A.M. Building neural representations of habits. Science 1999, 286, 1745–1749. [Google Scholar] [CrossRef]
- Keramati, M.; Durand, A.; Girardeau, P.; Gutkin, B.; Ahmed, S.H. Cocaine addiction as a homeostatic reinforcement learning disorder. Psychol. Rev. 2017, 124, 130–153. [Google Scholar] [CrossRef] [PubMed]
- Yager, L.M.; Garcia, A.F.; Wunsch, A.M.; Ferguson, S.M. The ins and outs of the striatum: Role in drug addiction. Neuroscience 2015, 301, 529–541. [Google Scholar] [CrossRef]
- Calabresi, P.; Gubellini, P.; Centonze, D.; Picconi, B.; Bernardi, G.; Chergui, K.; Svenningsson, P.; Fienberg, A.A.; Greengard, P. Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J. Neurosci. 2000, 20, 8443–8451. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.H.; Ostlund, S.B.; Knowlton, B.J.; Balleine, B.W. The role of the dorsomedial striatum in instrumental conditioning. Eur. J. Neurosci. 2005, 22, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Belin, D.; Everitt, B.J. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron 2008, 57, 432–441. [Google Scholar] [CrossRef]
- Everitt, B.J.; Robbins, T.W. From the ventral to the dorsal striatum: Devolving views of their roles in drug addiction. Neurosci. Biobehav. Rev. 2013, 37, 1946–1954. [Google Scholar] [CrossRef]
- Balleine, B.W.; Liljeholm, M.; Ostlund, S.B. The integrative function of the basal ganglia in instrumental conditioning. Behav. Brain Res. 2009, 199, 43–52. [Google Scholar] [CrossRef]
- Brennan, K.A.; Carati, C.; Lea, R.A.; Fitzmaurice, P.S.; Schenk, S. Effect of D1-like and D2-like receptor antagonists on methamphetamine and 3,4-methylenedioxymethamphetamine self-administration in rats. Behav. Pharm. 2009, 20, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Carati, C.; Schenk, S. Role of dopamine D1- and D2-like receptor mechanisms in drug-seeking following methamphetamine self-administration in rats. Pharm. Biochem. Behav. 2011, 98, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Bardo, M.T.; Valone, J.M.; Bevins, R.A. Locomotion and conditioned place preference produced by acute intravenous amphetamine: Role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacology 1999, 143, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, H.; Yamada, K.; Mizuno, M.; Mizuno, T.; Nitta, A.; Noda, Y.; Nabeshima, T. Regulations of methamphetamine reward by extracellular signal-regulated kinase 1/2/ets-like gene-1 signaling pathway via the activation of dopamine receptors. Mol. Pharm. 2004, 65, 1293–1301. [Google Scholar] [CrossRef]
- Gu, S.M.; Cha, H.J.; Seo, S.W.; Hong, J.T.; Yun, J. Dopamine D1 receptor antagonist reduces stimulant-induced conditioned place preferences and dopamine receptor supersensitivity. Naunyn Schmiedebergs Arch. Pharm. 2020, 393, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.D.; Aarde, S.M.; Cole, M.; Vandewater, S.A.; Grant, Y.; Taffe, M.A. Locomotor Stimulant and Rewarding Effects of Inhaling Methamphetamine, MDPV, and Mephedrone via Electronic Cigarette-Type Technology. Neuropsychopharmacology 2016, 41, 2759–2771. [Google Scholar] [CrossRef]
- Cheng, R.K.; Liao, R.M. Examination of the effects of SCH23390 and raclopride infused in the dorsal striatum on amphetamine-induced timing impulsivity measured on a differential reinforcement of low-rate responding (DRL) task in rats. Behav. Brain Res. 2020, 379, 112364. [Google Scholar] [CrossRef] [PubMed]
- Koshikawa, N.; Mori, E.; Oka, K.; Nomura, H.; Yatsushige, N.; Maruyama, Y. Effects of SCH23390 injection into the dorsal striatum and nucleus accumbens on methamphetamine-induced gnawing and hyperlocomotion in rats. J. Nihon Univ. Sch. Dent. 1989, 31, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Gross, N.B.; Duncker, P.C.; Marshall, J.F. Striatal dopamine D1 and D2 receptors: Widespread influences on methamphetamine-induced dopamine and serotonin neurotoxicity. Synapse 2011, 65, 1144–1155. [Google Scholar] [CrossRef]
- Gerdeman, G.; Lovinger, D.M. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J. Neurophysiol. 2001, 85, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Anggadiredja, K.; Nakamichi, M.; Hiranita, T.; Tanaka, H.; Shoyama, Y.; Watanabe, S.; Yamamoto, T. Endocannabinoid system modulates relapse to methamphetamine seeking: Possible mediation by the arachidonic acid cascade. Neuropsychopharmacology 2004, 29, 1470–1478. [Google Scholar] [CrossRef]
- Jing, L.; Qiu, Y.; Zhang, Y.; Li, J.X. Effects of the cannabinoid CB1 receptor allosteric modulator ORG 27569 on reinstatement of cocaine- and methamphetamine-seeking behavior in rats. Drug Alcohol Depend. 2014, 143, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Landa, L.; Sulcova, A.; Slais, K. Involvement of cannabinoid CB1 and CB2 receptor activity in the development of behavioural sensitization to methamphetamine effects in mice. Neuro Endocrinol. Lett. 2006, 27, 63–69. [Google Scholar]
- Nawata, Y.; Yamaguchi, T.; Fukumori, R.; Yamamoto, T. Inhibition of Monoacylglycerol Lipase Reduces the Reinstatement of Methamphetamine-Seeking and Anxiety-Like Behaviors in Methamphetamine Self-Administered Rats. Int. J. Neuropsychopharmacol. 2019, 22, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.S.; Boctor, S.Y.; Flores, L.C.; Phelix, C.F.; Martinez, J.L., Jr. Local pretreatment with the cannabinoid CB1 receptor antagonist AM251 attenuates methamphetamine intra-accumbens self-administration. Neurosci. Lett. 2011, 489, 187–191. [Google Scholar] [CrossRef]
- Yu, L.L.; Wang, X.Y.; Zhao, M.; Liu, Y.; Li, Y.Q.; Li, F.Q.; Wang, X.; Xue, Y.X.; Lu, L. Effects of cannabinoid CB1 receptor antagonist rimonabant in consolidation and reconsolidation of methamphetamine reward memory in mice. Psychopharmacology 2009, 204, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Zhou, S.J.; Wang, X.Y.; Liu, J.F.; Xue, Y.X.; Jiang, W.; Lu, L. Effects of cannabinoid CB1 receptor antagonist rimonabant on acquisition and reinstatement of psychostimulant reward memory in mice. Behav. Brain Res. 2011, 217, 111–116. [Google Scholar] [CrossRef]
- Steinkellner, T.; Mus, L.; Eisenrauch, B.; Constantinescu, A.; Leo, D.; Konrad, L.; Rickhag, M.; Sorensen, G.; Efimova, E.V.; Kong, E.; et al. In vivo amphetamine action is contingent on alphaCaMKII. Neuropsychopharmacology 2014, 39, 2681–2693. [Google Scholar] [CrossRef]
- Giese, K.P.; Fedorov, N.B.; Filipkowski, R.K.; Silva, A.J. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science 1998, 279, 870–873. [Google Scholar] [CrossRef]
- Partridge, J.G.; Tang, K.C.; Lovinger, D.M. Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J. Neurophysiol. 2000, 84, 1422–1429. [Google Scholar] [CrossRef]
- Devan, B.D.; White, N.M. Parallel information processing in the dorsal striatum: Relation to hippocampal function. J. Neurosci. 1999, 19, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- White, M.; Whittaker, R.; Gándara, C.; Stoll, E.A. A Guide to Approaching Regulatory Considerations for Lentiviral-Mediated Gene Therapies. Hum. Gene Ther. Methods 2017, 28, 163–176. [Google Scholar] [CrossRef]
- Pardo-Garcia, T.R.; Garcia-Keller, C.; Penaloza, T.; Richie, C.T.; Pickel, J.; Hope, B.T.; Harvey, B.K.; Kalivas, P.W.; Heinsbroek, J.A. Ventral Pallidum Is the Primary Target for Accumbens D1 Projections Driving Cocaine Seeking. J. Neurosci. 2019, 39, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, L.; Kim, J.E.; Miyanohara, A.; Kang, T.H.; Friedmann, T. Purinergic signaling in human pluripotent stem cells is regulated by the housekeeping gene encoding hypoxanthine guanine phosphoribosyltransferase. Proc. Natl. Acad. Sci. USA 2012, 109, 3377–3382. [Google Scholar] [CrossRef] [PubMed]
- Darvas, M.; Palmiter, R.D. Specific contributions of N-methyl-D-aspartate receptors in the dorsal striatum to cognitive flexibility. Neuroscience 2015, 284, 934–942. [Google Scholar] [CrossRef]
- Yam, P.Y.; Li, S.; Wu, J.; Hu, J.; Zaia, J.A.; Yee, J.K. Design of HIV vectors for efficient gene delivery into human hematopoietic cells. Mol. Ther. 2002, 5, 479–484. [Google Scholar] [CrossRef]
- Yee, J.K.; Miyanohara, A.; LaPorte, P.; Bouic, K.; Burns, J.C.; Friedmann, T. A general method for the generation of high-titer, pantropic retroviral vectors: Highly efficient infection of primary hepatocytes. Proc. Natl. Acad. Sci. USA 1994, 91, 9564–9568. [Google Scholar] [CrossRef]
- Galinato, M.H.; Lockner, J.W.; Fannon-Pavlich, M.J.; Sobieraj, J.C.; Staples, M.C.; Somkuwar, S.S.; Ghofranian, A.; Chaing, S.; Navarro, A.I.; Joea, A.; et al. A synthetic small-molecule Isoxazole-9 protects against methamphetamine relapse. Mol. Psychiatry 2018, 23, 629–638. [Google Scholar] [CrossRef]
- Galinato, M.H.; Orio, L.; Mandyam, C.D. Methamphetamine differentially affects BDNF and cell death factors in anatomically defined regions of the hippocampus. Neuroscience 2015, 286, 97–108. [Google Scholar] [CrossRef]
- Mandyam, C.D.; Wee, S.; Eisch, A.J.; Richardson, H.N.; Koob, G.F. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J. Neurosci. 2007, 27, 11442–11450. [Google Scholar] [CrossRef]
- Richardson, N.R.; Roberts, D.C. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J. Neurosci. Methods 1996, 66, 1–11. [Google Scholar] [CrossRef]
- Kang, S.; Cox, C.L.; Gulley, J.M. High frequency stimulation-induced plasticity in the prelimbic cortex of rats emerges during adolescent development and is associated with an increase in dopamine receptor function. Neuropharmacology 2018, 141, 158–166. [Google Scholar] [CrossRef]
- Adermark, L.; Talani, G.; Lovinger, D.M. Endocannabinoid-dependent plasticity at GABAergic and glutamatergic synapses in the striatum is regulated by synaptic activity. Eur. J. Neurosci. 2009, 29, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.P.; Moradpour, F.; Eadie, B.D.; Shin, J.D.; Kannangara, T.S.; Delaney, K.R.; Christie, B.R. Electrophysiological identification of medial and lateral perforant path inputs to the dentate gyrus. Neuroscience 2013, 252, 154–168. [Google Scholar] [CrossRef]
- Misgeld, U.; Okada, Y.; Hassler, R. Locally evoked potentials in slices of rat neostriatum: A tool for the investigation of intrinsic excitatory processes. Exp. Brain Res. 1979, 34, 575–590. [Google Scholar] [CrossRef]
- Avchalumov, Y.; Piña-Crespo, J.C.; Woodward, J.J.; Mandyam, C.D. Acute Ethanol Exposure Enhances Synaptic Plasticity in the Dorsal Striatum in Adult Male and Female Rats. Brain Plast. 2020, 6, 113–122. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avchalumov, Y.; Kreisler, A.D.; Trenet, W.; Nayak, M.; Head, B.P.; Piña-Crespo, J.C.; Mandyam, C.D. Caveolin-1 Expression in the Dorsal Striatum Drives Methamphetamine Addiction-Like Behavior. Int. J. Mol. Sci. 2021, 22, 8219. https://doi.org/10.3390/ijms22158219
Avchalumov Y, Kreisler AD, Trenet W, Nayak M, Head BP, Piña-Crespo JC, Mandyam CD. Caveolin-1 Expression in the Dorsal Striatum Drives Methamphetamine Addiction-Like Behavior. International Journal of Molecular Sciences. 2021; 22(15):8219. https://doi.org/10.3390/ijms22158219
Chicago/Turabian StyleAvchalumov, Yosef, Alison D. Kreisler, Wulfran Trenet, Mahasweta Nayak, Brian P. Head, Juan C. Piña-Crespo, and Chitra D. Mandyam. 2021. "Caveolin-1 Expression in the Dorsal Striatum Drives Methamphetamine Addiction-Like Behavior" International Journal of Molecular Sciences 22, no. 15: 8219. https://doi.org/10.3390/ijms22158219
APA StyleAvchalumov, Y., Kreisler, A. D., Trenet, W., Nayak, M., Head, B. P., Piña-Crespo, J. C., & Mandyam, C. D. (2021). Caveolin-1 Expression in the Dorsal Striatum Drives Methamphetamine Addiction-Like Behavior. International Journal of Molecular Sciences, 22(15), 8219. https://doi.org/10.3390/ijms22158219





