Regulatory Network Analysis in Estradiol-Treated Human Endothelial Cells
Abstract
:1. Introduction
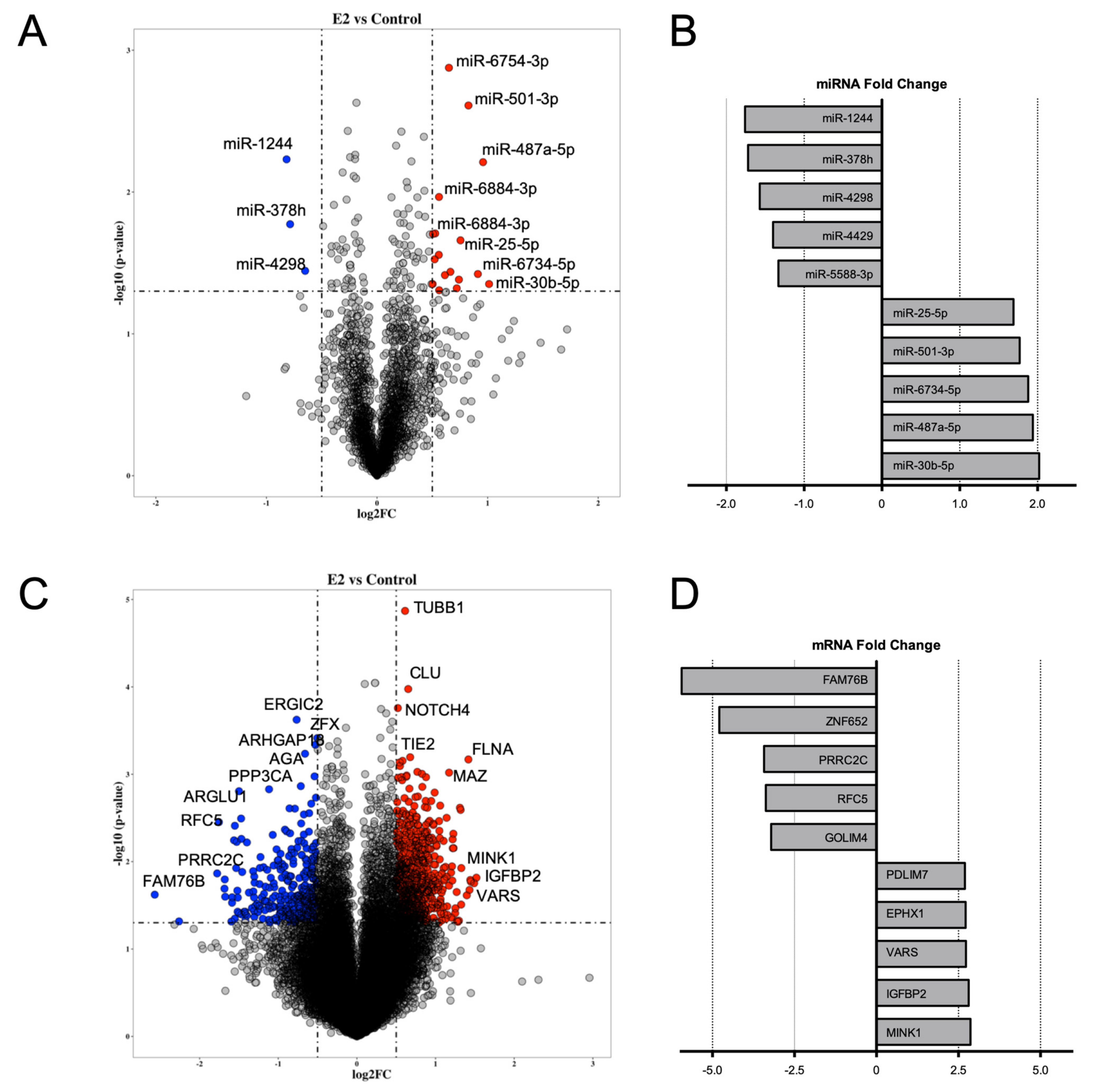
2. Results
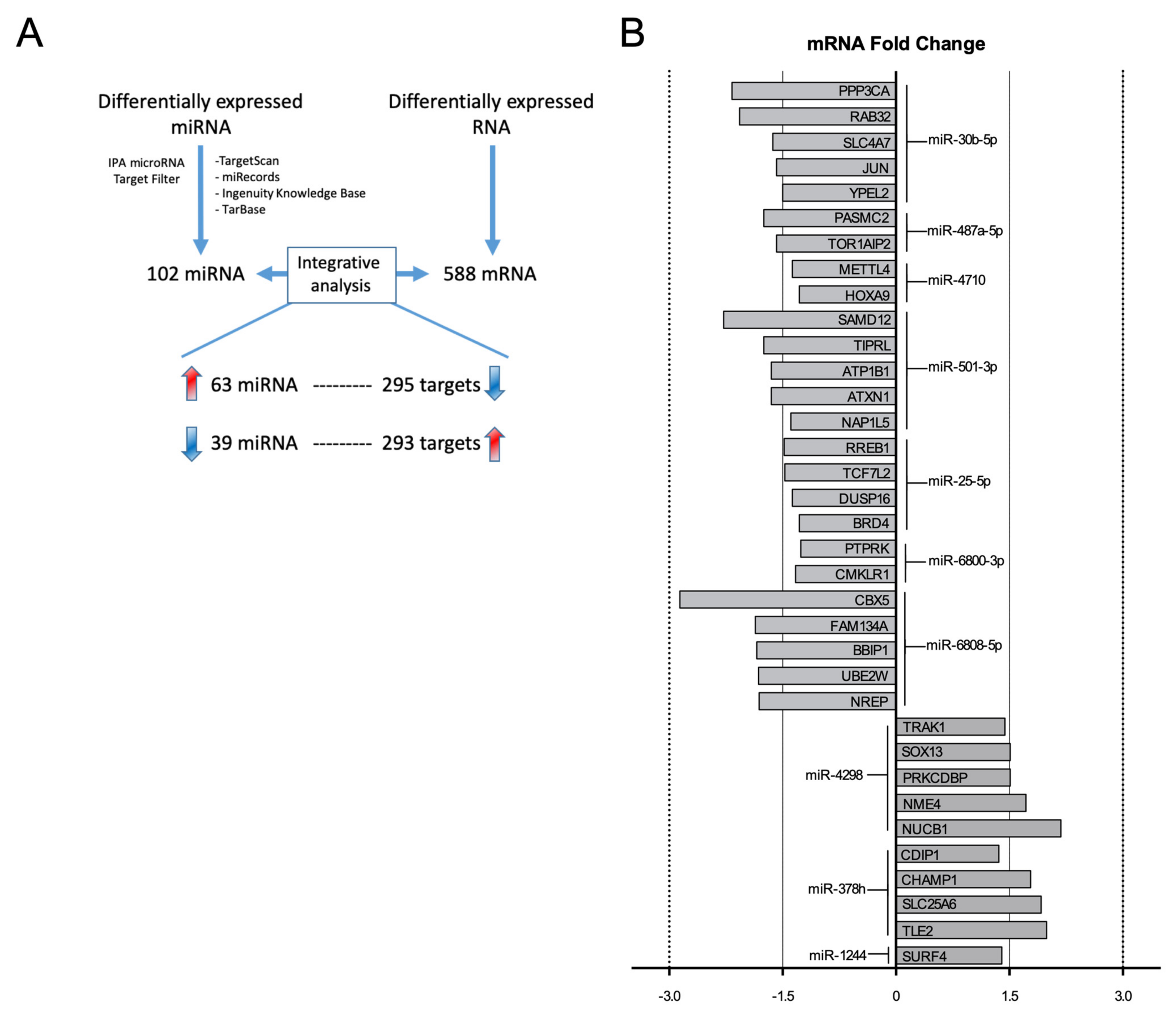
2.1. Integrative Analysis of miRNA–mRNA Expression Pairings in E2-Treated HUVEC
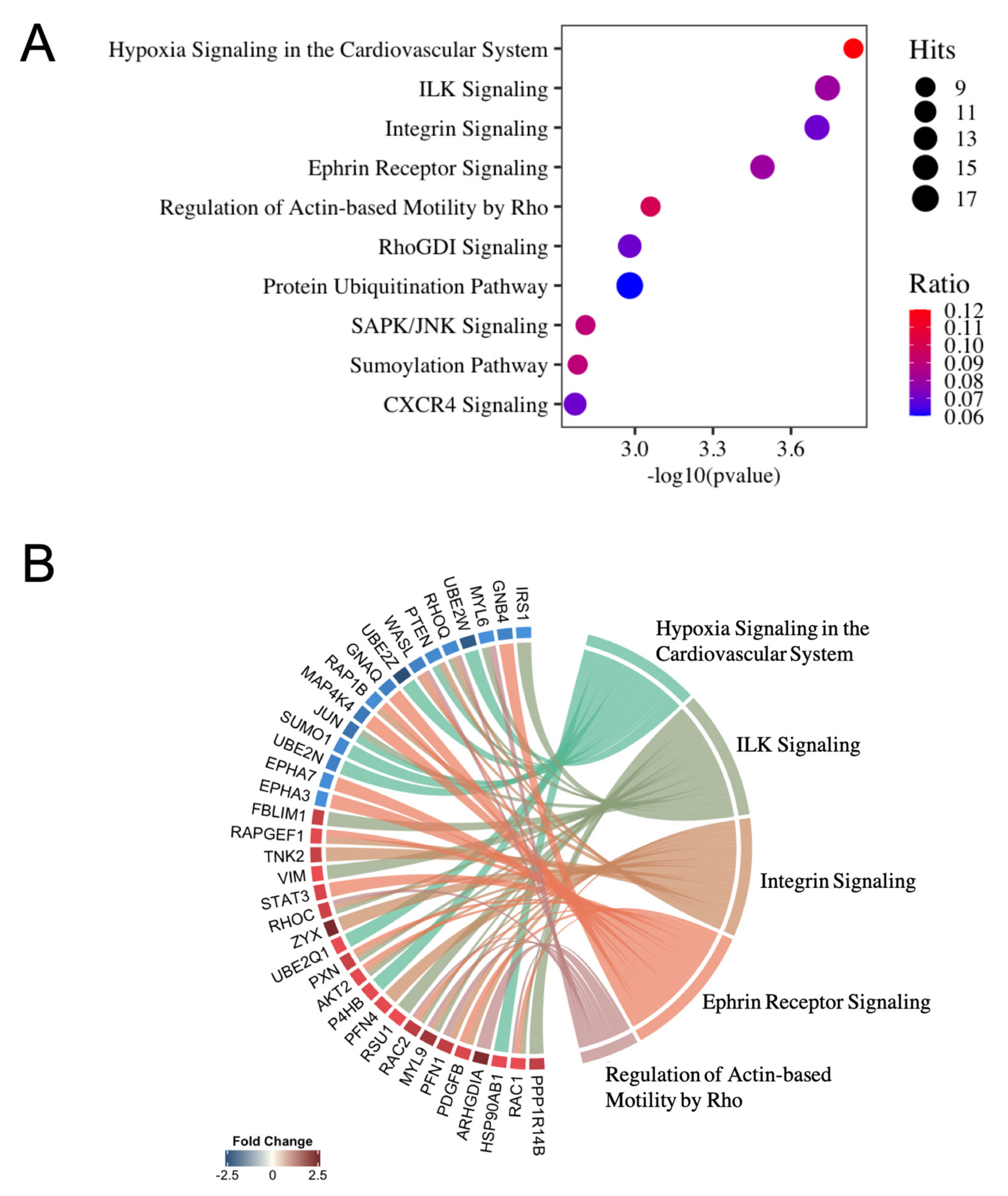
2.2. Enrichment Pathway Analysis of miRNA–mRNA Expression Pairings in E2-Treated HUVEC
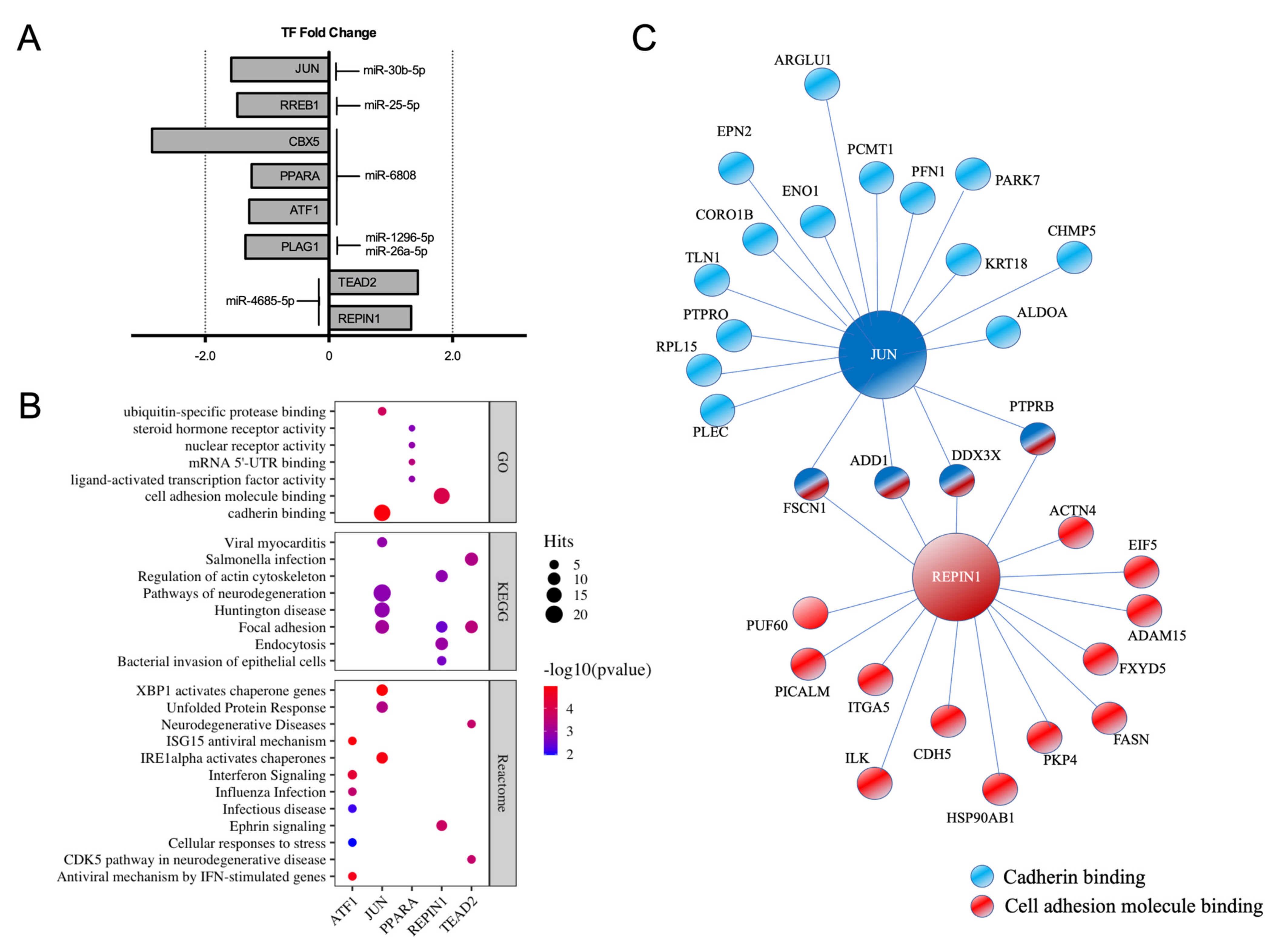
2.3. Identification of miRNA-Regulated Transcription Factors in E2-Treated HUVEC
3. Discussion
4. Material and Methods
4.1. Cell Culture and Experimental Design
4.2. Integrative Analysis of miRNA–mRNA Expression Profile
4.3. Pathway Enrichment Analysis
4.4. Identification of Transcription Factors—Target Networks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, V.M.; Duckles, S.P. Vascular actions of estrogens: Functional implications. Pharmacol. Rev. 2008, 60, 210–241. [Google Scholar] [CrossRef] [Green Version]
- Vitale, C.; Mendelsohn, M.E.; Rosano, G.M. Gender differences in the cardiovascular effect of sex hormones. Nat. Rev. Cardiol. 2009, 6, 532–542. [Google Scholar] [CrossRef]
- Arnal, J.F.; Fontaine, C.; Billon-Gales, A.; Favre, J.; Laurell, H.; Lenfant, F.; Gourdy, P. Estrogen receptors and endothelium. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Novella, S.; Pérez-Cremades, D.; Mompeón, A.; Hermenegildo, C. Mechanisms underlying the influence of oestrogen on cardiovascular physiology in women. J. Physiol. 2019, 597, 4873–4886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, V.N.; Han, J.; Siomi, M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S. Posttranscriptional upregulation by microRNAs. Wiley Interdiscip. Rev. RNA 2012, 3, 311–330. [Google Scholar] [CrossRef]
- Chamorro-Jorganes, A.; Araldi, E.; Suárez, Y. MicroRNAs as pharmacological targets in endothelial cell function and dysfunction. Pharmacol. Res. 2013, 75, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Eghbali, M. Influence of sex differences on microRNA gene regulation in disease. Biol. Sex Differ. 2014, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Zhang, Q.; Ma, X.; Wang, J.; Liang, T. miRNA and mRNA expression analysis reveals potential sex-biased miRNA expression. Sci. Rep. 2017, 7, 39812. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Cremades, D.; Mompeón, A.; Vidal-Gómez, X.; Hermenegildo, C.; Novella, S. miRNA as a New Regulatory Mechanism of Estrogen Vascular Action. Int. J. Biol. Sci. 2018, 19, 473. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Cremades, D.; Mompeón, A.; Vidal-Gómez, X.; Hermenegildo, C.; Novella, S. Role of miRNA in the Regulatory Mechanisms of Estrogens in Cardiovascular Ageing. Oxidative Med. Cell. Longev. 2018, 6082387. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Gomez, X.; Pérez-Cremades, D.; Mompeón, A.; Dantas, A.; Novella, S.; Hermenegildo, C. microRNA as crucial regulators of gene expression in estradiol-treated human endothelial cells. Cell. Physiol. Biochem. 2018, 45, 1878–1892. [Google Scholar] [CrossRef] [PubMed]
- Simoncini, T.; Maffei, S.; Basta, G.; Barsacchi, G.; Genazzani, A.R.; Liao, J.K.; de Caterina, R. Estrogens and glucocorticoids inhibit endothelial vascular cell adhesion molecule-1 expression by different transcriptional mechanisms. Circ. Res. 2000, 87, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, H.; Wallerath, T.; Euchenhofer, C.; Ihrig-Biedert, I.; Li, H.; Förstermann, U. Estrogens increase transcription of the human endothelial NO synthase gene: Analysis of the transcription factors involved. Hypertension 1998, 31, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Earley, S.; Resta, T.C. Estradiol attenuates hypoxia-induced pulmonary endothelin-1 gene expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L86–L93. [Google Scholar] [CrossRef] [Green Version]
- Sobrino, A.; Mata, M.; Laguna-Fernandez, A.; Novella, S.; Oviedo, P.J.; Garcia-Perez, M.A.; Tarin, J.J.; Cano, A.; Hermenegildo, C. Estradiol stimulates vasodilatory and metabolic pathways in cultured human endothelial cells. PLoS ONE 2009, 4, e8242. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zheng, C.; Wang, X.; Yun, S.; Zhao, Y.; Liu, L.; Lu, Y.; Ye, Y.; Zhu, X.; Zhang, C.; et al. MicroRNA-30 family members regulate calcium/calcineurin signaling in podocytes. J. Clin. Investig. 2015, 125, 4091–4106. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.Q.; Rao, C.L.; Tang, M.L.; Zhang, Y.; Lu, X.X.; Chen, J.G.; Mao, C.; Deng, L.; Li, Q.; Mao, X.H. Rab32 GTPase, as a direct target of miR-30b/c, controls the intracellular survival of Burkholderia pseudomallei by regulating phagosome maturation. PLoS Pathog. 2019, 15, e1007879. [Google Scholar] [CrossRef]
- Zhao, X.; Mohan, R.; Özcan, S.; Tang, X. MicroRNA-30d induces insulin transcription factor MafA and insulin production by targeting mitogen-activated protein 4 kinase 4 (MAP4K4) in pancreatic β-cells. J. Biol. Chem. 2012, 287, 31155–31164. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, C.; Eder, S.; Baker, C.; Aronovitz, M.J.; Weiss, A.D.; Hall-Porter, M.; Wang, F.; Ackerman, A.; Karas, R.H.; Molkentin, J.D.; et al. Estrogen attenuates left ventricular and cardiomyocyte hypertrophy by an estrogen receptor-dependent pathway that increases calcineurin degradation. Circ. Res. 2009, 104, 265–275. [Google Scholar] [CrossRef]
- Wu, J.; Dai, F.; Li, C.; Zou, Y. Gender Differences in Cardiac Hypertrophy. J. Cardiovasc. Transl. Res. 2020, 13, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Kong, N.; Ye, L.; Han, W.; Zhou, J.; Zhang, Q.; He, C.; Pan, H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014, 344, 174–179. [Google Scholar] [CrossRef]
- Roth Flach, R.J.; Skoura, A.; Matevossian, A.; Danai, L.V.; Zheng, W.; Cortes, C.; Bhattacharya, S.K.; Aouadi, M.; Hagan, N.; Yawe, J.C.; et al. Endothelial protein kinase MAP4K4 promotes vascular inflammation and atherosclerosis. Nat. Commun. 2015, 6, 8995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, J.; Liu, X.; Ren, J.; Chen, K.; Wang, H.L.; Miao, Y.Y.; Qi, M.M. How does estrogen work on autophagy? Autophagy 2019, 15, 197–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutter, C.H.; Laughner, E.; Semenza, G.L. Hypoxia-inducible factor 1alpha protein expression is controlled by oxygen-regulated ubiquitination that is disrupted by deletions and missense mutations. Proc. Natl. Acad. Sci. USA 2000, 97, 4748–4753. [Google Scholar] [CrossRef] [Green Version]
- George, A.L.; Rajoria, S.; Suriano, R.; Mittleman, A.; Tiwari, R.K. Hypoxia and estrogen are functionally equivalent in breast cancer-endothelial cell interdependence. Mol. Cancer 2012, 11, 80. [Google Scholar] [CrossRef] [Green Version]
- Pitulescu, M.E.; Adams, R.H. Eph/ephrin molecules—A hub for signaling and endocytosis. Genes Dev. 2010, 24, 2480–2492. [Google Scholar] [CrossRef] [Green Version]
- Luxán, G.; Stewen, J.; Díaz, N.; Kato, K.; Maney, S.K.; Aravamudhan, A.; Berkenfeld, F.; Nagelmann, N.; Drexler, H.C.; Zeuschner, D.; et al. Endothelial EphB4 maintains vascular integrity and transport function in adult heart. eLife 2019, 8. [Google Scholar] [CrossRef]
- Sottile, J. Regulation of angiogenesis by extracellular matrix. Biochim. Biophys. Acta 2004, 1654, 13–22. [Google Scholar] [CrossRef]
- Cid, M.C.; Esparza, J.; Schnaper, H.W.; Juan, M.; Yague, J.; Grant, D.S.; Urbano-Marquez, A.; Hoffman, G.S.; Kleinman, H.K. Estradiol enhances endothelial cell interactions with extracellular matrix proteins via an increase in integrin expression and function. Angiogenesis 1999, 3, 271–280. [Google Scholar] [CrossRef]
- Oviedo, P.J.; Sobrino, A.; Laguna-Fernandez, A.; Novella, S.; Tarín, J.J.; García-Pérez, M.-A.; Sanchís, J.; Cano, A.; Hermenegildo, C. Estradiol induces endothelial cell migration and proliferation through estrogen receptor-enhanced RhoA/ROCK pathway. Mol. Cell Endocrinol. 2011, 335, 96–103. [Google Scholar] [CrossRef]
- Krasinski, K.; Spyridopoulos, I.; Asahara, T.; van der Zee, R.; Isner, J.M.; Losordo, D.W. Estradiol accelerates functional endothelial recovery after arterial injury. Circulation 1997, 95, 1768–1772. [Google Scholar] [CrossRef]
- Masuda, H.; Kalka, C.; Takahashi, T.; Yoshida, M.; Wada, M.; Kobori, M.; Itoh, R.; Iwaguro, H.; Eguchi, M.; Iwami, Y.; et al. Estrogen-mediated endothelial progenitor cell biology and kinetics for physiological postnatal vasculogenesis. Circ. Res. 2007, 101, 598–606. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Flamini, M.I.; Zullino, S.; Gopal, S.; Genazzani, A.R.; Simoncini, T. Estrogen receptor-{alpha} promotes endothelial cell motility through focal adhesion kinase. Mol. Hum. Reprod. 2011, 17, 219–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groten, T.; Pierce, A.A.; Huen, A.C.; Schnaper, H.W. 17 beta-estradiol transiently disrupts adherens junctions in endothelial cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 1368–1370. [Google Scholar] [CrossRef]
- Qi, X.; Borowicz, S.; Pramanik, R.; Schultz, R.M.; Han, J.; Chen, G. Estrogen receptor inhibits c-Jun-dependent stress-induced cell death by binding and modifying c-Jun activity in human breast cancer cells. J. Biol. Chem. 2004, 279, 6769–6777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aranda, A.; Pascual, A. Nuclear hormone receptors and gene expression. Physiol. Rev. 2001, 81, 1269–1304. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, K.; Ono, T.; Sato, M.; Nishida, T.; Oguma, T.; Tadakuma, T. Regulation of the expression of caspase-9 by the transcription factor activator protein-4 in glucocorticoid-induced apoptosis. J. Biol. Chem. 2005, 280, 27638–27644. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.Y.; Jeong, B.C.; Lee, J.H.; Kee, H.J.; Kook, H.; Kim, N.S.; Kim, Y.H.; Kim, J.K.; Ahn, K.Y.; Kim, K.K. A repressor complex, AP4 transcription factor and geminin, negatively regulates expression of target genes in nonneuronal cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13074–13079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, P.; Menssen, A.; Mayr, D.; Hermeking, H. AP4 encodes a c-MYC-inducible repressor of p21. Proc. Natl. Acad. Sci. USA 2008, 105, 15046–15051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackstadt, R.; Röh, S.; Neumann, J.; Jung, P.; Hoffmann, R.; Horst, D.; Berens, C.; Bornkamm, G.W.; Kirchner, T.; Menssen, A.; et al. AP4 is a mediator of epithelial-mesenchymal transition and metastasis in colorectal cancer. J. Exp. Med. 2013, 210, 1331–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacic, J.C.; Dimmeler, S.; Harvey, R.P.; Finkel, T.; Aikawa, E.; Krenning, G.; Baker, A.H. Endothelial to Mesenchymal Transition in Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.S.; Chen, Y.J.; Chou, T.Y.; Chen, C.Y.; Li, H.Y.; Huang, B.S.; Tsai, H.W.; Lan, H.Y.; Chang, C.H.; Twu, N.F.; et al. Oestrogen-induced angiogenesis promotes adenomyosis by activating the Slug-VEGF axis in endometrial epithelial cells. J. Cell. Mol. Med. 2014, 18, 1358–1371. [Google Scholar] [CrossRef]
- Monsalve, E.; Oviedo, P.J.; Garcia-Perez, M.A.; Tarin, J.J.; Cano, A.; Hermenegildo, C. Estradiol counteracts oxidized LDL-induced asymmetric dimethylarginine production by cultured human endothelial cells. Cardiovasc. Res. 2007, 73, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M. A database for post-genome analysis. Trends Genet. TIG 1997, 13, 375–376. [Google Scholar] [CrossRef]
- Croft, D.; O’Kelly, G.; Wu, G.; Haw, R.; Gillespie, M.; Matthews, L.; Caudy, M.; Garapati, P.; Gopinath, G.; Jassal, B.; et al. Reactome: A database of reactions, pathways and biological processes. Nucleic Acids Res. 2011, 39, D691–D697. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. circlize Implements and enhances circular visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Cremades, D.; Paes, A.B.; Vidal-Gómez, X.; Mompeón, A.; Hermenegildo, C.; Novella, S. Regulatory Network Analysis in Estradiol-Treated Human Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 8193. https://doi.org/10.3390/ijms22158193
Pérez-Cremades D, Paes AB, Vidal-Gómez X, Mompeón A, Hermenegildo C, Novella S. Regulatory Network Analysis in Estradiol-Treated Human Endothelial Cells. International Journal of Molecular Sciences. 2021; 22(15):8193. https://doi.org/10.3390/ijms22158193
Chicago/Turabian StylePérez-Cremades, Daniel, Ana B. Paes, Xavier Vidal-Gómez, Ana Mompeón, Carlos Hermenegildo, and Susana Novella. 2021. "Regulatory Network Analysis in Estradiol-Treated Human Endothelial Cells" International Journal of Molecular Sciences 22, no. 15: 8193. https://doi.org/10.3390/ijms22158193
APA StylePérez-Cremades, D., Paes, A. B., Vidal-Gómez, X., Mompeón, A., Hermenegildo, C., & Novella, S. (2021). Regulatory Network Analysis in Estradiol-Treated Human Endothelial Cells. International Journal of Molecular Sciences, 22(15), 8193. https://doi.org/10.3390/ijms22158193






