Photoreceptor Compartment-Specific TULP1 Interactomes
Abstract
1. Introduction
2. Results
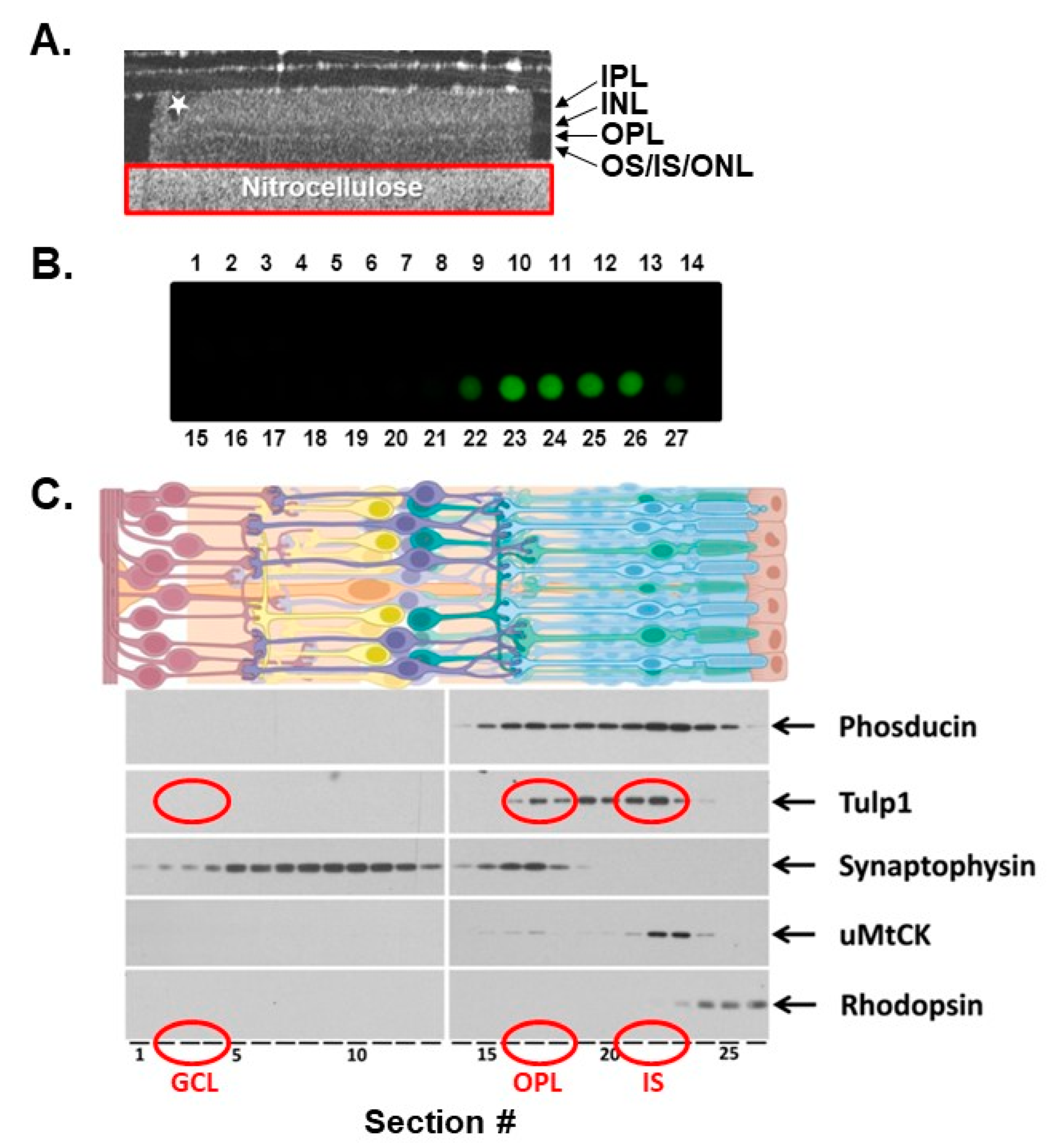
2.1. Photoreceptor Compartment Isolation
2.2. Tulp1 Photoreceptor Compartment-Specific Binding Partners
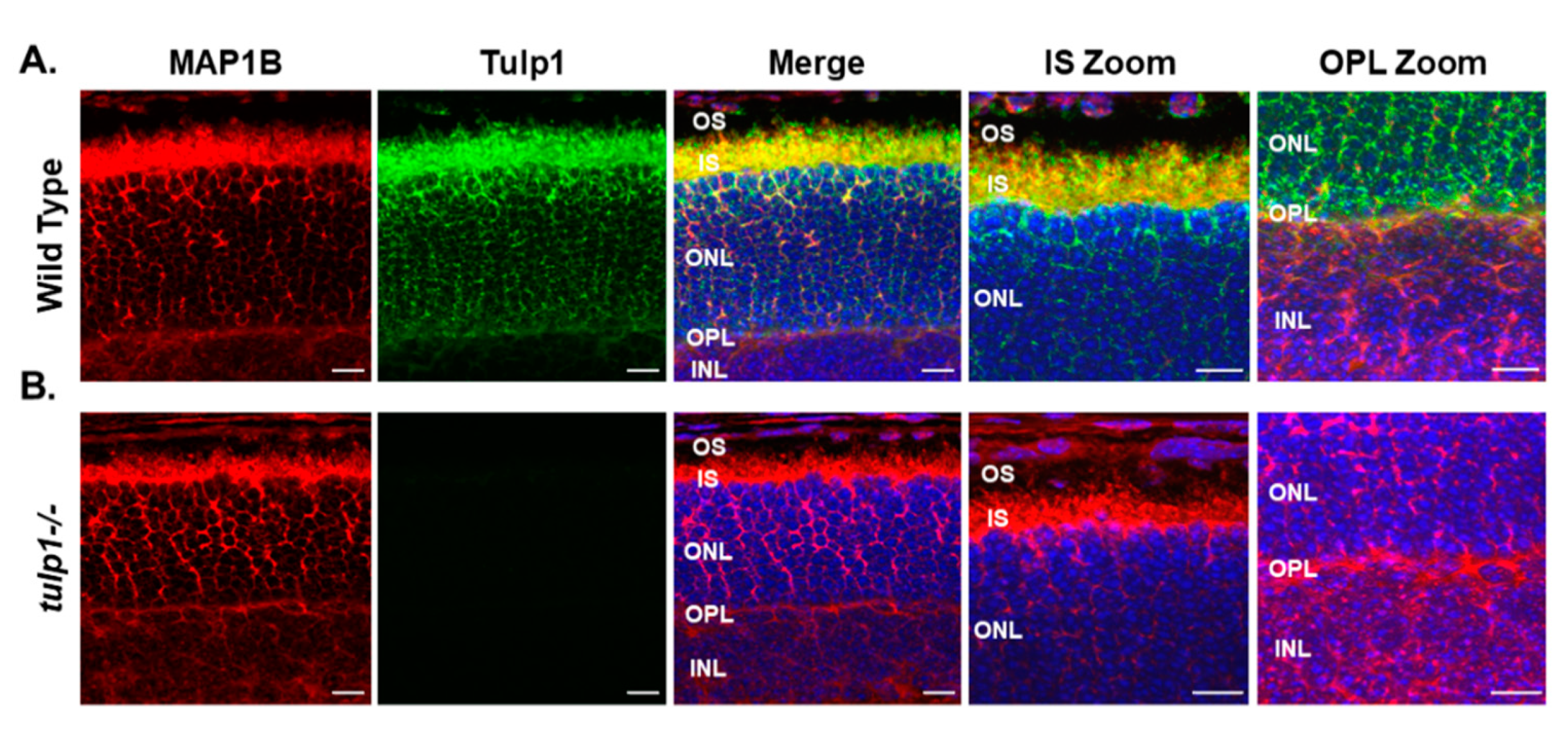
2.3. MAP1B Is a Tulp1-Interacting Partner in the IS and OPL
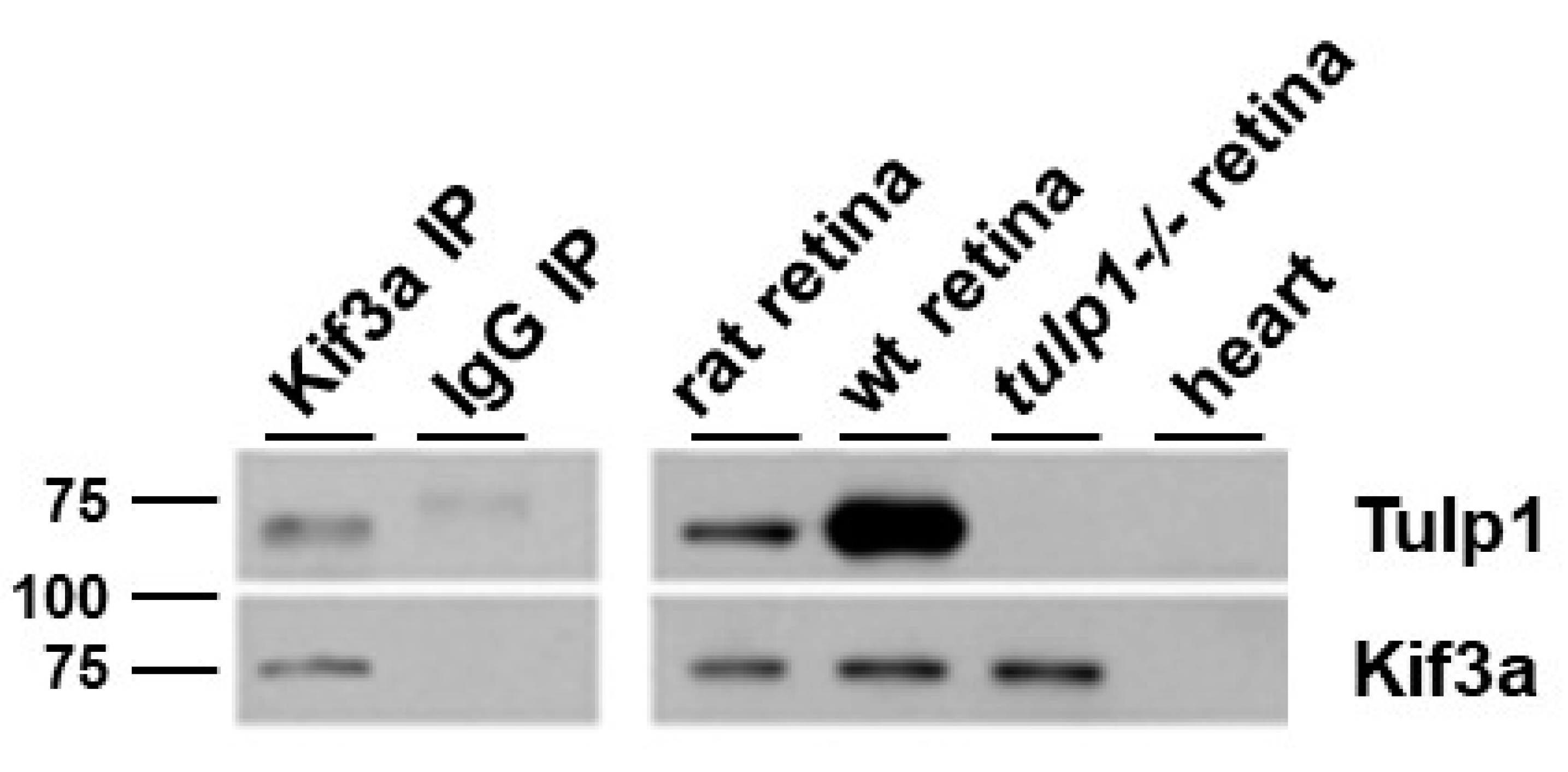
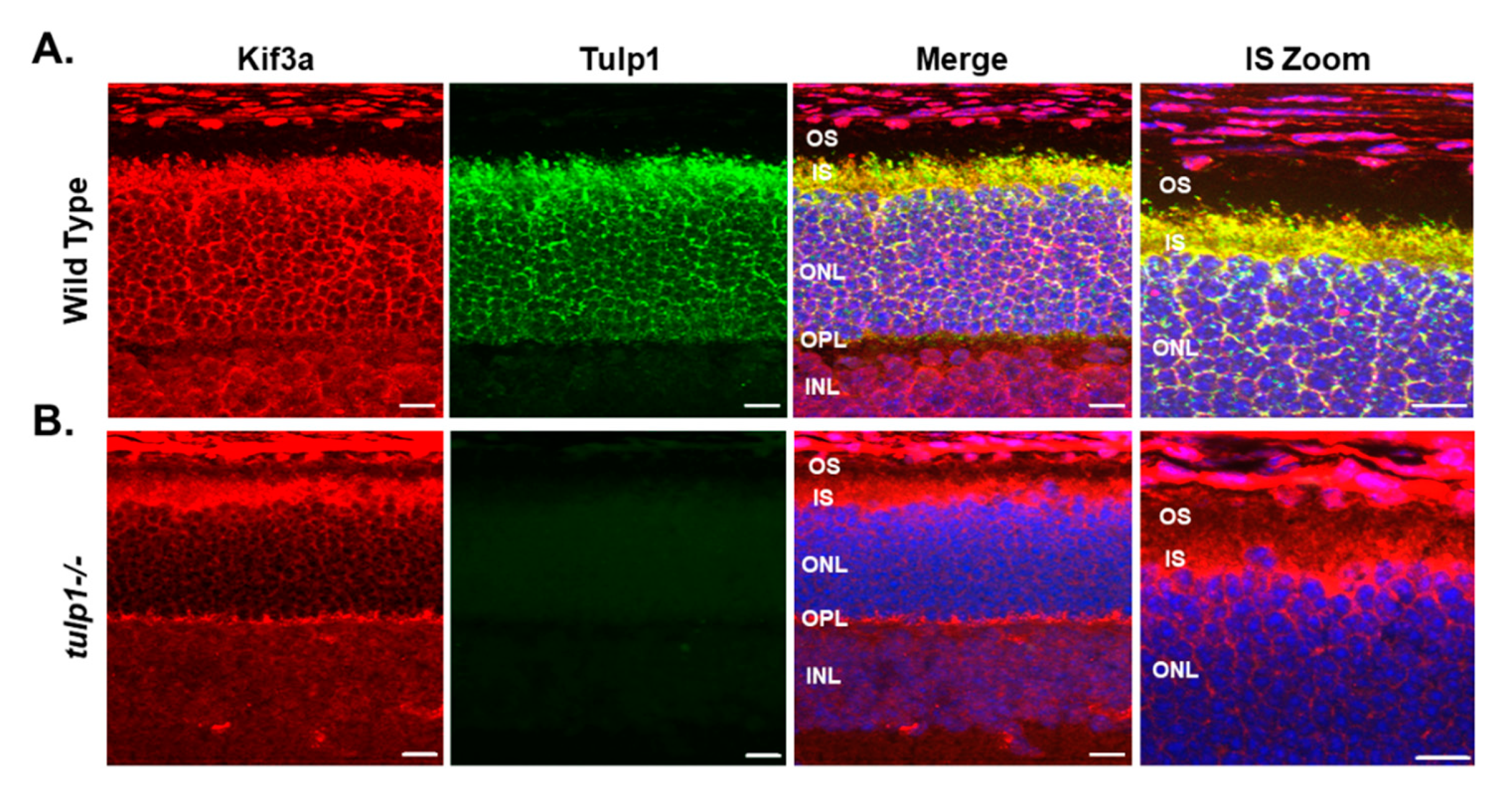
2.4. Kif3a Is a Tulp1-Interacting Partner in the IS
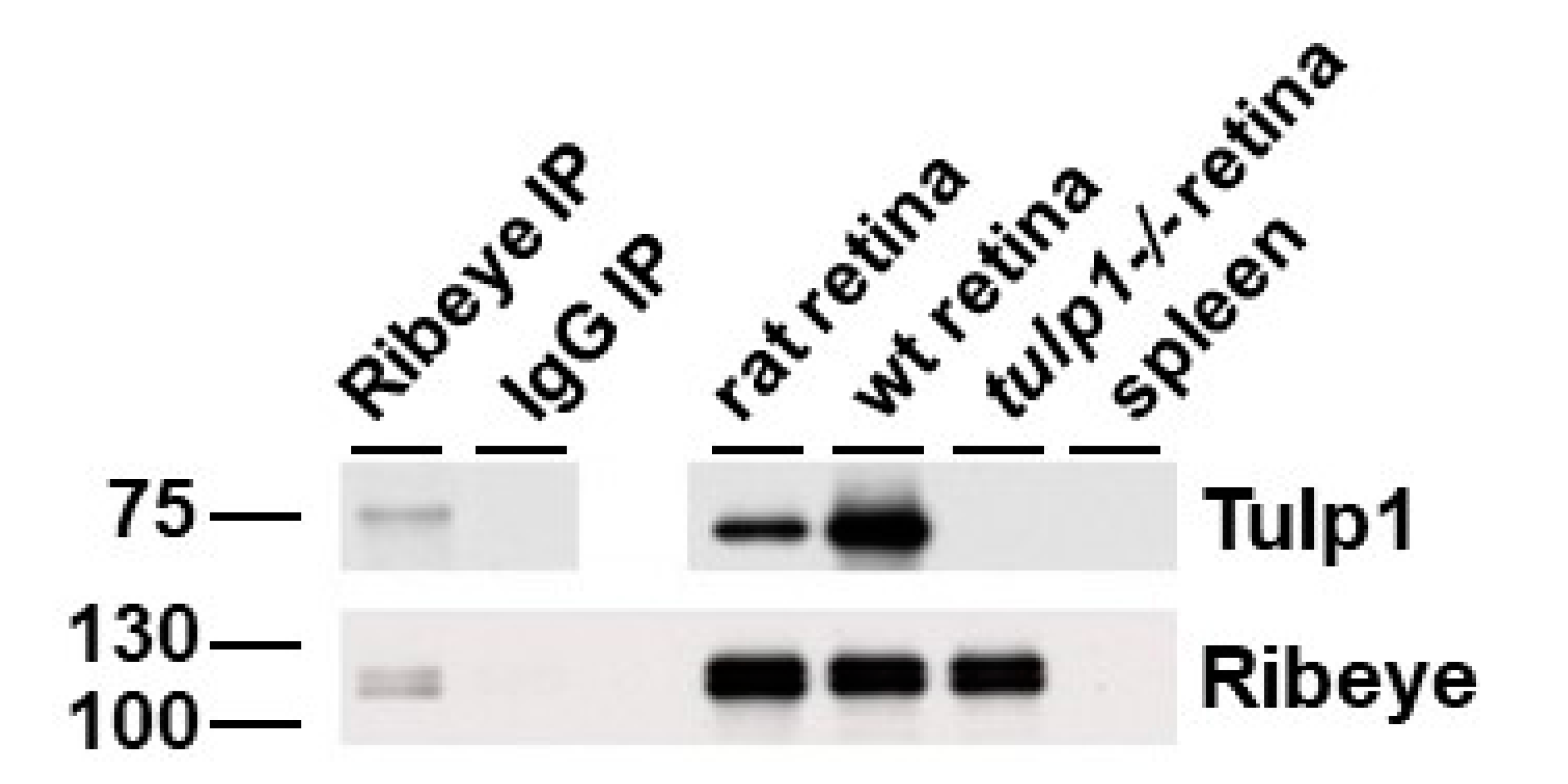
2.5. Ribeye Is a Tulp1-Interacting Partner in the OPL
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Serial Tangential Sectioning
4.3. Optical Coherance Tomography (OCT)
4.4. Dot Blot Analysis
4.5. SDS-PAGE and Western Blot Analysis
4.6. Immunoprecipitation (IP)
4.7. Liquid Chromatography Tandem Mass Spectrometry (LC–MS/MS)
4.8. Immunohistochemistry (IHC)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearring, J.N.; Salinas, R.Y.; Baker, S.A.; Arshavsky, V.Y. Protein sorting, targeting and trafficking in photoreceptor cells. Prog. Retin Eye Res. 2013, 36, 24–51. [Google Scholar] [CrossRef] [PubMed]
- Palczewski, K. Chemistry and biology of vision. J. Biol. Chem. 2012, 287, 1612–1619. [Google Scholar] [CrossRef]
- Arshavsky, V.Y.; Burns, M.E. Photoreceptor signaling: Supporting vision across a wide range of light intensities. J. Biol. Chem. 2012, 287, 1620–1626. [Google Scholar] [CrossRef]
- Young, R.W. The renewal of photoreceptor cell outer segments. J. Cell Biol. 1967, 33, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Young, R.W.; Bok, D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J. Cell Biol. 1969, 42, 392–403. [Google Scholar] [CrossRef]
- Taschner, M.; Bhogaraju, S.; Lorentzen, E. Architecture and function of IFT complex proteins in ciliogenesis. Differentiation 2012, 83, S12–S22. [Google Scholar] [CrossRef]
- Wang, J.; Deretic, D. Molecular complexes that direct rhodopsin transport to primary cilia. Prog. Retin. Eye Res. 2014, 38, 1–19. [Google Scholar] [CrossRef]
- Nemet, I.; Ropelewski, P.; Imanishi, Y. Rhodopsin Trafficking and Mistrafficking: Signals, Molecular Components, and Mechanisms. Prog. Mol. Biol. Transl. Sci. 2015, 132, 39–71. [Google Scholar] [CrossRef]
- Ahmari, S.E.; Buchanan, J.; Smith, S.J. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat. Neurosci. 2000, 3, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Voorn, R.A.; Vogl, C. Molecular Assembly and Structural Plasticity of Sensory Ribbon Synapses—A Presynaptic Perspective. Int. J. Mol. Sci. 2020, 21, 8758. [Google Scholar] [CrossRef]
- Hagstrom, S.A.; Duyao, M.; North, M.A.; Li, T. Retinal degeneration in tulp1−/− mice: Vesicular accumulation in the interphotoreceptor matrix. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2795–2802. [Google Scholar]
- Hagstrom, S.A.; Adamian, M.; Scimeca, M.; Pawlyk, B.S.; Yue, G.; Li, T. A role for the Tubby-like protein 1 in rhodopsin transport. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1955–1962. [Google Scholar]
- Ikeda, S.; Shiva, N.; Ikeda, A.; Smith, R.S.; Nusinowitz, S.; Yan, G.; Lin, T.R.; Chu, S.; Heckenlively, J.R.; North, M.A.; et al. Retinal degeneration but not obesity is observed in null mutants of the tubby-like protein 1 gene. Hum. Mol. Genet. 2000, 9, 155–163. [Google Scholar] [CrossRef]
- Grossman, G.H.; Pauer, G.J.; Narendra, U.; Peachey, N.S.; Hagstrom, S.A. Early synaptic defects in tulp1−/− mice. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3074–3083. [Google Scholar] [CrossRef] [PubMed]
- Grossman, G.H.; Watson, R.F.; Pauer, G.J.; Bollinger, K.; Hagstrom, S.A. Immunocytochemical evidence of Tulp1-dependent outer segment protein transport pathways in photoreceptor cells. Exp. Eye Res. 2011, 93, 658–668. [Google Scholar] [CrossRef]
- Hagstrom, S.A.; Watson, R.F.; Pauer, G.J.; Grossman, G.H. Tulp1 is involved in specific photoreceptor protein transport pathways. Adv. Exp. Med. Biol. 2012, 723, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Calvert, P.D. Transport and localization of signaling proteins in ciliated cells. Vis. Res. 2012, 75, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Xi, Q.; Pauer, G.J.; Marmorstein, A.D.; Crabb, J.W.; Hagstrom, S.A. Tubby- like protein 1 (TULP1) interacts with F-actin in photoreceptor cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4754–4761. [Google Scholar] [CrossRef] [PubMed]
- Xi, Q.; Pauer, G.J.; Ball, S.L.; Rayborn, M.; Hollyfield, J.G.; Peachey, N.S.; Crabb, J.W.; Hagstrom, S.A. Interaction between the photoreceptor-specific tubby-like protein 1 and the neuronal-specific GTPase dynamin-1. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2837–2844. [Google Scholar] [CrossRef]
- Grossman, G.H.; Beight, C.D.; Ebke, L.A.; Pauer, G.J.; Hagstrom, S.A. Interaction of tubby-like protein-1 (Tulp1) and microtubule-associated protein (MAP) 1A and MAP1B in the mouse retina. Adv. Exp. Med. Biol. 2014, 801, 511–518. [Google Scholar] [CrossRef]
- Sokolov, M.; Strissel, K.J.; Leskov, I.B.; Michaud, N.A.; Govardovskii, V.I.; Arshavsky, V.Y. Phosducin facilitates light-driven transducin translocation in rod photoreceptors. Evidence from the phosducin knockout mouse. J. Biol. Chem. 2004, 279, 19149–19156. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.J.; Li, X.; Yao, H.L.; Deng, J.X.; Liu, H.L.; Cui, Z.J.; Wang, Q.; Wu, P.; Deng, J.B. Neural differentiation and synaptogenesis in retinal development. Neural Regen Res. 2016, 11, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, G.; Huber, R.; Zanolla, E.; Eppenberger, H.M.; Wallimann, T. Differential expression and localization of brain-type and mitochondrial creatine kinase isoenzymes during development of the chicken retina: Mi-CK as a marker for differentiation of photoreceptor cells. Differentiation 1991, 46, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Eliuk, S.; Makarov, A. Evolution of Orbitrap Mass Spectrometry Instrumentation. Annu. Rev. Anal. Chem. 2015, 8, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Mellacheruvu, D.; Wright, Z.; Couzens, A.L.; Lambert, J.P.; St-Denis, N.A.; Li, T.; Miteva, Y.V.; Hauri, S.; Sardiu, M.E.; Low, T.Y.; et al. The CRAPome: A Contaminant Repository for Affinity Purification Mass Spectrometry Data. Nat. Methods 2013, 10, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Villarroel-Campos, D.; Gonzalez-Billault, C. The MAP1B case: An old MAP that is new again. Dev. Neurobiol. 2014, 74, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Atherton, J.; Houdusse, A.; Moores, C. MAPping out distribution routes for kinesin couriers. Biol. Cell. 2013, 105, 465–487. [Google Scholar] [CrossRef] [PubMed]
- Verhey, K.J.; Hammond, J.W. Traffic control: Regulation of kinesin motors. Nat. Rev. Mol. Cell Biol. 2009, 10, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F. The making of synaptic ribbons: How they are built and what they do. Neuroscientist 2009, 15, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Zanazzi, G.; Matthews, G. The molecular architecture of ribbon presynaptic terminals. Mol. Neurobiol. 2009, 39, 130–148. [Google Scholar] [CrossRef]
- Kantardzhieva, A.; Peppi, M.; Lane, W.S.; Sewell, W.F. Protein composition of immunoprecipitated synaptic ribbons. J. Proteome Res. 2012, 11, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Scholey, J.M. Kinesin-2: A family of heterotrimeric and homodimeric motors with diverse intracellular transport functions. Ann. Rev. Cell Dev. Biol. 2013, 29, 443–469. [Google Scholar] [CrossRef]
- Zhao, C.; Omori, Y.; Brodowska, K.; Kovach, P.; Malicki, J. Kinesin-2 family in vertebrate ciliogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 2388–2393. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wei, Y.; Ronquillo, C.C.; Marc, R.E.; Yoder, B.K.; Frederick, J.M.; Baehr, W. Heterotrimeric kinesin-2 (KIF3) mediates transition zone and axoneme formation of mouse photoreceptors. J. Biol. Chem. 2015, 290, 12765–12778. [Google Scholar] [CrossRef]
- Maxeiner, S.; Luo, F.; Tan, A.; Schmitz, F.; Südhof, T.C. How to make a synaptic ribbon: RIBEYE deletion abolishes ribbons in retinal synapses and disrupts neurotransmitter release. EMBO J. 2016, 35, 1098–1114. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.; Königstorfer, A.; Südhof, T.C. RIBEYE, a component of synaptic ribbons: A protein’s journey through evolution provides insight into synaptic ribbon function. Neuron 2000, 28, 857–872. [Google Scholar] [CrossRef]
- Wahl, S.; Magupalli, V.G.; Dembla, M.; Katiyar, R.; Schwarz, K.; Köblitz, L.; Alpadi, K.; Krause, E.; Rettig, J.; Sung, C.H.; et al. The Disease Protein Tulp1 Is Essential for Periactive Zone Endocytosis in Photoreceptor Ribbon Synapses. J. Neurosci. 2016, 36, 2473–2493. [Google Scholar] [CrossRef]
- North, M.A.; Naggert, J.K.; Yan, Y.; Noben-Trauth, K.; Nishina, P.M. Molecular characterization of TUB, TULP1, and TULP2, members of the novel tubby gene family and their possible relation to ocular diseases. Proc. Natl. Acad. Sci. USA 1997, 94, 3128–3133. [Google Scholar] [CrossRef]
- Lai, C.P.; Chen, P.H.; Huang, J.P.; Tzeng, Y.H.; Chaw, S.M.; Shaw, J.F. Functional diversification of the Tubby-like protein gene families (TULPs) during eukaryotic evolution. Biocatal. Agric. Biotechnol. 2012, 1, 2–8. [Google Scholar] [CrossRef]
- Hagstrom, S.A.; North, M.A.; Nishina, P.M.; Berson, E.L.; Dryja, T.P. Recessive mutations in the gene encoding the tubby-like protein TULP1 in patients with retinitis pigmentosa. Nat. Genet. 1998, 18, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Kleyn, P.W.; Knowles, J.A.; Lewis, C.A.; Ross, B.M.; Parano, E.; Kovats, S.G.; Lee, J.J.; Penchaszadeh, G.K.; Ott, J.; et al. TULP1 mutation in two extended Dominican kindreds with autosomal recessive retinitis pigmentosa. Nat. Genet. 1998, 18, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Mataftsi, A.; Schorderet, D.F.; Chachoua, L.; Boussalah, M.; Nouri, M.T.; Barthelmes, D.; Borruat, F.X.; Munier, F.L. Novel TULP1 mutation causing leber congenital amaurosis or early onset retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5160–5167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roosing, S.; van den Born, L.I.; Hoyng, C.B.; Thiadens, A.A.; de Baere, E.; Collin, R.W.; Koenekoop, R.K.; Leroy, B.P.; van Moll-Ramirez, N.; Venselaar, H.; et al. Maternal uniparental isodisomy of chromosome 6 reveals a TULP1 mutation as a novel cause of cone dysfunction. Ophthalmology 2013, 120, 1239–1246. [Google Scholar] [CrossRef]
- Lewis, C.A.; Batlle, I.R.; Batlle, K.G.; Banerjee, P.; Cideciyan, A.V.; Huang, J.; Alemán, T.S.; Huang, Y.; Ott, J.; Gilliam, T.C.; et al. Tubby-like protein 1 homozygous splice-site mutation causes early-onset severe retinal degeneration. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2106–2114. [Google Scholar]
- Santagata, S.; Boggon, T.J.; Baird, C.L.; Gomez, C.A.; Zhao, J.; Shan, W.S.; Myszka, D.G.; Shapiro, L. G-protein signaling through tubby proteins. Science 2001, 292, 2041–2050. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Wen, X.; Chih, B.; Nelson, C.D.; Lane, W.S.; Scales, S.J.; Jackson, P.K. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev. 2010, 24, 2180–2193. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, W.; Zervas, M.; Costello, P.; Roback, L.; Fischer, I.; Hammarback, J.A.; Cowan, N.; Davies, P.; Wainer, B.; Kucherlapati, R. Neuronal abnormalities in microtubule-associated protein 1B mutant mice. Proc. Natl. Acad. Sci. USA 1996, 93, 1270–1275. [Google Scholar] [CrossRef]
- Bodaleo, F.J.; Montenegro-Venegas, C.; Henríquez, D.R.; Court, F.A.; Gonzalez-Billault, C. Microtubule-associated protein 1B (MAP1B)-deficient neurons show structural presynaptic deficiencies in vitro and altered presynaptic physiology. Sci. Rep. 2016, 6, 30069. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Billault, C.; Jimenez-Mateos, E.M.; Caceres, A.; Diaz-Nido, J.; Wandosell, F.; Avila, J. Microtubule-associated protein 1B function during normal development, regeneration, and pathological conditions in the nervous system. J. Neurobiol. 2004, 58, 48–59. [Google Scholar] [CrossRef]
- Morthorst, S.K.; Christensen, S.T.; Pedersen, L.B. Regulation of ciliary membrane protein trafficking and signalling by kinesin motor proteins. FEBS J. 2018, 285, 4535–4564. [Google Scholar] [CrossRef]
- Baehr, W.; Hanke-Gogokhia, C.; Sharif, A.; Reed, M.; Dahl, T.; Frederick, J.M.; Ying, G. Insights into photoreceptor ciliogenesis revealed by animal models. Prog. Retin. Eye Res. 2019, 71, 26–56. [Google Scholar] [CrossRef]
- Marszalek, J.R.; Liu, X.; Roberts, E.A.; Chui, D.; Marth, J.D.; Williams, D.S.; Goldstein, L.S. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell 2000, 102, 175–187. [Google Scholar] [CrossRef]
- Lopes, V.S.; Jimeno, D.; Khanobdee, K.; Song, X.; Chen, B.; Nusinowitz, S.; Williams, D.S. Dysfunction of heterotrimeric kinesin-2 in rod photoreceptor cells and the role of opsin mislocalization in rapid cell death. Mol. Biol. Cell. 2010, 21, 4076–4088. [Google Scholar] [CrossRef]
- Jimeno, D.; Feiner, L.; Lillo, C.; Teofilo, K.; Goldstein, L.S.; Pierce, E.A.; Williams, D.S. Analysis of kinesin-2 function in photoreceptor cells using synchronous Cre-loxP knockout of Kif3a with RHO-Cre. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5039–5046. [Google Scholar] [CrossRef][Green Version]
- Avasthi, P.; Watt, C.B.; Williams, D.S.; Le, Y.Z.; Li, S.; Chen, C.K.; Marc, R.E.; Frederick, J.M.; Baehr, W. Trafficking of membrane proteins to cone but not rod outer segments is dependent on heterotrimeric kinesin-II. J. Neurosci. 2009, 29, 14287–14298. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.L.; Malhotra, H.; Calvert, P.D. Compartmentalization of Photoreceptor Sensory Cilia. Front. Cell Dev. Biol. 2021, 9, 636737. [Google Scholar] [CrossRef]
- Imanishi, Y. Protein Sorting in Healthy and Diseased Photoreceptors. Annu. Rev. Vis. Sci. 2019, 5, 73–98. [Google Scholar] [CrossRef]
- Muresan, V.; Lyass, A.; Schnapp, B.J. The kinesin motor KIF3A is a component of the presynaptic ribbon in vertebrate photoreceptors. J. Neurosci. 1999, 19, 1027–1037. [Google Scholar] [CrossRef]
- Thoreson, W.B. Transmission at rod and cone ribbon synapses in the retina. Pflugers Arch. 2021, 1–23. [Google Scholar] [CrossRef]
- Goldstein, A.Y.N.; Wang, X.; Schwarz, T.L. Axonal Transport and the Delivery of Presynaptic Components. Curr. Opin. Neurobiol. 2008, 18, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Yagensky, O.; Kalantary Dehaghi, T.; Chua, J.J. The Roles of Microtubule-Based Transport at Presynaptic Nerve Terminals. Front. Synaptic Neurosci. 2016, 8, 3. [Google Scholar] [CrossRef]
- Bury, L.A.; Sabo, S.L. Building a Terminal: Mechanisms of Presynaptic Development in the CNS. Neuroscientist 2016, 22, 372–391. [Google Scholar] [CrossRef] [PubMed]
- Fairless, R.; Williams, S.K.; Katiyar, R.; Maxeiner, S.; Schmitz, F.; Diem, R. ERG Responses in Mice with Deletion of the Synaptic Ribbon Component RIBEYE. Investig. Ophthalmol. Vis. Sci. 2020, 61, 7. [Google Scholar] [CrossRef]
- Badgandi, H.B.; Hwang, S.H.; Shimada, I.S.; Loriot, E.; Mukhopadhyay, S. Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins. J. Cell Biol. 2017, 216, 743–760. [Google Scholar] [CrossRef]
- Mirvis, M.; Stearns, T.; James Nelson, W. Cilium structure, assembly, and disassembly regulated by the cytoskeleton. Biochem. J. 2018, 475, 2329–2353. [Google Scholar] [CrossRef]
- Prevo, B.; Scholey, J.M.; Peterman, E.J.G. Intraflagellar transport: Mechanisms of motor action, cooperation, and cargo delivery. FEBS J. 2017, 284, 2905–2931. [Google Scholar] [CrossRef]
- Palfi, A.; Yesmambetov, A.; Humphries, P.; Hokamp, K.; Farrar, G.J. Non-photoreceptor Expression of Tulp1 May Contribute to Extensive Retinal Degeneration in Tulp1−/− Mice. Front. Neurosci. 2020, 14, 656. [Google Scholar] [CrossRef]
- Thuenauer, R.; Hsu, Y.C.; Carvajal-Gonzalez, J.M.; Deborde, S.; Chuang, J.Z.; Römer, W.; Sonnleitner, A.; Rodriguez-Boulan, E.; Sung, C.H. Four-dimensional live imaging of apical biosynthetic trafficking reveals a post-Golgi sorting role of apical endosomal intermediates. Proc. Natl. Acad. Sci. USA 2014, 111, 4127–4132. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Sokolov, M. Analysis of protein expression and compartmentalization in retinal neurons using serial tangential sec tioning of the retina. J. Proteome Res. 2009, 8, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Kinter, M.; Sherman, N.E. Protein Sequencing and Identification Using Tandem Mass Spectrometry; Desiderio, D., Nibbering, N., Eds.; John Wiley & Sons: New York, NY, USA, 2005. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebke, L.A.; Sinha, S.; Pauer, G.J.T.; Hagstrom, S.A. Photoreceptor Compartment-Specific TULP1 Interactomes. Int. J. Mol. Sci. 2021, 22, 8066. https://doi.org/10.3390/ijms22158066
Ebke LA, Sinha S, Pauer GJT, Hagstrom SA. Photoreceptor Compartment-Specific TULP1 Interactomes. International Journal of Molecular Sciences. 2021; 22(15):8066. https://doi.org/10.3390/ijms22158066
Chicago/Turabian StyleEbke, Lindsey A., Satyabrata Sinha, Gayle J. T. Pauer, and Stephanie A. Hagstrom. 2021. "Photoreceptor Compartment-Specific TULP1 Interactomes" International Journal of Molecular Sciences 22, no. 15: 8066. https://doi.org/10.3390/ijms22158066
APA StyleEbke, L. A., Sinha, S., Pauer, G. J. T., & Hagstrom, S. A. (2021). Photoreceptor Compartment-Specific TULP1 Interactomes. International Journal of Molecular Sciences, 22(15), 8066. https://doi.org/10.3390/ijms22158066




