A Review of the Neutrophil Extracellular Traps (NETs) from Cow, Sheep and Goat Models
Abstract
1. Introduction
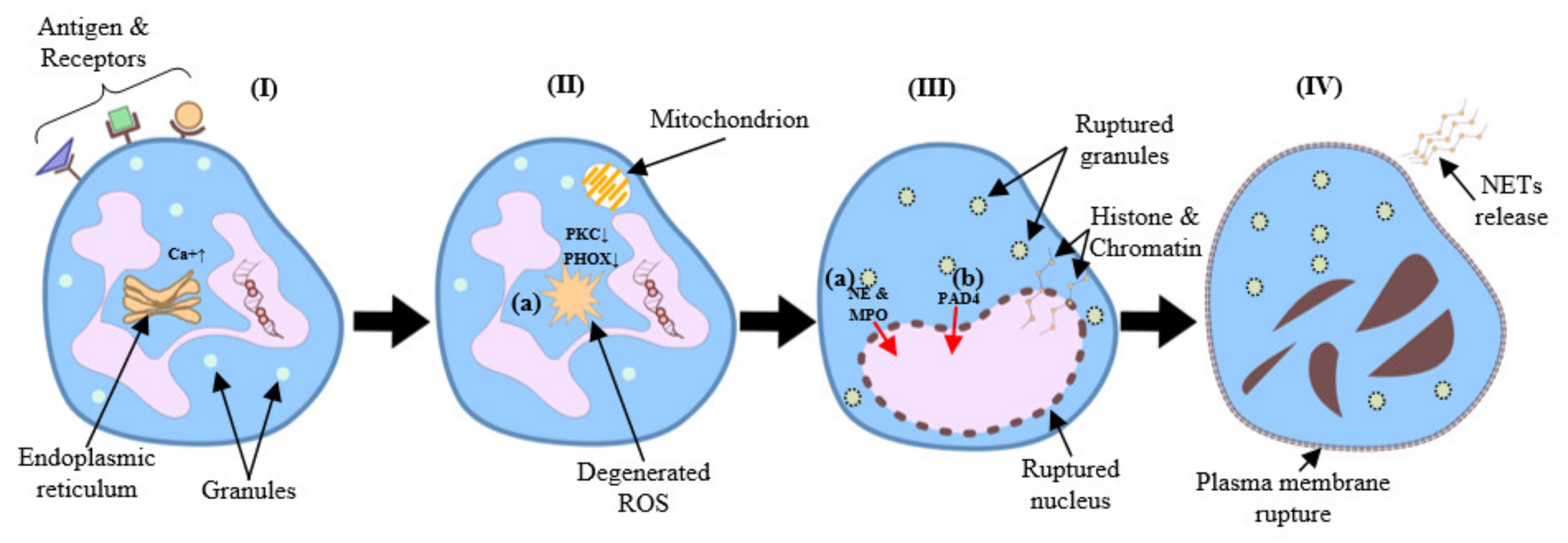
2. NETosis Mechanisms and Functions
3. Mammalian Neutrophil Extracellular Traps
3.1. Triggers and Phenotpes of Extracellular Traps
3.2. Extracellular Traps in Ruminants
NET Triggers in Cattle, Sheep and Goats
4. Prediction, Modulation and Therapy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IFN | interferon |
| IP-10 | IFN-γ-induced protein 10 |
| IFN-α | interferon α |
| IL | interleukin |
| MPO | myeloperoxidase |
| NE | neutrophil elastase |
| ETs | extracellular traps |
| NETs | neutrophil extracellular traps |
| Nox | NADPH-oxidase |
| PMA | phorbol-12-myristate-13-acetate |
| ROS | reactive oxygen species |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SLE | systemic lupus erythematosus |
| TNF-α | tumor necrosis factor-α |
| BPI | bactericidal permeability-increasing factor |
| ERK1/2 | extracellular signal-regulated kinases 1/2 |
| LPS | lipopolysaccharide |
| TLR | Toll-like receptor |
| C3 | complement 3 |
| PAD4 | peptidyl arginine deiminase 4 |
| PVL | Panto-Valentine leukocidin |
| HMGB1 | high-mobility group box 1 |
| MAPK | mitogen-activated protein kinase hi |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| PAMPs | pathogen-associated molecular patterns |
| CD 11b | cluster of differentiation molecule 11b |
| CD14 | cluster of differentiation antigen 14 |
| PMNs | polymorphonuclear leukocytes |
| CH3 | citrullinated Histone H3 |
| NEFAs | nonesterified fatty acids |
| ICAM-1 | intercellular Adhesion Molecule 1 |
| PVD | purulent vaginal discharge |
| MAC-1 | macrophage-1 antigen |
| FC | fold change |
| NFkB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| IL1B | interleukin 1 beta or lymphocyte activating factor (cytokine protein) |
| FZD | frizzled |
| PGLYRP1 | gene peptidoglycan recognition protein 1 gene |
| SERPINB4 | protein serpin family B member 4 protein |
| PMNL | polymorphonuclear leukocytes |
| Met | methionine |
| RPM | rumen-protected Met |
| GCSF | granulocyte colony stimulating factor |
| GMCSF | granulocyte macrophage colony stimulating factor |
| CoV | coronavirus |
| BCoV | bovine CoV |
| PRRs | pattern recognition receptors |
| CNN | convolutional neural network |
| NEFAs | nonesterified fatty acids |
| CCL3 | macrophage inflammatory protein-1 α, MIP-1α |
| CXCL6 | chemokine (C-X-C motif) ligand 6 |
| SOCE | store-operated calcium entry |
References
- Bailey, M.; Christoforidou, Z.; Lewis, M.C. The evolutionary basis for differences between the immune systems of man, mouse, pig and ruminants. Vet. Immunol. Immunopathol. 2013, 152, 13–19. [Google Scholar] [CrossRef]
- Döhrmann, S.; Cole, J.N.; Nizet, V. Conquering Neutrophils. PLoS Pathog. 2016, 12, e1005682. [Google Scholar] [CrossRef] [PubMed]
- George, J.W.; Snipes, J.; Lane, V.M. Comparison of bovine hematology reference intervals from 1957 to 2006. Vet. Clin. Pathol. 2010, 39, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Bassel, L.L.; Caswell, J.L. Bovine neutrophils in health and disease. Cell Tissue Res. 2018, 371, 617–637. [Google Scholar] [CrossRef]
- Tian, S.Z.; Chang, C.J.; Chiang, C.C.; Peh, H.C.; Huang, M.C.; Lee, J.-W.; Zhao, X. Comparison of morphology, viability, and function between blood and milk neutrophils from peak lactating goats. Can. J. Vet. Res. 2005, 69, 39–45. [Google Scholar] [PubMed]
- Janiuk, K.; Jabłońska, E.; Garley, M. Significance of NETs Formation in COVID-19. Cells 2021, 10, 151. [Google Scholar] [CrossRef]
- Mócsai, A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 2013, 210, 1283–1299. [Google Scholar] [CrossRef]
- Yang, H.; Biermann, M.H.; Brauner, J.; Liu, Y.; Zhao, Y.; Herrmann, M. New Insights into Neutrophil Extracellular Traps: Mechanisms of Formation and Role in Inflammation. Front. Immunol. 2016, 7, 302. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Steinberg, B.E.; Grinstein, S. Unconventional Roles of the NADPH Oxidase: Signaling, Ion Homeostasis, and Cell Death. Sci. STKE 2007, 2007, pe11. [Google Scholar] [CrossRef]
- Mendez, J.; Sun, D.; Tuo, W.; Xiao, Z. Bovine neutrophils form extracellular traps in response to the gastrointestinal parasite Ostertagia ostertagi. Sci. Rep. 2018, 8, 17598. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Hermosilla, C.; Caro, T.M.; Silva, L.M.R.; Ruiz, A.; Taubert, A. The intriguing host innate immune response: Novel anti-parasitic defence by neutrophil extracellular traps. Parasitology 2014, 141, 1489–1498. [Google Scholar] [CrossRef]
- De Buhr, N.; von Köckritz-Blickwede, M. How Neutrophil Extracellular Traps Become Visible. J. Immunol. Res. 2016, 2016, 4604713. [Google Scholar] [CrossRef] [PubMed]
- Li, R.H.L.; Tablin, F. A Comparative Review of Neutrophil Extracellular Traps in Sepsis. Front. Vet. Sci. 2018, 5, 291. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef]
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822. [Google Scholar] [CrossRef] [PubMed]
- Euler, M.; Hoffmann, M.H. The double-edged role of neutrophil extracellular traps in inflammation. Biochem. Soc. Trans. 2019, 47, 1921–1930. [Google Scholar] [CrossRef]
- Lapponi, M.J.; Carestia, A.; Landoni, V.I.; Rivadeneyra, L.; Etulain, J.; Negrotto, S.; Pozner, R.G.; Schattner, M. Regulation of Neutrophil Extracellular Trap Formation by Anti-Inflammatory Drugs. J. Pharmacol. Exp. Ther. 2013, 345, 430–437. [Google Scholar] [CrossRef]
- Kaplan, M.J.; Radic, M. Neutrophil Extracellular Traps: Double-Edged Swords of Innate Immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kaplan, M. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat. Rev. Nephrol. 2016, 12, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Rebernick, R.; Fahmy, L.; Glover, C.; Bawadekar, M.; Shim, D.; Holmes, C.L.; Rademacher, N.; Potluri, H.; Bartels, C.M.; Shelef, M.A. DNA Area and NETosis Analysis (DANA): A High-Throughput Method to Quantify Neutrophil Extracellular Traps in Fluorescent Microscope Images. Biol. Proced. Online 2018, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Garza, J.J.; Greiner, S.P.; Bowdridge, S.A. Ovine vital neutrophil extracellular traps bind and impair Haemonchus contortus L3 in a breed-dependent manner. Parasite Immunol. 2018, 40, e12572. [Google Scholar] [CrossRef] [PubMed]
- Swain, D.K.; Kushwah, M.S.; Kaur, M.; Patbandha, T.K.; Mohanty, A.K.; Dang, A. Formation of NET, phagocytic activity, surface architecture, apoptosis and expression of toll like receptors 2 and 4 (TLR2 and TLR4) in neutrophils of mastitic cows. Vet. Res. Commun. 2014, 38, 209–219. [Google Scholar] [CrossRef]
- Thiam, H.R.; Wong, S.L.; Wagner, D.D.; Waterman, C.M. Cellular Mechanisms of NETosis. Annu. Rev. Cell Dev. Biol. 2020, 36, 191–218. [Google Scholar] [CrossRef]
- Yipp, B.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.V.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Lippolis, J.D.; Reinhardt, T.; Goff, J.P.; Horst, R.L. Neutrophil extracellular trap formation by bovine neutrophils is not inhibited by milk. Vet. Immunol. Immunopathol. 2006, 113, 248–255. [Google Scholar] [CrossRef]
- Grinberg, N.; Elazar, S.; Rosenshine, I.; Shpigel, N.Y. Beta-hydroxybutyrate abrogates formation of bovine neutrophil extracellular traps and bactericidal activity against mammary pathogenic Escherichia coli. Infect. Immun. 2008, 76, 2802–2807. [Google Scholar] [CrossRef]
- Pieterse, E.; Rother, N.; Yanginlar, C.; Hilbrands, L.B.; Van Der Vlag, J. Neutrophils Discriminate between Lipopolysaccharides of Different Bacterial Sources and Selectively Release Neutrophil Extracellular Traps. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef]
- Hemmers, S.; Teijaro, J.R.; Arandjelovic, S.; Mowen, K.A. PAD4-Mediated Neutrophil Extracellular Trap Formation Is Not Required for Immunity against Influenza Infection. PLoS ONE 2011, 6, e22043. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Caro, T.; Mena Huertas, S.J.; Conejeros, I.; Alarcon, P.; Hidalgo, M.A.; Burgos, R.A.; Hermosilla, C.; Taubert, A. Eimeria bovis-triggered neutrophil extracellular trap formation is CD11b-, ERK 1/2-, p38 MAP kinase- and SOCE-dependent. Vet. Res. 2015, 46, 23. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, J.H.; Ruiz, A.; Zahner, H.; Taubert, A.; Hermosilla, C. Neutrophil extracellular trap formation as innate immune reactions against the apicomplexan parasite Eimeria bovis. Vet. Immunol. Immunopathol. 2010, 133, 1–8. [Google Scholar] [CrossRef]
- Silva, L.M.R.; Caro, T.M.; Gerstberger, R.; Vila-Viçosa, M.J.M.; Cortes, H.; Hermosilla, C.; Taubert, A. The apicomplexan parasite Eimeria arloingi induces caprine neutrophil extracellular traps. Parasitol. Res. 2014, 113, 2797–2807. [Google Scholar] [CrossRef]
- Muñoz-Caro, T.; Silva, L.M.R.; Ritter, C.; Taubert, A.; Hermosilla, C. Besnoitia besnoiti tachyzoites induce monocyte extracellular trap formation. Parasitol. Res. 2014, 113, 4189–4197. [Google Scholar] [CrossRef]
- Abdallah, D.S.A.; Lin, C.; Ball, C.J.; King, M.R.; Duhamel, G.E.; Denkers, E.Y. Toxoplasma gondii Triggers Release of Human and Mouse Neutrophil Extracellular Traps. Infect. Immun. 2011, 80, 768–777. [Google Scholar] [CrossRef]
- Neumann, A.; Brogden, G.; von Kockritz-Blickwede, M. Extracellular Traps: An Ancient Weapon of Multiple Kingdoms. Biology 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.; Leppkes, M.; Muñoz, L.E.; Schley, G.; Schett, G.; Herrmann, M. Extracellular DNA traps in inflammation, injury and healing. Nat. Rev. Nephrol. 2019, 15, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Wartha, F.; Henriques-Normark, B. ETosis: A Novel Cell Death Pathway. Sci. Signal. 2008, 1, pe25. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.H.; Fahey, C.; Yipp, B.; Sibley, C.D.; Robbins, S.; Green, F.H.Y.; Surette, M.G.; Sugai, M.; et al. A Novel Mechanism of Rapid Nuclear Neutrophil Extracellular Trap Formation in Response to Staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef]
- Byrd, A.S.; O’Brien, X.M.; Johnson, C.M.; Lavigne, L.M.; Reichner, J.S. An Extracellular Matrix–Based Mechanism of Rapid Neutrophil Extracellular Trap Formation in Response to Candida albicans. J. Immunol. 2013, 190, 4136–4148. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef]
- McIlroy, D.J.; Jarnicki, A.G.; Au, G.G.; Lott, N.; Smith, D.W.; Hansbro, P.; Balogh, Z.J. Mitochondrial DNA neutrophil extracellular traps are formed after trauma and subsequent surgery. J. Crit. Care 2014, 29, 1133.e1–1133.e5. [Google Scholar] [CrossRef]
- Yousefi, S.; Mihalache, C.C.; Kozlowski, E.O.; Schmid, I.; Simon, H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef]
- Seper, A.; Hosseinzadeh, A.; Gorkiewicz, G.; Lichtenegger, S.; Roier, S.; Leitner, D.R.; Röhm, M.; Grutsch, A.; Reidl, J.; Urban, C.F.; et al. Vibrio cholerae Evades Neutrophil Extracellular Traps by the Activity of Two Extracellular Nucleases. PLoS Pathog. 2013, 9, e1003614. [Google Scholar] [CrossRef] [PubMed]
- Wartha, F.; Beiter, K.; Albiger, B.; Fernebro, J.; Zychlinsky, A.; Normark, S.; Henriques-Normark, B. Capsule and d-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell. Microbiol. 2007, 9, 1162–1171. [Google Scholar] [CrossRef]
- Von Köckritz-Blickwede, M.; Goldmann, O.; Thulin, P.; Heinemann, K.; Norrby-Teglund, A.; Rohde, M.; Medina, E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 2008, 111, 3070–3080. [Google Scholar] [CrossRef]
- Beiter, K.; Wartha, F.; Albiger, B.; Normark, S.; Zychlinsky, A.; Henriques-Normark, B. An Endonuclease Allows Streptococcus pneumoniae to Escape from Neutrophil Extracellular Traps. Curr. Biol. 2006, 16, 401–407. [Google Scholar] [CrossRef]
- Juneau, R.A.; Pang, B.; Weimer, K.E.D.; Armbruster, C.E.; Swords, W.E. Nontypeable Haemophilus influenzae Initiates Formation of Neutrophil Extracellular Traps. Infect. Immun. 2011, 79, 431–438. [Google Scholar] [CrossRef]
- Munafo, D.B.; Johnson, J.L.; Brzezinska, A.A.; Ellis, B.A.; Wood, M.R.; Catz, S.D. DNase I Inhibits a Late Phase of Reactive Oxygen Species Production in Neutrophils. J. Innate Immun. 2009, 1, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Kichik, V.; Mondragón-Flores, R.; Mondragón-Castelán, M.; Gonzalez-Pozos, S.; Muñiz-Hernandez, S.; Rojas-Espinosa, O.; Chacon-Salinas, R.; Estrada-Parra, S.; Estrada-García, I. Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberculosis 2009, 89, 29–37. [Google Scholar] [CrossRef]
- McCormick, A.; Heesemann, L.; Wagener, J.; Marcos, V.; Hartl, D.; Loeffler, J.; Heesemann, J.; Ebel, F. NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microbes Infect. 2010, 12, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Niemiec, M.J.; Siler, U.; Urban, C.F.; Reichenbach, J. Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J. Allergy Clin. Immunol. 2011, 127, 1243–1252.e7. [Google Scholar] [CrossRef]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef]
- Delbosc, S.; Alsac, J.-M.; Journé, C.; Louedec, L.; Castier, Y.; Bonnaure-Mallet, M.; Ruimy, R.; Rossignol, P.; Bouchard, P.; Michel, J.-B.; et al. Porphyromonas gingivalis Participates in Pathogenesis of Human Abdominal Aortic Aneurysm by Neutrophil Activation. Proof of Concept in Rats. PLoS ONE 2011, 6, e18679. [Google Scholar] [CrossRef] [PubMed]
- Brogden, G.; von Köckritz-Blickwede, M.; Adamek, M.; Reuner, F.; Jung-Schroers, V.; Naim, H.Y.; Steinhagen, D. β-Glucan protects neutrophil extracellular traps against degradation by Aeromonas hydrophila in carp (Cyprinus carpio). Fish Shellfish Immunol. 2012, 33, 1060–1064. [Google Scholar] [CrossRef]
- Gondaira, S.; Higuchi, H.; Nishi, K.; Iwano, H.; Nagahata, H. Mycoplasma bovis escapes bovine neutrophil extracellular traps. Vet. Microbiol. 2017, 199, 68–73. [Google Scholar] [CrossRef]
- Gondaira, S.; Higuchi, H.; Iwano, H.; Nishi, K.; Nebu, T.; Nakajima, K.; Nagahata, H. Innate immune response of bovine mammary epithelial cells to Mycoplasma bovis. J. Vet. Sci. 2018, 19, 79–87. [Google Scholar] [CrossRef]
- Villagra-Blanco, R.; Silva, L.M.R.; Muñoz-Caro, T.; Yang, Z.; Li, J.; Gärtner, U.; Taubert, A.; Zhang, X.; Hermosilla, C. Bovine Polymorphonuclear Neutrophils Cast Neutrophil Extracellular Traps against the Abortive Parasite Neospora caninum. Front. Immunol. 2017, 8, 606. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, P.; Manosalva, C.; Conejeros, I.; Carretta, M.D.; Muñoz-Caro, T.; Silva, L.M.R.; Taubert, A.; Hermosilla, C.; Hidalgo, M.A.; Burgos, R.A. d(−) Lactic Acid-Induced Adhesion of Bovine Neutrophils onto Endothelial Cells Is Dependent on Neutrophils Extracellular Traps Formation and CD11b Expression. Front. Immunol. 2017, 8, 975. [Google Scholar] [CrossRef]
- Pérez, D.; Muñoz-Caro, T.; Silva, L.; Muñoz, M.; Molina, J.; Taubert, A.; Hermosilla, C.; Ruiz, A. Eimeria ninakohlyakimovae casts NOX-independent NETosis and induces enhanced IL-12, TNF-α, IL-6, CCL2 and iNOS gene transcription in caprine PMN. Exp. Parasitol. 2021, 220, 108034. [Google Scholar] [CrossRef]
- Pérez, D.; Munoz, M.; Molina, J.; Muñoz-Caro, T.; Silva, L.; Taubert, A.; Hermosilla, C.; Ruiz, A. Eimeria ninakohlyakimovae induces NADPH oxidase-dependent monocyte extracellular trap formation and upregulates IL-12 and TNF-α, IL-6 and CCL2 gene transcription. Vet. Parasitol. 2016, 227, 143–150. [Google Scholar] [CrossRef]
- Silva, L.M.R.; Muñoz-Caro, T.; Burgos, R.A.; Hidalgo, M.A.; Taubert, A.; Hermosilla, C. Far beyond phagocytosis: Phagocyte-derived extracellular traps act efficiently against protozoan parasites in vitro and in vivo. Mediat. Inflamm. 2016, 2016, 5898074. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Caro, T.; Rubio, R.M.; Silva, L.M.; Magdowski, G.; Gartner, U.; McNeilly, T.N.; Taubert, A.; Hermosilla, C. Leucocyte-derived extracellular trap formation significantly contributes to Haemonchus contortus larval entrapment. Parasites Vectors 2015, 8, 607. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Caro, T.; Lendner, M.; Daugschies, A.; Hermosilla, C.; Taubert, A. NADPH oxidase, MPO, NE, ERK1/2, p38 MAPK and Ca2+ influx are essential for Cryptosporidium parvum-induced NET formation. Dev. Comp. Immunol. 2015, 52, 245–254. [Google Scholar] [CrossRef]
- Muñoz-Caro, T.; Da Silva, L.M.R.; Rentería-Solis, Z.; Taubert, A.; Hermosilla, C. Neutrophil extracellular traps in the intestinal mucosa of Eimeria-infected animals. Asian Pac. J. Trop. Biomed. 2016, 6, 301–307. [Google Scholar] [CrossRef]
- Muñoz-Caro, T.; Gibson, A.; Conejeros, I.; Werling, D.; Taubert, A.; Hermosilla, C. The Role of TLR2 and TLR4 in Recognition and Uptake of the Apicomplexan Parasite Eimeria bovis and Their Effects on NET Formation. Pathogens 2021, 10, 118. [Google Scholar] [CrossRef]
- Yildiz, K.; Gokpinar, S.; Gazyagci, A.N.; Babur, C.; Sursal, N.; Azkur, A.K. Role of NETs in the difference in host susceptibility to Toxoplasma gondii between sheep and cattle. Vet. Immunol. Immunopathol. 2017, 189, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jerjomiceva, N.; Seri, H.; Völlger, L.; Wang, Y.; Zeitouni, N.; Naim, H.Y.; Von Köckritz-Blickwede, M. Enrofloxacin Enhances the Formation of Neutrophil Extracellular Traps in Bovine Granulocytes. J. Innate Immun. 2014, 6, 706–712. [Google Scholar] [CrossRef]
- Revelo, X.; Waldron, M. In vitro effects of Escherichia coli lipopolysaccharide on the function and gene expression of neutrophils isolated from the blood of dairy cows. J. Dairy Sci. 2012, 95, 2422–2441. [Google Scholar] [CrossRef] [PubMed]
- Vezina, B.; Al-Harbi, H.; Ramay, H.R.; Soust, M.; Moore, R.J.; Olchowy, T.W.J.; Alawneh, J.I. Sequence characterisation and novel insights into bovine mastitis-associated Streptococcus uberis in dairy herds. Sci. Rep. 2021, 11, 3046. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, S.; Cubeddu, T.; Pagnozzi, D.; Rocca, S.; Cacciotto, C.; Alberti, A.; Marogna, G.; Uzzau, S.; Addis, M.F. Neutrophil extracellular traps in sheep mastitis. Vet. Res. 2015, 46, 59. [Google Scholar] [CrossRef]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J. Small-Vessel Vasculitis. N. Engl. J. Med. 1997, 337, 1512–1523. [Google Scholar] [CrossRef]
- Söderberg, D.; Kurz, T.; Motamedi, A.; Hellmark, T.; Eriksson, P.; Segelmark, M. Increased levels of neutrophil extracellular trap remnants in the circulation of patients with small vessel vasculitis, but an inverse correlation to anti-neutrophil cytoplasmic antibodies during remission. Rheumatology 2015, 54, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Hanses, F.; Park, S.; Rich, J.; Lee, J.C. Reduced Neutrophil Apoptosis in Diabetic Mice during Staphylococcal Infection Leads to Prolonged Tnfα Production and Reduced Neutrophil Clearance. PLoS ONE 2011, 6, e23633. [Google Scholar] [CrossRef]
- Wong, S.L.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 2015, 21, 815–819. [Google Scholar] [CrossRef]
- Berger-Achituv, S.; Brinkmann, V.; Abu-Abed, U.; Kühn, L.I.; Ben-Ezra, J.; Elhasid, R.; Zychlinsky, A. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front. Immunol. 2013, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Rizo, V.; Martinez-Guzman, M.; Iñiguez-Gutierrez, L.; García-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front. Immunol. 2017, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Pelayo, R.; Monestier, M.; Ammollo, C.T.; Semeraro, F.; Taylor, F.B.; Esmon, N.L.; Lupu, F.; Esmon, C.T. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009, 15, 1318–1321. [Google Scholar] [CrossRef]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2012, 13, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.H.; Vrba, E.S. A complete estimate of the phylogenetic relationships in Ruminantia: A dated species-level supertree of the extant ruminants. Biol. Rev. 2005, 80, 269–302. [Google Scholar] [CrossRef]
- Simianer, H.; Reimer, C. COVID-19: A “black swan” and what animal breeding can learn from it. Anim. Front. 2021, 11, 57–59. [Google Scholar] [CrossRef]
- Leung, T.L.F.; Bates, A.E. More rapid and severe disease outbreaks for aquaculture at the tropics: Implications for food security. J. Appl. Ecol. 2012, 50, 215–222. [Google Scholar] [CrossRef]
- Roeber, F.; Jex, A.R.; Gasser, R.B. Impact of gastrointestinal parasitic nematodes of sheep, and the role of advanced molecular tools for exploring epidemiology and drug resistance—An Australian perspective. Parasites Vectors 2013, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Worku, M.; Franco, R.; Baldwin, K. Efficacy of garlic as an anthelmintic in adult Boer goats. Arch. Biol. Sci. 2009, 61, 135–140. [Google Scholar] [CrossRef]
- Gasbarre, L.C.; Leighton, E.A.; Sonstegard, T. Role of the bovine immune system and genome in resistance to gastrointestinal nematodes. Vet. Parasitol. 2001, 98, 51–64. [Google Scholar] [CrossRef]
- Paape, M.; Rautiainen, P.; Lilius, E.; Malstrom, C.; Elsasser, T. Development of Anti-Bovine TNF-α mAb and ELISA for Quantitating TNF-α in Milk after Intramammary Injection of Endotoxin. J. Dairy Sci. 2002, 85, 765–773. [Google Scholar] [CrossRef]
- Fingerhut, L.; Dolz, G.; De Buhr, N. What Is the Evolutionary Fingerprint in Neutrophil Granulocytes? Int. J. Mol. Sci. 2020, 21, 4523. [Google Scholar] [CrossRef]
- Asiamah, E.K.; Adjei-Fremah, S.; Ekwemalor, K.; Sordillo, L.; Worku, M. Parity and Periparturient Period Affects Galectin Gene Expression in Holstein Cow Blood. J. Appl. Biotechnol. 2018, 6, 19. [Google Scholar] [CrossRef]
- Asiamah, E.K.; Adjei-Fremah, S.; Osei, B.; Ekwemalor, K.; Worku, M. An Extract of Sericea Lespedeza Modulates Production of Inflammatory Markers in Pathogen Associated Molecular Pattern (PAMP) Activated Ruminant Blood. J. Agric. Sci. 2016, 8, 1. [Google Scholar] [CrossRef]
- Asiamah, E.K.; Ekwemalor, K.; Adjei-Fremah, S.; Osei, B.; Newman, R.; Worku, M. Natural and synthetic pathogen associated molecular patterns modulate galectin expression in cow blood. J. Anim. Sci. Technol. 2019, 61, 245–253. [Google Scholar] [CrossRef]
- Osei, B.; Worku, M.; Adjei-Fremah, S.; Asiamah, E.; Ekwemalor, K.; Eluka-Okoludoh, E.; Mulakala, B. Galectin Secretion and Modulation in Sheep Blood. J. Mol. Biol. Res. 2018, 8, p183. [Google Scholar] [CrossRef]
- Ekwemalor, K.; Asiamah, E.; Adjei-Fremah, S.; Worku, M. Effect of a Mushroom (Coriolus versicolor) Based Probiotic on Goat Health. Am. J. Anim. Vet. Sci. 2016, 11, 108–118. [Google Scholar] [CrossRef]
- Ekwemalor, K.; Asiamah, E.K.; Osei, B.; Ismail, H.; Worku, M. Evaluation of the Effect of Probiotic Administration on Gene Expression in Goat Blood. J. Mol. Biol. Res. 2017, 7, 88. [Google Scholar] [CrossRef][Green Version]
- Ekwemalor, K.; Adjei-Fremah, S.; Asiamah, E.; Eluka-Okoludoh, E.; Osei, B.; Worku, M. Systemic expression of galectin genes in periparturient goats. Small Rumin. Res. 2018, 168, 60–68. [Google Scholar] [CrossRef]
- Sarah, A.-F.; Jackai, L.E.; Worku, M. Analysis of Phenolic Content and Antioxidant Properties of Selected Cowpea Varieties Tested in Bovine Peripheral Blood. Am. J. Anim. Vet. Sci. 2015, 10, 235–245. [Google Scholar]
- Erpenbeck, L.; Gruhn, A.L.; Kudryasheva, G.; Günay, G.; Meyer, D.; Busse, J.; Neubert, E.; Schön, M.P.; Rehfeldt, F.; Kruss, S. Effect of adhesion and substrate elasticity on neutrophil extracellular trap formation. Front. Immunol. 2019, 10, 2320. [Google Scholar] [CrossRef] [PubMed]
- Sabbione, F.; Keitelman, I.A.; Iula, L.; Ferrero, M.; Giordano, M.N.; Baldi, P.; Rumbo, M.; Jancic, C.; Trevani, A.S. Neutrophil Extracellular Traps Stimulate Proinflammatory Responses in Human Airway Epithelial Cells. J. Innate Immun. 2017, 9, 387–402. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.; Wang, Y.; Wang, C.; Liu, X.; Han, Z.; Fu, Y.; Yang, Z. Effects of Neutrophil Extracellular Traps on Bovine Mammary Epithelial Cells in vitro. Front. Immunol. 2019, 10, 1003. [Google Scholar] [CrossRef]
- Fichtner, T.; Kotarski, F.; Gärtner, U.; Conejeros, I.; Hermosilla, C.; Wrenzycki, C.; Taubert, A. Bovine sperm samples induce different NET phenotypes in a NADPH oxidase-, PAD4-, and Ca++-dependent process. Biol. Reprod. 2020, 102, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, F.; Silva, L.; Uribe, P.; Gärtner, U.; Taubert, A.; Schulz, M.; Sánchez, R.; Hermosilla, C. SOCE-inhibitor reduced human sperm-induced formation of neutrophil extracellular traps. Reproduction 2021, 161, 21–29. [Google Scholar] [CrossRef]
- Bean, A.G.D.; Baker, M.L.; Stewart, C.R.; Deffrasnes, C.; Wang, L.-F.; Lowenthal, J.W. Studying immunity to zoonotic diseases in the natural host - keeping it real. Nat. Rev. Immunol. 2013, 13, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.M.E.; Shehata, A.M.; Alzahrani, S.O.; Shafi, M.E.; Mesalam, N.M.; Taha, A.E.; Swelum, A.A.; Arif, M.; Fayyaz, M.; Abd El-Hack, M.E. The role of polyphenols in poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1851–1866. [Google Scholar] [CrossRef]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allergy Immunol. 2020, 1–18. [Google Scholar] [CrossRef]
- Zappulli, V.; Ferro, S.; Bonsembiante, F.; Brocca, G.; Calore, A.; Cavicchioli, L.; Centelleghe, C.; Corazzola, G.; De Vreese, S.; Gelain, M.E.; et al. Pathology of Coronavirus Infections: A Review of Lesions in Animals in the One-Health Perspective. Animals 2020, 10, 2377. [Google Scholar] [CrossRef]
- Ekwemalor, K.; Adjei-Fremah, S.; Asiamah, E.; Worku, M. Molecular Genetics and Genome Biology of Goats. In Goat Science; IntechOpen Limited: London, UK, 2018. [Google Scholar]
- Paape, M.J.; Bannerman, D.D.; Zhao, X.; Lee, J.-W. The bovine neutrophil: Structure and function in blood and milk. Vet. Res. 2003, 34, 597–627. [Google Scholar] [CrossRef]
- Vong, L.; Lorentz, R.J.; Assa, A.; Glogauer, M.; Sherman, P. Probiotic Lactobacillus rhamnosus Inhibits the Formation of Neutrophil Extracellular Traps. J. Immunol. 2014, 192, 1870–1877. [Google Scholar] [CrossRef] [PubMed]
- Cacciotto, C.; Cubeddu, T.; Addis, M.F.; Anfossi, A.G.; Tedde, V.; Tore, G.; Carta, T.; Rocca, S.; Chessa, B.; Pittau, M.; et al. Mycoplasma lipoproteins are major determinants of neutrophil extracellular trap formation. Cell. Microbiol. 2016, 18, 1751–1762. [Google Scholar] [CrossRef]
- Aulik, N.A.; Hellenbrand, K.M.; Czuprynski, C.J. Mannheimia haemolytica and Its Leukotoxin Cause Macrophage Extracellular Trap Formation by Bovine Macrophages. Infect. Immun. 2012, 80, 1923–1933. [Google Scholar] [CrossRef]
- Aulik, N.A.; Hellenbrand, K.M.; Klos, H.; Czuprynski, C.J. Mannheimia haemolytica and Its Leukotoxin Cause Neutrophil Extracellular Trap Formation by Bovine Neutrophils. Infect. Immun. 2010, 78, 4454–4466. [Google Scholar] [CrossRef]
- Hellenbrand, K.M.; Forsythe, K.M.; Rivera-Rivas, J.J.; Czuprynski, C.J.; Aulik, N.A. Histophilus somni causes extracellular trap formation by bovine neutrophils and macrophages. Microb. Pathog. 2013, 54, 67–75. [Google Scholar] [CrossRef]
- Maksimov, P.; Hermosilla, C.; Kleinertz, S.; Hirzmann, J.; Taubert, A. Besnoitia besnoiti infections activate primary bovine endothelial cells and promote PMN adhesion and NET formation under physiological flow condition. Parasitol. Res. 2016, 115, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Grob, D.; Conejeros, I.; Velásquez, Z.D.; Preußer, C.; Gärtner, U.; Alarcón, P.; Burgos, R.A.; Hermosilla, C.; Taubert, A. Trypanosoma brucei brucei Induces Polymorphonuclear Neutrophil Activation and Neutrophil Extracellular Traps Release. Front. Immunol. 2020, 11, 559561. [Google Scholar] [CrossRef] [PubMed]
- Villagra-Blanco, R.; Silva, L.; Gärtner, U.; Wagner, H.; Failing, K.; Wehrend, A.; Taubert, A.; Hermosilla, C. Molecular analyses on Neospora caninum-triggered NETosis in the caprine system. Dev. Comp. Immunol. 2017, 72, 119–127. [Google Scholar] [CrossRef]
- Yang, Z.; Wei, Z.; Hermosilla, C.; Taubert, A.; He, X.; Wang, X.; Gong, P.; Li, J.; Zhang, X. Caprine Monocytes Release Extracellular Traps against Neospora caninum In Vitro. Front. Immunol. 2018, 8, 2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Stadler, S.C.; Correll, S.; Li, P.; Wang, D.; Hayama, R.; Leonelli, L.; Han, H.; Grigoryev, S.A.; et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 2009, 184, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Wu, Z.; Zhu, X.; Li, P.; Wang, J.; Yang, Z. Characterization of Bovine Neutrophil Extracellular Traps Induced by Histamine. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Hahn, S.; Giaglis, S.; Chowdury, C.S.; Hösli, I.; Hasler, P. Modulation of neutrophil NETosis: Interplay between infectious agents and underlying host physiology. Semin. Immunopathol. 2013, 35, 439–453. [Google Scholar] [CrossRef]
- Peixoto, R.; Silva, L.M.R.; López-Osório, S.; Zhou, E.; Gärtner, U.; Conejeros, I.; Taubert, A.; Hermosilla, C. Fasciola hepatica induces weak NETosis and low production of intra- and extracellular ROS in exposed bovine polymorphonuclear neutrophils. Dev. Comp. Immunol. 2021, 114, 103787. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, P.; Manosalva, C.; Quiroga, J.; Belmar, I.; Álvarez, K.; Díaz, G.; Taubert, A.; Hermosilla, C.; Carretta, M.D.; Burgos, R.A.; et al. Oleic and Linoleic Acids Induce the Release of Neutrophil Extracellular Traps via Pannexin 1-Dependent ATP Release and P2X1 Receptor Activation. Front. Vet. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2010, 7, 75–77. [Google Scholar] [CrossRef]
- Bannerman, D.D.; Paape, M.J.; Lee, J.-W.; Zhao, X.; Hope, J.C.; Rainard, P. Escherichia coli and Staphylococcus aureus Elicit Differential Innate Immune Responses following Intramammary Infection. Clin. Vaccine Immunol. 2004, 11, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Worku, M.; Morris, A. Binding of different forms of lipopolysaccharide and gene expression in bovine blood neutrophils. J. Dairy Sci. 2009, 92, 3185–3193. [Google Scholar] [CrossRef] [PubMed]
- Elsherif, L.; Sciaky, N.; Metts, C.A.; Modasshir, M.; Rekleitis, I.; Burris, C.A.; Walker, J.A.; Ramadan, N.; Leisner, T.M.; Holly, S.P.; et al. Machine Learning to Quantitate Neutrophil NETosis. Sci. Rep. 2019, 9, 16891. [Google Scholar] [CrossRef]
- Adjei-Fremah, S.; Everett, A.; Franco, R.; Moultone, K.; Asiamah, E.; Ekwemalor, K.; Jackai, L.E.; Whitley, N.; Schimmel, K.; Worku, M. Health and production benefits of feeding cowpeas to goats. J. Anim. Sci. 2016, 94, 80–81. [Google Scholar] [CrossRef]
- Fisher, J.; Mohanty, T.; Karlsson, C.; Khademi, S.M.H.; Malmström, E.; Frigyesi, A.; Nordenfelt, P.; Malmstrom, J.; Linder, A. Proteome profiling of recombinant DNase therapy in reducing NETs and aiding recovery in COVID-19 patients. Mol. Cell. Proteom. MCP 2021, 100113. [Google Scholar] [CrossRef]
- Adjei-Fremah, S.; Asiamah, E.K.; Ekwemalor, K.; Jackai, L.; Schimmel, K.; Worku, M. Modulation of Bovine Wnt Signaling Pathway Genes by Cowpea Phenolic Extract. J. Agric. Sci. 2016, 8, 21. [Google Scholar] [CrossRef][Green Version]
- Worku, M.; Franco, R.; Miller, J. Evaluation of the Activity of Plant Extracts in Boer Goats. Am. J. Anim. Vet. Sci. 2009, 4, 72–79. [Google Scholar] [CrossRef]
- Worku, M.; Abdalla, A.; Adjei-Fremah, S.; Ismail, H. The Impact of Diet on Expression of Genes Involved in Innate Immunity in Goat Blood. J. Agric. Sci. 2016, 8, 1. [Google Scholar] [CrossRef][Green Version]
- Adjei-Fremah, S.; Ekwemalor, K.; Asiamah, E.; Ismail, H.; Worku, M. Transcriptional profiling of the effect of lipopolysaccharide (LPS) pretreatment in blood from probiotics-treated dairy cows. Genom. Data 2016, 10, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Adjei-Fremah, S.; Ekwemalor, K.; Worku, M.; Ibrahim, S. Probiotics and Ruminant Health; IntechOpen Limited: London, UK, 2018. [Google Scholar]
- Vorobjeva, N.V.; Pinegin, B.V. Effects of the antioxidants Trolox, Tiron and Tempol on neutrophil extracellular trap formation. Immunobiology 2016, 221, 208–219. [Google Scholar] [CrossRef]
- Kirchner, T.; Hermann, E.; Möller, S.; Klinger, M.; Solbach, M.D.W.; Laskay, T.; Behnen, M. Flavonoids and 5-Aminosalicylic Acid Inhibit the Formation of Neutrophil Extracellular Traps. Mediat. Inflamm. 2013, 2013, 710239. [Google Scholar] [CrossRef]
- Adjei-Fremah, S.; Worku, M. Cowpea polyphenol extract regulates galectin gene expression in bovine blood. Anim. Biotechnol. 2021, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Adjei-Fremah, S.; Jackai, L.E.N.; Schimmel, K.; Worku, M. Microarray analysis of the effect of Cowpea (Vigna unguiculata) phenolic extract in bovine peripheral blood. J. Appl. Anim. Res. 2016, 46, 100–106. [Google Scholar] [CrossRef]
- Adjei-Fremah, S.; Jackai, L.E.; Schimmel, K.; Worku, M. Immunomodulatory activities of polyphenol extract from cowpea (Vigna unguiculata) on bovine polymorphonuclear neutrophils. J. Anim. Sci. 2016, 94, 86–87. [Google Scholar] [CrossRef]
- Adjei-Fremah, S.; Asiamah, E.; Ekwemalor, K.; Osei, B.; Ismail, H.; Jackai, L.E.; Worku, M. The anti-inflammatory effect of cowpea polyphenol in bovine blood. J. Anim. Sci. 2017, 95, 27. [Google Scholar] [CrossRef][Green Version]
- Worku, M.; Adjei-Fremah, S.; Whitley, N.; Jackai, L. Effect of Cowpea (Vigna unguiculata) Pasture Grazing on Growth, Gastrointestinal Parasite Infection and Immune Response Biomarkers of Goat. J. Agric. Sci. 2017, 10, 27. [Google Scholar] [CrossRef]
- Ekwemalor, K.; Asiamah, E.; Worku, M. Effect of a Mushroom (Coriolus versicolor) Based Probiotic on the Expression of Toll-like Receptors and Signal Transduction in Goat Neutrophils. J. Mol. Biol. Res. 2015, 6, 71. [Google Scholar] [CrossRef][Green Version]
- Gyenai, K.; Worku, M.; Tajkarimi, M.; Ibrahim, S. Influence of Probiotics on Coccidia, H. Contortus and Markers of Infection in Goats. Am. J. Anim. Vet. Sci. 2016, 11, 91–99. [Google Scholar] [CrossRef][Green Version]
- Salvesen, Ø.; Reiten, M.R.; Heegaard, P.M.H.; Tranulis, M.A.; Espenes, A.; Skovgaard, K.; Ersdal, C. Activation of innate immune genes in caprine blood leukocytes after systemic endotoxin challenge. BMC Vet. Res. 2016, 12, 241. [Google Scholar] [CrossRef]
- Asiamah, E.K. Galectin Gene Expression and Secretion in Dairy Cow Blood; North Carolina Agricultural and Technical State University: Greensboro, NC, USA, 2018. [Google Scholar]
- Meglia, G.E.; Johannisson, A.; Petersson, L.; Waller, K.P. Investigation in blood Leukocytes and Neutrophils in Periparturient Dairy Cow. Sci. J. Anim. Vet. Sci. 2018, 1, 001–009. [Google Scholar]
- Li, C.; Batistel, F.; Osorio, J.S.; Drackley, J.K.; Luchini, D.; Loor, J.J. Peripartal rumen-protected methionine supplementation to higher energy diets elicits positive effects on blood neutrophil gene networks, performance and liver lipid content in dairy cows. J. Anim. Sci. Biotechnol. 2016, 7, 18. [Google Scholar] [CrossRef]
- Riboni, M.V.; Zhou, Z.; Jacometo, C.; Minuti, A.; Trevisi, E.; Luchini, D.; Loor, J. Supplementation with rumen-protected methionine or choline during the transition period influences whole-blood immune response in periparturient dairy cows. J. Dairy Sci. 2017, 100, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.S.; Ji, P.; Drackley, J.K.; Luchini, D.; Loor, J.J. Supplemental Smartamine M or MetaSmart during the transition period benefits postpartal cow performance and blood neutrophil function. J. Dairy Sci. 2013, 96, 6248–6263. [Google Scholar] [CrossRef] [PubMed]
- Stella, S.; Velasco-Acosta, D.; Skenandore, C.; Zhou, Z.; Steelman, A.; Luchini, D.; Cardoso, F. Improved uterine immune mediators in Holstein cows supplemented with rumen-protected methionine and discovery of neutrophil extracellular traps (NET). Theriogenology 2018, 114, 116–125. [Google Scholar] [CrossRef]
- Parker, H.; Albrett, A.M.; Kettle, A.J.; Winterbourn, C.C. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J. Leukoc. Biol. 2011, 91, 369–376. [Google Scholar] [CrossRef]
- Petretto, A.; Bruschi, M.; Pratesi, F.; Croia, C.; Candiano, G.; Ghiggeri, G.M.; Migliorini, P. Neutrophil extracellular traps (NET) induced by different stimuli: A comparative proteomic analysis. PLoS ONE 2019, 14, e0218946. [Google Scholar] [CrossRef]
- Asiamah, E.K.; Vailati-Riboni, M.; Zhou, Z.; Xu, T.; Loor, J.; Schimmel, K.; Worku, M. Rumen-protected methionine supplementation during the peripartal period alters the expression of galectin genes associated with inflammation in peripheral neutrophils and secretion in plasma of Holstein cows. J. Dairy Res. 2019, 86, 394–398. [Google Scholar] [CrossRef]
- Cooper, D.; Iqbal, A.J.; Gittens, B.R.; Cervone, C.; Perretti, M. The effect of galectins on leukocyte trafficking in inflammation: Sweet or sour? Ann. N. Y. Acad. Sci. 2012, 1253, 181–192. [Google Scholar] [CrossRef]
- Schorn, C.; Janko, C.; Krenn, V.; Zhao, Y.; Munoz, L.E.; Schett, G.; Herrmann, M. Bonding the foe—NETting neutrophils immobilize the pro-inflammatory monosodium urate crystals. Front. Immunol. 2012, 3, 376. [Google Scholar] [CrossRef] [PubMed]
- Nishi, N.; Shoji, H.; Seki, M.; Itoh, A.; Miyanaka, H.; Yuube, K.; Hirashima, M.; Nakamura, T. Galectin-8 modulates neutrophil function via interaction with integrin αM. Glycobiology 2003, 13, 755–763. [Google Scholar] [CrossRef]
- Thurston, T.; Wandel, M.P.; Von Muhlinen, N.; Foeglein, Á.; Randow, F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nat. Cell Biol. 2012, 482, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Godínez, C.; Carrero, J.C. The state of art of neutrophil extracellular traps in protozoan and helminthic infections. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
| Microorganisms | Species | Mechanism | Types of NETosis | References |
|---|---|---|---|---|
| BACTERIA | ||||
| Staphylococcus aureus | Mice Humans Bovine | Dependent on TLR2 and Complement C3 in mice PAD4 dependent Response to virulence factor, PVL in a ROS independent manner DNA extruded via vesicles Unknown | Vital Vital Unknown | [26] [40] [27] |
| E. coli | Humans * Mice * Bovine | * Mediated via platelet TLR4 Histone H3 citrullination by PAD4 | Vital in the presence of platelets | [39] [28] |
| E. coli LPS | Humans * | * Mediated via platelet TLR4 and present HMGB1 to neutrophils | Vital in vivo | [29] |
| VIRUS | ||||
| Influenza A Influenza H1N1 | Mice Humans | Not dependent on PAD4 ROS and PAD4 dependent | Suicidal Suicidal | [30] |
| PARASITES | ||||
| Eimeria bovis | Bovine | Recognition by CD11b Dependent on NAPDH oxidase, NE and MPO Requires p38 MAPK and ERK1/2 phosphorylation | Unknown | [31] [32] |
| Eimeria arloingi | Goat | NADPH oxidase dependent | Unknown | [33] |
| Besnoitia besnoiti | Bovine | Dependent on NAPDH oxidase, NE and MPO | Unknown | [34] |
| Toxoplasma gondii | Humans Mice | ERK-MEK dependent * NADPH oxidase/ROS dependent | Suicidal | [35] |
| Inducer Type | Reference |
|---|---|
| Staphylococcus aureus | [40,47] |
| Streptococcus sp. | [48] |
| Haemophilus influenzae | [49] |
| Klebsiella pneumoniae | [16] |
| Listeria monocytogenes | [50] |
| Mycobacterium tuberculosis | [51] |
| Shigella flexneri | [9] |
| Aspergillus nidulans | [52,53,54] |
| Aspergillus fumigatus | |
| Candida albicans | |
| Porphyromonas gingivalis | [55] |
| V. cholera | [45] |
| Aeromonas hydrophila | [56] |
| E. arloingi sporozoites, B. besnoiti, C. parvum, Spermatozoa, H. contortus, N. caninum, D (-) lactic acid, M. bovis, E. ninakohlyakimovae, T. gondii, S. uberis | [24,31,32,33,34,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72] |
| Modulator (s) | Sample Type (s) | Cytokines | Innate Immune Response | Reference |
|---|---|---|---|---|
| Probiotics | Whole blood, serum | IL2, IL5, IL10, IL8, IL18 | TLR4, TLR6, TLR7, TLR9 | [96,143] |
| Cowpea | Whole blood, serum, plasma | TNFα, IL1α, ILβ, IL8 IL10RA, IL15, IP10, GCSF, Rantes and IFNγ | TLR2 | [139] |
| Sericea lespedeza | Whole blood, Serum | TNF-α, IFNr, GCSF, GMCSF, IL-1α, IP-10 | TLR2 and TLR4 | [132] |
| Mushroom | Neutrophils, Whole blood, serum | IFNr, Rantes, GCSF, and GM-CSF | TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10 | [142] |
| Lipopolysaccharide | Mammary epithelial cells, whole blood, blood leukocytes | IL1B, CCL3 and IL8, CCL2, CXCL6, IL6, CXCL8 | PTGS2, IFIT3, MYD88, NFKB1, and TLR4 | [92,144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worku, M.; Rehrah, D.; Ismail, H.D.; Asiamah, E.; Adjei-Fremah, S. A Review of the Neutrophil Extracellular Traps (NETs) from Cow, Sheep and Goat Models. Int. J. Mol. Sci. 2021, 22, 8046. https://doi.org/10.3390/ijms22158046
Worku M, Rehrah D, Ismail HD, Asiamah E, Adjei-Fremah S. A Review of the Neutrophil Extracellular Traps (NETs) from Cow, Sheep and Goat Models. International Journal of Molecular Sciences. 2021; 22(15):8046. https://doi.org/10.3390/ijms22158046
Chicago/Turabian StyleWorku, Mulumebet, Djaafar Rehrah, Hamid D. Ismail, Emmanuel Asiamah, and Sarah Adjei-Fremah. 2021. "A Review of the Neutrophil Extracellular Traps (NETs) from Cow, Sheep and Goat Models" International Journal of Molecular Sciences 22, no. 15: 8046. https://doi.org/10.3390/ijms22158046
APA StyleWorku, M., Rehrah, D., Ismail, H. D., Asiamah, E., & Adjei-Fremah, S. (2021). A Review of the Neutrophil Extracellular Traps (NETs) from Cow, Sheep and Goat Models. International Journal of Molecular Sciences, 22(15), 8046. https://doi.org/10.3390/ijms22158046






