Jasmonic Acid Plays a Pivotal Role in Pollen Development and Fertility Regulation in Different Types of P(T)GMS Rice Lines
Abstract
:1. Introduction
2. Results
2.1. Photoperiod and Temperature Experimental System for Pollen Fertility Transformations of Both PGMS and PTGMS Rice Lines
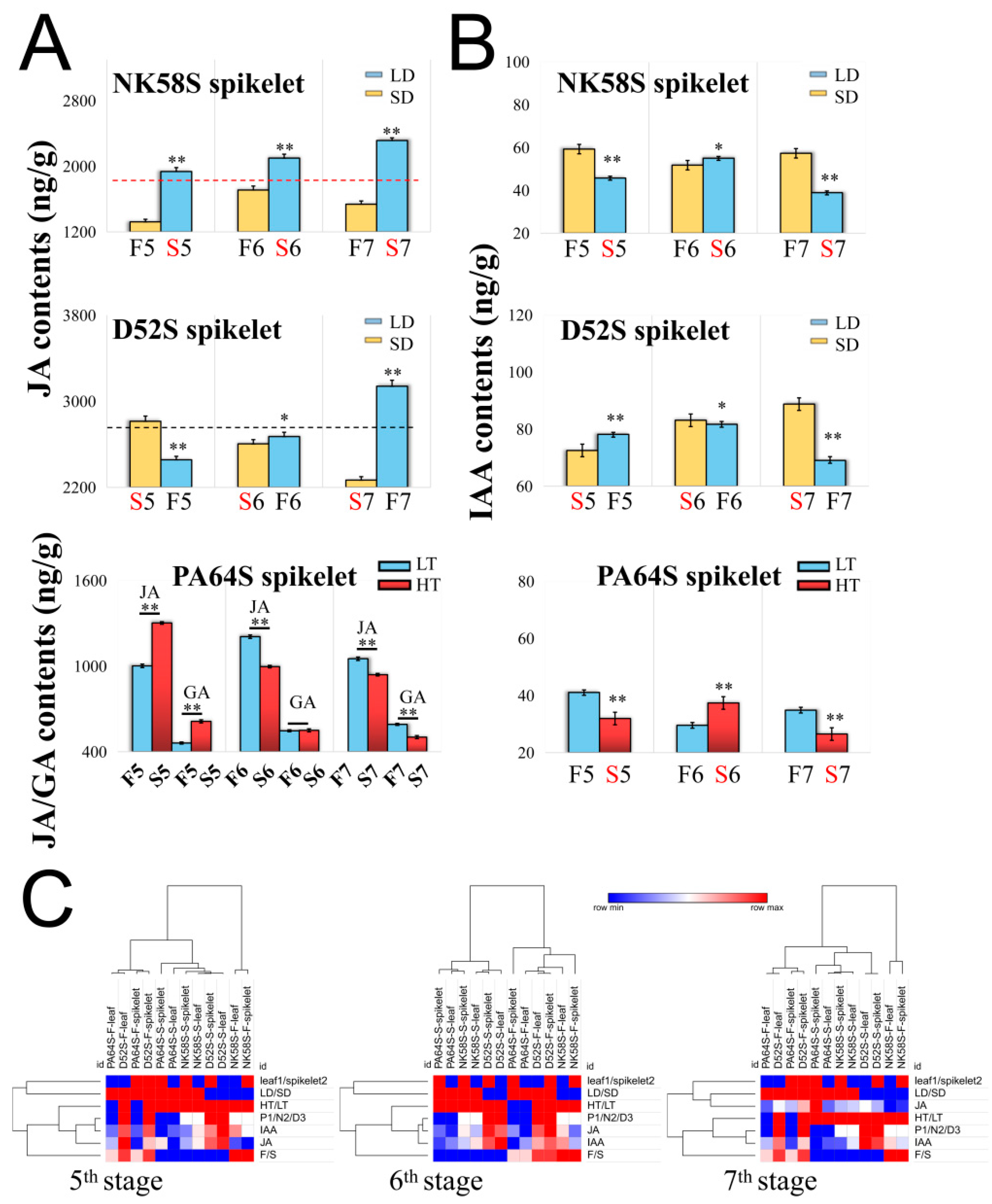
2.2. JA regulates the Pollen Fertility of Both PGMS and PTGMS Rice Lines
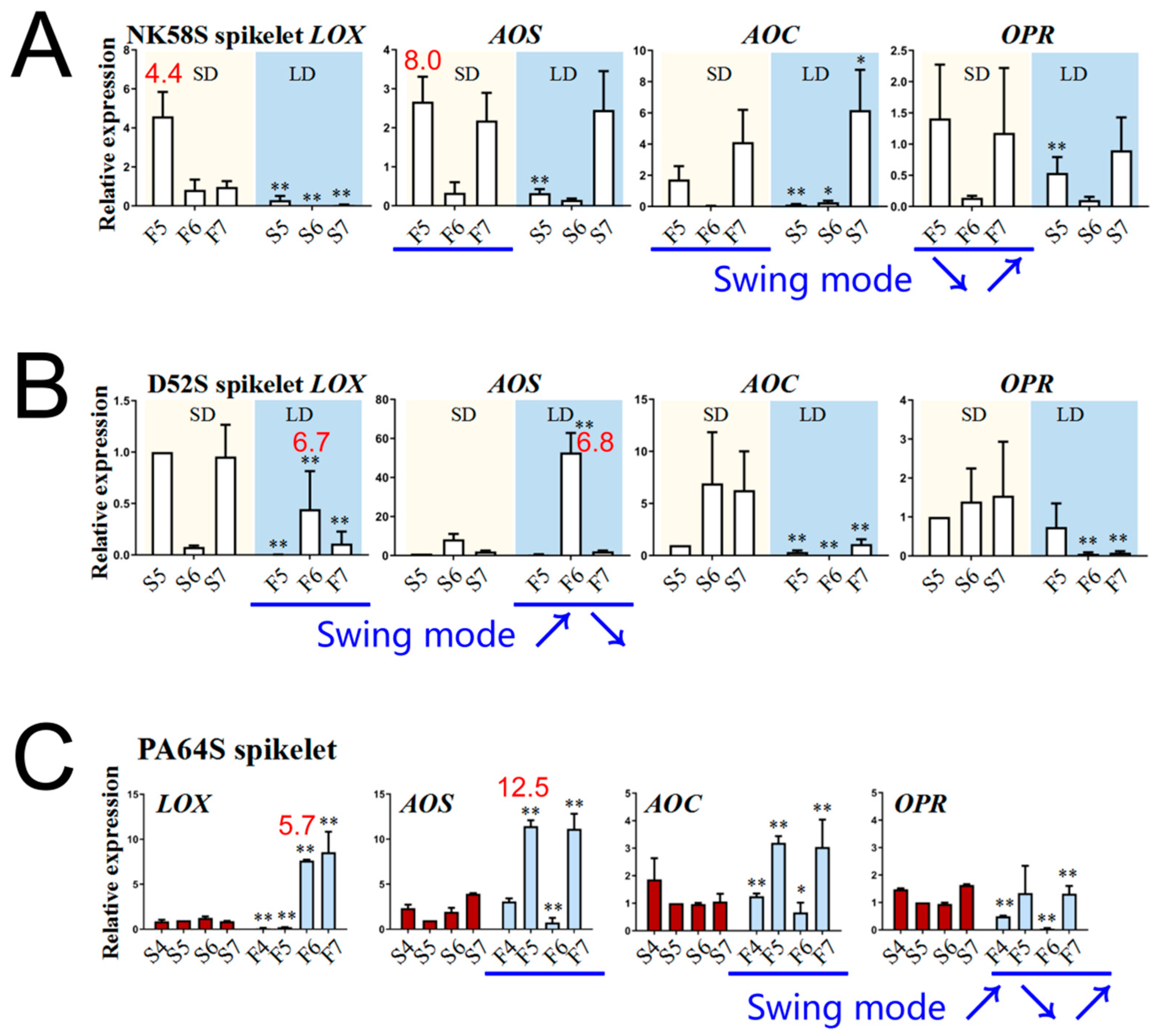
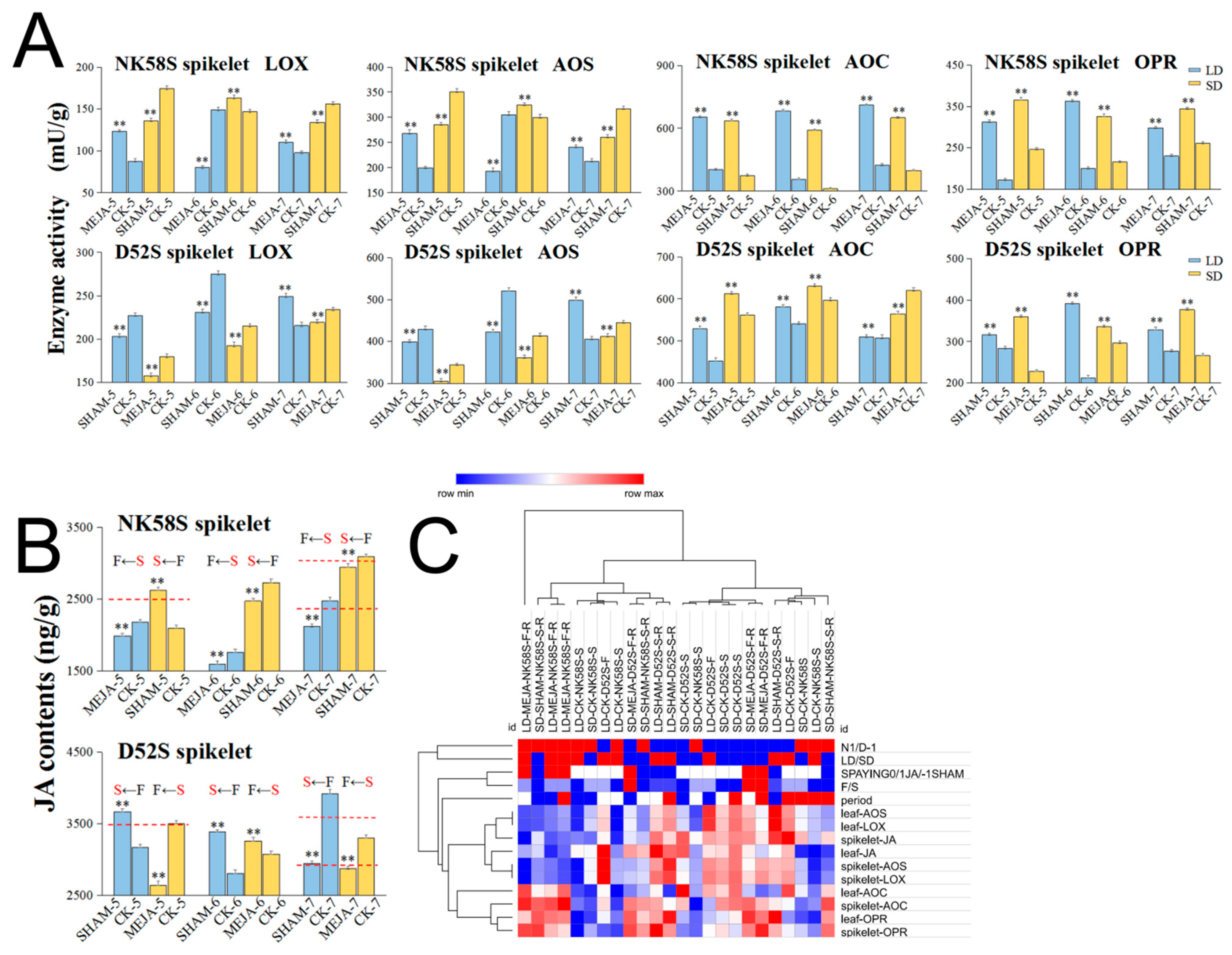
2.3. Analysis of JA Synthetic Enzyme Activities in Pollen Fertility for Both PGMS and PTGMS Rice Lines
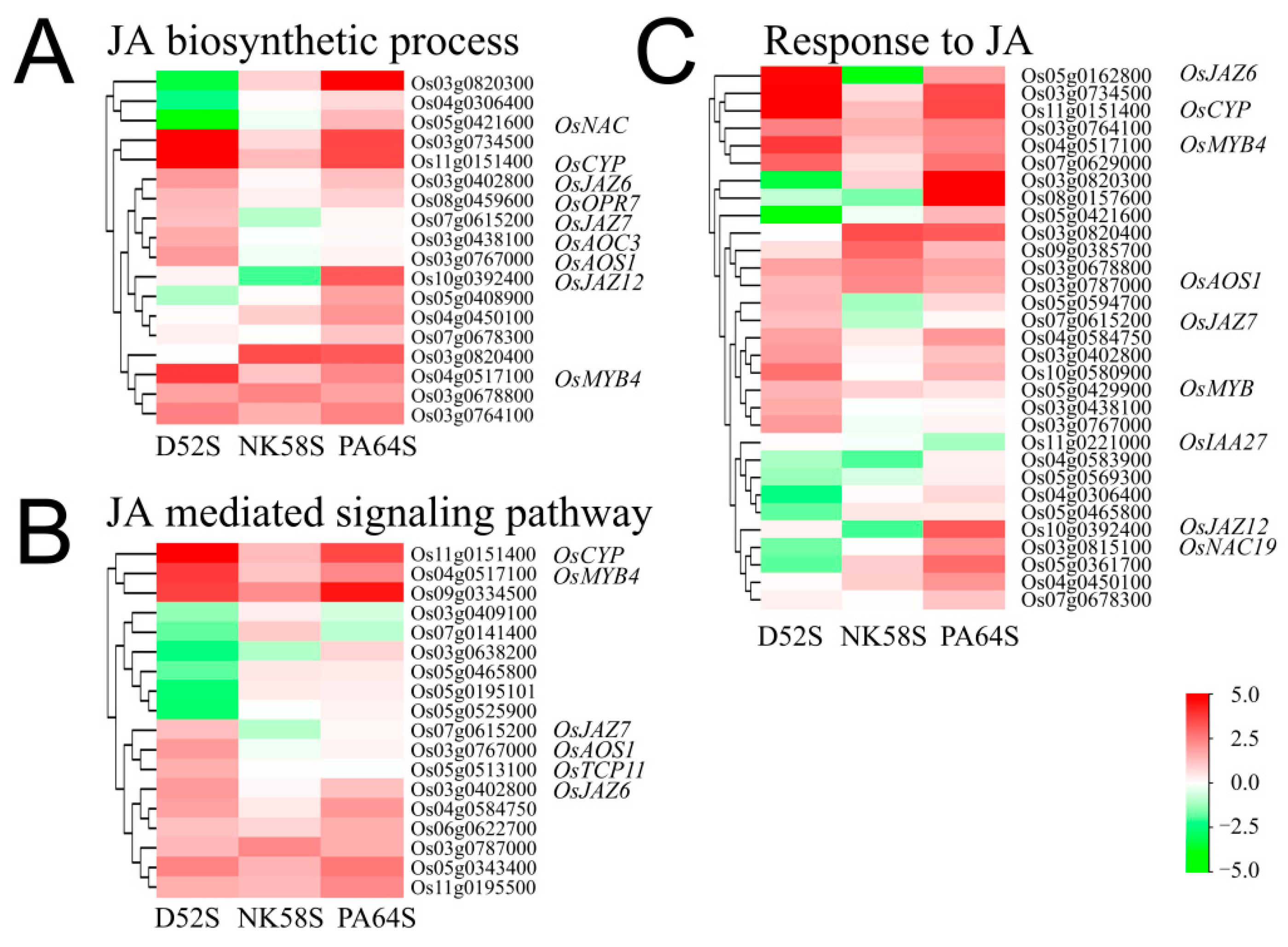
2.4. Analysis of JA Synthesis Gene Expression in Sterile and Fertile Lines by Transcriptome and qPCR Validation
2.5. Methyl Jasmonate/Salicylhydroxamic Acid Spraying Treatment for Fertility Reversal in PGMS Rice
3. Discussion
3.1. The Key Role of JA in Male Sterility in Rice
3.2. GA Specifically Regulates PA64S Pollen Fertility
3.3. A Possible Model for miRNAs and Phytohormones (JA, GA) Crosstalk Network in Rice Fertility Regulation
4. Materials and Methods
4.1. Plant Materials
4.2. Rice Planting and Treatment under Sterile/Fertile Photoperiod and Temperature Conditions
4.3. MEJA and SHAM Spraying Treatment for Fertility Reversal
4.4. Phenotype Identification and Characterization and Sampling of Sterile and Fertile Plants
4.5. RNA Extraction and RNA Sequencing
4.6. Quantitative Real-Time PCR Validation of Gene Expression
4.7. Measurement of LOX, AOS, AOC and OPR Enzyme Activities and Plant Hormone Contents
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Kim, Y.-J.; Zhang, D. Molecular Control of Male Fertility for Crop Hybrid Breeding. Trends Plant Sci. 2018, 23, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L. Purification and production of foundation seed of rice PGMS and TGMS lines. Hybrid Rice 1994, 6, 3. [Google Scholar]
- Chen, L.-Y.; Lei, D.-Y.; Tang, W.-B.; Xiao, Y.-H. Thoughts and Practice on Some Problems about Research and Application of Two-Line Hybrid Rice. Rice Sci. 2011, 18, 79–85. [Google Scholar] [CrossRef]
- Shi, M.S. The discovery and study of the photosensitive recessive male-sterile rice (Oryza sativa L. subsp. japonica). Sci. Agric. Sin. 1985, 2, 44–48. [Google Scholar]
- Chen, L.-B.; Zhou, G.-Q.; Huang, Y.-X. Effects of Temperature and Photoperiod on Fertility and Physiological Activities of Rice Annong S-1 and Hengnong S-1. J. Integr. Plant Biol. 1994, 36, 119–123. [Google Scholar]
- Zeng, H.; Zhang, D. Developing near isogenic lines of different critical male sterile temperature of thermo-photoperiod sensitive male sterile rice Peiai 64S. Acta Agron. Sin. 2001, 27, 351–355. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, J.; Qian, Q.; Chen, H.; Jin, J.; Ding, Y. Small RNA Profiles of the Rice PTGMS Line Wuxiang S Reveal miRNAs Involved in Fertility Transition. Front. Plant Sci. 2016, 7, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Shen, B.Z.; Dai, X.K.; Mei, M.H.; Maroof, M.S.; Li, Z.B. Using bulked extremes and recessive class to map genes for photoperiod-sensitive genic male sterility in rice. Proc. Natl. Acad. Sci. USA 1994, 91, 8675–8679. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Xu, W.W.; Wang, J.Z.; Wu, W.; Zheng, H.G.; Yang, Z.Y.; Ray, J.D.; Nguyen, H.T. Tagging and mapping the thermo-sensitive genic male-sterile gene in rice (Oryza sativa L.) with molecular markers. Theor. Appl. Genet. 1995, 91, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Lkeda, R.; Hirasawa, H.; Minami, M.; Ujihara, A. Linkage Analysis of Thermosensitive Genic Male Sterility Gene, tms-2 in Rice (Oryza sativa L.). Jpn. J. Breed. 1997, 47, 371–373. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wang, B.; Wu, Y.; Du, P.; Wang, J.; Wang, M.; Yi, C.; Gu, M.; Liang, G. Fine mapping and candidate gene analysis of ptgms2-1, the photoperiod-thermo-sensitive genic male sterile gene in rice (Oryza sativa L.). Theor. Appl. Genet. 2011, 122, 365–372. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Q.; Li, J.; Jiang, D.; Zhou, L.; Wu, P.; Lu, S.; Li, F.; Zhu, L.; Liu, Z.; et al. Photoperiod- and thermo-sensitive genic male sterility in rice are caused by a point mutation in a novel noncoding RNA that produces a small RNA. Cell Res. 2012, 22, 649–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, Z.; Wei, X.; Shao, G.; Chen, M.; Song, J.; Tang, S.; Luo, J.; Hu, Y.; Hu, P.; Chen, L. Genetic analysis and fine mapping oftms9, a novel thermosensitive genic male-sterile gene in rice (Oryza sativa L.). Plant Breed. 2013, 132, 159–164. [Google Scholar] [CrossRef]
- Dobritsa, A.A.; Shrestha, J.; Morant, M.; Pinot, F.; Matsuno, M.; Swanson, R.; Møller, B.L.; Preuss, D. CYP704B1 Is a Long-Chain Fatty Acid ω-Hydroxylase Essential for Sporopollenin Synthesis in Pollen of Arabidopsis. Plant Physiol. 2009, 151, 574–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Pinot, F.; Sauveplane, V.; Werck-Reichhart, D.; Diehl, P.; Schreiber, L.; Franke, R.; Zhang, P.; Chen, L.; Gao, Y.; et al. Cytochrome P450 Family Member CYP704B2 Catalyzes the ω-Hydroxylation of Fatty Acids and Is Required for Anther Cutin Biosynthesis and Pollen Exine Formation in Rice. Plant Cell 2010, 22, 173–190. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Liang, W.; Yin, C.; Zong, J.; Gu, F.; Zhang, D. OsC6, Encoding a Lipid Transfer Protein, Is Required for Postmeiotic Anther Development In Rice. Plant Physiol. 2010, 154, 149–162. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Tan, H.; Yu, X.-H.; Liu, Y.; Liang, W.; Ranathunge, K.; Franke, R.B.; Schreiber, L.; Wang, Y.; Kai, G.; et al. Defective Pollen Wall Is Required for Anther and Microspore Development in Rice and Encodes a Fatty Acyl Carrier Protein Reductase. Plant Cell 2011, 23, 2225–2246. [Google Scholar] [CrossRef] [Green Version]
- Qin, P.; Tu, B.; Wang, Y.; Deng, L.; Quilichini, T.D.; Li, T.; Wang, H.; Ma, B.; Li, S. ABCG15 Encodes an ABC Transporter Protein, and is Essential for Post-Meiotic Anther and Pollen Exine Development in Rice. Plant Cell Physiol. 2012, 54, 138–154. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wu, D.; Shi, J.; He, Y.; Pinot, F.; Grausem, B.; Yin, C.; Zhu, L.; Chen, M.; Luo, Z.; et al. Rice CYP703A3, a cytochrome P450 hydroxylase, is essential for development of anther cuticle and pollen exine. J. Integr. Plant Biol. 2014, 56, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Shi, J.; Liang, W.; Xue, F.; Luo, Q.; Zhu, L.; Qu, G.; Chen, M.; Schreiber, L.; Zhang, D.-B. Two ATP Binding Cassette G (ABCG) Transporters, OsABCG26 and OsABCG15, Collaboratively Regulate Rice Male Reproduction. Plant Physiol. 2015, 169, 2064–2079. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, D.; Guo, Z.; Shi, Q.; Xiong, S.-X.; Zhang, C.; Zhu, J.; Yang, Z. OsACOS12, an orthologue of Arabidopsis acyl-CoA synthetase5, plays an important role in pollen exine formation and anther development in rice. BMC Plant Biol. 2016, 16, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Yu, J.; Shi, J.; Tohge, T.; Fernie, A.R.; Meir, S.; Aharoni, A.; Xu, D.; Zhang, D.; Liang, W. The polyketide synthase OsPKS2 is essential for pollen exine and Ubisch body patterning in rice. J. Integr. Plant Biol. 2017, 59, 612–628. [Google Scholar] [CrossRef]
- Pak, H.; Wang, H.; Kim, Y.; Song, U.; Tu, M.; Wu, D.; Jiang, L. Creation of male-sterile lines that can be restored to fertility by exogenous methyl jasmonate for the establishment of a two-line system for the hybrid production of rice (Oryza sativa L.). Plant Biotechnol. J. 2021, 19, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, Z.; Li, H.; Sun, Y.; Wang, L.; Zeng, H.; He, Y. Lipid metabolism is involved in male fertility regulation of the photoperiod- and thermo sensitive genic male sterile rice line Peiai 64S. Plant Sci. 2020, 299, 110581. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, Z.; Li, Q.; Sun, Y.; Jin, J.; Chen, H.; Zou, Y.; Huang, X.; Ding, Y. Circular RNA profiling of the rice photo-thermosensitive genic male sterile line Wuxiang S reveals circRNA involved in the fertility transition. BMC Plant Biol. 2019, 19, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, H.; Li, Q.; Jin, J.; Chen, H.; Zou, Y.; Huang, X.; Ding, Y. Genome-Wide Identification of lncRNAs Involved in Fertility Transition in the Photo-Thermosensitive Genic Male Sterile Rice Line Wuxiang S. Front. Plant Sci. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Z.; Zhi-Guo, E.; Zhang, H.L.; Shu, Q.Y. Workable male sterility systems for hybrid rice: Genetics, biochemistry, molecular biology, and utilization. Rice 2014, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Cai, Q.; Yuan, Z.; Chen, M.; Yin, C.; Luo, Z.; Zhao, X.; Liang, W.; Hu, J.; Zhang, D. Jasmonic acid regulates spikelet development in rice. Nat. Commun. 2014, 5, 3476. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zou, Z.; Qian, K.; Xia, C.; He, Y.; Zeng, H.; Zhou, X.; Riemann, M.; Yin, C. Jasmonic acid deficiency leads to scattered floret opening time in cytoplasmic male sterile rice Zhenshan 97A. J. Exp. Bot. 2017, 68, 4613–4625. [Google Scholar] [CrossRef] [Green Version]
- Creelman, R.A.; Mullet, J.E. Biosynthesis and action of jasmonates in plants. Annu. Rev. Plant Biol. 1997, 48, 355–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellinger, D.; Stingl, N.; Kubigsteltig, I.I.; Bals, T.; Juenger, M.; Pollmann, S.; Berger, S.; Schuenemann, D.; Mueller, M.J. Dongle and defective in anther dehiscence1 Lipases Are Not Essential for Wound- and Pathogen-Induced Jasmonate Biosynthesis: Redundant Lipases Contribute to Jasmonate Formation. Plant Physiol. 2010, 153, 114–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laudert, D.; Pfannschmidt, U.; Lottspeich, F.; Weiler, E.W. Cloning, molecular and functional characterization of Arabidopsis thaliana allene oxide synthase (CYP 74), the first enzyme of the octadecanoid pathway to jasmonates. Plant Mol. Biol. 1996, 31, 323–335. [Google Scholar] [CrossRef]
- Stenzel, I.; Hause, B.; Miersch, O.; Kurz, T.; Maucher, H.; Weichert, H.; Ziegler, J.; Feussner, I.; Wasternack, C. Jasmonate biosynthesis and the allene oxide cyclase family of Arabidopsis thaliana. Plant Mol. Biol. 2003, 51, 895–911. [Google Scholar] [CrossRef] [PubMed]
- Bannenberg, G.; Martínez, M.; Hamberg, M.; Castresana, C. Diversity of the Enzymatic Activity in the Lipoxygenase Gene Family of Arabidopsis thaliana. Lipids 2008, 44, 85–95. [Google Scholar] [CrossRef]
- Schaller, A.; Stintzi, A. Enzymes in jasmonate biosynthesis—Structure, function, regulation. Phytochemistry 2009, 70, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Stintzi, A.; Browse, J. The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc. Natl. Acad. Sci. USA 2000, 97, 10625–10630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiguro, S.; Kawai-Oda, A.; Ueda, J.; Nishida, I.; Okada, K. The Defective in another dehiscence1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 2001, 13, 2191–2209. [Google Scholar] [CrossRef] [Green Version]
- Deng, Q.; Ou, A.; Fu, Z.; Zhu, Q. A Preliminary Study on the Method for Identifying the Practlcal Photo and Thermo sensitive Genic Male Sterile RiceI. Analysis on Fertility Reaction of Photo and Thermo sensitive Genic Male Sterile Rice to Photoperiod and Temperature. Hybrid Rice 1996, 23–27. [Google Scholar] [CrossRef]
- Yan, Y.; Stolz, S.; Chételat, A.; Reymond, P.; Pagni, M.; Dubugnon, L.; Farmer, E.E. A Downstream Mediator in the Growth Repression Limb of the Jasmonate Pathway. Plant Cell 2007, 19, 2470–2483. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.-H.; Lenglet-Hilfiker, A.; Stolz, S.; Glauser, G.; Farmer, E.E. Jasmonate Precursor Biosynthetic Enzymes LOX3 and LOX4 Control Wound-Response Growth Restriction. Plant Physiol. 2020, 184, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Schommer, C.; Palatnik, J.F.; Aggarwal, P.; Chételat, A.; Cubas, P.; Farmer, E.E.; Nath, U.; Weigel, D. Control of Jasmonate Biosynthesis and Senescence by miR319 Targets. PLoS Biol. 2008, 6, e230. [Google Scholar] [CrossRef] [Green Version]
- Jewell, J.B.; Browse, J. Epidermal jasmonate perception is sufficient for all aspects of jasmonate-mediated male fertility in Arabidopsis. Plant J. 2016, 85, 634–647. [Google Scholar] [CrossRef] [Green Version]
- Shaban, M.; Ahmed, M.M.; Sun, H.; Ullah, A.; Zhu, L. Genome-wide identification of lipoxygenase gene family in cotton and functional characterization in response to abiotic stresses. BMC Genom. 2018, 19, 599. [Google Scholar] [CrossRef]
- Pysh, L.D.; Wysocka-Diller, J.W.; Camilleri, C.; Bouchez, D.; Benfey, P.N. The GRAS gene family in Arabidopsis: Sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. Plant J. 1999, 18, 111–119. [Google Scholar] [CrossRef]
- Sun, Y.; Xiong, X.; Wang, Q.; Zhu, L.; Wang, L.; He, Y.; Zeng, H. Integrated Analysis of Small RNA, Transcriptome, and Degradome Sequencing Reveals the MiR156, MiR5488 and MiR399 Are Involved in the Regulation of Male Sterility in PTGMS Rice. Int. J. Mol. Sci. 2021, 22, 2260. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Galvao, V.C.; Zhang, Y.-C.; Horrer, D.; Zhang, T.-Q.; Hao, Y.-H.; Feng, Y.-Q.; Wang, S.; Schmid, M.; Wang, J.-W. Gibberellin Regulates the Arabidopsis Floral Transition through miR156-Targeted SQUAMOSA PROMOTER BINDING–LIKE Transcription Factors. Plant Cell 2012, 24, 3320–3332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, S.; Salinas, M.; Höhmann, S.; Berndtgen, R.; Huijser, P. miR156-Targeted and Nontargeted SBP-Box Transcription Factors Act in Concert to Secure Male Fertility in Arabidopsis. Plant Cell 2011, 22, 3935–3950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curaba, J.; Singh, M.; Bhalla, P.L. miRNAs in the crosstalk between phytohormone signalling pathways. J. Exp. Bot. 2014, 65, 1425–1438. [Google Scholar] [CrossRef]
- Schwab, R.; Palatnik, J.; Riester, M.; Schommer, C.; Schmid, M.; Weigel, D. Specific Effects of MicroRNAs on the Plant Transcriptome. Dev. Cell 2005, 8, 517–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.-H.; Seo, P.J.; Kang, S.K.; Park, C.-M. miR172 signals are incorporated into the miR156 signaling pathway at the SPL3/4/5 genes in Arabidopsis developmental transitions. Plant Mol. Biol. 2011, 76, 35–45. [Google Scholar] [CrossRef]
- Wu, G.; Park, M.Y.; Conway, S.R.; Wang, J.-W.; Weigel, D.; Poethig, R.S. The Sequential Action of miR156 and miR172 Regulates Developmental Timing in Arabidopsis. Cell 2009, 138, 750–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio-Somoza, I.; Weigel, D. MicroRNA networks and developmental plasticity in plants. Trends Plant Sci. 2011, 16, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, T.; Ren, M.-Y.; Zhu, J.; Shi, Q.-S.; Zhang, Y.-F.; Qi, Y.-W.; Huang, M.-J.; Song, L.; Xu, P.; et al. Slow Development Restores the Fertility of Photoperiod-Sensitive Male-Sterile Plant Lines. Plant Physiol. 2020, 184, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Hewezi, T.; Baum, T.J. Complex feedback regulations govern the expression of miRNA396 and its GRF target genes. Plant Signal. Behav. 2012, 7, 749–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhao, G.; Li, Y.; Mo, N.; Zhang, J.; Liang, Y. Transcriptomic Analysis Implies That GA Regulates Sex Expression via Ethylene-Dependent and Ethylene-Independent Pathways in Cucumber (Cucumis sativus L.). Front. Plant Sci. 2017, 8, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanurdzic, M. Sex-Determining Mechanisms in Land Plants. Plant Cell 2004, 16, S61–S71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Li, H.; Ma, X.; Dong, H.; Ma, D.; Zeng, H. Proteome alterations of reverse photoperiod-sensitive genic male sterile rice (Oryza sativa L.) at fertility transformation stage. Genes Genom. 2014, 36, 711–726. [Google Scholar] [CrossRef]
- He, Y.; Luo, Y.; Wang, Q.; Sun, Y.; Duan, N.; Chen, Z.; Zeng, H. Spray treatment of leaves with Fe2+ promotes procyanidin biosynthesis by upregulating the expression of the F3H and ANS genes in red rice grains (Oryza sativa L.). J. Cereal Sci. 2021, 100, 103231. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.M.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Que, F.; Khadr, A.; Wang, G.-L.; Li, T.; Wang, Y.-H.; Xu, Z.-S.; Xiong, A.-S. Exogenous brassinosteroids altered cell length, gibberellin content, and cellulose deposition in promoting carrot petiole elongation. Plant Sci. 2018, 277, 110–120. [Google Scholar] [CrossRef] [PubMed]


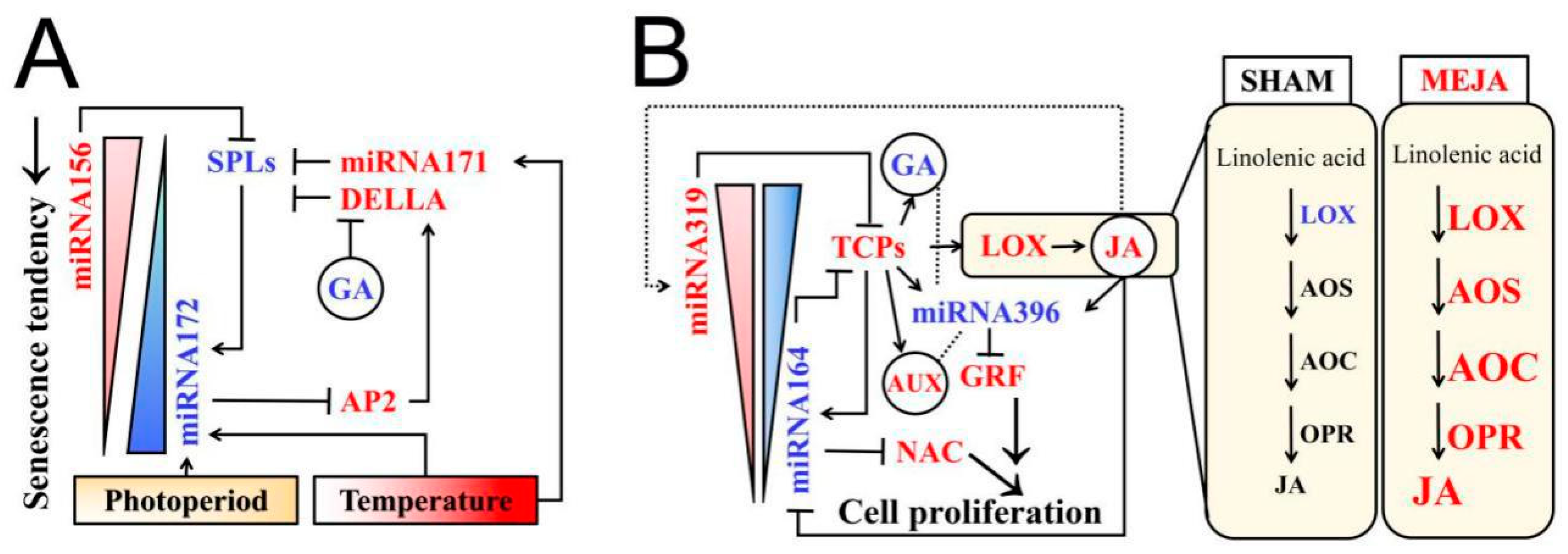
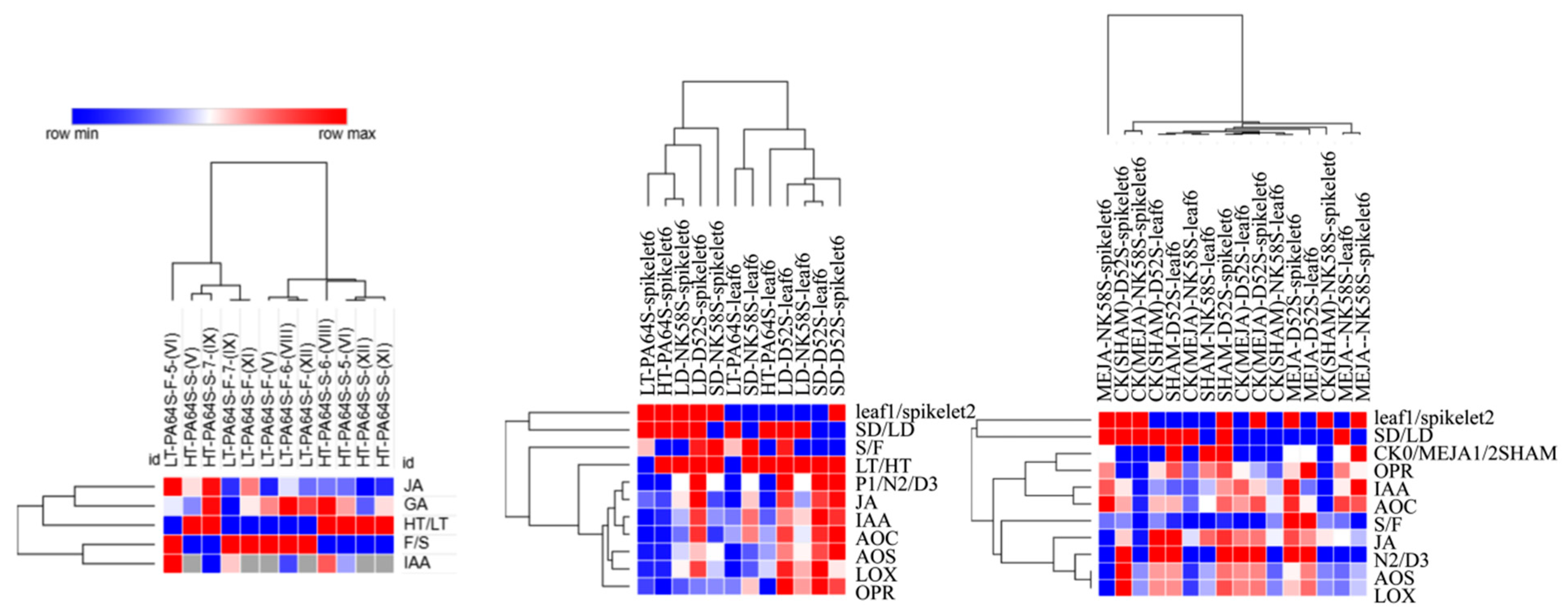
| Photoperiod and Temperature Condition | S/F Plant | Pollen Fertility (%) |
|---|---|---|
| LD-NT (about 27 °C) | NK58S-S | 4.63 ± 1.87 ** |
| SD-NT (about 27 °C) | NK58S-F | 70.61 ± 5.49 |
| SD-NT (about 27 °C) | D52S-S | 1.34 ± 0.30 ** |
| LD-NT (about 27 °C) | D52S-F | 48.93 ± 2.01 |
| LD-HT (28 °C) | PA64S-S | 0.00 ± 0.00 ** |
| LD-LT (21 °C) | PA64S-F | 41.35 ± 1.76 |
| Photoperiod Condition | D52S | NK58S | ||||
|---|---|---|---|---|---|---|
| Spraying | S/F Plant | Pollen Fertility (%) | Spraying | S/F Plant | Pollen Fertility (%) | |
| LD | Control | D52S-F | 21.54 ± 4.91 | Control | NK58S-S | 0.25 ± 0.01 |
| 0.5 mM SHAM | D52S-S-R | 8.63 ± 1.78 ** | 1 mM MEJA | NK58S-F-R | 19.01 ± 2.66 ** | |
| SD | Control | D52S-S | 0.54 ± 0.01 | Control | NK58S-F | 21.54 ± 6.11 |
| 1 mM MEJA | D52S-F-R | 64.07 ± 7.00 ** | 0.5 mM SHAM | NK58S-S-R | 0.19 ± 0.01 ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Liu, C.; Zhu, L.; Fu, M.; Sun, Y.; Zeng, H. Jasmonic Acid Plays a Pivotal Role in Pollen Development and Fertility Regulation in Different Types of P(T)GMS Rice Lines. Int. J. Mol. Sci. 2021, 22, 7926. https://doi.org/10.3390/ijms22157926
He Y, Liu C, Zhu L, Fu M, Sun Y, Zeng H. Jasmonic Acid Plays a Pivotal Role in Pollen Development and Fertility Regulation in Different Types of P(T)GMS Rice Lines. International Journal of Molecular Sciences. 2021; 22(15):7926. https://doi.org/10.3390/ijms22157926
Chicago/Turabian StyleHe, Ying, Chen Liu, Lan Zhu, Ming Fu, Yujun Sun, and Hanlai Zeng. 2021. "Jasmonic Acid Plays a Pivotal Role in Pollen Development and Fertility Regulation in Different Types of P(T)GMS Rice Lines" International Journal of Molecular Sciences 22, no. 15: 7926. https://doi.org/10.3390/ijms22157926
APA StyleHe, Y., Liu, C., Zhu, L., Fu, M., Sun, Y., & Zeng, H. (2021). Jasmonic Acid Plays a Pivotal Role in Pollen Development and Fertility Regulation in Different Types of P(T)GMS Rice Lines. International Journal of Molecular Sciences, 22(15), 7926. https://doi.org/10.3390/ijms22157926






