A Glimpse at the Size of the Fetal Liver—Is It Connected with the Evolution of Gestational Diabetes?
Abstract
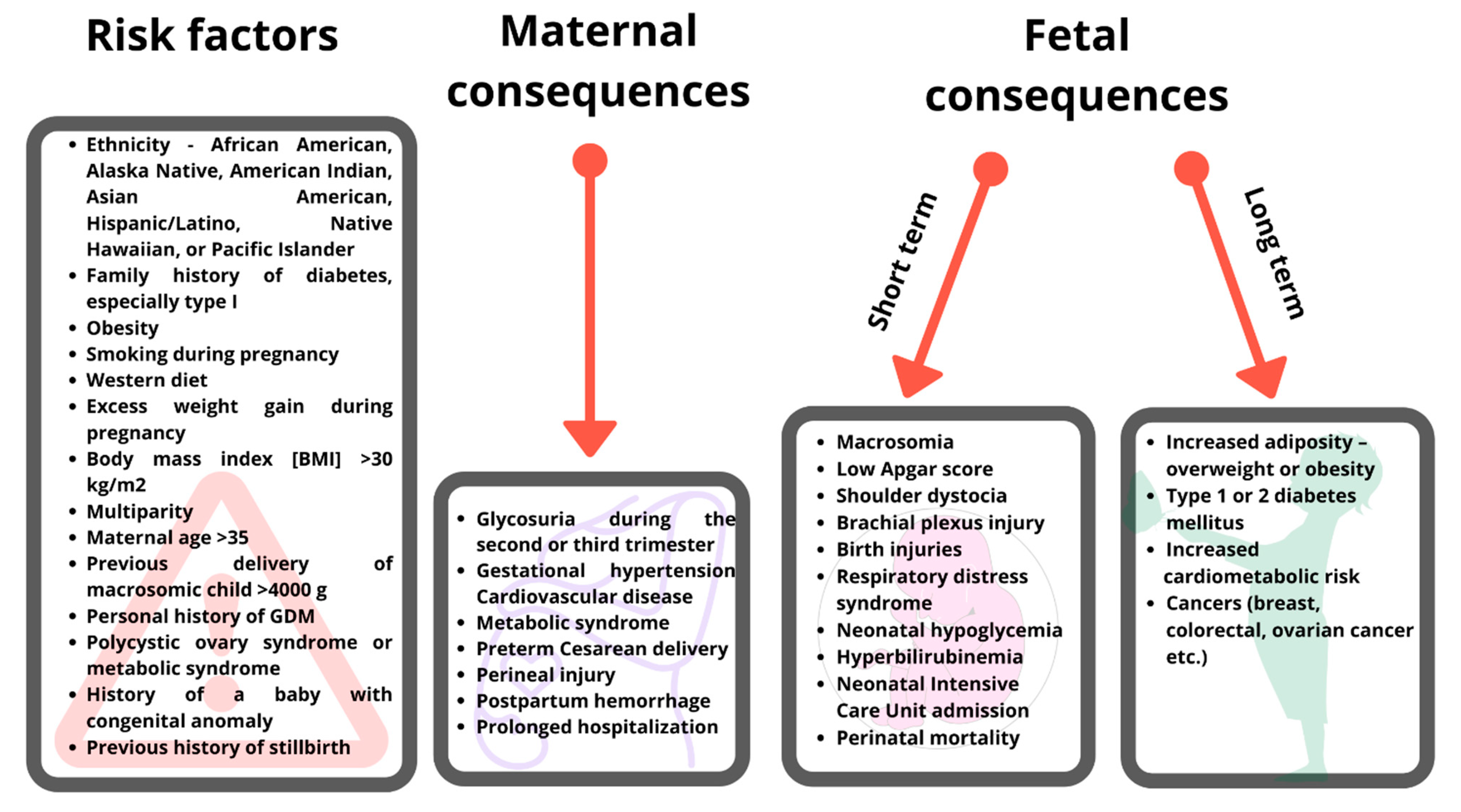
1. Introduction
2. Dietary Factors and Dietary Patterns Associated with the Development of GDM
3. Fetal Consequences of Maternal Gestational Diabetes Mellitus and Maternal Diet
4. The Size of Fetal Liver as a Predictive Parameter for the Evolution of GDM
5. Fetal Liver Length Measurements by Ultrasound—Any Value?
| Liver Ultrasound Timing (Weeks of Gestation) | No. of Subjects | Condition | Evaluated Parameters | Main Results | Reference |
|---|---|---|---|---|---|
| 18, 28, 36 | 104 | T1D, T2D, obesity | FL, WC, FLL, LS | FL↑, WC↑, FLL↑ versus reference values (p < 0.001) FLL↑ at all-time points during pregnancy (p < 0.001) Mean excess size of FL, WC: steady between 18–36 weeks ↑LS: 12.0% (18 weeks) → 16.7% (24 weeks) → 19.3% (36 weeks) (p < 0.02) T1D versus T2D: no differences at 18, 28, 36 weeks Postpartum: weight of newborns from diabetic mothers = 1.79 x controls | Roberts et al. (1994) [85] |
| 21–24 | 123 | GDM, healthy women | SFL, LRLL, CM, PT, WJA | LRLL↑ (p < 0.01) in GDM females FLV and maternal HbA1c were connected: liver volume is increased by 8.1% for each unit increase in HbA1c (95% CI 3.5–13.0%) and by 14% (95% CI 13.0–15.8%) per week of gestational age | Mirghani et al. (2006) [69] |
| 18–36 (median 26) | 64 | IDDM, healthy women | FWC, FLV, FLV/EFWR, UEFW, UVV/kg FW | IDMM: ↑FLL, ↑FLV/EFWR = 1.20 x controls IDMM: ↑FWC, ↑FLV, ↑UEFW, ↑FLV/EFWR IDDM: ↓UVV/kg FW No differences in FLV at 32 and 36 weeks in NGT versus GDM if appropriate treatment | Boito et al. (2007) [86] |
| 32, 36 | 27 | GDM, NGT | FLV, FW | GDM versus NGT: no difference in FLV, FW FLV (32 weeks)-BW correlation (ρ = 0.42, p = 0.03) FLV (36 weeks)-BW correlation (ρ = 0.61, p < 0.001) | Dubé et al. (2011) [24] |
| 23 | 331 | GDM, healthy women | FLL | GDM: ↑BMI, ↑second parity, ↑ fetal liver measurements (p < 0.001) FLL-FPG positive correlation during OGTT (p < 0.001) FLL-BMI correlation (r = 0.586; p < 0.001) no FLL-parity correlation FLL-GDM association (OR = 1.401; 95% CI 1.308–1.501; p < 0.001; R2 = 0.597) independent of BMI/parity FLL = 39 mm, cutoff value for predicting GDM (sensitivity: 71.76%, specificity: 97.56%, positive predictive value: 91.0%, negative predictive value: 90.9%) | Perovic et al. (2014) [37] |
| 24–28 | 97 | GDM, healthy women | FLV, EFW | no differences in standard fetal biometric measurements, EFW GDM: ↑FLV (p < 0.01), ↑BMI, ↑BW no FLV-BMI correlation BW-FLV positive correlation (p < 0.05) | g et al. (2018) [4] |
| 24 | 120 | GDM, healthy women | FLL | midtrimester connection of FLL and FPG (OGTT)GDM: ↑FLL [37.2 (3.4)] versus controls [33.1 (2.7)], p < 0.001 FLL (GDM) = 1.6 x controls (OR 1.6; 95% CI 1.305–1.962), specificity 95.9%, negative predictive value 95.9% | Showman et al. (2019) [5] |
| 28, 37 | 60 | PGM, GDM, healthy women | FLL | PGM, GDM vs. controls: ↑FLL (28 weeks), 48.9 ± 3.4 mm vs. 41.7 ± 3.3 mm, p < 0.001 PGM, GDM vs. controls: ↑FLL (37 weeks), 65.6 ± 4.8 mm vs. 54.5 ± 3.4 mm, p < 0.001 PGD vs. GDM: ↑FLL (28 weeks), 50.55 ± 2.35 mm vs. 46.15 ± 2.1 mm, p = 0.01 PGD vs. GDM: ↑FLL (37 weeks), 66 ± 2.65 mm vs. 59.69 ± 2.7 mm, p = 0.01 FLL correlated with WC (r = 0.82), AFI (r = 0.86), HbA1c levels (r = 0.83), EFBW (r = 0.82), BW (r = 0.80) | Gharib et al. (2019) [68] |
| 18–21, 22–25, 26–30 | 69 | Healthy human fetuses | FLV | ↑FLV 6.57 cm3 (18–21 weeks) → 14.36 cm3 (22–25) → 20.77 cm3 (26–30 weeks) ↑FLV by 20%/week of gestation vs. normal | Szpinda et al. (2015) [87] |
| 24–36 | 49 | PGM: T1D, T2D | LPVFV, TVSPFL, UVLF | ↑LPVFV, ↑TVSPFL vs. reference no difference in PVF ↑UVLF in GDM vs. reference mean | Lund et al. (2019) [89] |
| 18, 20 | 137 | Healthy women | LF, UVLF | postprandial ↑ liver flow in NW postprandial ↓ liver flow if ↑BMI prepregnancy ↑UVLF in NW regardless of fetal size ↓UVLF in the overweight | Opheim et al. (2019) [90] |
6. The Value of Nutrition Therapy in GDM
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes:Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Baz, B.; Riveline, J.-P.; Gautier, J.-F. Endocrinology of pregnancy: Gestational Diabetes Mellitus: Definition, Aetiological and Clinical Aspects. Eur. J. Endocrinol. 2016, 174, R43–R51. [Google Scholar] [CrossRef] [PubMed]
- İlhan, G.; Gültekin, H.; Kubat, A.; Gokmen Karasu, A.F.; Güngör, E.S.; Zebitay, G.A.; Verit Atmaca, F.F. Preliminary Evaluation of Foetal Liver Volume by Three-Dimensional Ultrasound in Women with Gestational Diabetes Mellitus. J. Obstet. Gynaecol. 2018, 38, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Showman, H.A.K.; Al-Rawi, H.A.G.; Zghair, M.A.G. The Value of Mid-Trimester Fetal Liver Length Measurement in Prediction of Gestational Diabetes in Iraqi Women. S. Afr. J. Obstet. Gynaecol. 2020, 25, 100. [Google Scholar] [CrossRef]
- Chamberlain, C.; McNamara, B.; Williams, E.D.; Yore, D.; Oldenburg, B.; Oats, J.; Eades, S. Diabetes in Pregnancy among Indigenous Women in Australia, Canada, New Zealand and the United States: Indigenous Diabetes in Pregnancy. Diabetes. Metab. Res. Rev. 2013, 29, 241–256. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef]
- Kim, W.; Park, S.K.; Kim, Y.L. Gestational Diabetes Mellitus Diagnosed at 24 to 28 Weeks of Gestation in Older and Obese Women: Is It Too Late? PLoS ONE 2019, 14, e0225955. [Google Scholar] [CrossRef]
- Turi, V.; Iurciuc, S.; Crețu, O.M.; Tit, D.M.; Bungau, S.; Apostol, A.; Moleriu, R.D.; Bustea, C.; Behl, T.; Diaconu, C.C.; et al. Arterial Function in Hypertensive Pregnant Women. Is Arterial Stiffness a Marker for the Outcomes in Pregnancy? Life Sci. 2021, 264, 118723. [Google Scholar] [CrossRef]
- Diaconu, C.; Bălăceanu, A.; Bartoş, D. Venous Thromboembolism in Pregnant Woman—A Challenge for the Clinician. Cent. Eur. J. Med. 2013, 8, 548–552. [Google Scholar] [CrossRef]
- Ferrara, A. Increasing Prevalence of Gestational Diabetes Mellitus: A Public Health Perspective. Diabetes Care. 2007, 30, S141–S146. [Google Scholar] [CrossRef] [PubMed]
- Yogev, Y.; Visser, G.H.A. Obesity, Gestational Diabetes and Pregnancy Outcome. Semin. Fetal Neonatal Med. 2009, 14, 77–84. [Google Scholar] [CrossRef]
- Guariguata, L.; Linnenkamp, U.; Beagley, J.; Whiting, D.R.; Cho, N.H. Global Estimates of the Prevalence of Hyperglycaemia in Pregnancy. Diabetes Res. Clin. Pract. 2014, 103, 176–185. [Google Scholar] [CrossRef]
- Caissutti, C.; Berghella, V. Scientific Evidence for Different Options for GDM Screening and Management: Controversies and Review of the Literature. Biomed Res. Int. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet. Gynecol. 2018, 131, e49–e64. [Google Scholar] [CrossRef] [PubMed]
- Society of Maternal-Fetal Medicine (SMFM) Publications Committee. Electronic address: Pubs@smfm.org. SMFM Statement: Pharmacological Treatment of Gestational Diabetes. Am. J. Obstet. Gynecol. 2018, 218, B2–B4. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef]
- Eades, C.E.; Cameron, D.M.; Evans, J.M.M. Prevalence of Gestational Diabetes Mellitus in Europe: A Meta-Analysis. Diabetes Res. Clin. Pract. 2017, 129, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Ching, S.M.; Ramachandran, V.; Yee, A.; Hoo, F.K.; Chia, Y.C.; Wan Sulaiman, W.A.; Suppiah, S.; Mohamed, M.H.; Veettil, S.K. Prevalence and Risk Factors of Gestational Diabetes Mellitus in Asia: A Systematic Review and Meta-Analysis. BMC Pregnancy Childbirth 2018, 18, 494. [Google Scholar] [CrossRef] [PubMed]
- Muche, A.A.; Olayemi, O.O.; Gete, Y.K. Prevalence of Gestational Diabetes Mellitus and Associated Factors among Women Attending Antenatal Care at Gondar Town Public Health Facilities, Northwest Ethiopia. BMC Pregnancy Childbirth 2019, 19, 334. [Google Scholar] [CrossRef] [PubMed]
- Sert, U.Y.; Ozgu-Erdinc, A.S. Gestational Diabetes Mellitus Screening and Diagnosis. Adv. Exp. Med. Biol. 2021, 1307, 231–255. [Google Scholar] [PubMed]
- Gestational Diabetes. Available online: https://www.idf.org/our-activities/care-prevention/gdm (accessed on 21 June 2021).
- Liu, B.; Lamerato, L.E.; Misra, D.P. A Retrospective Analysis of the Relationship between Race/Ethnicity, Age at Delivery and the Risk of Gestational Diabetes Mellitus. J. Matern. Fetal. Neonatal Med. 2020, 33, 2961–2969. [Google Scholar] [CrossRef]
- Dubé, M.-C.; Girard, M.; Morisset, A.-S.; Tchernof, A.; John Weisnagel, S.; Bujold, E. Evaluation of Fetal Liver Volume by Tridimensional Ultrasound in Women with Gestational Diabetes Mellitus. J. Obstet. Gynaecol. Can. 2011, 33, 1095–1098. [Google Scholar] [CrossRef]
- IDF Diabetes Atlas 9th Edition. 2019. Available online: https://diabetesatlas.org/en/ (accessed on 21 June 2021).
- Pons, R.S.; Rockett, F.C.; de Almeida Rubin, B.; Oppermann, M.L.R.; Bosa, V.L. Risk Factors for Gestational Diabetes Mellitus in a Sample of Pregnant Women Diagnosed with the Disease. Diabetol. Metab. Syndr. 2015, 7, A80. [Google Scholar] [CrossRef][Green Version]
- Kaseva, N.; Vääräsmäki, M.; Matinolli, H.-M.; Sipola-Leppänen, M.; Tikanmäki, M.; Heinonen, K.; Lano, A.; Wolke, D.; Andersson, S.; Järvelin, M.-R.; et al. Pre-Pregnancy Overweight or Obesity and Gestational Diabetes as Predictors of Body Composition in Offspring Twenty Years Later: Evidence from Two Birth Cohort Studies. Int. J. Obes. 2018, 42, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Reece, E.A. The Fetal and Maternal Consequences of Gestational Diabetes Mellitus. J. Matern. Fetal. Neonatal Med. 2010, 23, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Getahun, D.; Fassett, M.J.; Jacobsen, S.J. Gestational Diabetes: Risk of Recurrence in Subsequent Pregnancies. Am. J. Obstet. Gynecol. 2010, 203, 467.e1–467.e6. [Google Scholar] [CrossRef]
- Mirghani Dirar, A.; Doupis, J. Gestational Diabetes from A to Z. World J. Diabetes 2017, 8, 489–511. [Google Scholar] [CrossRef]
- Lo, J.C.; Feigenbaum, S.L.; Escobar, G.J.; Yang, J.; Crites, Y.M.; Ferrara, A. Increased Prevalence of Gestational Diabetes Mellitus among Women with Diagnosed Polycystic Ovary Syndrome: A Population-Based Study. Diabetes Care 2006, 29, 1915–1917. [Google Scholar] [CrossRef]
- Hedderson, M.M.; Williams, M.A.; Holt, V.L.; Weiss, N.S.; Ferrara, A. Body Mass Index and Weight Gain Prior to Pregnancy and Risk of Gestational Diabetes Mellitus. Am. J. Obstet. Gynecol. 2008, 198, 409.e1–409.e7. [Google Scholar] [CrossRef]
- Hedderson, M.M.; Ferrara, A. High Blood Pressure before and during Early Pregnancy Is Associated with an Increased Risk of Gestational Diabetes Mellitus. Diabetes Care 2008, 31, 2362–2367. [Google Scholar] [CrossRef]
- Dandjinou, M.; Sheehy, O.; Bérard, A. Antidepressant Use during Pregnancy and the Risk of Gestational Diabetes Mellitus: A Nested Case-Control Study. BMJ Open. 2019, 9, e025908. [Google Scholar] [CrossRef]
- Bodén, R.; Lundgren, M.; Brandt, L.; Reutfors, J.; Kieler, H. Antipsychotics during Pregnancy: Relation to Fetal and Maternal Metabolic Effects: Relation to Fetal and Maternal Metabolic Effects. Arch. Gen. Psychiatry 2012, 69, 715–721. [Google Scholar] [CrossRef]
- Fisher, J.E.; Smith, R.S.; Lagrandeur, R.; Lorenz, R.P. Gestational Diabetes Mellitus in Women Receiving Beta-Adrenergics and Corticosteroids for Threatened Preterm Delivery. Obstet. Gynecol. 1997, 90, 880–883. [Google Scholar] [CrossRef]
- Perovic, M.; Gojnic, M.; Arsic, B.; Pantic, I.; Stefanovic, T.; Kovacevic, G.; Kovacevic, M.; Garalejic, E.; Dugalic, S.; Radakovic, J.; et al. Relationship between Mid-Trimester Ultrasound Fetal Liver Length Measurements and Gestational Diabetes Mellitus: Fetal Liver Length Predicts GDM. J. Diabetes 2015, 7, 497–505. [Google Scholar] [CrossRef]
- Perović, M.; Garalejić, E.; Gojnić, M.; Arsić, B.; Pantić, I.; Bojović, D.J.; Fazlagić, A.; Gardiner, H. Sensitivity and Specificity of Ultrasonography as a Screening Tool for Gestational Diabetes Mellitus. J. Matern. Fetal. Neonatal Med. 2012, 25, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Gojnic, M.; Stefanovic, T.; Perovic, M.; Arsic, B.; Garalejic, E.; Micic, J.; Maricic, Z.; Ratkovic, R.; Ljubic, A. Prediction of Fetal Macrosomia with Ultrasound Parameters and Maternal Glycemic Controls in Gestational Diabetes Mellitus. Clin. Exp. Obstet. Gynecol. 2012, 39, 512–515. [Google Scholar] [PubMed]
- Jin, D.; Rich-Edwards, J.W.; Chen, C.; Huang, Y.; Wang, Y.; Xu, X.; Liu, J.; Liu, Z.; Gao, Y.; Zou, S.; et al. Gestational Diabetes Mellitus: Predictive Value of Fetal Growth Measurements by Ultrasonography at 22–24 Weeks: A Retrospective Cohort Study of Medical Records. Nutrients 2020, 12, 3645. [Google Scholar] [CrossRef]
- Rasmussen, L.; Christensen, M.L.; Poulsen, C.W.; Rud, C.; Christensen, A.S.; Andersen, J.R.; Kampmann, U.; Ovesen, P.G. Effect of High versus Low Carbohydrate Intake in the Morning on Glycemic Variability and Glycemic Control Measured by Continuous Blood Glucose Monitoring in Women with Gestational Diabetes Mellitus-A Randomized Crossover Study. Nutrients 2020, 12, 475. [Google Scholar] [CrossRef] [PubMed]
- Atakora, L.; Poston, L.; Hayes, L.; Flynn, A.C.; White, S.L. Influence of GDM Diagnosis and Treatment on Weight Gain, Dietary Intake and Physical Activity in Pregnant Women with Obesity: Secondary Analysis of the UPBEAT Study. Nutrients 2020, 12, 359. [Google Scholar] [CrossRef]
- Thorkelson, S.J.; Anderson, K.R. Oral Medications for Diabetes in Pregnancy: Use in a Rural Population. Diabetes Spectr. 2016, 29, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Barbour, L.A.; Feig, D.S. Metformin for Gestational Diabetes Mellitus: Progeny, Perspective, and a Personalized Approach. Diabetes Care 2019, 42, 396–399. [Google Scholar] [CrossRef]
- Balani, J.; Hyer, S.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H.; Johnson, A.; Shehata, H. Association between Insulin Resistance and Preeclampsia in Obese Non-Diabetic Women Receiving Metformin. Obstet. Med. 2017, 10, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Szmuilowicz, E.D.; Josefson, J.L.; Metzger, B.E. Gestational Diabetes Mellitus. Endocrinol. Metab. Clin. N. Am. 2019, 48, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Jorquera, G.; Echiburú, B.; Crisosto, N.; Sotomayor-Zárate, R.; Maliqueo, M.; Cruz, G. Metformin during Pregnancy: Effects on Offspring Development and Metabolic Function. Front. Pharmacol. 2020, 11, 653. [Google Scholar] [CrossRef]
- Găman, M.-A.; Cozma, M.-A.; Dobrică, E.-C.; Bacalbașa, N.; Bratu, O.G.; Diaconu, C.C. Dyslipidemia: A Trigger for Coronary Heart Disease in Romanian Patients with Diabetes. Metabolites 2020, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Palacios, M.; Ruiz-Alcaraz, A.J.; Sanchez-Campillo, M.; Larqué, E. Role of Insulin in Placental Transport of Nutrients in Gestational Diabetes Mellitus. Ann. Nutr. Metab. 2017, 70, 16–25. [Google Scholar] [CrossRef]
- Shin, D.; Lee, K.W.; Song, W.O. Dietary Patterns during Pregnancy Are Associated with Risk of Gestational Diabetes Mellitus. Nutrients 2015, 7, 9369–9382. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, F.; Akhoondan, M.; Ehteshami, M.; Aghamohammadi, V.; Ghanei, N.; Mirmiran, P.; Rashidkhani, B. Maternal Dietary Patterns and Gestational Diabetes Risk: A Case-Control Study. J. Diabetes Res. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic-Vukas, J.; Capling, L.; Cheng, S.; Stamatakis, E.; Louie, J.; Cheung, N.; Markovic, T.; Ross, G.; Senior, A.; Brand-Miller, J.; et al. Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 698. [Google Scholar] [CrossRef]
- Hu, J.; Oken, E.; Aris, I.M.; Lin, P.-I.D.; Ma, Y.; Ding, N.; Gao, M.; Wei, X.; Wen, D. Dietary Patterns during Pregnancy Are Associated with the Risk of Gestational Diabetes Mellitus: Evidence from a Chinese Prospective Birth Cohort Study. Nutrients 2019, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Moses, R.G.; Brand-Miller, J.C. Dietary Risk Factors for Gestational Diabetes Mellitus: Are Sugar-Sweetened Soft Drinks Culpable or Guilty by Association? Diabetes Care 2009, 32, 2314–2315. [Google Scholar] [CrossRef][Green Version]
- Dominguez, L.J.; Martínez-González, M.A.; Basterra-Gortari, F.J.; Gea, A.; Barbagallo, M.; Bes-Rastrollo, M. Fast Food Consumption and Gestational Diabetes Incidence in the SUN Project. PLoS ONE 2014, 9, e106627. [Google Scholar] [CrossRef]
- Wen, L.; Ge, H.; Qiao, J.; Zhang, L.; Chen, X.; Kilby, M.D.; Zhou, Y.; Gan, J.; Saffery, R.; Yan, J.; et al. Maternal Dietary Patterns and Risk of Gestational Diabetes Mellitus in Twin Pregnancies: A Longitudinal Twin Pregnancies Birth Cohort Study. Nutr. J. 2020, 19, 13. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, R.; Zhong, C.; Wu, J.; Li, X.; Li, Q.; Cui, W.; Yi, N.; Xiao, M.; Yin, H.; et al. Maternal Dietary Pattern Characterised by High Protein and Low Carbohydrate Intake in Pregnancy Is Associated with a Higher Risk of Gestational Diabetes Mellitus in Chinese Women: A Prospective Cohort Study. Br. J. Nutr. 2018, 120, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Ponzo, V.; Fedele, D.; Goitre, I.; Leone, F.; Lezo, A.; Monzeglio, C.; Finocchiaro, C.; Ghigo, E.; Bo, S. Diet-Gut Microbiota Interactions and Gestational Diabetes Mellitus (GDM). Nutrients 2019, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Yoo, J.Y.; Valeria Ozorio Dutra, S.; Morgan, K.H.; Groer, M. The Association between Early-Life Gut Microbiota and Long-Term Health and Diseases. J. Clin. Med. 2021, 10, 459. [Google Scholar] [CrossRef]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Gestational Diabetes Is Associated with Change in the Gut Microbiota Composition in Third Trimester of Pregnancy and Postpartum. Microbiome 2018, 6, 89. [Google Scholar] [CrossRef]
- Hasain, Z.; Mokhtar, N.M.; Kamaruddin, N.A.; Mohamed Ismail, N.A.; Razalli, N.H.; Gnanou, J.V.; Raja Ali, R.A. Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front. Cell. Infect. Microbiol. 2020, 10, 188. [Google Scholar] [CrossRef]
- Astbury, S.; Song, A.; Zhou, M.; Nielsen, B.; Hoedl, A.; Willing, B.P.; Symonds, M.E.; Bell, R.C. High Fructose Intake during Pregnancy in Rats Influences the Maternal Microbiome and Gut Development in the Offspring. Front. Genet. 2018, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.; Ojo, O.O.; Wang, X.-H.; Adegboye, A.R.A. The Effects of a Low GI Diet on Cardiometabolic and Inflammatory Parameters in Patients with Type 2 and Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2019, 11, 1584. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, Y.; Xue, H.; Xiong, J.; Cheng, G. Vitamin D and Gestational Diabetes Mellitus: A Systematic Review Based on Data Free of Hawthorne Effect. BJOG 2018, 125, 784–793. [Google Scholar] [CrossRef]
- Jamilian, M.; Mirhosseini, N.; Eslahi, M.; Bahmani, F.; Shokrpour, M.; Chamani, M.; Asemi, Z. The Effects of Magnesium-Zinc-Calcium-Vitamin D Co-Supplementation on Biomarkers of Inflammation, Oxidative Stress and Pregnancy Outcomes in Gestational Diabetes. BMC Pregnancy Childbirth 2019, 19, 107. [Google Scholar] [CrossRef]
- Lai, J.S.; Pang, W.W.; Cai, S.; Lee, Y.S.; Chan, J.K.Y.; Shek, L.P.C.; Yap, F.K.P.; Tan, K.H.; Godfrey, K.M.; van Dam, R.M.; et al. High Folate and Low Vitamin B12 Status during Pregnancy Is Associated with Gestational Diabetes Mellitus. Clin. Nutr. 2018, 37, 940–947. [Google Scholar] [CrossRef]
- Mardali, F.; Fatahi, S.; Alinaghizadeh, M.; Kord Varkaneh, H.; Sohouli, M.H.; Shidfar, F.; Găman, M.-A. Association between Abnormal Maternal Serum Levels of Vitamin B12 and Preeclampsia: A Systematic Review and Meta-Analysis. Nutr. Rev. 2021, 79, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Gharib, W.F.; Huissen, W.M. Fetal Liver Length and State of Maternal Glycemic Control. Available online: https://austinpublishinggroup.com/obstetrics-gynecology/fulltext/ajog-v6-id1144.php (accessed on 21 June 2021).
- Mirghani, H.; Zayed, R.; Thomas, L.; Agarwal, M. Gestational Diabetes Mellitus: Fetal Liver Length Measurements between 21and 24 Weeks’ Gestation. J. Clin. Ultrasound. 2007, 35, 34–37. [Google Scholar] [CrossRef]
- Castori, M. Diabetic Embryopathy: A Developmental Perspective from Fertilization to Adulthood. Mol. Syndromol. 2013, 4, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Zabihi, S.; Loeken, M.R. Understanding Diabetic Teratogenesis: Where Are We Now and Where Are We Going? Molecular Causes of Diabetic Teratogenesis. Birth Defects Res. A Clin. Mol. Teratol. 2010, 88, 779–790. [Google Scholar] [CrossRef]
- Dobjanschi, C. Actualitati in Diabetul Gestational; Medicala: Bucharest, Romania, 2015. [Google Scholar]
- Catalano, P.M.; Hauguel-De Mouzon, S. Is It Time to Revisit the Pedersen Hypothesis in the Face of the Obesity Epidemic? Am. J. Obstet. Gynecol. 2011, 204, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Usta, A.; Usta, C.S.; Yildiz, A.; Ozcaglayan, R.; Dalkiran, E.S.; Savkli, A.; Taskiran, M. Frequency of Fetal Macrosomia and the Associated Risk Factors in Pregnancies without Gestational Diabetes Mellitus. Pan Afr. Med. J. 2017, 26, 62. [Google Scholar] [CrossRef]
- Desoye, G.; Nolan, C.J. The Fetal Glucose Steal: An Underappreciated Phenomenon in Diabetic Pregnancy. Diabetologia 2016, 59, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Reiher, H.; Fuhrmann, K.; Jutzi, E.; Hahn, H.J. Fetal hyperinsulinism in early pregnancy—A cause of diabetic fetopathy? Zentralbl. Gynakol. 1983, 105, 889–893. [Google Scholar] [PubMed]
- Moreli, J.B.; Santos, J.H.; Rocha, C.R.; Damasceno, D.C.; Morceli, G.; Rudge, M.V.; Bevilacqua, E.; Calderon, I.M.P. DNA Damage and Its Cellular Response in Mother and Fetus Exposed to Hyperglycemic Environment. Biomed. Res. Int. 2014, 2014, 676758. [Google Scholar] [CrossRef]
- McFarland, M.B.; Trylovich, C.G.; Langer, O. Anthropometric Differences in Macrosomic Infants of Diabetic and Nondiabetic Mothers. J. Matern. Fetal. Med. 1998, 7, 292–295. [Google Scholar]
- Naeye, R.L. Infants of Diabetic Mothers: A Quantitative, Morphologic Study. Pediatrics 1965, 35, 980–988. [Google Scholar]
- Sinclair, K.J.; Friesen-Waldner, L.J.; McCurdy, C.M.; Wiens, C.N.; Wade, T.P.; de Vrijer, B.; Regnault, T.R.H.; McKenzie, C.A. Quantification of Fetal Organ Volume and Fat Deposition Following in Utero Exposure to Maternal Western Diet Using MRI. PLoS ONE 2018, 13, e0192900. [Google Scholar] [CrossRef]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Pardo, Z.; Heras-Molina, A.; Encinas, T.; Torres-Rovira, L.; Astiz, S.; Nieto, R.; Ovilo, C.; Gonzalez-Bulnes, A.; et al. Polyphenols and IUGR Pregnancies: Effects of Maternal Hydroxytyrosol Supplementation on Hepatic Fat Accretion and Energy and Fatty Acids Profile of Fetal Tissues. Nutrients 2019, 11, 1534. [Google Scholar] [CrossRef]
- Xue, Y.; Guo, C.; Hu, F.; Zhu, W.; Mao, S. Maternal Undernutrition Induces Fetal Hepatic Lipid Metabolism Disorder and Affects the Development of Fetal Liver in a Sheep Model. FASEB J. 2019, 33, 9990–10004. [Google Scholar] [CrossRef]
- Bloomfield, F.H.; Spiroski, A.-M.; Harding, J.E. Fetal Growth Factors and Fetal Nutrition. Semin. Fetal Neonatal Med. 2013, 18, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, M.A.; Gardner, D.S.; Sebert, S.; Wilson, V.; Davidson, N.; Nigmatullina, Y.; Chan, L.L.Y.; Budge, H.; Symonds, M.E. Suboptimal Maternal Nutrition, during Early Fetal Liver Development, Promotes Lipid Accumulation in the Liver of Obese Offspring. J. Reprod. Fertil. 2011, 141, 119–126. [Google Scholar] [CrossRef]
- Roberts, A.B.; Mitchell, J.; Murphy, C.; Koya, H.; Cundy, T. Fetal Liver Length in Diabetic Pregnancy. Am. J. Obstet. Gynecol. 1994, 170, 1308–1312. [Google Scholar] [CrossRef]
- Boito, S.M.; Struijk, P.C.; Ursem, N.T.C.; Stijnen, T.; Wladimiroff, J.W. Assessment of Fetal Liver Volume and Umbilical Venous Volume Flow in Pregnancies Complicated by Insulin-Dependent Diabetes Mellitus. BJOG 2003, 110, 1007–1013. [Google Scholar] [CrossRef]
- Szpinda, M.; Paruszewska-Achtel, M.; Woźniak, A.; Mila-Kierzenkowska, C.; Elminowska-Wenda, G.; Dombek, M.; Szpinda, A.; Badura, M. Volumetric Growth of the Liver in the Human Fetus: An Anatomical, Hydrostatic, and Statistical Study. Biomed. Res. Int. 2015, 2015, 858162. [Google Scholar] [CrossRef] [PubMed]
- Tchirikov, M.; Kertschanska, S.; Stürenberg, H.J.; Schröder, H.J. Liver Blood Perfusion as a Possible Instrument for Fetal Growth Regulation. Placenta 2002, 23, S153–S158. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.; Ebbing, C.; Rasmussen, S.; Kiserud, T.; Hanson, M.; Kessler, J. Altered Development of Fetal Liver Perfusion in Pregnancies with Pregestational Diabetes. PLoS ONE 2019, 14, e0211788. [Google Scholar] [CrossRef]
- Opheim, G.L.; Henriksen, T.; Haugen, G. The Effect of a Maternal Meal on Fetal Liver Blood Flow. PLoS ONE 2019, 14, e0216176. [Google Scholar] [CrossRef] [PubMed]
- Haugen, G.; Hanson, M.; Kiserud, T.; Crozier, S.; Inskip, H.; Godfrey, K.M. Fetal Liver-Sparing Cardiovascular Adaptations Linked to Mother’s Slimness and Diet. Circ. Res. 2005, 96, 12–14. [Google Scholar] [CrossRef]
- Jichitu, A.; Bungau, S.; Stanescu, A.M.A.; Vesa, C.M.; Toma, M.M.; Bustea, C.; Iurciuc, S.; Rus, M.; Bacalbasa, N.; Diaconu, C.C. Non-Alcoholic Fatty Liver Disease and Cardiovascular Comorbidities: Pathophysiological Links, Diagnosis, and Therapeutic Management. Diagnostics 2021, 11, 689. [Google Scholar] [CrossRef]
- Opheim, G.L.; Moe Holme, A.; Blomhoff Holm, M.; Melbye Michelsen, T.; Muneer Zahid, S.; Paasche Roland, M.C.; Henriksen, T.; Haugen, G. The Impact of Umbilical Vein Blood Flow and Glucose Concentration on Blood Flow Distribution to the Fetal Liver and Systemic Organs in Healthy Pregnancies. FASEB J. 2020, 34, 12481–12491. [Google Scholar] [CrossRef] [PubMed]
- Kamimae-Lanning, A.N.; Krasnow, S.M.; Goloviznina, N.A.; Zhu, X.; Roth-Carter, Q.R.; Levasseur, P.R.; Jeng, S.; McWeeney, S.K.; Kurre, P.; Marks, D.L. Maternal High-Fat Diet and Obesity Compromise Fetal Hematopoiesis. Mol. Metab. 2015, 4, 25–38. [Google Scholar] [CrossRef]
- Ikenoue, S.; Waffarn, F.; Ohashi, M.; Tanaka, M.; Gillen, D.L.; Buss, C.; Entringer, S.; Wadhwa, P.D. Placental Corticotrophin-Releasing Hormone Is a Modulator of Fetal Liver Blood Perfusion. J. Clin. Endocrinol. Metab. 2021, 106, 646–653. [Google Scholar] [CrossRef]
- Reader, D.M. Medical Nutrition Therapy and Lifestyle Interventions. Diabetes Care 2007, 30, S188–S193. [Google Scholar] [CrossRef]
- Halperin, I.J.; Feig, D.S. The Role of Lifestyle Interventions in the Prevention of Gestational Diabetes. Curr. Diab. Rep. 2014, 14, 452. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, G.; Feig, D.S. Pharmacological Management of Gestational Diabetes Mellitus. Drugs 2017, 77, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.W.; Carroll, D.G.; Meyer, A. A Review of Current Treatment Strategies for Gestational Diabetes Mellitus. Drugs Context. 2015, 4, 212282. [Google Scholar] [CrossRef] [PubMed]
- Turi, V.; Dragan, S.; Iurciuc, M.; Moleriu, L.; Bungau, S.; Tit, D.M.; Toader, D.-O.; Diaconu, C.C.; Behl, T.; Petre, I. Arterial Function in Healthy Pregnant Women vs. Non-Pregnant Women-A 10-Year Study. Diagnostics 2020, 10, 374. [Google Scholar] [CrossRef]
- Epingeac, M.E.; Gaman, M.A.; Diaconu, C.C.; Gaman, A.M. Crosstalk between Oxidative Stress and Inflammation in Obesity. Rev. Chim. 2020, 71, 228–232. [Google Scholar] [CrossRef]
- Bergel, R.; Hadar, E.; Toledano, Y.; Hod, M. Pharmacological Management of Gestational Diabetes Mellitus. Curr. Diab. Rep. 2016, 16, 118. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozma, M.-A.; Găman, M.-A.; Dobrică, E.-C.; Boroghină, S.C.; Iancu, M.A.; Crețoiu, S.M.; Simionescu, A.A. A Glimpse at the Size of the Fetal Liver—Is It Connected with the Evolution of Gestational Diabetes? Int. J. Mol. Sci. 2021, 22, 7866. https://doi.org/10.3390/ijms22157866
Cozma M-A, Găman M-A, Dobrică E-C, Boroghină SC, Iancu MA, Crețoiu SM, Simionescu AA. A Glimpse at the Size of the Fetal Liver—Is It Connected with the Evolution of Gestational Diabetes? International Journal of Molecular Sciences. 2021; 22(15):7866. https://doi.org/10.3390/ijms22157866
Chicago/Turabian StyleCozma, Matei-Alexandru, Mihnea-Alexandru Găman, Elena-Codruța Dobrică, Steluța Constanța Boroghină, Mihaela Adela Iancu, Sanda Maria Crețoiu, and Anca Angela Simionescu. 2021. "A Glimpse at the Size of the Fetal Liver—Is It Connected with the Evolution of Gestational Diabetes?" International Journal of Molecular Sciences 22, no. 15: 7866. https://doi.org/10.3390/ijms22157866
APA StyleCozma, M.-A., Găman, M.-A., Dobrică, E.-C., Boroghină, S. C., Iancu, M. A., Crețoiu, S. M., & Simionescu, A. A. (2021). A Glimpse at the Size of the Fetal Liver—Is It Connected with the Evolution of Gestational Diabetes? International Journal of Molecular Sciences, 22(15), 7866. https://doi.org/10.3390/ijms22157866









