Structural Insights for the Stronger Ability of Shrimp Ferritin to Coordinate with Heavy Metal Ions as Compared to Human H-Chain Ferritin
Abstract
:1. Introduction
2. Results and Discussion
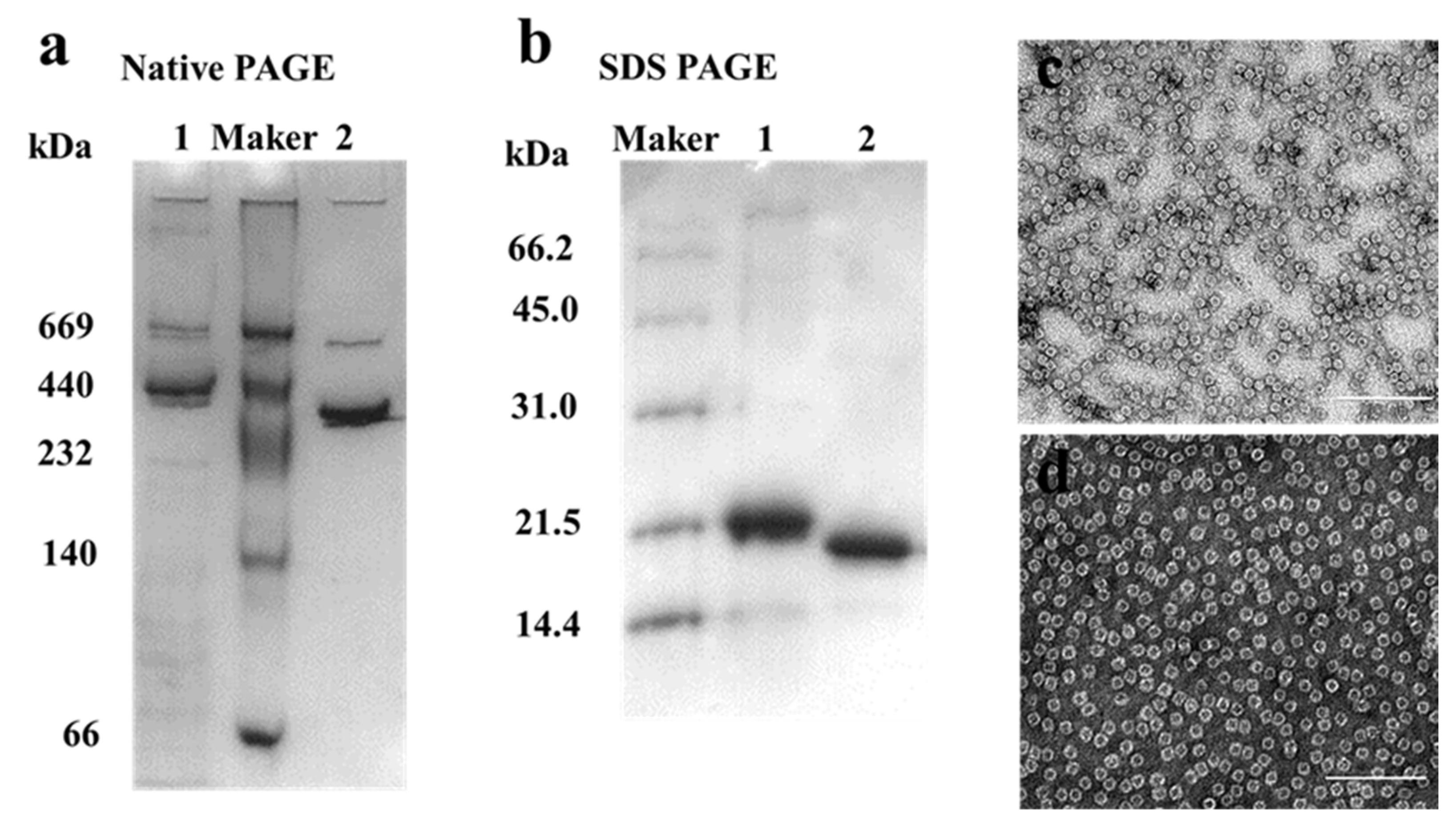
2.1. Preparation and Characterization of HuHF and MjF
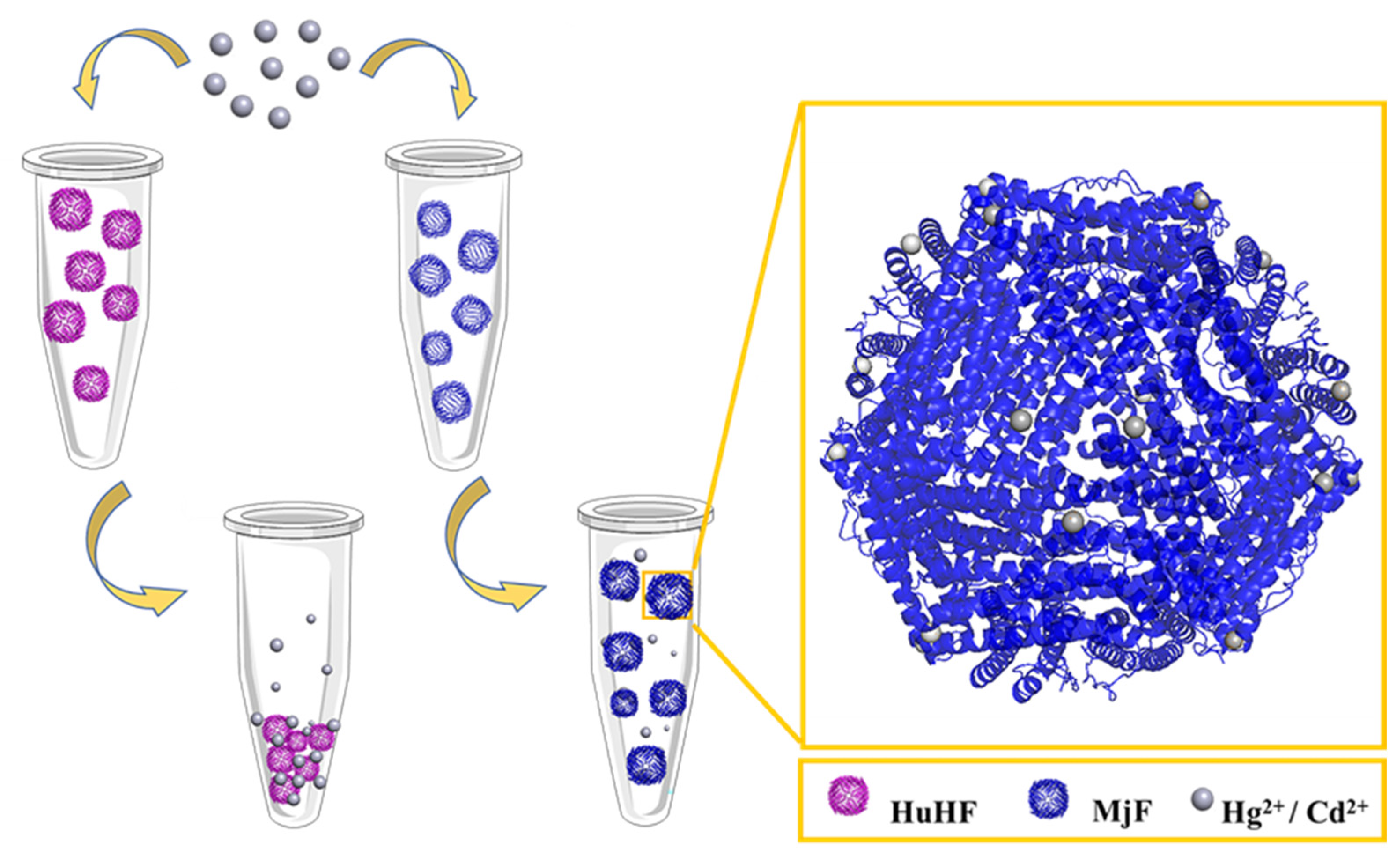
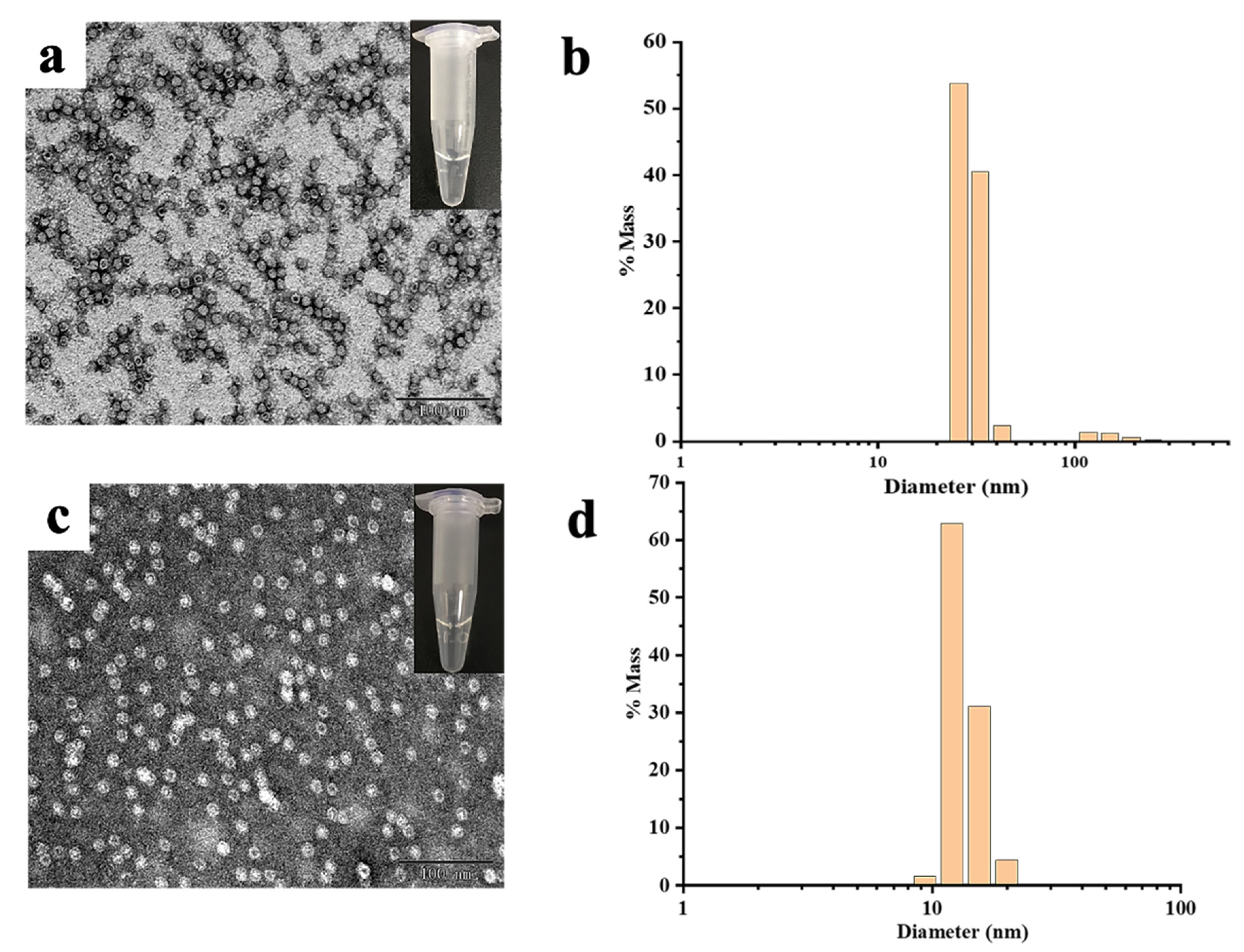
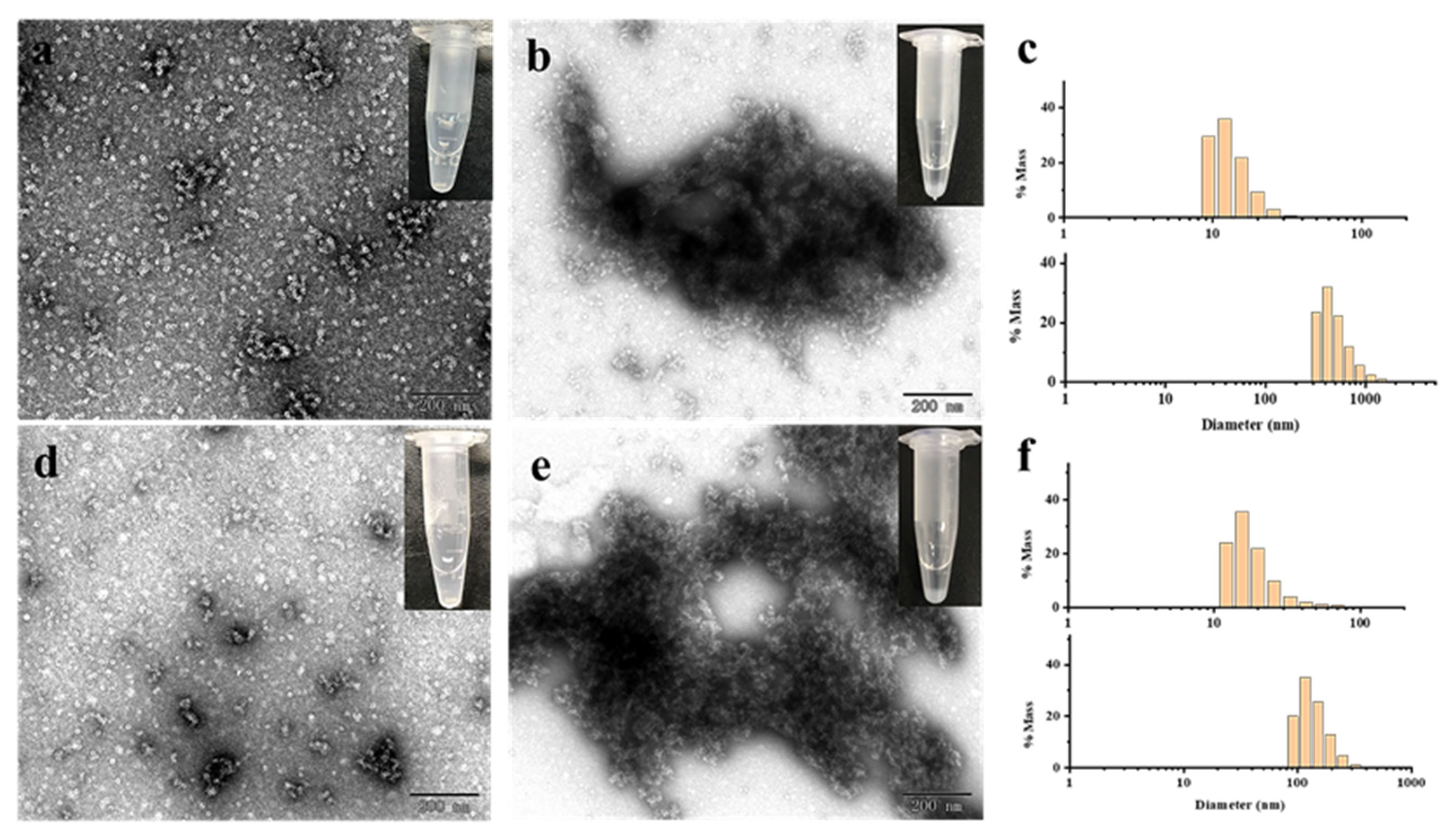
2.2. Morphological and Aggregation Characterization of MjF and HuHF upon Treatment with Hg2+ or Cd2+
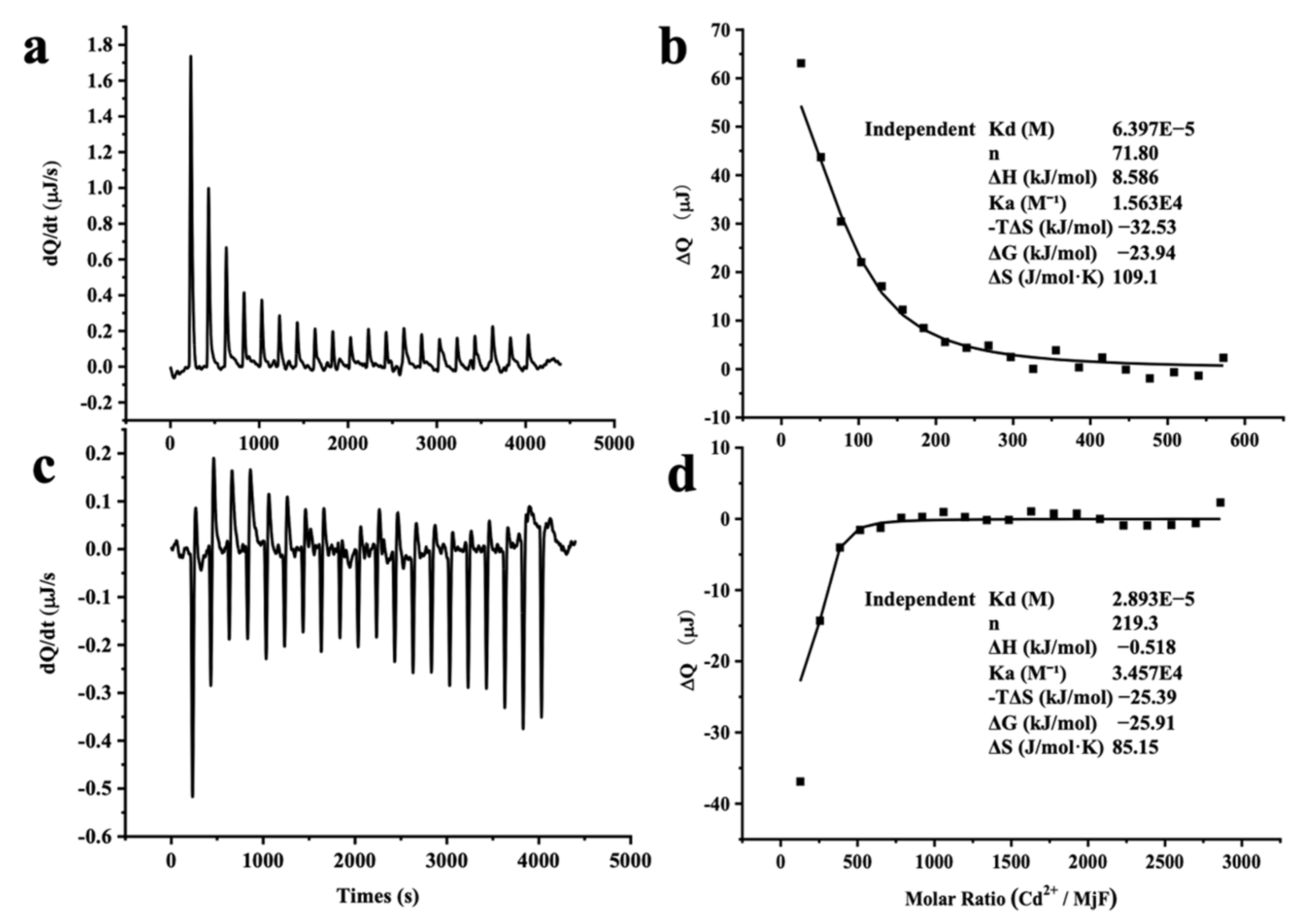
2.3. Thermodynamic Characteristics of Cd2+ and Hg2+ Binding to MjF
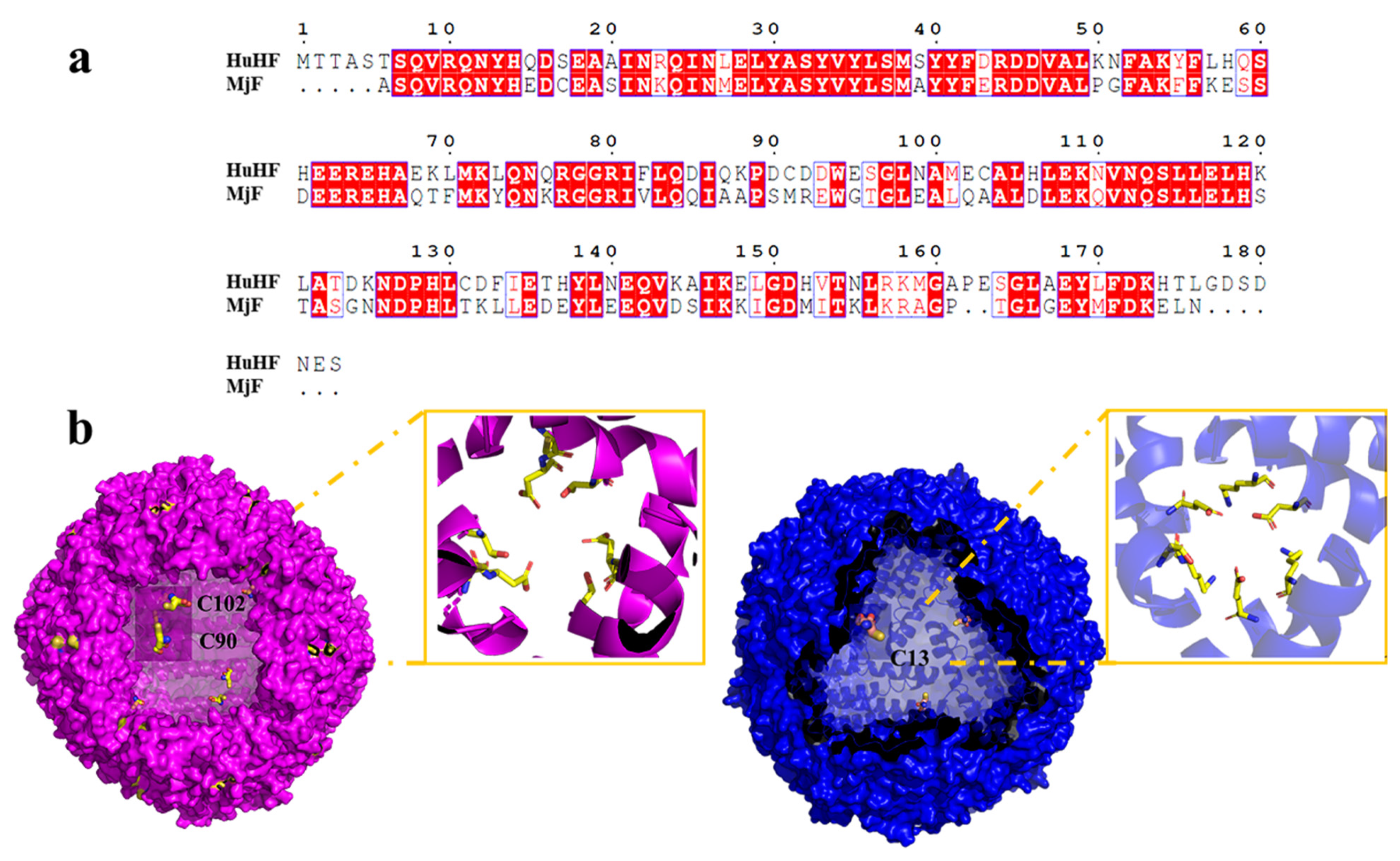
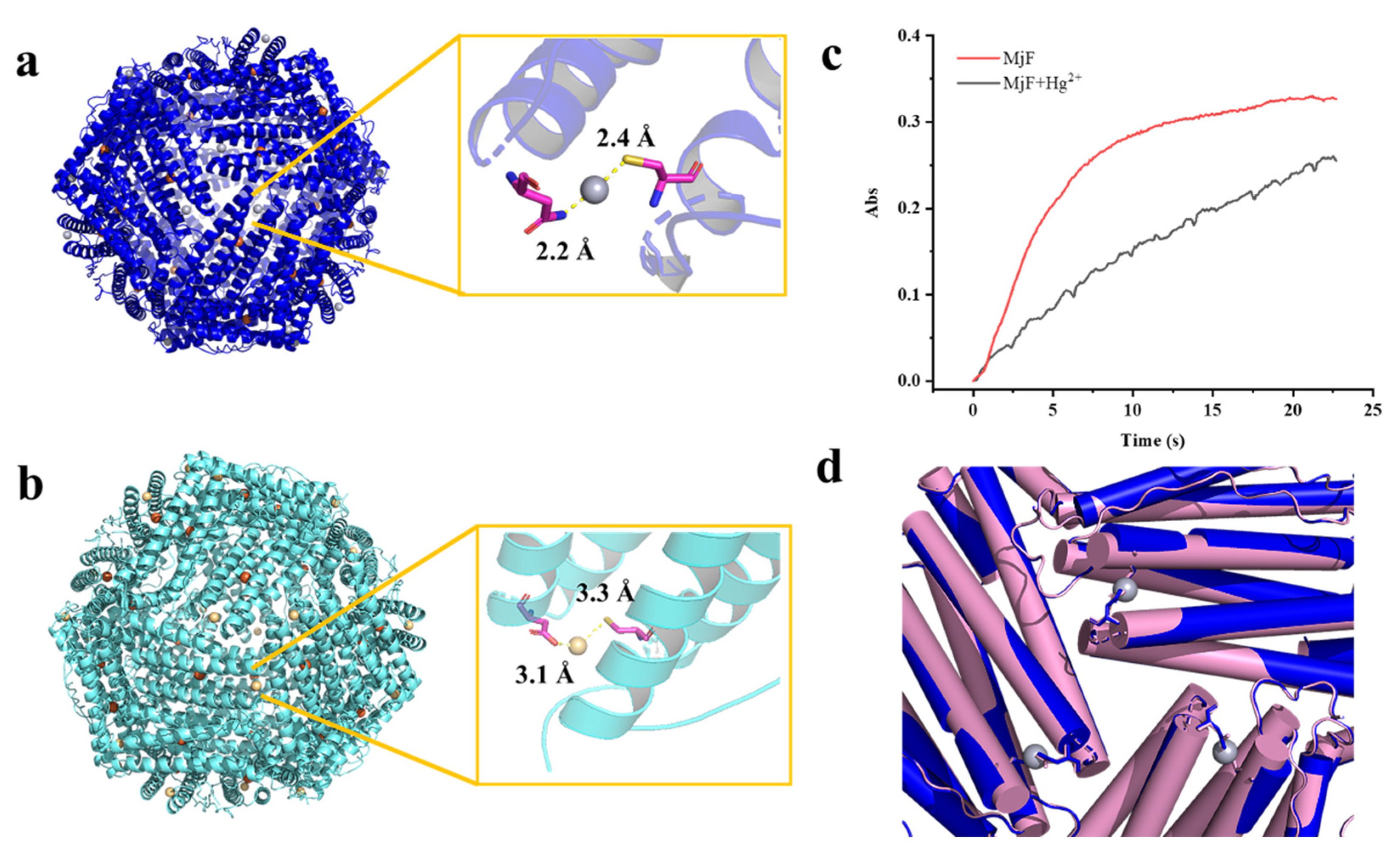
2.4. The Crystal Structure of the Complexes of MjF Plus Cd2+ or Hg2+
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Apparatus
3.3. Preparation of HuHF and MjF
3.4. SDS-PAGE and Native PAGE
3.5. Dynamic Light Scattering (DLS) Experiments
3.6. Transmission Electron Microscopy (TEM)
3.7. Binding Assay of Ferritin to Cd2+ and Hg2+ by Isothermal Titration Calorimetry (ITC)
3.8. Crystallization, Data Collection, and Structure Determination
3.9. Fe2+ Oxidative Deposition in Ferritin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Suturović, Z.; Kravić, S.; Milanović, S.; Đurović, A.; Brezo, T. Determination of heavy metals in milk and fermented milk products by potentiometric stripping analysis with constant inverse current in the analytical step. Food Chem. 2014, 155, 120–125. [Google Scholar] [CrossRef]
- Yan, H.; Li, Q.; Yuan, Z.; Jin, S.; Jing, M. Research Progress of Mercury Bioaccumulation in the Aquatic Food Chain, China: A Review. Bull. Environ. Contam. Toxicol. 2019, 102, 612–620. [Google Scholar] [CrossRef]
- Jusoh, A.; Shiung, L.S.; Ali, N.; Noor, M. A simulation study of the removal efficiency of granular activated carbon on cadmium and lead. Desalination 2007, 206, 9–16. [Google Scholar] [CrossRef]
- Sasaki, T.; Araki, R.; Michihata, T.; Kozawa, M.; Tokuda, K.; Koyanagi, T.; Enomoto, T. Removal of cadmium from fish sauce using chelate resin. Food Chem. 2015, 173, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Bolisetty, S.; Mezzenga, R. Amyloid–carbon hybrid membranes for universal water purification. Nat. Nanotechnol. 2016, 11, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Peydayesh, M.; Bolisetty, S.; Mohammadi, T.; Mezzenga, R. Assessing the Binding Performance of Amyloid–Carbon Membranes toward Heavy Metal Ions. Langmuir 2019, 35, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Mohanasrinivasan, V.; Mishra, M.; Paliwal, J.S.; Singh, S.K.; Selvarajan, E.; Suganthi, V.; Devi, C.S. Studies on heavy metal removal efficiency and antibacterial activity of chitosan prepared from shrimp shell waste. 3 Biotech 2014, 4, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wei, M.; Huang, Y. Biosorption of Multifold Toxic Heavy Metal Ions from Aqueous Water onto Food Residue Eggshell Membrane Functionalized with Ammonium Thioglycolate. J. Agric. Food Chem. 2013, 61, 4988–4996. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Pešić, M.B.; Barać, M.B.; Stanojević, S.P.; Lačnjevac, Č.M.; Maćej, O.D.; Stojanović, M.D. The Influence of The Addition of Polyacrylic Hydrogel on the Content of Proteins, Minerals and Trace Elements in Milk Protein Solutions. Food Technol. Biotech. 2014, 52, 128–134. [Google Scholar]
- Xiao, F.; Cheng, J.; Cao, W.; Yang, C.; Chen, J.; Luo, Z. Removal of heavy metals from aqueous solution using chitosan-combined magnetic biochars. J. Colloid Interface Sci. 2019, 540, 579–584. [Google Scholar] [CrossRef]
- Shen, C.; Peng, B.; Wang, Y.; Meng, Q. Pb 2+ and Hg 2+ removal from polluted milk by di-acrylated Pluronic P123 hydrogels. Food Chem. 2018, 258, 331–336. [Google Scholar] [CrossRef]
- Zang, J.; Li, C.; Zhou, K.; Dong, H.; Chen, B.; Wang, F.; Zhao, G. Nanomolar Hg2+ Detection Using β-Lactoglobulin-Stabilized Fluorescent Gold Nanoclusters in Beverage and Biological Media. Anal. Chem. 2016, 88, 10275–10283. [Google Scholar] [CrossRef]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta BBA Bioenerg. 1996, 1275, 161–203. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zang, J.; Wang, W.; Chen, H.; Zhang, X.; Wang, F.; Wang, H.; Zhao, G. Conversion of the Native 24-mer Ferritin Nanocage into Its Non-Native 16-mer Analogue by Insertion of Extra Amino Acid Residues. Angew. Chem. Int. Ed. 2016, 55, 16064–16070. [Google Scholar] [CrossRef]
- Ensign, D.; Young, M.; Douglas, T. Photocatalytic Synthesis of Copper Colloids from Cu(II) by the Ferrihydrite Core of Ferritin. Inorg. Chem. 2004, 43, 3441–3446. [Google Scholar] [CrossRef]
- Behera, R.; Theil, E.C. Moving Fe2+ from ferritin ion channels to catalytic OH centers depends on conserved protein cage carboxylates. Proc. Natl. Acad. Sci. USA 2014, 111, 7925–7930. [Google Scholar] [CrossRef] [Green Version]
- Chandramouli, B.; del Galdo, S.; Mancini, G.; Barone, V. Mechanistic insights into metal ions transit through threefold ferritin channel. Biochim. Biophys. Acta BBA Gen. Subj. 2019, 1863, 472–480. [Google Scholar] [CrossRef]
- Stefanini, S.; Chiancone, E.; Vecchini, P.; Antonini, E. Studies on iron uptake and micelle formation in ferritin and apoferritin. Mol. Cell. Biochem. 1976, 13, 55–61. [Google Scholar] [CrossRef]
- Jiang, B.; Fang, L.; Wu, K.; Yan, X.; Fan, K. Ferritins as natural and artificial nanozymes for theranostics. Theranostics 2020, 10, 687–706. [Google Scholar] [CrossRef]
- Masuda, T.; Zang, J.; Zhao, G.; Mikami, B. The first crystal structure of crustacean ferritin that is a hybrid type of H and L ferritin. Protein Sci. 2018, 27, 1955–1960. [Google Scholar] [CrossRef]
- Lv, C.; Yin, S.; Zhang, X.; Hu, J.; Zhang, T.; Zhao, G. 16-Mer ferritin-like protein templated gold nanoclusters for bioimaging detection of methylmercury in the brain of living mice. Anal. Chim. Acta 2020, 1127, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Jutz, G.; van Rijn, P.; Miranda, B.S.; Böker, A. Ferritin: A Versatile Building Block for Bionanotechnology. Chem. Rev. 2015, 115, 1653–1701. [Google Scholar] [CrossRef]
- Stefanini, S.; Cavallo, S.; Wang, C.-Q.; Tataseo, P.; Vecchini, P.; Giartosio, A.; Chiancone, E. Thermal Stability of Horse Spleen Apoferritin and Human Recombinant H Apoferritin. Arch. Biochem. Biophys. 1996, 325, 58–64. [Google Scholar] [CrossRef]
- Bou-Abdallah, F.; Arosio, P.; Levi, S.; Janus-Chandler, C.; Chasteen, N.D. Defining metal ion inhibitor interactions with recombinant human H- and L-chain ferritins and site-directed variants: An isothermal titration calorimetry study. JBIC J. Biol. Inorg. Chem. 2003, 8, 489–497. [Google Scholar] [CrossRef]
- Tolbert, A.E.; Ervin, C.S.; Ruckthong, L.; Paul, T.; Jayasinghe-Arachchige, V.M.; Neupane, K.P.; Stuckey, J.A.; Prabhakar, R.; Pecoraro, V.L. Heteromeric three-stranded coiled coils designed using a Pb(ii)(Cys)3 template mediated strategy. Nat. Chem. 2020, 12, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, C.; Ciambellotti, S.; Bernacchioni, C.; Di Pisa, F.; Mangani, S.; Turano, P. Chemistry at the protein–mineral interface in L-ferritin assists the assembly of a functional (μ3-oxo)Tris[(μ2-peroxo)] triiron(III) cluster. Proc. Natl. Acad. Sci. USA 2017, 114, 2580–2585. [Google Scholar] [CrossRef] [Green Version]
- Gallois, B.; D’Estaintot, B.L.; Michaux, M.-A.; Dautant, A.; Granier, T.; Précigoux, G.; Soruco, J.-A.; Roland, F.; Chavas-Alba, O.; Herbas, A.; et al. X-ray structure of recombinant horse L-chain apoferritin at 2.0 Å resolution: Implications for stability and function. JBIC J. Biol. Inorg. Chem. 1997, 2, 360–367. [Google Scholar] [CrossRef]
- Lawson, D.M.; Artymiuk, P.J.; Yewdall, S.J.; Smith, J.M.A.; Livingstone, J.C.; Treffry, A.; Luzzago, A.; Levi, S.; Arosio, P.; Cesareni, G.; et al. Solving the structure of human H ferritin by genetically engineering intermolecular crystal contacts. Nat. Cell Biol. 1991, 349, 541–544. [Google Scholar] [CrossRef]
- Welch, K.D.; Reilly, C.A.; Aust, S.D. The role of cysteine residues in the oxidation of ferritin. Free. Radic. Biol. Med. 2002, 33, 399–408. [Google Scholar] [CrossRef]
- Johnson, L.E.; Wilkinson, T.; Arosio, P.; Melman, A.; Bou-Abdallah, F. Effect of chaotropes on the kinetics of iron release from ferritin by flavin nucleotides. Biochim. Biophys. Acta BBA Gen. Subj. 2017, 1861, 3257–3262. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; Preston, G.M.; Smith, B.L.; Jung, J.S.; Raina, S.; Moon, C.; Guggino, W.B.; Nielsen, S. Aquaporin CHIP: The archetypal molecular water channel. Am. J. Physiol. Physiol. 1993, 265, F463–F476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preston, G.; Jung, J.; Guggino, W.; Agre, P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J. Biol. Chem. 1993, 268, 17–20. [Google Scholar] [CrossRef]
- Masuda, T.; Goto, F.; Yoshihara, T.; Mikami, B. Crystal Structure of Plant Ferritin Reveals a Novel Metal Binding Site That Functions as a Transit Site for Metal Transfer in Ferritin. J. Biol. Chem. 2010, 285, 4049–4059. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Chen, L.; Zhang, T.; Yang, S.; Leng, X.; Zhao, G. Self-assembly of ferritin nanocages into linear chains induced by poly(α,l-lysine). Chem. Commun. 2013, 50, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.A.; Manley, O.; Spuches, A.M.; Grossoehme, N.E. Dissecting ITC data of metal ions binding to ligands and proteins. Biochim. Biophys. Acta BBA Gen. Subj. 2016, 1860, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Bai, G.; Chen, L.; Shen, Q.; Diao, X.; Zhao, G. The interaction of phenolic acids with Fe(III) in the presence of citrate as studied by isothermal titration calorimetry. Food Chem. 2014, 157, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, X.; Zhao, G. Two different H-type subunits from pea seed (Pisum sativum) ferritin that are responsible for fast Fe(II) oxidation. Biochimie 2009, 91, 230–239. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zang, J.; Wang, C.; Zhang, X.; Zhao, G. Structural Insights for the Stronger Ability of Shrimp Ferritin to Coordinate with Heavy Metal Ions as Compared to Human H-Chain Ferritin. Int. J. Mol. Sci. 2021, 22, 7859. https://doi.org/10.3390/ijms22157859
Wang Y, Zang J, Wang C, Zhang X, Zhao G. Structural Insights for the Stronger Ability of Shrimp Ferritin to Coordinate with Heavy Metal Ions as Compared to Human H-Chain Ferritin. International Journal of Molecular Sciences. 2021; 22(15):7859. https://doi.org/10.3390/ijms22157859
Chicago/Turabian StyleWang, Yingjie, Jiachen Zang, Chengtao Wang, Xiuqing Zhang, and Guanghua Zhao. 2021. "Structural Insights for the Stronger Ability of Shrimp Ferritin to Coordinate with Heavy Metal Ions as Compared to Human H-Chain Ferritin" International Journal of Molecular Sciences 22, no. 15: 7859. https://doi.org/10.3390/ijms22157859
APA StyleWang, Y., Zang, J., Wang, C., Zhang, X., & Zhao, G. (2021). Structural Insights for the Stronger Ability of Shrimp Ferritin to Coordinate with Heavy Metal Ions as Compared to Human H-Chain Ferritin. International Journal of Molecular Sciences, 22(15), 7859. https://doi.org/10.3390/ijms22157859







