The Sodium-Glucose Cotransporter-2 Inhibitor Canagliflozin Alleviates Endothelial Dysfunction Following In Vitro Vascular Ischemia/Reperfusion Injury in Rats
Abstract
1. Introduction
2. Results
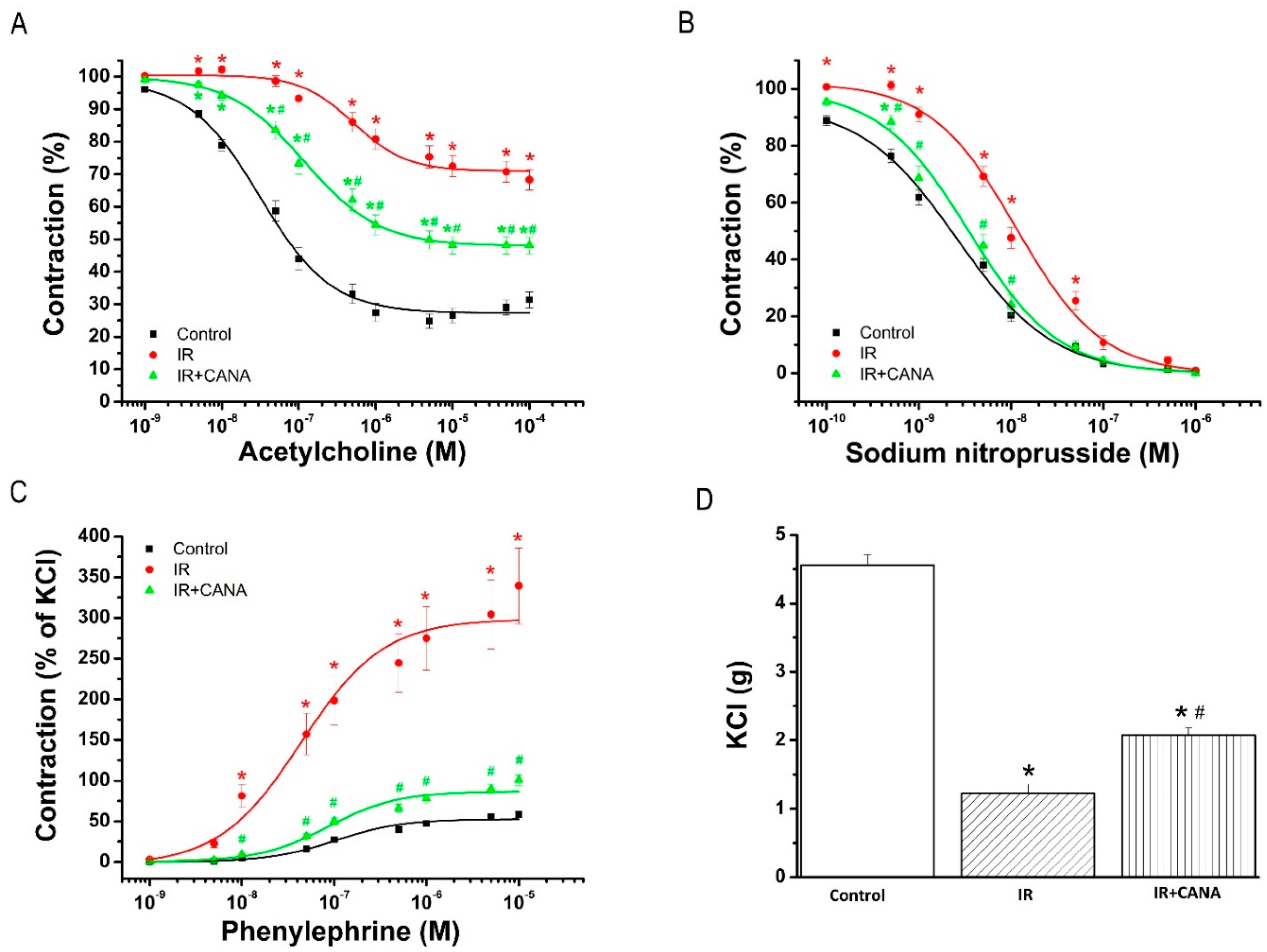
2.1. Aortic Vasoreactivity Following Vascular IRI
2.1.1. Effect of CANA on Endothelial Function after Vascular IRI
2.1.2. Effects of CANA on Smooth Muscle Relaxation after Vascular IRI
2.1.3. Effects of CANA on Contractility after Vascular IRI
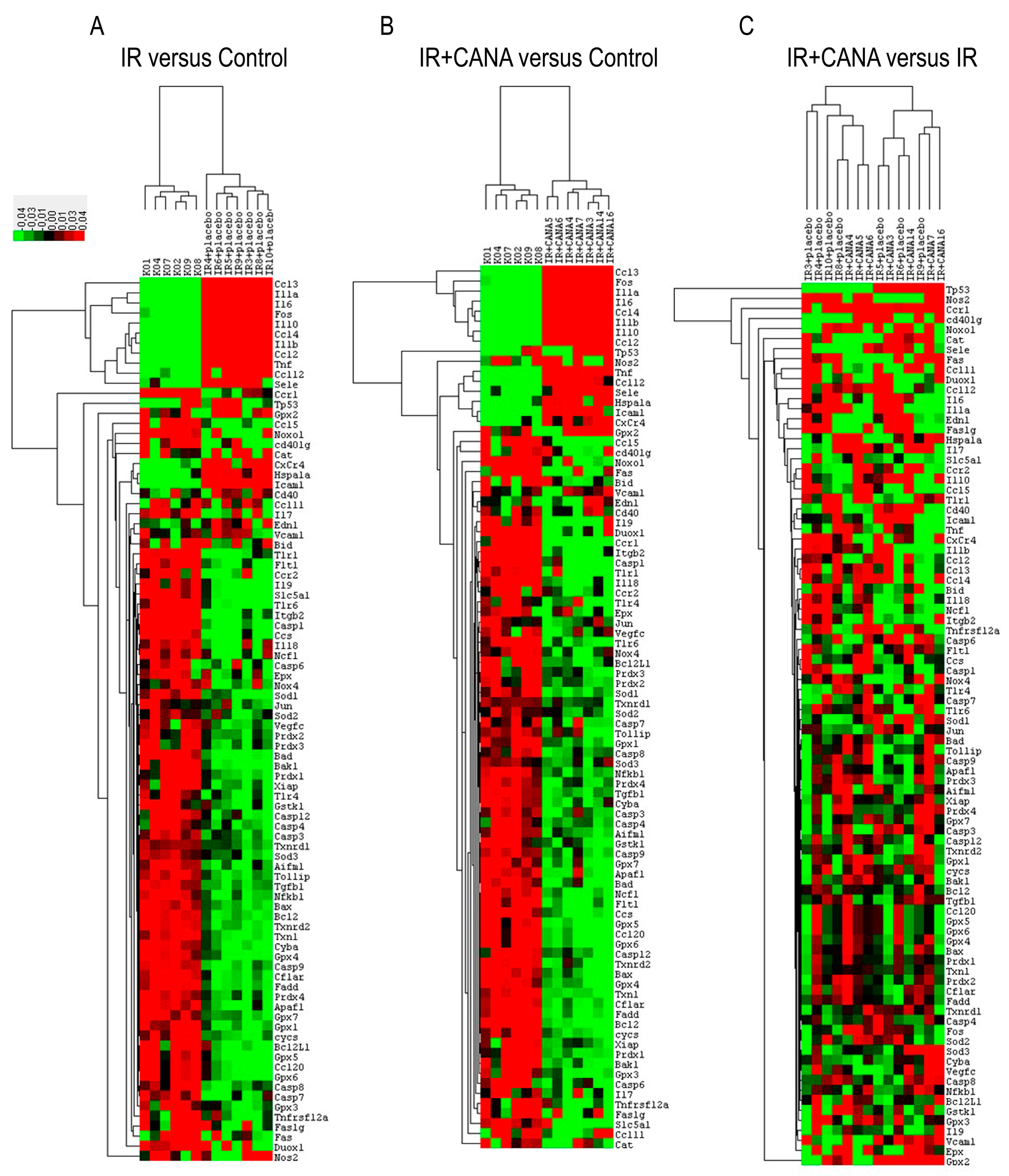
2.2. Effects of CANA on Aortic Gene Expression Following Vascular IRI
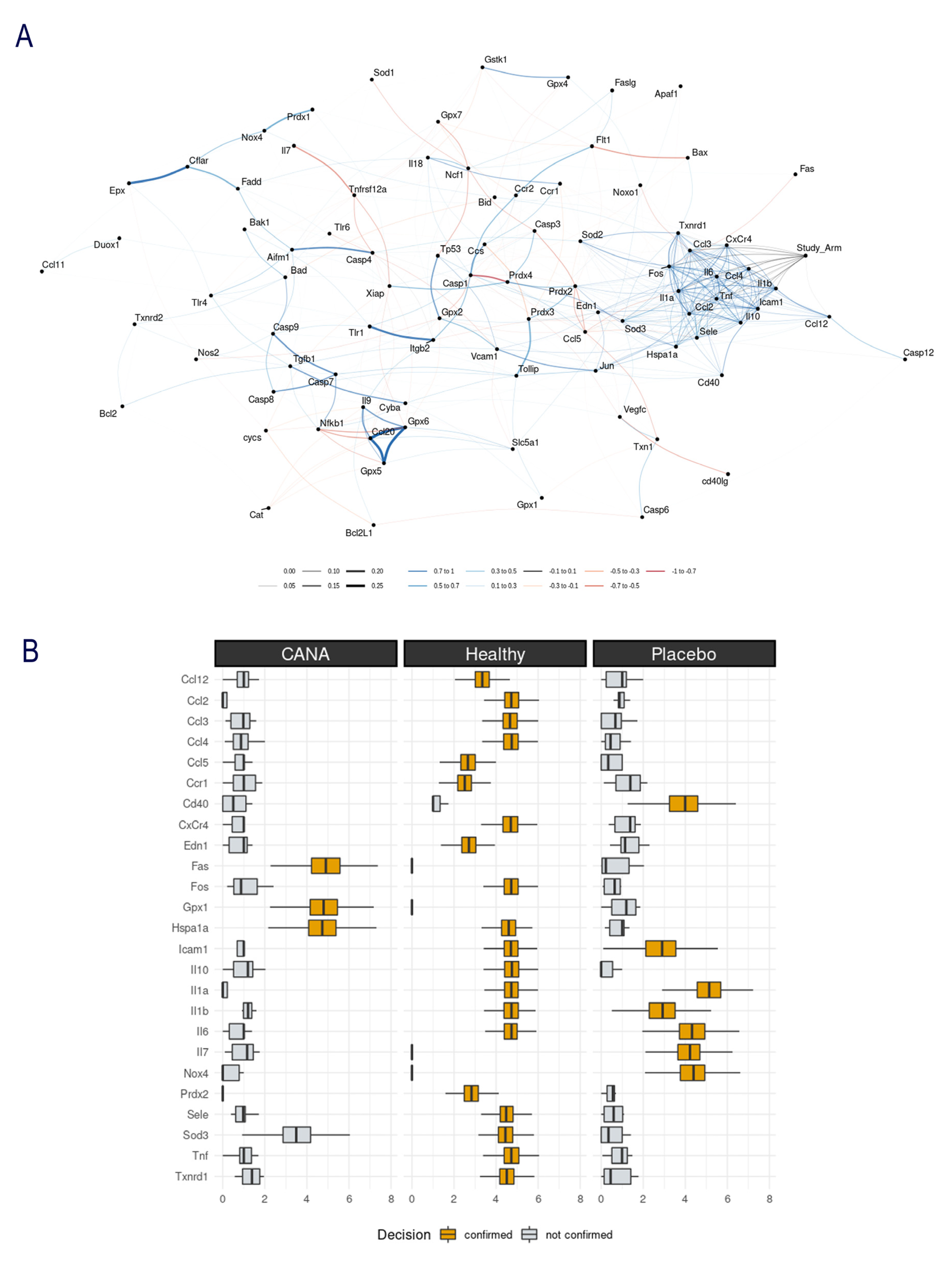
2.3. Aortic Gene Expression Explored by Machine Learning Algorithms
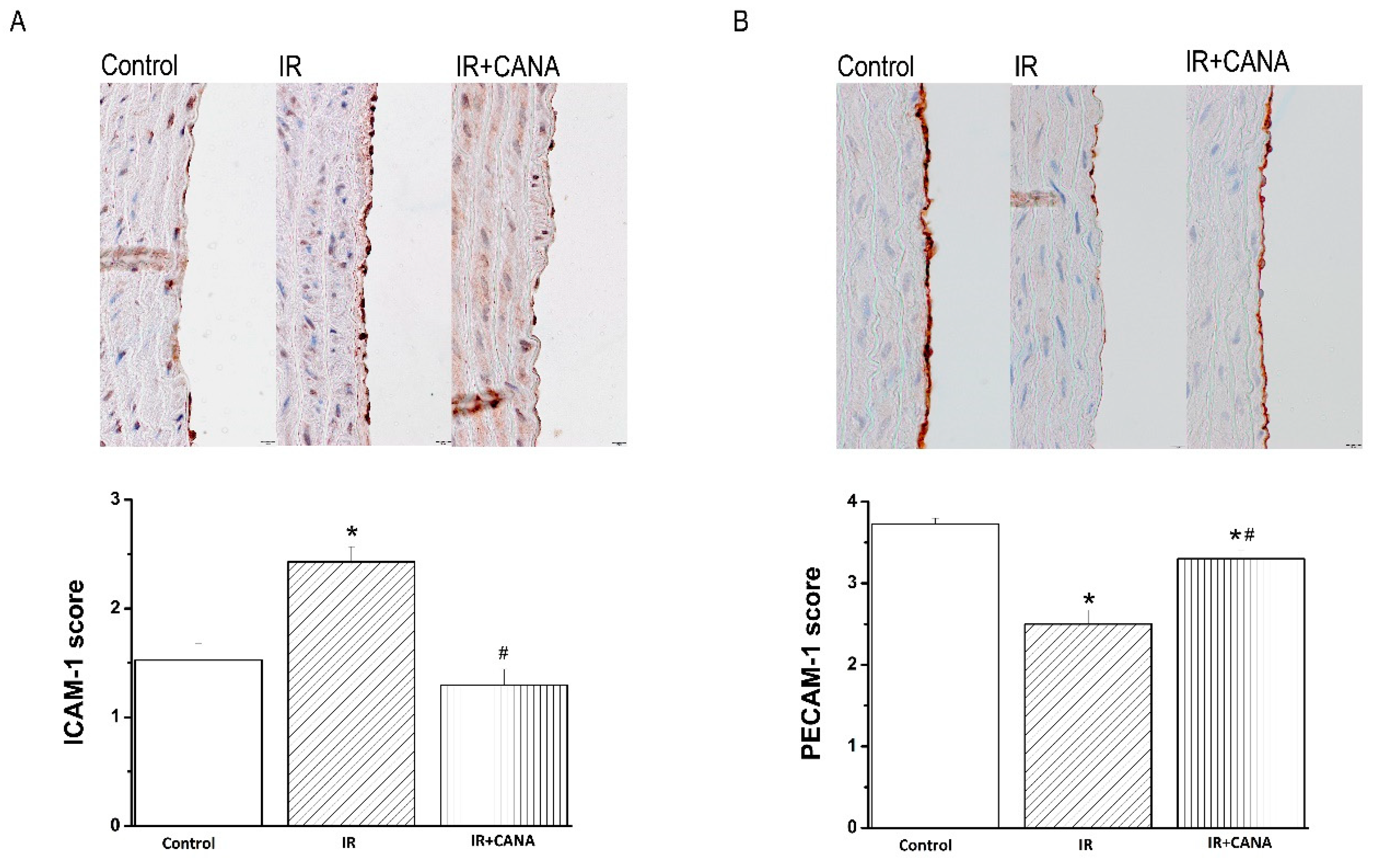
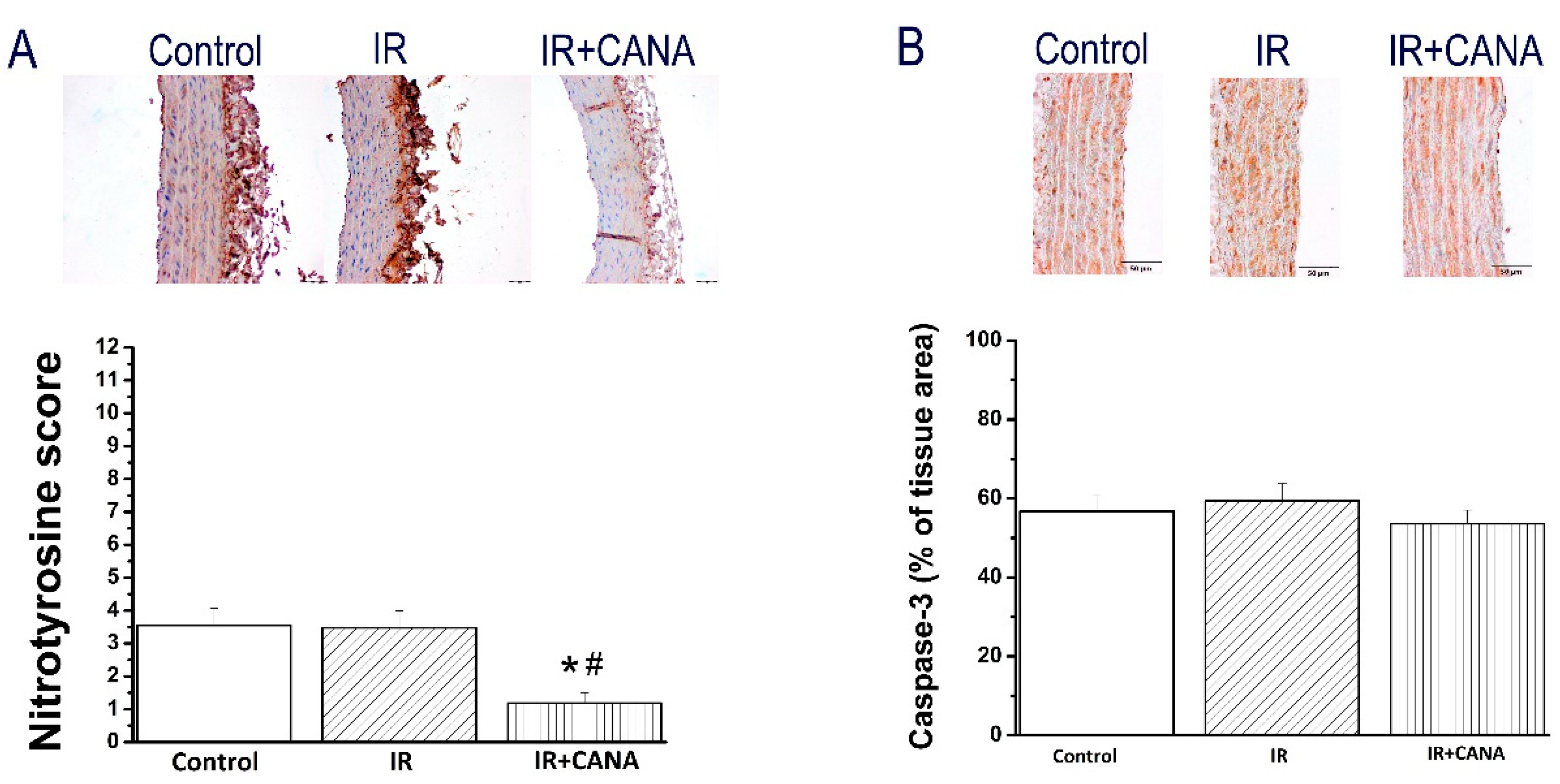
2.4. Effects of CANA on Aortic Intercellular Adhesion Molecule (ICAM)-1, Platelet Endothelial Cell Adhesion Molecule (PECAM)-1, Nitrotyrosine, and Caspase-3 Immunoreactivity after Vascular IRI
3. Discussion
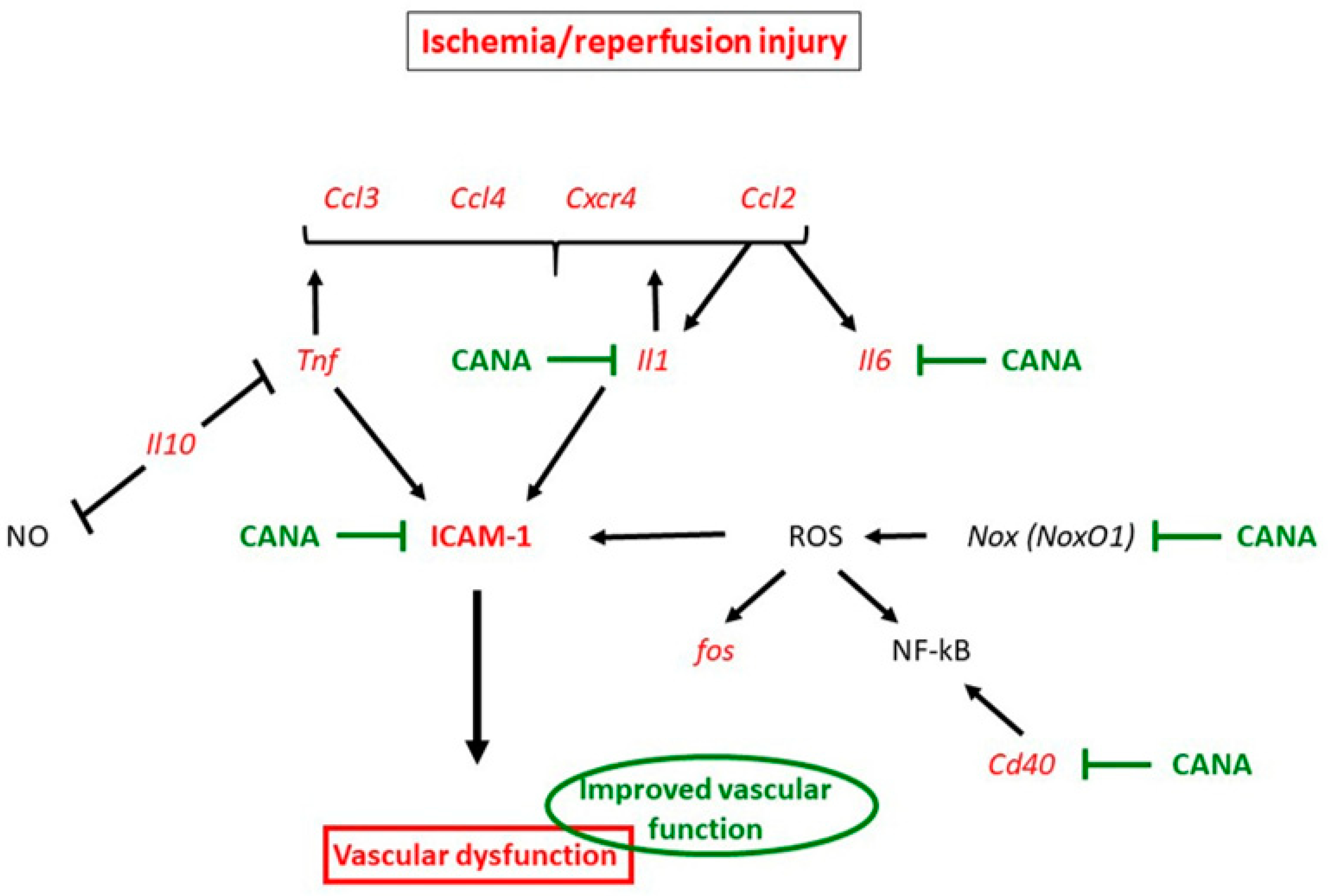
3.1. Mechanisms Underlying Protective Effects of CANA against IRI in Vasculature
3.2. Study Limitations
4. Materials and Methods
4.1. Animals
4.2. Rat Model of Vascular IRI
4.2.1. Preparation of Aortic Rings
4.2.2. Conservation of Aortic Rings and Experimental Groups
4.2.3. Ex Vivo Organ Baths Functional Experiments
4.3. Gene Expression Analysis
4.4. Machine Learning Algorithms
4.5. Immunohistochemical Staining for ICAM-1, PECAM-1, Nitrotyrosine, and Caspase-3
4.6. Canagliflozin
4.7. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carden, D.L.; Granger, D.N. Pathophysiology of ischaemia-reperfusion injury. J. Pathol. 2000, 190, 255–266. [Google Scholar] [CrossRef]
- Harskamp, R.E.; Lopes, R.D.; Baisden, C.E.; de Winter, R.J.; Alexander, J.H. Saphenous vein graft failure after coronary artery bypass surgery: Pathophysiology, management, and future directions. Ann. Surg. 2013, 257, 824–833. [Google Scholar] [CrossRef]
- Veres, G.; Hegedus, P.; Barnucz, E.; Schmidt, H.; Radovits, T.; Zoller, R.; Karck, M.; Szabo, G. TiProtec preserves endothelial function in a rat model. J. Surg. Res. 2016, 200, 346–355. [Google Scholar] [CrossRef]
- Wilbring, M.; Tugtekin, S.M.; Zatschler, B.; Ebner, A.; Reichenspurner, H.; Matschke, K.; Deussen, A. Even short-time storage in physiological saline solution impairs endothelial vascular function of saphenous vein grafts. Eur. J. Cardiothorac. Surg. 2011, 40, 811–815. [Google Scholar] [CrossRef]
- Tomlinson, B.; Hu, M.; Zhang, Y.; Chan, P.; Liu, Z.M. Evaluation of the pharmacokinetics, pharmacodynamics and clinical efficacy of empagliflozin for the treatment of type 2 diabetes. Expert Opin. Drug Metab. Toxicol. 2017, 13, 211–223. [Google Scholar] [CrossRef]
- Devineni, D.; Curtin, C.R.; Polidori, D.; Gutierrez, M.J.; Murphy, J.; Rusch, S.; Rothenberg, P.L. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J. Clin. Pharmacol. 2013, 53, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Dixon, D.L. The CANVAS Program: Implications of canagliflozin on reducing cardiovascular risk in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2019, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S. Canagliflozin and renal outcomes in type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and cardiovascular and renal events in type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Lim, V.G.; Bell, R.M.; Arjun, S.; Kolatsi-Joannou, M.; Long, D.A.; Yellon, D.M. SGLT2 Inhibitor, Canagliflozin, attenuates myocardial infarction in the diabetic and nondiabetic heart. JACC Basic Transl. Sci. 2019, 4, 15–26. [Google Scholar] [CrossRef]
- Sayour, A.A.; Celeng, C.; Olah, A.; Ruppert, M.; Merkely, B.; Radovits, T. Sodium-glucose cotransporter 2 inhibitors reduce myocardial infarct size in preclinical animal models of myocardial ischaemia-reperfusion injury: A meta-analysis. Diabetologia 2021, 64, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Sayour, A.A.; Korkmaz-Icoz, S.; Loganathan, S.; Ruppert, M.; Sayour, V.N.; Olah, A.; Benke, K.; Brun, M.; Benko, R.; Horváth, E.M.; et al. Acute canagliflozin treatment protects against in vivo myocardial ischemia-reperfusion injury in non-diabetic male rats and enhances endothelium-dependent vasorelaxation. J. Transl. Med. 2019, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Uthman, L.; Kuschma, M.; Romer, G.; Boomsma, M.; Kessler, J.; Hermanides, J.; Hollmann, M.W.; Preckel, B.; Zuurbier, C.J.; Weber, N.C. Novel anti-inflammatory effects of Canagliflozin involving Hexokinase II in Lipopolysaccharide-stimulated human coronary artery Endothelial cells. Cardiovasc. Drugs Ther. 2020. [Google Scholar] [CrossRef]
- Mancini, S.J.; Boyd, D.; Katwan, O.J.; Strembitska, A.; Almabrouk, T.A.; Kennedy, S.; Palmer, T.M.; Salt, I.P. Canagliflozin inhibits interleukin-1beta-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Sci. Rep. 2018, 8, 5276. [Google Scholar] [CrossRef]
- Rahadian, A.; Fukuda, D.; Salim, H.M.; Yagi, S.; Kusunose, K.; Yamada, H.; Soeki, T.; Sata, M. Canagliflozin prevents diabetes-induced vascular dysfunction in ApoE-deficient mice. J. Atheroscler. Thromb. 2020, 27, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Fischer, J.; Ley, K.; Sarembock, I.J. The role of inflammation in vascular injury and repair. J. Thromb. Haemost. 2003, 1, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Khemais-Benkhiat, S.; Belcastro, E.; Idris-Khodja, N.; Park, S.H.; Amoura, L.; Abbas, M.; Auger, C.; Kessler, L.; Mayoux, E.; Toti, F.; et al. Angiotensin II-induced redox-sensitive SGLT1 and 2 expression promotes high glucose-induced endothelial cell senescence. J. Cell Mol. Med. 2020, 24, 2109–2122. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. Endothelial nitric oxide synthase and endothelial dysfunction. Curr. Hypertens Rep. 2003, 5, 473–480. [Google Scholar] [CrossRef]
- Bagi, Z.; Frangos, J.A.; Yeh, J.C.; White, C.R.; Kaley, G.; Koller, A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1590–1595. [Google Scholar] [CrossRef]
- Newman, P.J. The biology of PECAM-1. J. Clin. Investig. 1997, 99, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Jevnikar, A.M.; Wuthrich, R.P.; Takei, F.; Xu, H.W.; Brennan, D.C.; Glimcher, L.H.; Rubin-Kelley, V.E. Differing regulation and function of ICAM-1 and class II antigens on renal tubular cells. Kidney Int. 1990, 38, 417–425. [Google Scholar] [CrossRef]
- Koong, A.C.; Chen, E.Y.; Giaccia, A.J. Hypoxia causes the activation of nuclear factor kappa B through the phosphorylation of I kappa B alpha on tyrosine residues. Cancer Res. 1994, 54, 1425–1430. [Google Scholar] [PubMed]
- Clark, P.R.; Manes, T.D.; Pober, J.S.; Kluger, M.S. Increased ICAM-1 expression causes endothelial cell leakiness, cytoskeletal reorganization and junctional alterations. J. Investig. Dermatol. 2007, 127, 762–774. [Google Scholar] [CrossRef] [PubMed]
- El-Daly, M.; Pulakazhi Venu, V.K.; Saifeddine, M.; Mihara, K.; Kang, S.; Fedak, P.W.M.; Alston, L.A.; Hirota, S.A.; Ding, H.; Triggle, C.R. Hyperglycaemic impairment of PAR2-mediated vasodilation: Prevention by inhibition of aortic endothelial sodium-glucose-co-Transporter-2 and minimizing oxidative stress. Vascul. Pharmacol. 2018, 109, 56–71. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Tsogka, K.; Nikolaou, E.; Liakopoulos, V.; Stefanidis, I. A unifying model of glucotoxicity in human renal proximal tubular epithelial cells and the effect of the SGLT2 inhibitor dapagliflozin. Int. Urol. Nephrol. 2020, 52, 1179–1189. [Google Scholar] [CrossRef]
- Umino, H.; Hasegawa, K.; Minakuchi, H.; Muraoka, H.; Kawaguchi, T.; Kanda, T.; Tokuyama, H.; Wakino, S.; Itoh, H. High Basolateral Glucose Increases Sodium-Glucose Cotransporter 2 and Reduces Sirtuin-1 in renal tubules through Glucose Transporter-2 Detection. Sci. Rep. 2018, 8, 6791. [Google Scholar] [CrossRef]
- Nespoux, J.; Patel, R.; Zhang, H.; Huang, W.; Freeman, B.; Sanders, P.W.; Kim, Y.C.; Vallon, V. Gene knockout of the Na(+)-glucose cotransporter SGLT2 in a murine model of acute kidney injury induced by ischemia-reperfusion. Am. J. Physiol. Renal Physiol. 2020, 318, F1100–F1112. [Google Scholar] [CrossRef]
- Nespoux, J.; Patel, R.; Hudkins, K.L.; Huang, W.; Freeman, B.; Kim, Y.C.; Koepsell, H.; Alpers, C.E.; Vallon, V. Gene deletion of the Na(+)-glucose cotransporter SGLT1 ameliorates kidney recovery in a murine model of acute kidney injury induced by ischemia-reperfusion. Am. J. Physiol. Renal Physiol. 2019, 316, F1201–F1210. [Google Scholar] [CrossRef]
- Li, Z.; Agrawal, V.; Ramratnam, M.; Sharma, R.K.; D’Auria, S.; Sincoular, A.; Jakubiak, M.; Music, M.L.; Kutschke, W.J.; Huang, X.N. Cardiac sodium-dependent glucose cotransporter 1 is a novel mediator of ischaemia/reperfusion injury. Cardiovasc. Res. 2019, 115, 1646–1658. [Google Scholar] [CrossRef] [PubMed]
- Ange, M.; De Poortere, J.; Ginion, A.; Battault, S.; Dechamps, M.; Muccioli, G.G.; Roumain, R.; Morelle, J.; Druart, S.; Mathivet, T. Canagliflozin protects against sepsis capillary leak syndrome by activating endothelial alpha1AMPK. Sci. Rep. 2021, 11, 13700. [Google Scholar] [CrossRef]
- Hristov, M.; Gumbel, D.; Lutgens, E.; Zernecke, A.; Weber, C. Soluble CD40 ligand impairs the function of peripheral blood angiogenic outgrowth cells and increases neointimal formation after arterial injury. Circulation 2010, 121, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Weissmann, N.; Schroder, K. NADPH oxidases in cardiovascular disease. Free Radic. Biol. Med. 2010, 49, 687–706. [Google Scholar] [CrossRef] [PubMed]
- Barnucz, E.; Veres, G.; Hegedus, P.; Klein, S.; Zoller, R.; Radovits, T.; Korkmaz, S.; Horkay, F.; Merkely, B.; Karck, M. Prolyl-hydroxylase inhibition preserves endothelial cell function in a rat model of vascular ischemia reperfusion injury. J. Pharmacol. Exp. Ther. 2013, 345, 25–31. [Google Scholar] [CrossRef]
- Radovits, T.; Lin, L.N.; Zotkina, J.; Koch, A.; Rauen, U.; Kohler, G.; Karck, M.; Szabo, G. Endothelial dysfunction after long-term cold storage in HTK organ preservation solutions: Effects of iron chelators and N-alpha-acetyl-L-histidine. J. Heart Lung Transplant. 2008, 27, 208–216. [Google Scholar] [CrossRef]
- Veres, G.; Hegedus, P.; Barnucz, E.; Zoller, R.; Radovits, T.; Korkmaz, S.; Kolonics, F.; Weymann, A.; Karck, M.; Szabo, G. Addition of vardenafil into storage solution protects the endothelium in a hypoxia-reoxygenation model. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 242–248. [Google Scholar] [CrossRef][Green Version]
- Korkmaz, S.; Loganathan, S.; Mikles, B.; Radovits, T.; Barnucz, E.; Hirschberg, K.; Li, S.; Hegedus, P.; Pali, S.; Weymann, A.; et al. Nitric oxide- and heme-independent activation of soluble guanylate cyclase attenuates peroxynitrite-induced endothelial dysfunction in rat aorta. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 70–77. [Google Scholar] [CrossRef]
- Korkmaz-Icoz, S.; Radovits, T.; Loganathan, S.; Li, S.; Ruppert, M.; Benke, K.; Brlecic, P.; Szabo, C.; Karck, M.; Szabo, G. Prolonging hypothermic ischaemic cardiac and vascular storage by inhibiting the activation of the nuclear enzyme poly (adenosine diphosphate-ribose) polymerase. Eur. J. Cardiothorac. Surg. 2017, 51, 829–835. [Google Scholar] [CrossRef][Green Version]
- Korkmaz-Icoz, S.; Vater, A.; Li, S.; Lehner, A.; Radovits, T.; Hegedus, P.; Ruppert, M.; Brlecic, P.; Zorn, M.; Karck, M. Mild type 2 diabetes mellitus improves remote endothelial dysfunction after acute myocardial infarction. J. Diabetes Complicat. 2015, 29, 1253–1260. [Google Scholar] [CrossRef]
- Korkmaz-Icoz, S.; Li, S.; Huttner, R.; Ruppert, M.; Radovits, T.; Loganathan, S.; Sayour, A.A.; Brlecic, P.; Lasitschka, F.; Karck, M. Hypothermic perfusion of donor heart with a preservation solution supplemented by mesenchymal stem cells. J. Heart Lung Transplant. 2019, 38, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz-Icoz, S.; Li, S.; Loganathan, S.; Radovits, T.; Ruppert, M.; Brlecic, P.; Sayour, A.A.; Veres, G.; Fleming, T.; Brune, M.; et al. Impairment of the Akt pathway in transplanted Type 1 diabetic hearts is associated with post-transplant graft injury. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 13. [Google Scholar] [CrossRef]
- Gansner, E.R.; Koren, Y.; North, S. Graph drawing by stress majorization. In International Symposium on Graph Drawing; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
| Control | IR | IR + CANA | |
|---|---|---|---|
| PE (% of KCl) | 2.6 ± 0.2 | 2.5 ± 0.1 | 1.9 ± 0.1 *,# |
| pD2 to PE | 6.77 ± 0.07 | 7.20 ± 0.14 * | 6.84 ± 0.06 # |
| KCl (g) | 4.6 ± 0.2 | 1.2 ± 0.1 * | 2.1 ± 0.1 *,# |
| Rmax to ACh (%) | 75.2 ± 2.3 | 31.7 ± 3.2 * | 51.9 ± 2.5 *,# |
| pD2 to ACh | 7.35 ± 0.08 | 6.28 ± 0.15 * | 6.86 ± 0.09 *,# |
| Rmax to SNP (%) | 99.4 ± 0.2 | 99.0 ± 0.6 | 100.0 ± 0.0 |
| pD2 to SNP | 8.65 ± 0.06 | 7.97 ± 0.07 * | 8.49 ± 0.09 # |
| IR vs. Control | IR + CANA vs. Control | IR + CANA vs. IR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Symbol | p-Value | Regulation | Comments | Symbol | p-Value | Regulation | Comments | Symbol | p-Value | Regulation | Comments |
| Aifm1 | 0.527 | 1.06 | Aifm1 | 0.277 | 1.09 | Aifm1 | 0.764 | 1.03 | |||
| Apaf1 | 0.716 | 1.03 | Apaf1 | 0.361 | 1.06 | Apaf1 | 0.67 | 1.04 | |||
| Bad | 0.059 | −1.17 | Bad | 0.894 | −1.01 | Bad | 0.05 | 1.16 | |||
| Bak1 | 0.374 | −1.1 | Bak1 | 0.532 | −1.07 | Bak1 | 0.545 | 1.03 | |||
| Bax | 0.023 | 1.1 | Bax | 0.168 | 1.06 | Bax | 0.259 | −1.04 | |||
| Bcl2 | 0.123 | 1.06 | Bcl2 | 0.791 | −1.00 | Bcl2 | 0.071 | −1.06 | |||
| Bcl2L1 | 0.321 | 1.09 | Bcl2L1 | 0.453 | 1.08 | Bcl2L1 | 0.919 | −1.01 | |||
| Bid | 0.068 | 1.83 | Bid | 0.252 | 1.41 | Bid | 0.041 | −1.29 | |||
| Casp1 | 0.018 | −1.28 | Casp1 | 0.061 | −1.27 | Casp1 | 0.945 | 1.01 | |||
| Casp12 | 0.055 | 1.18 | Casp12 | 0.229 | 1.11 | Casp12 | 0.241 | −1.07 | |||
| Casp3 | 0.256 | 1.1 | Casp3 | 0.247 | 1.11 | Casp3 | 0.91 | 1.01 | |||
| Casp4 | 0.146 | 1.18 | Casp4 | 0.156 | 1.19 | Casp4 | 0.814 | 1.01 | |||
| Casp6 | 0.276 | 1.17 | Casp6 | 0.863 | −1.03 | Casp6 | 0.191 | −1.21 | |||
| Casp7 | 0.607 | 1.06 | Casp7 | 0.026 | 1.26 | Casp7 | 0.128 | 1.19 | |||
| Casp8 | 0.461 | 1.08 | Casp8 | 0.059 | 1.20 | Casp8 | 0.266 | 1.1 | |||
| Casp9 | 0.74 | −1.03 | Casp9 | 0.44 | 1.07 | Casp9 | 0.272 | 1.1 | |||
| Cat | 0.767 | 1.14 | B | Cat | 0.818 | −1.10 | B | Cat | 0.581 | −1.25 | B |
| Ccl11 | 0.171 | 1.64 | Ccl11 | 0.841 | 1.07 | Ccl11 | 0.246 | −1.52 | |||
| Ccl12 | 0 | 8.55 | Ccl12 | 0 | 10.09 | Ccl12 | 0.658 | 1.18 | |||
| Ccl2 | 0 | 24.21 | Ccl2 | 0 | 21.75 | Ccl2 | 0.53 | −1.11 | |||
| Ccl20 | 0.787 | −1.01 | C | Ccl20 | 0.137 | −1.08 | C | Ccl20 | 0.089 | −1.06 | C |
| Ccl3 | 0.003 | 71.33 | Ccl3 | 0.003 | 75.18 | Ccl3 | 0.73 | 1.05 | |||
| Ccl4 | 0 | 34.85 | Ccl4 | 0 | 36.45 | Ccl4 | 0.806 | 1.05 | |||
| Ccl5 | 0.011 | −2.81 | Ccl5 | 0.012 | −2.82 | Ccl5 | 0.985 | −1 | |||
| Ccr1 | 0.153 | −4.21 | Ccr1 | 0.028 | −1.65 | Ccr1 | 0.326 | 2.55 | |||
| Ccr2 | 0.461 | −1.18 | Ccr2 | 0.776 | −1.05 | Ccr2 | 0.556 | 1.12 | |||
| Ccs | 0.113 | −1.22 | Ccs | 0.163 | −1.1 | Ccs | 0.349 | 1.11 | |||
| Cd40 | 0.004 | 2.13 | Cd40 | 0.04 | 1.6 | Cd40 | 0.206 | −1.34 | |||
| cd40lg | 0.281 | −1.56 | B | cd40lg | 0.882 | −1.07 | B | cd40lg | 0.428 | 1.46 | B |
| Cflar | 0.894 | 1.01 | Cflar | 0.675 | 1.02 | Cflar | 0.885 | 1.01 | |||
| CxCr4 | 0 | 4.47 | CxCr4 | 0 | 3.37 | CxCr4 | 0.144 | −1.32 | |||
| Cyba | 0.092 | 1.07 | Cyba | 0.281 | 1.1 | Cyba | 0.706 | 1.03 | |||
| cycs | 0.825 | 1.02 | cycs | 0.972 | 1 | cycs | 0.807 | −1.01 | |||
| Duox1 | 0.998 | −1 | B | Duox1 | 0.666 | −1.1 | B | Duox1 | 0.798 | −1.09 | B |
| Edn1 | 0.002 | 2.44 | Edn1 | 0.017 | 2.06 | Edn1 | 0.556 | −1.19 | |||
| Epx | 0.746 | 1.06 | Epx | 0.587 | 1.1 | Epx | 0.806 | 1.04 | |||
| Fadd | 0.954 | −1 | Fadd | 0.955 | −1 | Fadd | 0.978 | 1 | |||
| Fas | 0.973 | −1.01 | B | Fas | 0.081 | −1.87 | B | Fas | 0.06 | −1.85 | B |
| Faslg | 0.926 | 1.02 | Faslg | 0.932 | −1.02 | Faslg | 0.877 | −1.05 | |||
| Flt1 | 0.143 | −1.22 | Flt1 | 0.242 | −1.09 | Flt1 | 0.352 | 1.12 | |||
| Fos | 0 | 51.6 | Fos | 0 | 54.88 | Fos | 0.348 | 1.06 | |||
| Gpx1 | 0.738 | 1.02 | Gpx1 | 0.065 | 1.11 | Gpx1 | 0.054 | 1.09 | |||
| Gpx2 | 0.279 | 2.01 | B | Gpx2 | 0.144 | 2.12 | B | Gpx2 | 0.922 | 1.06 | B |
| Gpx3 | 0.696 | 1.05 | Gpx3 | 0.909 | 1.01 | Gpx3 | 0.678 | −1.04 | |||
| Gpx4 | 0.697 | 1.02 | Gpx4 | 0.748 | 1.01 | Gpx4 | 0.897 | −1 | |||
| Gpx5 | 0.428 | −1.09 | B | Gpx5 | 0.198 | −1.17 | B | Gpx5 | 0.089 | −1.06 | C |
| Gpx6 | 0.787 | −1.01 | C | Gpx6 | 0.137 | −1.08 | C | Gpx6 | 0.089 | −1.06 | C |
| Gpx7 | 0.647 | 1.03 | Gpx7 | 0.286 | 1.08 | Gpx7 | 0.453 | 1.05 | |||
| Gstk1 | 0.305 | 1.11 | Gstk1 | 0.453 | 1.07 | Gstk1 | 0.655 | −1.04 | |||
| Hspa1a | 0 | 4.63 | A | Hspa1a | 0 | 5.65 | A | Hspa1a | 0.376 | 1.22 | |
| Icam1 | 0 | 4.34 | Icam1 | 0 | 3.66 | Icam1 | 0.233 | −1.19 | |||
| Il10 | 0 | 35.06 | A | Il10 | 0 | 38.69 | A | Il10 | 0.63 | −1.1 | |
| Il18 | 0.817 | 1.03 | Il18 | 0.316 | −1.16 | Il18 | 0.306 | −1.2 | |||
| Il1a | 0 | 210.71 | A | Il1a | 0 | 138.48 | A | Il1a | 0.193 | −1.52 | |
| Il1b | 0 | 28.95 | Il1b | 0 | 24.13 | Il1b | 0.386 | −1.2 | |||
| Il6 | 0 | 95.21 | Il6 | 0 | 53.87 | Il6 | 0.086 | −1.77 | |||
| Il7 | 0.238 | −1.38 | Il7 | 0.693 | 1.08 | Il7 | 0.099 | 1.48 | |||
| Il9 | 0.381 | −1.22 | B | Il9 | 0.659 | −1.12 | B | Il9 | 0.591 | 1.09 | B |
| Itgb2 | 0.028 | −1.44 | Itgb2 | 0.015 | −1.55 | Itgb2 | 0.668 | −1.08 | |||
| Jun | 0.005 | 1.34 | Jun | 0.027 | 1.31 | Jun | 0.813 | −1.02 | |||
| Ncf1 | 0.827 | 1.03 | Ncf1 | 0.136 | −1.12 | Ncf1 | 0.247 | −1.16 | |||
| Nfkb1 | 0.046 | 1.11 | Nfkb1 | 0.074 | 1.11 | Nfkb1 | 0.96 | −1 | |||
| Nos2 | 0.891 | −1.08 | B | Nos2 | 0.686 | 1.29 | B | Nos2 | 0.567 | 1.39 | B |
| Nox4 | 0.023 | 1.35 | Nox4 | 0.372 | 1.11 | Nox4 | 0.14 | −1.21 | |||
| Noxo1 | 0.127 | −1.9 | B | Noxo1 | 0.036 | −2.27 | B | Noxo1 | 0.674 | −1.2 | B |
| Prdx1 | 0.239 | 1.12 | Prdx1 | 0.376 | 1.09 | Prdx1 | 0.178 | −1.03 | |||
| Prdx2 | 0.01 | 1.23 | Prdx2 | 0.026 | 1.21 | Prdx2 | 0.593 | −1.02 | |||
| Prdx3 | 0.099 | 1.19 | Prdx3 | 0.088 | 1.21 | Prdx3 | 0.841 | 1.01 | |||
| Prdx4 | 0.179 | 1.09 | Prdx4 | 0.027 | 1.14 | Prdx4 | 0.49 | 1.05 | |||
| Sele | 0 | 8.3 | A | Sele | 0 | 9.81 | A | Sele | 0.644 | 1.18 | |
| Slc5a1 | 0.241 | −1.23 | B | Slc5a1 | 0.847 | −1.06 | B | Slc5a1 | 0.516 | 1.16 | B |
| Sod1 | 0.352 | −1.09 | Sod1 | 0.948 | −1.01 | Sod1 | 0.234 | 1.09 | |||
| Sod2 | 0.005 | 1.32 | Sod2 | 0.001 | 1.33 | Sod2 | 0.991 | 1 | |||
| Sod3 | 0 | 1.24 | Sod3 | 0.002 | 1.36 | Sod3 | 0.217 | 1.1 | |||
| Tgfb1 | 0.129 | 1.11 | Tgfb1 | 0.176 | 1.09 | Tgfb1 | 0.764 | −1.02 | |||
| Tlr1 | 0.082 | −1.49 | Tlr1 | 0.183 | −1.27 | Tlr1 | 0.468 | 1.18 | |||
| Tlr4 | 0.83 | 1.03 | Tlr4 | 0.284 | 1.19 | Tlr4 | 0.267 | 1.16 | |||
| Tlr6 | 0.095 | −1.23 | Tlr6 | 0.131 | −1.16 | Tlr6 | 0.575 | 1.06 | |||
| Tnf | 0 | 17.56 | Tnf | 0 | 14.7 | Tnf | 0.483 | −1.19 | |||
| Tnfrsf12a | 0.947 | 1.01 | Tnfrsf12a | 0.609 | −1.1 | Tnfrsf12a | 0.462 | −1.12 | |||
| Tollip | 0.06 | 1.1 | Tollip | 0.027 | 1.2 | Tollip | 0.204 | 1.1 | |||
| Tp53 | 0.051 | 3.7 | B | Tp53 | 0.076 | 4.79 | B | Tp53 | 0.762 | 1.3 | B |
| Txn1 | 0.202 | 1.05 | Txn1 | 0.386 | 1.03 | Txn1 | 0.437 | −1.02 | |||
| Txnrd1 | 0 | 1.28 | Txnrd1 | 0 | 1.34 | Txnrd1 | 0.325 | 1.05 | |||
| Txnrd2 | 0.428 | 1.04 | Txnrd2 | 0.773 | 1.02 | Txnrd2 | 0.772 | −1.02 | |||
| Vcam1 | 0.011 | 1.73 | Vcam1 | 0.024 | 1.75 | Vcam1 | 0.934 | 1.01 | |||
| Vegfc | 0.156 | 1.24 | Vegfc | 0.154 | 1.26 | Vegfc | 0.87 | 1.02 | |||
| Xiap | 0.381 | 1.1 | Xiap | 0.14 | 1.21 | Xiap | 0.116 | 1.09 | |||
| Name/Gene ID/Symbol | Gene Name/Description (for Rattus Norvegicus (=Wistar rat)) |
|---|---|
| Aifm1 | apoptosis inducing factor, mitochondria associated 1 |
| Apaf1 | apoptotic peptidase activating factor 1 |
| Bad | BCL2-associated agonist of cell death |
| Bak1 | BCL2-antagonist/killer 1 |
| Bax | BCL2 associated X, apoptosis regulator |
| Bcl2 | BCL2, apoptosis regulator |
| Bcl2L1 | Bcl2-like 1 |
| Bid | BH3 interacting domain death agonist |
| Casp1 | caspase 1 |
| Casp12 | caspase 12 |
| Casp3 | caspase 3 |
| Casp4 | caspase 4 |
| Casp6 | caspase 6 |
| Casp7 | caspase 7 |
| Casp8 | caspase 8 |
| Casp9 | caspase 9 |
| Cat | catalase |
| Ccl11 | C-C motif chemokine ligand 11 |
| Ccl12 | chemokine (C-C motif) ligand 12 |
| Ccl2 | C-C motif chemokine ligand 2 |
| Ccl20 | C-C motif chemokine ligand 20 |
| Ccl3 | C-C motif chemokine ligand 3 |
| Ccl4 | C-C motif chemokine ligand 4 |
| Ccl5 | C-C motif chemokine ligand 5 |
| Ccr1 | C-C motif chemokine receptor 1 |
| Ccr2 | C-C motif chemokine receptor 2 |
| Ccs | copper chaperone for superoxide dismutase |
| Cd40 | CD40 molecule |
| cd40lg | CD40 ligand |
| Cflar | CASP8 and FADD-like apoptosis regulator |
| CxCr4 | C-X-C motif chemokine receptor 4 |
| Cyba | cytochrome b-245 alpha chain |
| cycs | cytochrome c, somatic |
| Duox1 | dual oxidase 1 |
| Edn1 | endothelin 1 |
| Epx | eosinophil peroxidase |
| Fadd | Fas associated via death domain |
| Fas | Fas cell surface death receptor |
| Faslg | Fas ligand |
| Flt1 | Fms related receptor tyrosine kinase 1 |
| Fos | Fos proto-oncogene, AP-1 transcription factor subunit |
| Gpx1 | glutathione peroxidase 1 |
| Gpx2 | glutathione peroxidase 2 |
| Gpx3 | glutathione peroxidase 3 |
| Gpx4 | glutathione peroxidase 4 |
| Gpx5 | glutathione peroxidase 5 |
| Gpx6 | glutathione peroxidase 6 |
| Gpx7 | glutathione peroxidase 7 |
| Gstk1 | glutathione S-transferase kappa 1 |
| Hspa1a | heat shock protein family A (Hsp70) member 1A |
| Icam1 | intercellular adhesion molecule 1 |
| Il10 | interleukin 10 |
| Il18 | interleukin 18 |
| Il1a | interleukin 1 alpha |
| Il1b | interleukin 1 beta |
| Il6 | interleukin 6 |
| Il7 | interleukin 7 |
| Il9 | interleukin 9 |
| Itgb2 | integrin subunit beta 2 |
| Jun | Jun proto-oncogene, AP-1 transcription factor subunit |
| Ncf1 | neutrophil cytosolic factor 1 |
| Nfkb1 | nuclear factor kappa B subunit 1 |
| Nos2 | nitric oxide synthase 2 |
| Nox4 | NADPH oxidase 4 |
| Noxo1 | NADPH oxidase organizer 1 |
| Prdx1 | peroxiredoxin 1 |
| Prdx2 | peroxiredoxin 2 |
| Prdx3 | peroxiredoxin 3 |
| Prdx4 | peroxiredoxin 4 |
| Sele | selectin E |
| Slc5a1 | solute carrier family 5 member 1 |
| Sod1 | superoxide dismutase 1 |
| Sod2 | superoxide dismutase 2 |
| Sod3 | superoxide dismutase 3 |
| Tgfb1 | transforming growth factor, beta 1 |
| Tlr1 | toll-like receptor 1 |
| Tlr4 | toll-like receptor 4 |
| Tlr6 | toll-like receptor 6 |
| Tnf | tumor necrosis factor |
| Tnfrsf12a | TNF receptor superfamily member 12A |
| Tollip | toll interacting protein |
| Tp53 | tumor protein p53 |
| Txn1 | thioredoxin 1 |
| Txnrd1 | thioredoxin reductase 1 |
| Txnrd2 | thioredoxin reductase 2 |
| Vcam1 | vascular cell adhesion molecule 1 |
| Vegfc | vascular endothelial growth factor C |
| Xiap | X-linked inhibitor of apoptosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korkmaz-Icöz, S.; Kocer, C.; Sayour, A.A.; Kraft, P.; Benker, M.I.; Abulizi, S.; Georgevici, A.-I.; Brlecic, P.; Radovits, T.; Loganathan, S.; et al. The Sodium-Glucose Cotransporter-2 Inhibitor Canagliflozin Alleviates Endothelial Dysfunction Following In Vitro Vascular Ischemia/Reperfusion Injury in Rats. Int. J. Mol. Sci. 2021, 22, 7774. https://doi.org/10.3390/ijms22157774
Korkmaz-Icöz S, Kocer C, Sayour AA, Kraft P, Benker MI, Abulizi S, Georgevici A-I, Brlecic P, Radovits T, Loganathan S, et al. The Sodium-Glucose Cotransporter-2 Inhibitor Canagliflozin Alleviates Endothelial Dysfunction Following In Vitro Vascular Ischemia/Reperfusion Injury in Rats. International Journal of Molecular Sciences. 2021; 22(15):7774. https://doi.org/10.3390/ijms22157774
Chicago/Turabian StyleKorkmaz-Icöz, Sevil, Cenk Kocer, Alex A. Sayour, Patricia Kraft, Mona I. Benker, Sophia Abulizi, Adrian-Iustin Georgevici, Paige Brlecic, Tamás Radovits, Sivakkanan Loganathan, and et al. 2021. "The Sodium-Glucose Cotransporter-2 Inhibitor Canagliflozin Alleviates Endothelial Dysfunction Following In Vitro Vascular Ischemia/Reperfusion Injury in Rats" International Journal of Molecular Sciences 22, no. 15: 7774. https://doi.org/10.3390/ijms22157774
APA StyleKorkmaz-Icöz, S., Kocer, C., Sayour, A. A., Kraft, P., Benker, M. I., Abulizi, S., Georgevici, A.-I., Brlecic, P., Radovits, T., Loganathan, S., Karck, M., & Szabó, G. (2021). The Sodium-Glucose Cotransporter-2 Inhibitor Canagliflozin Alleviates Endothelial Dysfunction Following In Vitro Vascular Ischemia/Reperfusion Injury in Rats. International Journal of Molecular Sciences, 22(15), 7774. https://doi.org/10.3390/ijms22157774






