Cardiomyopathies: An Overview
Abstract
1. Introduction
2. Methods
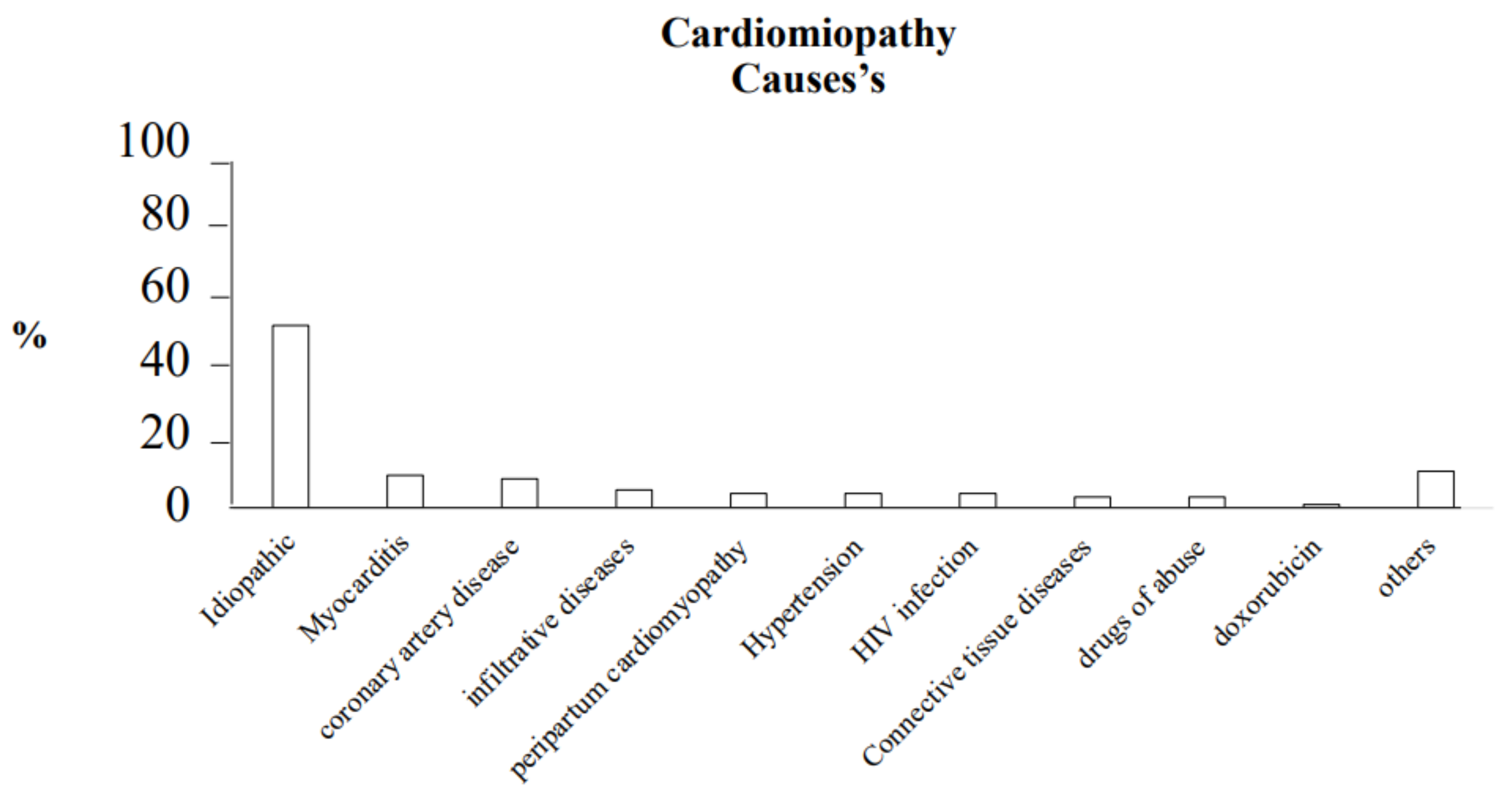
2.1. Dilated Cardiomyopathy (DCM)
- (a)
- Enteroviruses (coxsackievirus B2);
- (b)
- Adenoviruses;
- (c)
- Parvovirus B19;
- (d)
- Herpesviruses;
- (e)
- Epstein–Barr virus;
- (f)
- Rarely, hepatitis viruses;
- (g)
2.1.1. Causes
- ○
- TTN gene.
- ○
- LMNA gene.
- ○
- Mutations in the Phospholamban (PLN) and Filamin C (FLNC) genes.
- ○
- Other mutations that can cause dilated cardiomyopathies are those related to the following:
2.1.2. Clinical Manifestation
2.1.3. Diagnosis
Echocardiography
EKG
Laboratory Test
Cardiac Magnetic Resonance Imaging (MRI)
Coronary Angiography
Endomyocardial Biopsy
Genetic Testing
2.1.4. Management
- ○
- Patients who have survived a ventricular tachycardia, or;
- ○
- Patients who have had symptomatic ventricular tachycardia, and;
- ○
- In primary prevention in post-ischemic dilated cardiomyopathy.
2.2. Hypertrophic Cardiomyopathy (HCM)
2.2.1. Causes
- ○
- MYBPC3 gene (locus 11p11.2). This encodes cardiac myosin-binding protein C of the intermediate filament. Several mutations of this gene have been identified as missense, nonsense, splicing, deletion, and insertion. It is the most common gene involved, representing up to 40% of mutations [53].
- ○
- ○
- TNNT2 gene (locus 1q32.1). This encodes cardiac muscle troponin T of thin filament. It represents 5–10% of cases [55].
- ○
- TNNI3 gene (locus 19q13.4). This encodes cardiac troponin I of thin filament and is present in 4–8% of cases [56].
- ○
- Rare genes involved are:
- ○
- MYL2 gene (locus 12q23-q24) that encodes regulatory myosin light chain of thick filament [57].
- ○
- MYL3 gene (locus 3p21.3) that encodes essential myosin light chain of thick filament [57].
- ○
- TPM1 gene (locus 15q22.1) that encodes α-tropomyosin of thin filament [58].
- ○
- ACTC1 gene (locus 15q11q14) that encodes α-cardiac actin of thin filament [59].
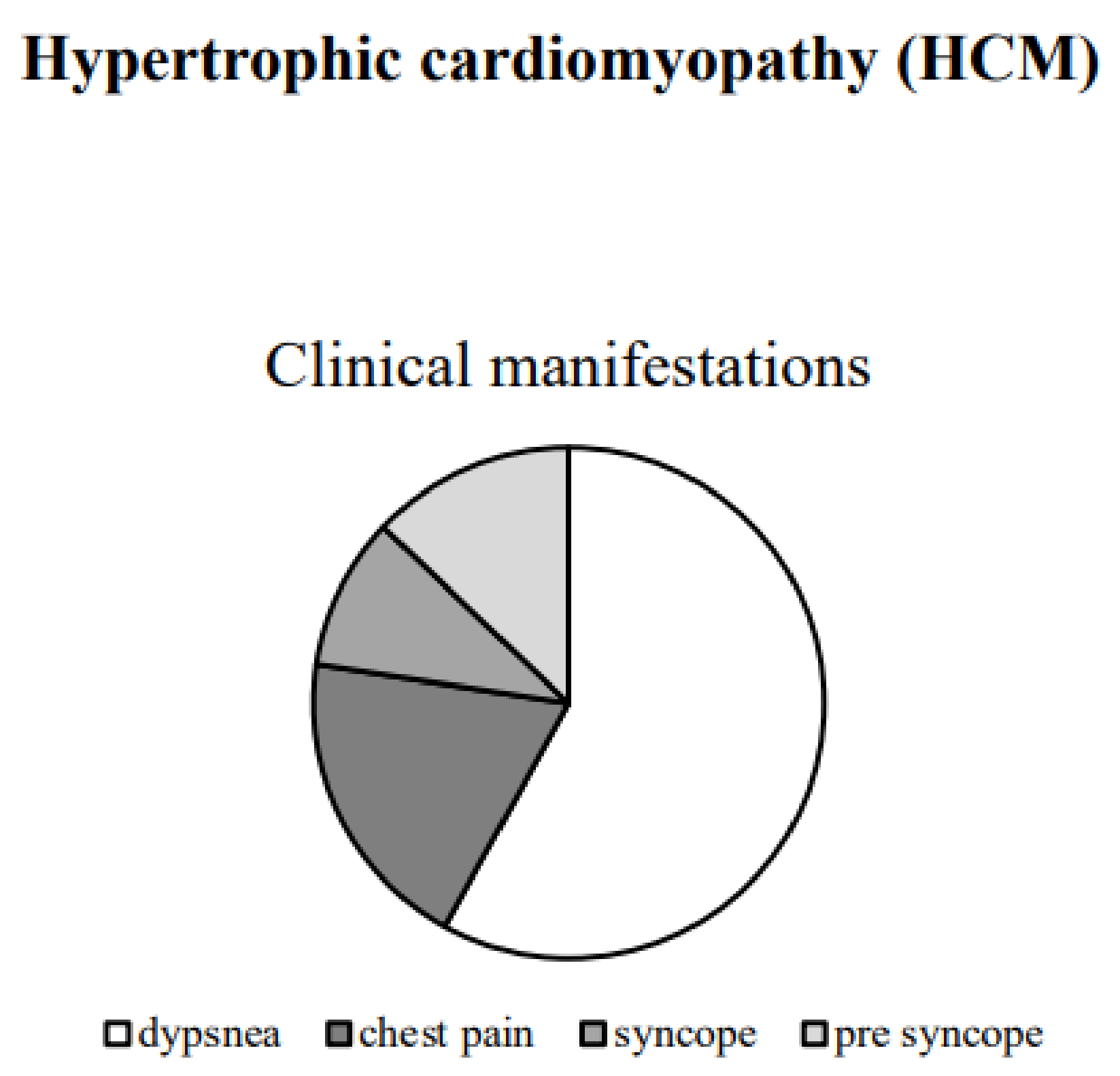
2.2.2. Clinical Manifestations
- ○
- A systolic murmur that begins slightly after S1 and is heard best at the apex and lower left sternal border, due to LVOTO obstruction.
- ○
- A holosystolic murmur heard loudest at the apex which radiates to the axilla, due to mitral regurgitation.
2.2.3. Diagnosis
EKG
Dynamic EKG Holter
Echocardiography
- ○
- Diastolic dysfunction;
- ○
- Enlargement of left atrium that is associated with increased risk of atrial fibrillation;
- ○
- Systolic dysfunction evaluated with global longitudinal strain (GLS), which is associated with major risk of heart failure, even with a normal LV ejection fraction [67].
- ○
- LVOTO obstruction due to systolic anterior motion (SAM) of the mitral valve. The obstruction is defined as a maximal left ventricular gradient >30 mmHg at rest or during exercise or provocative manoeuvers (such as Valsalva) [56].
- ○
- Exercise stress testing;
Cardiovascular Magnetic Resonance (CMR)
Genetic Test
2.2.4. Management
2.3. Sudden Cardiac Death (SCD)
- ○
- Family history of HCM-related sudden death;
- ○
- Massive LVH (≥30 mm);
- ○
- Unexplained syncope;
- ○
- End stage HF (ejection fraction <50%);
- ○
- Multiple, repetitive NSVT;
- ○
- Extensive LGE;
- ○
- LV apical aneurysm.
- ○
- Marked LV outflow obstruction at rest;
- ○
- Hypotensive response to exercise;
- ○
- Age ≥ 60 years (reduced risk);
- ○
- Alcohol septal ablation.
2.4. Arrhythmogenic Cardiomyopathy (ARCV)
2.4.1. Causes
- ○
- JUP,
- ○
- DSP,
- ○
- PKP2,
- ○
- DSG2,
- ○
- DSC2.
- ○
- LMNA and TMEM43 genes.
2.4.2. Clinical Manifestation
- ○
- The concealed phase, in which there are no or subtle structural changes in the right ventricle, with or without minor ventricular arrhythmias. In this case, sudden cardiac death may occur even at this early stage as the first manifestation of the disease in previously asymptomatic young individuals.
- ○
- The second phase is characterized by the occurrence of arrhythmias in association with manifest functional and structural abnormalities in the right ventricle, which are detectable by current imaging tests. Patients may experience arrhythmic symptoms such as palpitations, syncope, or cardiac arrest.
- ○
- The third phase is characterized by right ventricular (RV) failure with a relatively preserved LV function.
- ○
- The end stage is characterized by parallel significant left ventricular (LV) involvement with systolic dysfunction. At this stage, AC can mimic dilated cardiomyopathy of other causes with its related complications, such as atrial fibrillation and thromboembolic events.
2.4.3. Diagnosis
Echocardiography
EKG
Biopsy
Family History
2.4.4. Management
- ○
- The high-risk category (estimated event rate >10% per year) includes either patients with a history of cardiac arrest or sustained VT or patients with severe dysfunction of the RV, LV, or both. The indication for ICD implantation in this subset of patients is a class I recommendation.
- ○
- For the intermediate-risk category (estimated event rate of 1–10% per year), which includes patients with ≥1 risk factor and no previous malignant arrhythmic events, the indications for ICD therapy for primary prevention of sudden cardiac death (SCD) are the following:
- ○
- In the presence of major risk factors such as syncope, non-sustained VT, or moderate ventricular dysfunction, an ICD can be recommended (class IIa).
- ○
- In selected patients with ≥1 minor risk factor, where the arrhythmic risk is not sufficiently high or defined, ICD therapy may also be considered (class IIb).
- ○
- Prophylactic ICD implantation is not recommended (class III) in asymptomatic patients with no risk factors and in healthy gene carriers (low-risk category), event rate <10% per year [103].
2.5. Restrictive Cardiomyopathy (RCM)
2.5.1. Causes
- ○
- Infiltrative:
- (a)
- Amyloidosis (acquired/inherited);
- (b)
- Genes: TTR gene variants (V122I; I68L; L111M; T60A; S23N; P24S; W41L; V30M; V20I), APOA1;
- (c)
- Sarcoidosis (acquired);
- (d)
- Primary hyperoxaluria (inherited).
- ○
- Storage disease:
- (a)
- Fabry disease (inherited). Gene: GLA;
- (b)
- Gaucher disease (inherited). Gene: GBA;
- (c)
- Hereditary hemochromatosis (inherited). Genes: HAMP, HFE, HFE2, HJV, PNPLA3, SLC40A1, TfR2;
- (d)
- Glycogen storage disease (inherited);
- (e)
- Mucopolysaccharidosis type I (Hurler syndrome) (inherited). Gene: IDUA;
- (f)
- Mucopolysaccharidosis type II (Hunter syndrome) (inherited). Gene: IDS;
- (g)
- Niemann–Pick disease (inherited). Genes: NPC1, NPC2, SMPD1.
- ○
- Non-infiltrative:
- (a)
- Idiopathic (acquired);
- (b)
- Diabetic cardiomyopathy (acquired);
- (c)
- Scleroderma (acquired);
- (d)
- Myofibrillar myopathies (inherited). Genes: BAG3, CRYAB, DES, DNAJB6, FHL1, FLNC, LDB3, MYOT;
- (e)
- Pseudoxanthoma elasticum (inherited). Gene: ABCC6;
- (f)
- Sarcomeric protein disorders (inherited). Genes: ACTC, β-MHC, TNNT2, TNNI3, TNNC1, DES, MYH, MYL3, CRYAB;
- (g)
- Werner’s syndrome (inherited). Gene: WRN.
- ○
- Endomyocardial:
- (a)
- Carcinoid heart disease (acquired);
- (b)
- Endomyocardial fibrosis idiopathic (acquired);
- (c)
- Hypereosinophilic syndrome (acquired);
- (d)
- Chronic eosinophilic leukemia (acquired);
- (e)
- Drugs (serotonin, methysergide, ergotamine, mercurial agents, busulfan) (acquired);
- (f)
- Endocardial fibroelastosis (inherited). Genes: BMP5, BMP7, TAZ;
- (g)
- Consequence of cancer/cancer therapy: metastatic cancer, drugs (anthracyclines), radiation (acquired).
2.5.2. Clinical Presentation
2.5.3. Diagnosis
EKG
Chest Radiography
Echocardiography
- ○
- Atrial left (LA) maximum volume index >34 mL/m;
- ○
- Tricuspid regurgitation peak velocity (TRV) >2.8 m/s;
- ○
- Average E/e’ ratio >14;
- ○
- Annular e’ velocity (septal e’ <7 cm/s, lateral e’ <10 cm/s).
Cardiac Magnetic Resonance (CMR)
Endomyocardial Biopsy
2.5.4. Treatment
2.6. Takotsubo Cardiomyopathy
2.7. Peripartum Cardiomyopathy
- ○
- Development of heart failure (HF) toward the end of pregnancy or within five months following delivery.
- ○
- Absence of another identifiable cause for the HF.
- ○
- Left ventricular (LV) systolic dysfunction with an LV ejection fraction (LVEF) of less than 45 percent. The LV may or may not be dilated [126].
- ○
- Age greater than 30 years;
- ○
- African descent;
- ○
- Pregnancy with multiple fetus;
- ○
- Preeclampsia, eclampsia;
- ○
- Cocaine abuse;
- ○
- Long-term use (>4 weeks) of tocolytics (terbutaline).
- ○
- EKG: in 50%, it presents anomalies such as sinus tachycardia, repolarization anomalies, Q waves [141];
- ○
- BNP: BNP is typically high [142];
- ○
- Chest X-ray: Enlargement of the cardiac silhouette, redistribution of flow, and pleural effusion may be found [143];
- ○
- Echocardiography: Reduction in left ventricular ejection fraction (<45%) and frequent left ventricle dilatation [144].
2.8. Cardiotoxicity and Chemotherapy Drugs
- ○
- Old age (<65 years) or young (>4 years);
- ○
- Female gender;
- ○
- Pre-existing heart disease;
- ○
- Hypertension;
- ○
- Smoke;
- ○
- Hyperlipidemia;
- ○
- Obesity;
- ○
- Diabetes;
- ○
- High cumulative anthracycline exposure.
- ○
- Over 50 years of age;
- ○
- Previous or concomitant use of anthracyclines;
- ○
- Obesity;
- ○
- Preexisting cardiac dysfunction;
- ○
- Hypertension.
- ○
- ○
- ○
- Cisplatin: Cardiotoxicity due to cisplatin can be manifested by supraventricular tachycardia, bradycardia, ST-T wave changes, left bundle branch block, acute ischemic events, myocardial infarction, and ischemic cardiomyopathy. This toxicity may be related to electrolyte abnormalities secondary to cisplatin-induced nephrotoxicity [169,170].
3. Conclusions
4. Key Messages
- The American Heart Association describes a classification system that categorizes cardiomyopathy as primary or secondary. In primary cases, the disease process is chiefly confined to the heart. Secondary cardiomyopathy describes conditions in which cardiac involvement occurs as part of a systemic condition. This classification system is imperfect, and there is often overlap.
- Hypertrophic cardiomyopathy (HCM) is the most common primary cardiomyopathy, with a prevalence of 1:500 persons. Many patients with HCM are asymptomatic and are diagnosed during family screening,
- Dilated cardiomyopathy (DCM) has a prevalence of 1:2.500 and is the leading indication for heart transplantation. DCM can occur at any age, but is most common in patients 40 to 59 years of age. Symptom’s characteristic of DCM includes arrhythmias and thromboembolic events. Pathogenic or likely pathogenic variants were found in eight genes, (20%) of which are not included in a standard commercially available dilated cardiomyopathy panel.
- LV involvement in ARVC is characterized by clinical and cardiac magnetic resonance features which differ from those seen in DCM. The most distinctive feature of ARVC-LV phenotype is the large amount of fibrosis, which directly and negatively impacts the LV systolic function.
- Restrictive cardiomyopathy is the least common of the major cardiomyopathies, representing 2% to 5% of cases. The restrictive category includes many underlying etiologies and is defined by physiologic function rather than anatomy. Genetic-based RCM might be induced by mutations in genes of nonsarcomeric, sarcomeric, and sarcomere-associated proteins.
- Takotsubo cardiomyopathy is defined as an abrupt onset of left ventricular dysfunction in response to severe emotional or physiologic stress. Postmenopausal women are most commonly affected.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B.; American Heart Association. Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef]
- Arbustini, E.; Narula, N.; Tavazzi, L.; Serio, A.; Grasso, M.; Favalli, V.; Bellazzi, R.; Tajik, J.A.; Bonow, R.O.; Fuster, V.; et al. The MOGE(S) classification of cardiomyopathy for clinicians. J. Am. Coll. Cardiol. 2014, 64, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. Br. Heart J. 1980, 44, 672. [CrossRef] [PubMed]
- Richardson, P.; McKenna, W.; Bristow, M.; Maisch, B.; Mautner, B.; O’Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J.; Gyarfas, I.; et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar] [PubMed]
- Mahmaljy, H.; Yelamanchili, V.S.; Singhal, M. Dilated Cardiomyopathy; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Reichart, D.; Magnussen, C.; Zeller, T.; Blankenberg, S. Dilated cardiomyopathy: From epidemiologic to genetic phenotypes: A translational review of current literature. J. Intern. Med. 2019, 286, 362–372. [Google Scholar] [CrossRef]
- Weintraub, R.G.; Semsarian, C.; Macdonald, P. Dilated cardiomyopathy. Lancet 2017, 390, 400–414. [Google Scholar] [CrossRef]
- Manolio, T.A.; Baughman, K.L.; Rodeheffer, R.; Pearson, T.A.; Bristow, J.; Michels, V.V.; Abelmann, W.H.; Harlan, W.R. Prevalence and etiology of idiopathic dilated cardiomyopathy (summary of a National Heart, Lung, and Blood Institute workshop). Am. J. Cardiol. 1992, 69, 1458. [Google Scholar] [CrossRef]
- Towbin, J.A.; Lowe, A.M.; Colan, S.D.; Sleeper, L.A.; Orav, E.J.; Clunie, S.; Messere, J.; Cox, G.F.; Lurie, P.R.; Hsu, D.; et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA 2006, 296, 1867–1876. [Google Scholar] [CrossRef]
- Nugent, A.W.; Daubeney, P.E.; Chondros, P.; Carlin, J.; Cheung, M.; Wilkinson, L.C.; Davis, A.; Kahler, S.G.; Chow, C.; Wilkinson, J.L.; et al. The epidemiology of childhood cardiomyopathy in Australia. N. Engl. J. Med. 2003, 348, 1639. [Google Scholar] [CrossRef]
- Felker, G.M.; Thompson, R.E.; Hare, J.M.; Hruban, R.H.; Clemetson, D.E.; Howard, D.L.; Baughman, K.L.; Kasper, E.K. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N. Engl. J. Med. 2000, 342, 1077. [Google Scholar] [CrossRef]
- Japp, A.G.; Gulati, A.; Cook, S.A.; Cowie, M.R.; Prasad, S.K. The Diagnosis and Evaluation of Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2996–3010. [Google Scholar] [CrossRef]
- Laonigro, I.; Correale, M.; di Biase, M.; Altomare, E. Alcohol abuse and heart failure. Eur. J. Heart Fail. 2009, 11, 453–462. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Adams, M.J.; Colan, S.D.; Constine, L.S.; Herman, E.H.; Hsu, D.; Hudson, M.M.; Kremer, L.C.; Landy, D.; Miller, T.L.; et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions. Circulation 2013, 128, 1927–1995. [Google Scholar] [CrossRef]
- Ozcelik, C.; Erdmann, B.; Pilz, B.; Wettschureck, N.; Britsch, S.; Hübner, N.; Chien, K.R.; Birchmeier, C.; Garratt, A.N. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc. Natl. Acad. Sci. USA 2002, 99, 8880. [Google Scholar] [CrossRef]
- McNally, E.M.; Golbus, J.R.; Puckelwartz, M.J. Genetic mutations and mechanisms in dilated cardiomyopathy. J. Clin. Investig. 2013, 123, 19–26. [Google Scholar] [CrossRef]
- LeWinter, M.M.; Granzier, H.L. Cardiac titin and heart disease. J. Cardiovasc. Pharmacol. 2014, 63, 207–212. [Google Scholar] [CrossRef]
- Herman, D.S.; Lam, L.; Taylor, M.R.; Wang, L.; Teekakirikul, P.; Christodoulou, D.; Conner, L.; DePalma, S.R.; McDonough, B.; Sparks, E.; et al. Truncations of titin causing dilated cardiomyopathy. N. Engl. J. Med. 2012, 366, 619–628. [Google Scholar] [CrossRef]
- Schäfer, S.; De Marvao, A.; Adami, E.; Fiedler, L.; Ng, B.; Khin, E.; Rackham, O.; van Heesch, S.; Pua, C.J.; Kui, M.; et al. Titin-truncating variants affect heart function in disease cohorts and the general population. Nat. Genet. 2017, 49, 46–53. [Google Scholar] [CrossRef]
- Ware, J.; Li, J.; Mazaika, E.; Yasso, C.M.; DeSouza, T.; Cappola, T.P.; Tsai, E.J.; Hilfiker-Kleiner, D.; Kamiya, C.A.; Mazzarotto, F.; et al. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N. Engl. J. Med. 2016, 374, 233–241. [Google Scholar] [CrossRef]
- Taylor, M.R.; Fain, P.R.; Sinagra, G.; Robinson, M.L.; Robertson, A.D.; Carniel, E.; di Lenarda, A.; Bohlmeyer, T.J.; Ferguson, D.A.; Brodsky, G.L.; et al. Natural history of dilated cardiomyopathy due to lamin A/C gene mutations. J. Am. Coll Cardiol. 2003, 41, 771–780. [Google Scholar] [CrossRef]
- di Barletta, M.R.; Ricci, E.; Galluzzi, G.; Tonali, P.; Mora, M.; Morandi, L.; Romorini, A.; Voit, T.; Orstavik, K.H.; Merlini, L.; et al. Different mutations in the LMNA gene cause autosomal dominant and autosomal recessive Emery-Dreifuss muscular dystrophy. Am. J. Hum. Genet. 2000, 66, 1407–1412. [Google Scholar] [CrossRef]
- Truszkowska, G.T.; Bilinska, Z.T.; Kosińska, J.; Śleszycka, J.; Rydzanicz, M.; Sobieszczańska-Małek, M.; Franaszczyk, M.; Bilińska, M.; Stawiński, P.; Michalak, E.; et al. A study in Polish patients with cardiomyopathy emphasizes pathogenicity of phospholamban (PLN) mutations at amino acid position 9 and low penetrance of heterozygous null PLN mutations. BMC Med. Genet. 2015, 16, 21. [Google Scholar] [CrossRef]
- Dec, G.W.; Fuster, V. Idiopathic dilated cardiomyopathy. N. Engl. J. Med. 1994, 331, 1564–1575. [Google Scholar] [CrossRef]
- Merlo, M.; Cannatà, A.; Gobbo, M.; Stolfo, D.; Elliott, P.M.; Sinagra, G. Evolving concepts in dilated cardiomyopathy. Eur. J. Heart Fail. 2018, 20, 228–239. [Google Scholar] [CrossRef]
- Lakdawala, N.K.; Winterfield, J.R.; Funke, B.H. Dilated cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2013, 6, 228–237. [Google Scholar] [CrossRef]
- Rapezzi, C.; Arbustini, E.; Caforio, A.L.P.; Charron, P.; Blanes, J.G.; Heliö, T.; Linhart, A.; Mogensen, J.; Pinto, Y.; Ristic, A.; et al. Diagnostic work-up in cardiomyopathies: Bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 1448–1458. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular magnetic resonance in myocarditis: AJACC white paper. J. Am. Coll Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Marcotte, F. Cardiac magnetic resonance assessment of myocarditis. Circ. Cardiovasc. Imaging 2013, 6, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Hershberger, R.E. Genetic evaluation of dilated cardiomyopathy. Curr. Cardiol. Rep. 2013, 15, 375. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [PubMed]
- Beckermann, T.M.; McLeod, K.; Murday, V.; Potet, F.; George, A.L., Jr. Novel SCN5A mutation in amiodarone-responsive multifocal ventricular ectopy-associated cardiomyopathy. Heart Rhythm. 2014, 11, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Køber, L.; Thune, J.J.; Nielsen, J.C.; Haarbo, J.; Videbæk, L.; Korup, E.; Jensen, G.; Hildebrandt, P.; Steffensen, F.H.; Bruun, N.E.; et al. Defi brillator implantation in patients with nonischemic systolic heart failure. N. Engl. J. Med. 2016, 375, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Wilkoff, B.L.; Cook, J.R.; Epstein, A.E.; Greene, H.L.; Hallstrom, A.P.; Hsia, H.; Kutalek, S.P.; Sharma, A. Dual Chamber and VVI Implantable Defibrillator Trial Investigators. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA 2002, 288, 3115–3123. [Google Scholar] [PubMed]
- Yeoh, T.; Hayward, C.; Benson, V.; Sheu, A.; Richmond, Z.; Feneley, M.P.; Keogh, A.M.; Macdonald, P.; Fatkin, D. A randomised, placebo-controlled trial of carvedilol in early familial dilated cardiomyopathy. Heart Lung Circ. 2011, 20, 566–573. [Google Scholar] [CrossRef]
- Raman, S.V.; Hor, K.N.; Mazur, W.; Halnon, N.J.; Kissel, J.T.; He, X.; Tran, T.; Smart, S.; McCarthy, B.; Taylor, M.D.; et al. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2015, 14, 153–161. [Google Scholar] [CrossRef]
- Martino, H.; Brofman, P.; Greco, O.; Bueno, R.; Bodanese, L.; Clausell, N.; Maldonado, J.A.; Mill, J.; Braile, D.; Moraes, J.; et al. Multicentre, randomized, double-blind trial of intracoronary autologous mononuclear bone marrow cell injection in non-ischaemic dilated cardiomyopathy (the dilated cardiomyopathy arm of the MiHeart study). Eur. Heart J. 2015, 36, 2898–2904. [Google Scholar] [CrossRef]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar]
- Gersh, B.J.; Maron, B.J.; Bonow, R.O.; Dearani, J.A.; Fifer, M.A.; Link, M.S.; Naidu, S.S.; Nishimura, R.A.; Ommen, S.R.; Rakowski, H.; et al. ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation 2011, 124, e783–e831. [Google Scholar]
- Vulpian, A. Contribution à l’étude des rétrécissements de l’orifi ce ventriculo-aortique. Arch. Physiol. 1868, 3, 456–457. [Google Scholar]
- Brock, R. Functional obstruction of the left ventricle; acquired aortic subvalvar stenosis. Guys Hosp. Rep. 1957, 106, 221–238. [Google Scholar]
- Teare, D. Asymmetrical hypertrophy of the heart in young adults. Br. Heart J. 1958, 20, 1–8. [Google Scholar] [CrossRef]
- Maron, B.J. Clinical Course and Management of Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2018, 379, 655. [Google Scholar] [CrossRef]
- Maron, B.J.; Gardin, J.M.; Flack, J.M.; Gidding, S.S.; Kurosaki, T.T.; Bild, D.E. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 1995, 92, 785. [Google Scholar] [CrossRef]
- Maron, B.J.; Spirito, P.; Roman, M.J.; Paranicas, M.; Okin, P.M.; Best, L.G.; Lee, E.T.; Devereux, R.B. Prevalence of hypertrophic cardiomyopathy in a population-based sample of American Indians aged 51 to 77 years (the Strong Heart Study). Am. J. Cardiol. 2004, 93, 1510–1514. [Google Scholar] [CrossRef]
- Zou, Y.; Song, L.; Wang, Z.; Ma, A.; Liu, T.; Gu, H.; Lu, S.; Wu, P.; Zhang, Y.; Shen, L.; et al. Prevalence of idiopathic hypertrophic cardiomyopathy in China: A population-based echocardiographic analysis of 8080 adults. Am. J. Med. 2004, 116, 14. [Google Scholar] [CrossRef]
- Maron, M.S.; Rowin, E.J.; Lin, D.; Appelbaum, E.; Chan, R.H.; Gibson, C.M.; Lesser, J.R.; Lindberg, J.; Haas, T.S.; Udelson, J.E.; et al. Prevalence and clinical profile of myocardial crypts in hypertrophic cardiomyopathy. Circ. Cardiovasc. Imaging 2012, 5, 441–447. [Google Scholar] [CrossRef]
- Maron, M.S.; Maron, B.J.; Harrigan, C. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J. Am. Coll Cardiol. 2009, 54, 220–228. [Google Scholar] [CrossRef]
- Semsarian, C.; Ingles, J.; Maron, M.S.; Maron, B.J. New Perspectives on the Prevalence of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 1249–1254. [Google Scholar] [CrossRef]
- Olivotto, I.; Maron, M.S.; Adabag, A.S.; Casey, S.A.; Vargiu, D.; Link, M.S.; Udelson, J.E.; Cecchi, F.; Maron, B.J. Gender-related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2005, 46, 480. [Google Scholar] [CrossRef]
- Geske, J.B.; Ong, K.C.; Siontis, K.C.; Hebl, V.B.; Ackerman, M.J.; Hodge, O.D.; Miller, V.M.; Nishimura, A.R.; Oh, J.K.; Schaff, H.; et al. Women with hypertrophic cardiomyopathy have worse survival. Eur. Heart J. 2017, 38, 3434–3440. [Google Scholar] [CrossRef]
- Marian, A.J.; Braunwald, E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ. Res. 2017, 121, 749–770. [Google Scholar] [CrossRef]
- Van Driest, S.L.; Ommen, S.R. Sarcomeric genotyping in hypertrophic cardiomyopathy. Mayo Clin. Proc. 2005, 80, 463. [Google Scholar] [CrossRef]
- Richard, P.; Charron, P.; Carrier, L.; Ledeuil, C.; Cheav, T.; Pichereau, C.; Benaiche, A.; Isnard, R.; Dubourg, O.; Burban, M.; et al. Hypertrophic cardiomyopathy: Distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 2003, 107, 2227. [Google Scholar] [CrossRef]
- Van Driest, S.L.; Jaeger, M.A.; Ommen, S.R.; Will, M.L.; Gersh, B.J.; Tajik, A.J.; Ackerman, M.J. Comprehensive analysis of the beta-myosin heavy chain gene in 389 unrelated patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2004, 44, 602. [Google Scholar] [CrossRef]
- Veselka, J.; Anavekar, N.S.; Charron, P. Hypertrophic obstructive cardiomyopathy. Lancet 2017, 389, 1253–1267. [Google Scholar] [CrossRef]
- Poetter, K.; Jiang, H.; Master, S.R.; Chang, A.; Dalakas, M.C.; Rayment, I.; Sellers, J.R.; Fananapazir, L.; Epstein, N.D. Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nat. Genet. 1996, 13, 63. [Google Scholar] [CrossRef]
- Coviello, D.A.; Maron, B.J.; Spirito, P.; Watkins, H.; Vosberg, H.P.; Thierfelder, L.; Schoen, F.J.; Seidman, J.G.; Seidman, C.E. Clinical features of hypertrophic cardiomyopathy caused by mutation of a “hot spot” in the alpha-tropomyosin gene. J. Am. Coll. Cardiol. 1997, 29, 635. [Google Scholar] [CrossRef]
- Monserrat, L.; Hermida-Prieto, M.; Fernández, X.-G.F.; Rodríguez, I.; Dumont, C.; Cazón, L.; Cuesta, M.G.; Gonzalez-Juanatey, C.; Peteiro, J.; Álvarez, N.; et al. Mutation in the alpha-cardiac actin gene associated with apical hypertrophic cardiomyopathy, left ventricular non-compaction, and septal defects. Eur. Heart J. 2007, 28, 1953–1961. [Google Scholar] [CrossRef]
- Wigle, E.D.; Rakowski, H.; Kimball, B.P.; Williams, W.G. Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation 1995, 92, 1680. [Google Scholar] [CrossRef]
- Elliott, P.M.; Kaski, J.C.; Prasad, K.; Seo, H.; Slade, A.K.; Goldman, J.H.; McKenna, W.J. Chest pain during daily life in patients with hypertrophic cardiomyopathy: An ambulatory electrocardiographic study. Eur. Heart J. 1996, 17, 1056. [Google Scholar] [CrossRef]
- Nienaber, C.A.; Hiller, S.; Spielmann, R.P.; Geiger, M.; Kuck, K.H. Syncope in hypertrophic cardiomyopathy: Multivariate analysis of prognostic determinants. J. Am. Coll. Cardiol. 1990, 15, 948. [Google Scholar] [CrossRef]
- Garg, L.; Gupta, M.; Sabzwari, S.R.A.; Agrawal, S.; Agarwal, M.; Nazir, T.; Gordon, J.; Bozorgnia, B.; Martinez, M.W. Atrial fibrillation in hypertrophic cardiomyopathy: Prevalence, clinical impact, and management. Heart Fail. Rev. 2019, 24, 189-–197. [Google Scholar] [CrossRef] [PubMed]
- Weissler-Snir, A.; Chan, R.H.; Adler, A.; Care, M.; Chauhan, V.; Gollob, M.H.; Ziv-Baran, T.; Fourey, D.; Hindieh, W.; Rakowski, H.; et al. Usefulness of 14-Day Holter for Detection of Nonsustained Ventricular Tachycardia in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2016, 118, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Marstrand, P.; Han, L.; Day, S.M.; Olivotto, I.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Wittekind, S.G.; Helms, A.; Saberi, S.; et al. Hypertrophic Cardiomyopathy with Left Ventricular Systolic Dysfunction: Insights from the SHaRe Registry. Circulation 2020, 141, 1371. [Google Scholar] [CrossRef]
- McLeod, C.J.; Ackerman, M.J.; Nishimura, R.A.; Tajik, A.J.; Gersh, B.J.; Ommen, S.R. Outcome of patients with hypertrophic cardiomyopathy and a normal electrocardiogram. J. Am. Coll. Cardiol. 2009, 54, 229–233. [Google Scholar] [CrossRef]
- Hiemstra, Y.L.; Debonnaire, P. Development of and Progression of Overt Heart Failure in Nonobstructive Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2018, 122, 656. [Google Scholar] [CrossRef]
- Morise, A.P. Exercise Testing in Nonatherosclerotic Heart Disease: Hypertrophic Cardiomyopathy, Valvular Heart Disease, and Arrhythmias. Circulation 2011, 123, 216–225. [Google Scholar] [CrossRef]
- Gimeno, J.R.; Tome-Esteban, M.; Lofiego, C.; Hurtado, J.; Pantazis, A.; Mist, B.; Lambiase, P.; McKenna, W.J.; Elliott, P.M. Exercise-induced ventricular arrhythmias and risk of sudden cardiac death in patients with hypertrophic cardiomyopathy. Eur. Heart J. 2009, 30, 2599–2605. [Google Scholar] [CrossRef]
- Bogaert, J.; Olivotto, I. MR Imaging in Hypertrophic Cardiomyopathy: From Magnet to Bedside. Radiology 2014, 273, 329. [Google Scholar] [CrossRef]
- Maron, M.S.; Maron, B.J. Clinical Impact of Contemporary Cardiovascular Magnetic Resonance Imaging in Hypertrophic Cardiomyopathy. Circulation 2015, 132, 292–298. [Google Scholar] [CrossRef]
- Spirito, P.; Binaco, I.; Nishimura, R.A.; Tajik, A.J.; Gersh, B.J.; Ommen, S.R. Role of Preoperative Cardiovascular Magnetic Resonance in Planning Ventricular Septal Myectomy in Patients with Obstructive Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2019, 123, 1517. [Google Scholar] [CrossRef]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014, 130, 484. [Google Scholar] [CrossRef]
- Adabag, A.S.; Maron, B.J.; Appelbaum, E.; Harrigan, C.J.; Buros, J.; Gibson, C.M.; Lesser, J.R.; Hanna, C.A.; Udelson, J.E.; Manning, W.J.; et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2008, 51, 1369. [Google Scholar] [CrossRef]
- Ackerman, M.J.; Priori, S.G.; Willems, S.; Berul, C.; Brugada, R.; Calkins, H.; Camm, A.J.; Ellinor, P.; Gollob, M.; Hamilton, R.; et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011, 8, 1308. [Google Scholar] [CrossRef]
- Chimenti, C.; Pieroni, M.; Morgante, E.; Antuzzi, D.; Russo, A.; Russo, M.A.; Maseri, A.; Frustaci, A. Prevalence of Fabry disease in female patients with late-onset hypertrophic cardiomyopathy. Circulation 2004, 110, 1047. [Google Scholar] [CrossRef]
- Wu, X.; Simpson, J.; Hong, J.H.; Kim, K.H.; Thavarajah, N.K.; Backx, P.H.; Neel, B.G.; Araki, T. MEK-ERK pathway modulation ameliorates disease phenotypes in a mouse model of Noonan syndrome associated with the Raf1(L613V) mutation. J. Clin. Investig. 2011, 121, 1009–1025. [Google Scholar] [CrossRef]
- Arad, M.; Maron, B.J.; Gorham, J.M.; Johnson, W.H.; Saul, J.P.; Perez-Atayde, A.R.; Spirito, P.; Wright, G.B.; Kanter, R.J.; Seidman, C.E.; et al. Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N. Engl. J. Med. 2005, 352, 362. [Google Scholar] [CrossRef]
- Morrow, A.G.; Reitz, B.A.; Epstein, S.E.; Henry, W.L.; Conkle, D.M.; Itscoitz, S.B.; Redwood, D.R. Operative treatment in hypertrophic subaortic stenosis. Techniques, and the results of preand postoperative assessments in 83 patients. Circulation 1975, 52, 88–102. [Google Scholar] [CrossRef]
- Sigwart, U. Non-surgical myocardial reductionfor hypertrophic obstructivecardiomyopathy. Lancet 1995, 346, 211–214. [Google Scholar] [CrossRef]
- Geske, J.B.; Sorajja, P.; Nishimura, R.A.; Ommen, S.R. Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy: Correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation 2007, 116, 2702–2708. [Google Scholar] [CrossRef]
- MacIntyre, C.; Lakdawala, N.K. Management of Atrial Fibrillation in Hypertrophic Cardiomyopathy. Circulation 2016, 133, 1901–1905. [Google Scholar] [CrossRef]
- Olivotto, I.; Oreziak, A.; Barriales-Villa, R.; Abraham, T.P.; Masri, A.; Garcia-Pavia, P.; Saberi, S.; Lakdawala, N.K.; Wheeler, M.T.; Owens, A.; et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): A randomised, double-blind, placebo-controlled, phase 3 trial.; EXPLORER-HCM study investigators. Lancet 2020, 396, 759–769. [Google Scholar] [CrossRef]
- Towbin, J.A.; McKenna, W.J.; Abrams, D.; Ackerman, M.J.; Calkins, H.; Darrieux, F.; Daubert, J.P.; De Chillou, C.; DePasquale, E.C.; Desai, M.Y.; et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019, 16, e301–e372. [Google Scholar] [CrossRef]
- Marrone, D.; Zampieri, F.; Basso, C.; Zanatta, A.; Thiene, G. History of the discovery of Arrhythmogenic Cardiomyopathy. Eur. Heart J. 2019, 40, 1100–1104. [Google Scholar] [CrossRef]
- Perry, M.; Elliott, A.A.; Asimaki, A.; Basso, C.; Bauce, B.; Brooke, M.A.; Calkins, H.; Corrado, D.; Duru, F.; Green, K.J.; et al. Definition and treatment of arrhythmogenic cardiomyopathy: An updated expert panel report. Eur. J. Heart Fail. 2019, 21, 955–964. [Google Scholar]
- Basso, C.; Thiene, G.; Corrado, D.; Angelini, A.; Nava, A.; Valente, M. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation 1996, 94, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.G.P.J.; Daubert, J.P.; et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the task force criteria. Circulation 2010, 121, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.; Simpson, M.; Mogensen, J.; Shaw, A.; Hughes, S.; Syrris, P.; Sen-Chowdhry, S.; Rowland, E.; Crosby, A.; McKenna, W.J. Novel mutation in desmoplakin causes arrhythmogenic left ventricular cardiomyopathy. Circulation 2005, 112, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Trümmel, M.; Meyners, W. Prevalence of right ventricular dysplasia-cardiomyopathy in a non-referral hospital. Int. J. Cardiol. 2004, 97, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Groeneweg, J.A.; Bhonsale, A.; James, C.A.; Riele, A.S.t.; Dooijes, D.; Tichnell, C.; Murray, B.; Wiesfeld, A.C.P.; Sawant, A.C.; Kassamali, B. Clinical presentation, longterm follow-up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ. Cardiovasc. Genet. 2015, 8, 437–446. [Google Scholar] [CrossRef]
- Corrado, D.; Basso, C.; Judge, D.P. Arrhythmogenic Cardiomyopathy. Circ. Res. 2017, 121, 784–802. [Google Scholar] [CrossRef]
- Thiene, G.; Nava, A.; Corrado, D.; Rossi, L.; Pennelli, N. Right ventricular cardiomyopathy and sudden death in young people. N. Engl. J. Med. 1988, 318, 129–133. [Google Scholar] [CrossRef]
- Corrado, D.; Basso, C.; Thiene, G.; McKenna, W.J.; Davies, M.J.; Fontaliran, F.; Nava, A.; Silvestri, F.; Blomstrom-Lundqvist, C.; Wlodarska, E.K. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: A multicenter study. J. Am. Coll. Cardiol. 1997, 30, 1512–1520. [Google Scholar] [CrossRef]
- Calabrese, F.; Basso, C.; Thiene, G.; Carturan, E.; Valente, M.; Thiene, G. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: Is there a role for viruses? Cardiovasc. Pathol. 2006, 15, 11–17. [Google Scholar] [CrossRef]
- Zorzi, A.; Rigato, I.; Pilichou, K.; Marra, M.P.; Migliore, F.; Mazzotti, E.; Gregori, D.; Thiene, G.; Daliento, L.; Iliceto, S. Phenotypic expression is a prerequisite for malignant arrhythmic events and sudden cardiac death in arrhythmogenic right ventricular cardiomyopathy. Europace 2016, 18, 1086–1094. [Google Scholar] [CrossRef]
- Thiene, G.; Corrado, D.; Nava, A.; Rossi, L.; Poletti, A.; Boffa, G.M.; Daliento, L.; Pennelli, N. Right ventricular cardiomyopathy: Is there evidence of an inflammatory aetiology? Eur Heart J. 1991, 12 (Suppl. D), 22–25. [Google Scholar] [CrossRef]
- Corrado, D.; Zorzi, A.; Cerrone, M.; Rigato, I.; Mongillo, M.; Bauce, B.; Delmar, M. Relationship between arrhythmogenic right ventricular cardiomyopathy and brugada syndrome: New insights from molecular biology and clinical implications. Circ. Arrhythm. Electrophysiol. 2016, 9, e003631. [Google Scholar] [CrossRef]
- Cerrone, M.; Noorman, M.; Lin, X.; Chkourko, H.; Liang, F.; van der Nagel, R.; Hund, T.; Birchmeier, W.; Mohler, P.; van Veen, T.A. Sodium current deficit and arrhythmogenesis in a murine model of plakophilin-2 haploinsufficiency. Cardiovasc. Res. 2012, 95, 460–468. [Google Scholar] [CrossRef]
- Basso, C.; Corrado, D.; Thiene, G.; Marcus, F.I.; Nava, A.; Thiene, G. Arrhythmogenic right ventricular cardiomyopathy. Lancet 2009, 373, 1289–1300. [Google Scholar] [CrossRef]
- Cruz, F.M.; Sanz-Rosa, D.; Roche-Molina, M.; García-Prieto, J.; García-Ruiz, J.M.; Pizarro, G.; Jiménez-Borreguero, L.J.; Torres, M.; Bernad, A.; Ruíz-Cabello, J.; et al. Exercise triggers ARVC phenotype in mice expressing a disease-causing mutated version of human plakophilin-2. J. Am. Coll. Cardiol. 2015, 65, 1438–1450. [Google Scholar] [CrossRef]
- Corrado, D.; Wichter, T.; Link, M.S.; Hauer, R.N.W.; Marchlinski, F.E.; Anastasakis, A.; Bauce, B.; Basso, C.; Brunckhorst, C.; Tsatsopoulou, A. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: An International Task Force Consensus Statement. Circulation 2015, 132, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Marcus, G.M.; Glidden, D.; Polonsky, B.; Zareba, W.; Smith, L.M.; Cannom, D.S.; Estes, N.M.; Marcus, F.; Scheinman, M.M. Multidisciplinary Study of Right Ventricular Dysplasia Investigators. Multidisciplinary Study of Right Ventricular Dysplasia Investigators. Efficacy of antiarrhythmic drugs in arrhythmogenic right ventricular cardiomyopathy: A report from the North American ARVC Registry. J. Am. Coll. Cardiol. 2009, 54, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Dalal, D.; Jain, R.; Tandri, H.; Dong, J.; Eid, S.M.; Prakasa, K.; Tichnell, C.; James, C.; Abraham, T.; Russell, S.D. Long-term efficacy of catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J. Am. Coll. Cardiol. 2007, 50, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Asimaki, A.; Kapoor, S.; Plovie, E.; Arndt, A.K.; Adams, E.; Liu, Z.; James, C.A.; Judge, D.P.; Calkins, H.; Churko, J. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci. Transl. Med. 2014, 6, 240ra74. [Google Scholar] [CrossRef] [PubMed]
- Chelko, S.P.; Asimaki, A.; Andersen, P.; Bedja, D.; Amat-Alarcon, N.; DeMazumder, D.; Jasti, R.; MacRae, C.A.; Leber, R.; Kleber, A.G. Central role for GSK3β in the pathogenesis of arrhythmogenic cardiomyopathy. JCI Insight 2016, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Friedland, S.N.; Leong, A.; Filion, K.B.; Genest, J.; Lega, I.C.; Mottillo, S.; Poirier, P.; Reoch, J.; Eisenberg, M.J. The cardiovascular effects of peroxisome proliferator-activated receptor agonists. Am. J. Med. 2012, 125, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Broughton, K.M.; Sussman, M.A. Empowering adult stem cells for myocardial regeneration v2.0: Success in small steps. Circ. Res. 2016, 118, 867–880. [Google Scholar] [CrossRef]
- Ammash, N.M.; Seward, J.B.; Bailey, K.R.; Edwards, W.D.; Tajik, A.J. Clinical Profile and Outcome of Idiopathic Restrictive Cardiomyopathy. Circulation 2000, 101, 2490–2496. [Google Scholar] [CrossRef]
- Lewis, A.B. Clinical profile and outcome of restrictive cardiomyopathy in children. Am. Heart J. 1992, 123, 1589–1593. [Google Scholar] [CrossRef]
- Sayegh, A.L.C.; Dos Santos, M.R.; Sarmento, A.O.; de Souza, F.R.; Salemi, V.M.C.; Hotta, V.T.; Marques, A.C.D.B.; Krämer, H.H.; Trombetta, I.C.; Mady, C.; et al. Cardiac and peripheral autonomic control in restrictive cardiomyopathy. ESC Heart Fail. 2017, 4, 341. [Google Scholar] [CrossRef]
- Leya, F.S.; Arab, D.; Joyal, D.; Shioura, K.M.; Lewis, B.E.; Steen, L.H.; Cho, L. The efficacy of brain natriuretic peptide levels in differentiating constrictive pericarditis from restrictive cardiomyopathy. J. Am. Coll. Cardiol. 2005, 45, 1900–1902. [Google Scholar] [CrossRef]
- Amaki, M.; Savino, J.; Ain, D.L.; Sanz, J.; Pedrizzetti, G.; Kulkarni, H.; Narula, J.; Sengupta, P.P. Diagnostic concordance of echocardiography and cardiac magnetic resonance-based tissue tracking for differentiating constrictive pericarditis from restrictive cardiomyopathy. Circ. Cardiovasc. Imaging 2014, 7, 819–827. [Google Scholar] [CrossRef]
- Selvaganesh, M.; Arul, A.S.; Balasubramanian, S.; Ganesan, N.; Mohammed, S.N.; Sivakumar, G.S.; Veeramani, S.R.; Jeyasingh, P.; Sathishkumar, S.; Selvaraju, S. An unusual ECG pattern in restrictive cardiomyopathy. Indian Heart J. 2015, 67, 362–367. [Google Scholar] [CrossRef][Green Version]
- Habib, G.; Bucciarelli-Ducci, C.; Caforio, A.L.P.; Cardim, N.; Charron, P.; Cosyns, B.; Dehaene, A.; Derumeaux, G.; Donal, E.; Dweck, M.R. Multimodality Imaging in Restrictive Cardiomyopathies: An EACVI expert consensus document in collaboration with the “Working Group on myocardial and pericardial diseases” of the European Society of Cardiology Endorsed by The Indian Academy of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1090–1121. [Google Scholar]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Seward, J.B.; Casaclang-Verzosa, G. Infiltrative cardiovascular diseases: Cardiomyopathies that look alike. J. Am. Coll. Cardiol. 2010, 55, 1769–1779. [Google Scholar] [CrossRef]
- Patel, A.R.; Kramer, C.M. Role of Cardiac Magnetic Resonance in the Diagnosis and Prognosis of Nonischemic Cardiomyopathy. JACC Cardiovasc. Imaging 2017, 10, 1180–1193. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Francis, J.M.; Selvanayagam, J.B.; Neubauer, S. The Role of Cardiovascular Magnetic Resonance Imaging in Heart Failure. J. Am. Coll. Cardiol. 2009, 54, 1407–1424. [Google Scholar] [CrossRef]
- Kushwaha, S.S.; Fallon, J.T.; Fuster, V. Restrictive Cardiomyopathy. N. Engl. J. Med. 1997, 336, 267–276. [Google Scholar] [CrossRef]
- Zangwill, S.; Hamilton, R. Restrictive Cardiomyopathy. Pacing Clin. Electrophysiol. 2009, 32, S41–S43. [Google Scholar] [CrossRef]
- Denfield, S.W.; Webber, S.A. Restrictive Cardiomyopathy in Childhood. Heart Fail. Clin. 2010, 6, 445–452. [Google Scholar] [CrossRef]
- Deshmukh, A.; Kumar, G.; Pant, S.; Pant, S.; Rihal, C.; Murugiah, K.; Mehta, J.L. Prevalence of Takotsubo cardiomyopathy in the United States. Am. Heart J. 2012, 164, 66–71.e1. [Google Scholar] [CrossRef]
- Ono, R.; Falcão, L.M. Takotsubo cardiomyopathy systematic review: Pathophysiologic process, clinical presentation and diagnostic approach to Takotsubo cardiomyopathy. Int. J. Cardiol. 2016, 209, 196–205. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomström-Lundqvist, C.; Cífková, R.; de Bonis, M.; Iung, B.; Johnson, M.R.; Kintscher, U.; Kranke, P. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 2018, 39, 3165. [Google Scholar] [CrossRef]
- Kolte, D.; Khera, S.; Aronow, W.S.; Palaniswamy, C.; Mujib, M.; Ahn, C.; Jain, D.; Gass, A.; Ahmed, A.; Panza, J.A. Temporal trends in incidence and outcomes of peripartum cardiomyopathy in the United States: A nationwide population-based study. J. Am. Heart Assoc. 2014, 3, e001056. [Google Scholar] [CrossRef]
- Barasa, A.; Rosengren, A.; Sandström, T.Z.; Ladfors, L.; Schaufelberger, M. Heart Failure in Late Pregnancy and Postpartum: Incidence and Long-Term Mortality in Sweden from 1997 to 2010. J. Card. Fail. 2017, 23, 370. [Google Scholar] [CrossRef]
- Ersbøll, A.S.; Johansen, M.; Damm, P.; Rasmussen, S.; Vejlstrup, N.G.; Gustafsson, F. Peripartum cardiomyopathy in Denmark: A retrospective, population-based study of incidence, management and outcome. Eur. J. Heart Fail. 2017, 19, 1712. [Google Scholar] [CrossRef]
- Patten, I.S.; Rana, S.; Shahul, S.; Rowe, G.C.; Jang, C.; Liu, L.; Hacker, M.R.; Rhee, J.S.; Mitchell, J.; Mahmood, F. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012, 485, 33. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker-Kleiner, D.; Kaminski, K.; Podewski, E.; Bonda, T.; Schaefer, A.; Sliwa, K.; Forster, O.; Quint, A.; Landmesser, U.; Doerries, C. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 2007, 128, 589. [Google Scholar] [CrossRef] [PubMed]
- Halkein, J.; Tabruyn, S.P.; Ricke-Hoch, M.; Haghikia, A.; Nguyen, N.; Scherr, M.; Castermans, K.; Malvaux, L.; Lambert, V.; Thiry, M. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J. Clin. Investig. 2013, 123, 2143. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, K.; Förster, O.; Libhaber, E.; Fett, J.D.; Sundstrom, J.B.; Hilfiker-Kleiner, D.; Ansari, A.A. Peripartum cardiomyopathy: Inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur. Heart J. 2006, 27, 441. [Google Scholar] [CrossRef]
- Elkayam, U.; Akhter, M.W.; Singh, H.; Khan, S.; Bitar, F.; Hameed, A.; Shotan, A. Pregnancy-associated cardiomyopathy: Clinical characteristics and a comparison between early and late presentation. Circulation 2005, 111, 2050. [Google Scholar] [CrossRef]
- Homans, D.C. Peripartum cardiomyopathy. N. Engl. J. Med. 1985, 312, 1432. [Google Scholar] [CrossRef]
- Mendelson, M.A.; Chandler, J. Postpartum cardiomyopathy associated with maternal cocaine abuse. Am. J. Cardiol. 1992, 70, 1092. [Google Scholar] [CrossRef]
- Lampert, M.B.; Hibbard, J.; Weinert, L.; Briller, J.; Lindheimer, M.; Lang, R.M. Peripartum heart failure associated with prolonged tocolytic therapy. Am. J. Obstet. Gynecol. 1993, 168, 493. [Google Scholar] [CrossRef]
- Mandal, D.; Mandal, S.; Mukherjee, D.; Biswas, S.C.; Maiti, T.K.; Chattopadhaya, N.; Majumdar, B.; Panja, M. Pregnancy and subsequent pregnancy outcomes in peripartum cardiomyopathy. J. Obstet. Gynaecol. Res. 2011, 37, 222. [Google Scholar] [CrossRef]
- Mallikethi-Reddy, S.; Akintoye, E.; Trehan, N.; Sharma, S.; Briasoulis, A.; Jagadeesh, K.; Rubenfire, M.; Grines, C.L.; Afonso, L. Burden of arrhythmias in peripartum cardiomyopathy: Analysis of 9841 hospitalizations. Int. J. Cardiol. 2017, 235, 114. [Google Scholar] [CrossRef]
- Kane, A.; Mbaye, M.; Ndiaye, M.B.; Diao, M.; Moreira, P.-M.; Mboup, C.; Diop, I.B.; Sarr, M.; Kane, A.; Moreau, J.-C.; et al. Evolution and thromboembolic complications of the idiopathic peripartal cardiomyopathy at Dakar University Hospital: Forward-looking study about 33 cases. J. Gynecol. Obstet. Biol. Reprod. 2010, 39, 484. [Google Scholar] [CrossRef]
- Honigberg, M.C.; Elkayam, U.; Rajagopalan, N.; Modi, K.; Briller, J.E.; Drazner, M.H.; Wells, G.L.; McNamara, D.M.; Givertz, M.M. IPAC Investigators Electrocardiographic findings in peripartum cardiomyopathy. Clin. Cardiol. 2019, 42, 524. [Google Scholar] [CrossRef]
- Forster, O.; Hilfiker-Kleiner, D.; Ansari, A.A.; Sundstrom, J.B.; Libhaber, E.; Tshani, W.; Becker, A.; Yip, A.; Klein, G.; Sliwa, K. Reversal of IFN-gamma, oxLDL and prolactin serum levels correlate with clinical improvement in patients with peripartum cardiomyopathy. Eur. J. Heart Fail. 2008, 10, 861. [Google Scholar] [CrossRef]
- Blauwet, L.A.; Cooper, L.T. Diagnosis and management of peripartum cardiomyopathy. Heart 2011, 97, 1970. [Google Scholar] [CrossRef]
- Simeon, I.A. Echocardiographic profile of peripartum cardiomyopathy in a tertiary care hospital in sokoto, Nigeria. Indian Heart J. 2006, 58, 234. [Google Scholar]
- Mebazaa, A.; Seronde, M.F.; Gayat, E.; Gayat, E.; Tibazarwa, K.; Anumba, D.O.C.; Akrout, N.; Sadoune, M.; Sarb, J.; Arrigo, M.; et al. Imbalanced Angiogenesis in Peripartum Cardiomyopathy- Diagnostic Value of Placenta Growth Factor. Circ. J. 2017, 81, 1654. [Google Scholar] [CrossRef]
- Sliwa, K.; Hilfiker-Kleiner, D.; Petrie, M.C.; Mebazaa, A.; Pieske, B.; Buchmann, E.; Regitz-Zagrosek, V.; Schaufelberger, M.; Tavazzi, L.; van Veldhuisen, D.J.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2010, 12, 767. [Google Scholar] [CrossRef]
- Sliwa, K.; Blauwet, L.; Tibazarwa, K.; Libhaber, E.; Smedema, J.-P.; Becker, A.; McMurray, J.; Yamac, H.; Labidi, S.; Struman, I.; et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: A proof-of-concept pilot study. Circulation 2010, 121, 1465. [Google Scholar] [CrossRef]
- Bozkurt, B.; Villaneuva, F.S.; Holubkov, R.; Tokarczyk, T.; Alvarez, R.J., Jr.; MacGowan, G.A.; Murali, S.; Rosenblum, W.D.; Feldman, A.M.; McNamara, D.M. Intravenous immune globulin in the therapy of peripartum cardiomyopathy. J. Am. Coll. Cardiol. 1999, 34, 177. [Google Scholar] [CrossRef]
- Łasińska-Kowaraa, M.; Lango, R.; Kowalik, M.; Jarmoszewicz, K. Accelerated heart function recovery after therapeutic plasma exchange in patient treated with biventricular mechanical circulatory support for severe peripartum cardiomyopathy. Eur. J. Cardiothorac. Surg. 2014, 46, 1035. [Google Scholar] [CrossRef]
- Elkayam, U. Clinical characteristics of peripartum cardiomyopathy in the United States: Diagnosis, prognosis, and management. J. Am. Coll. Cardiol. 2011, 58, 659. [Google Scholar] [CrossRef]
- Bauersachs, J.; König, T.; van der Meer, P.; Petrie, M.C.; Hilfiker-Kleiner, D.; Mbakwem, A.; Hamdan, R.; Jackson, A.M.; Forsyth, P.; de Boer, R.A. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2019, 21, 827. [Google Scholar] [CrossRef]
- Pillarisetti, J.; Kondur, A.; Alani, A.; Reddy, M.; Reddy, M.; Vacek, J.; Weiner, C.P.; Ellerbeck, E.; Schreiber, T.; Lakkireddy, D. Peripartum cardiomyopathy: Predictors of recovery and current state of implantable cardioverter-defibrillator use. J. Am. Coll. Cardiol. 2014, 63, 2831. [Google Scholar] [CrossRef] [PubMed]
- McNamara, D.M.; Elkayam, U.; Alharethi, R.; Damp, J.; Hsich, E.; Ewald, G.; Modi, K.; Alexis, J.D.; Ramani, G.V.; Semigran, M.J. Clinical Outcomes for Peripartum Cardiomyopathy in North America: Results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). J. Am. Coll. Cardiol. 2015, 66, 905. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.C.; Neilan, T.G.; Francis, S.; Plana, J.C.; Scherrer-Crosbie, M. Anthracycline-Induced Cardiomyopathy in Adults. Compr. Physiol. 2015, 5, 1517. [Google Scholar] [PubMed]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911. [Google Scholar] [CrossRef]
- Qin, A.; Thompson, C.L.; Silverman, P. Predictors of late-onset heart failure in breast cancer patients treated with doxorubicin. J. Cancer Surviv. 2015, 9, 252. [Google Scholar] [CrossRef]
- Fiúza, M. Cardiotoxicity associated with trastuzumab treatment of HER2+ breast cancer. Adv. Ther. 2009, 26 (Suppl. 1), S9. [Google Scholar] [CrossRef]
- Ewer, M.S.; Lippman, S.M. Type II chemotherapy-related cardiac dysfunction: Time to recognize a new entity. J. Clin. Oncol. 2005, 23, 2900. [Google Scholar] [CrossRef]
- Bowles, E.J.; Wellman, R.; Feigelson, H.S.; Onitilo, A.A.; Freedman, A.N.; Delate, T.; Allen, L.A.; Nekhlyudov, L.; Goddard, K.A.B.; Davis, R.L.; et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: A retrospective cohort study. J. Natl. Cancer Inst. 2012, 104, 1293. [Google Scholar] [CrossRef]
- Guenancia, C.; Lefebvre, A.; Cardinale, D.; Yu, A.F.; Ladoire, S.; Ghiringhelli, F.; Zeller, M.; Rochette, L.; Cottin, Y.; Vergely, C. Obesity as a Risk Factor for Anthracyclines and Trastuzumab Cardiotoxicity in Breast Cancer: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2016, 34, 3157. [Google Scholar] [CrossRef]
- Steinberg, J.S.; Cohen, A.J.; Wasserman, A.G.; Cohen, P.; Ross, A.M. Acute arrhythmogenicity of doxorubicin administration. Cancer 1987, 60, 1213. [Google Scholar] [CrossRef]
- Rudzinski, T.; Ciesielczyk, M.; Religa, W.; Bednarkiewicz, Z.; Krzeminska-Pakula, M. Doxorubicin-induced ventricular arrhythmia treated by implantation of an automatic cardioverter-defibrillator. Europace 2007, 9, 278. [Google Scholar] [CrossRef]
- Kilickap, S.; Akgul, E.; Aksoy, S.; Aytemir, K.; Barista, I. Doxorubicin-induced second degree and complete atrioventricular block. Europace 2005, 7, 227. [Google Scholar] [CrossRef]
- Wang, L.; Tan, T.C.; Halpern, E.F.; Neilan, T.G.; Francis, S.A.; Picard, M.H.; Fei, H.; Hochberg, E.P.; Abramson, J.S.; Weyman, A.E. Major Cardiac Events and the Value of Echocardiographic Evaluation in Patients Receiving Anthracycline-Based Chemotherapy. Am. J. Cardiol. 2015, 116, 442. [Google Scholar] [CrossRef]
- Procter, M.; Suter, T.M.; de Azambuja, E.; Dafni, U.; van Dooren, V.; Muehlbauer, S.; Climent, M.A.; Rechberger, E.; Liu, W.T.-W.; Toi, M.; et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J. Clin. Oncol. 2010, 28, 3422. [Google Scholar] [CrossRef]
- Romond, E.H.; Jeong, J.H.; Rastogi, P.; Swain, S.M.; Geyer, C.E., Jr.; Ewer, M.S.; Rathi, V.; Fehrenbacher, L.; Brufsky, A.; Azar, C.A.; et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 2012, 30, 3792. [Google Scholar]
- Nieto, Y.; Cagnoni, P.J.; Bearman, S.I.; Shpall, E.J.; Matthes, S.; Jones, R.B. Cardiac toxicity following high-dose cyclophosphamide, cisplatin, and BCNU (STAMP-I) for breast cancer. Biol. Blood Marrow Transpl. 2000, 6, 198. [Google Scholar] [CrossRef]
- Brockstein, B.E.; Smiley, C.; Al-Sadir, J.; Williams, S.F. Cardiac and pulmonary toxicity in patients undergoing high-dose chemotherapy for lymphoma and breast cancer: Prognostic factors. Bone Marrow Transpl. 2000, 25, 885. [Google Scholar] [CrossRef] [PubMed]
- Tomirotti, M.; Riundi, R.; Pulici, S.; Ungaro, A.; Pedretti, D.; Villa, S.; Scanni, A. Ischemic cardiopathy from cis-diamminedichloroplatinum (CDDP). Tumori 1984, 70, 235. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, J.E.; Crowley, J.; Eyre, H.; Weiden, P.; Eltringham, J.; Stuckey, W.J. A phase II randomized study comparing sequential and combined intraarterial cisplatin and radiation therapy in primary brain tumors. A Southwest Oncology Group study. Cancer 1992, 69, 1220. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Sbaizero, O.; Taylor, M.R.; Mestroni, L. Lamin A/C Cardiomyopathy: Implications for Treatment. Curr. Cardiol. Rep. 2019, 21, 160. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciarambino, T.; Menna, G.; Sansone, G.; Giordano, M. Cardiomyopathies: An Overview. Int. J. Mol. Sci. 2021, 22, 7722. https://doi.org/10.3390/ijms22147722
Ciarambino T, Menna G, Sansone G, Giordano M. Cardiomyopathies: An Overview. International Journal of Molecular Sciences. 2021; 22(14):7722. https://doi.org/10.3390/ijms22147722
Chicago/Turabian StyleCiarambino, Tiziana, Giovanni Menna, Gennaro Sansone, and Mauro Giordano. 2021. "Cardiomyopathies: An Overview" International Journal of Molecular Sciences 22, no. 14: 7722. https://doi.org/10.3390/ijms22147722
APA StyleCiarambino, T., Menna, G., Sansone, G., & Giordano, M. (2021). Cardiomyopathies: An Overview. International Journal of Molecular Sciences, 22(14), 7722. https://doi.org/10.3390/ijms22147722




