Neural Stem Cells for Early Ischemic Stroke
Abstract
1. Introduction
2. Biology of Neural Stem Cells
2.1. Endogenous Neural Stem Cells
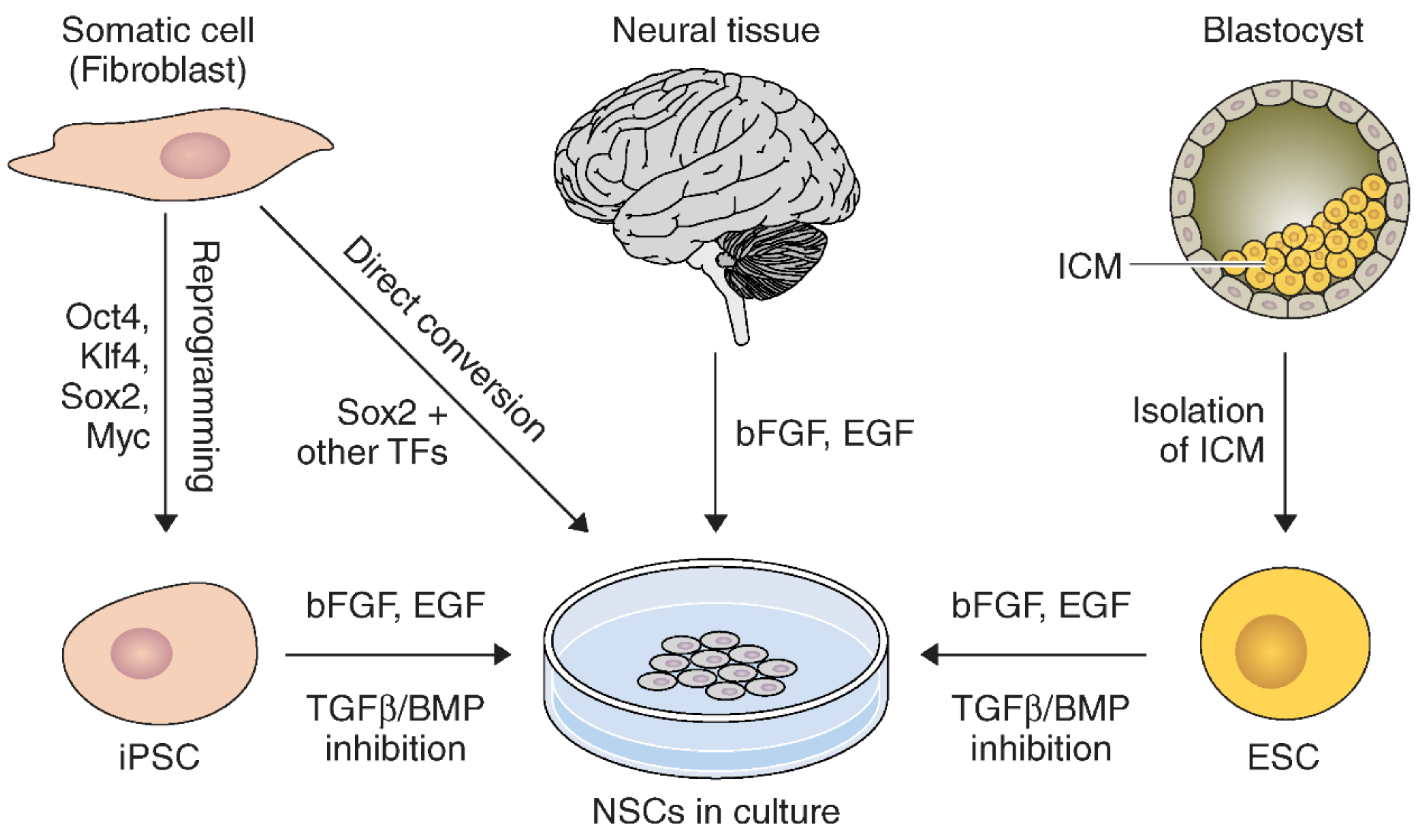
2.2. NSC Derivation
2.3. Labeling and Tracking Exogenous NSCs
2.4. Stem Cell Migration
3. Pathophysiology of Early Ischemic Stroke
3.1. Blood-Brain Barrier and Ischemia-Reperfusion Injury
3.2. MMPs
3.2.1. MMP-2 and MMP-9
3.2.2. MMP-3 (Stromelysin-1)
3.3. Inflammatory and Immune Responses after Stroke
3.4. Ischemic Tissue Loss and Neurological Dysfunction
4. Endogenous Repair Mechanisms
4.1. Angiogenesis
4.2. Endogenous Neurogenesis
5. Transplantation of Pleiotropic Neural Stem Cells for Ischemic Stroke
5.1. Transplantation of NSCs for Early Stroke Intervention
5.1.1. BBB Support and MMPs
5.1.2. Inflammation
5.2. Long-Term Outcome of NSC Transplantation at the Early Stroke Phase
5.2.1. Angiogenesis
5.2.2. Cell Replacement
5.2.3. Improved Neurological Outcome
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Description |
| AMPA | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| BBB | Blood-brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| CNS | Central nervous system |
| CXCR4 | C-X-C chemokine type 4 receptor |
| ECASS-4 | European Cooperative Acute Stroke Study-4 |
| ECM | Extracellular matrix |
| EGF | Endothelial growth factor |
| ESC | Embryonic stem cell |
| FGF | Fibroblast growth factor |
| GFP | Green fluorescent protein |
| ICH | Intracerebral hemorrhage |
| IL-1β | Interleukin-1β |
| iPSC | Induced pluripotent stem cell |
| IR | Ischemic reperfusion |
| LRP | Lipoprotein receptor-related protein |
| MCAO | Middle cerebral artery occlusion |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MIP-1α | Macrophage inflammatory proteins |
| MMP | Matrix metalloproteinase |
| NF-κB | Nuclear factor kappa B |
| NMDA | N-Methyl-D-aspartic acid or N-methyl-D-aspartate |
| NSC | Neural stem cell |
| NTF | Neurotrophic factor |
| NVU | Neurovascular unit |
| ROS | Reactive oxygen species |
| SDF-1α | Stromal cell derived factor 1-α |
| STAT | Signal transducer and activator of transcription |
| SVZ | Subventricular zone |
| TIMP-2 | Tissue inhibitor of metalloproteinases 2 |
| TJPs | Tight junction proteins |
| TNF-α | Tumor necrosis factor |
| tPA | Tissue plasminogen activator |
| VEGF | Vascular endothelial growth factor |
| vWF | von Willebrand factor |
| ZO-1 | Zonula occludens-1 (ZO-1) |
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T.; et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2014, 383, 245–254. [Google Scholar] [CrossRef]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The Science of Stroke: Mechanisms in Search of Treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef]
- Thom, T.; Haase, N.; Rosamond, W.; Howard, V.J.; Rumsfeld, J.; Manolio, T.; Zheng, Z.J.; Flegal, K.; O’Donnell, C.; Kittner, S.; et al. Heart disease and stroke statistics--2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006, 113, e85–e151. [Google Scholar] [CrossRef] [PubMed]
- Sporns, P.B.; Minnerup, J.; Warneke, N.; Dziewas, R.; Hanning, U.; Berkemeyer, S.; Zoubi, T.; Heindel, W.; Schwindt, W.; Niederstadt, T. Impact of the Implementation of Thrombectomy with Stent Retrievers on the Frequency of Hemicraniectomy in Patients with Acute Ischemic Stroke. Clin. Neuroradiol. 2017, 27, 193–197. [Google Scholar] [CrossRef]
- Wahlgren, N.; Moreira, T.; Michel, P.; Steiner, T.; Jansen, O.; Cognard, C.; Mattle, H.P.; van Zwam, W.; Holmin, S.; Tatlisumak, T.; et al. Mechanical thrombectomy in acute ischemic stroke: Consensus statement by ESO-Karolinska Stroke Update 2014/2015, supported by ESO, ESMINT, ESNR and EAN. Int. J. Stroke 2016, 11, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Deguchi, K.; Nagotani, S.; Abe, K. Vascular protection and restorative therapy in ischemic stroke. Cell Transplant 2011, 20, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.M. Recombinant tissue plasminogen activator for the treatment of acute ischemic stroke. Proc. Baylor Univ. Med. Center 2011, 24, 257–259. [Google Scholar] [CrossRef]
- Kilic, E.; Bahr, M.; Hermann, D.M. Effects of recombinant tissue plasminogen activator after intraluminal thread occlusion in mice: Role of hemodynamic alterations. Stroke 2001, 32, 2641–2647. [Google Scholar] [CrossRef] [PubMed]
- Amiri, H.; Bluhmki, E.; Bendszus, M.; Eschenfelder, C.C.; Donnan, G.A.; Leys, D.; Molina, C.; Ringleb, P.A.; Schellinger, P.D.; Schwab, S.; et al. European Cooperative Acute Stroke Study-4: Extending the time for thrombolysis in emergency neurological deficits ECASS-4: ExTEND. Int. J. Stroke 2016, 11, 260–267. [Google Scholar] [CrossRef]
- Ringleb, P.; Bendszus, M.; Bluhmki, E.; Donnan, G.; Eschenfelder, C.; Fatar, M.; Kessler, C.; Molina, C.; Leys, D.; Muddegowda, G.; et al. Extending the time window for intravenous thrombolysis in acute ischemic stroke using magnetic resonance imaging-based patient selection. Int. J. Stroke 2019, 14, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Albers, G.W.; Marks, M.P.; Lansberg, M.G. Thrombectomy for Stroke with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 1849–1850. [Google Scholar] [CrossRef]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Ting, P.; Martinez, H.; Klatzo, I. The biphasic opening of the blood-brain barrier to proteins following temporary middle cerebral artery occlusion. Acta Neuropathol. 1985, 68, 122–129. [Google Scholar] [CrossRef]
- Yandava, B.D.; Billinghurst, L.L.; Snyder, E.Y. “Global” cell replacement is feasible via neural stem cell transplantation: Evidence from the dysmyelinated shiverer mouse brain. Proc. Natl. Acad. Sci. USA 1999, 96, 7029–7034. [Google Scholar] [CrossRef]
- Lee, J.P.; Jeyakumar, M.; Gonzalez, R.; Takahashi, H.; Lee, P.J.; Baek, R.C.; Clark, D.; Rose, H.; Fu, G.; Clarke, J.; et al. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat. Med. 2007, 13, 439–447. [Google Scholar] [CrossRef]
- Park, K.I.; Teng, Y.D.; Snyder, E.Y. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat. Biotechnol. 2002, 20, 1111–1117. [Google Scholar] [CrossRef]
- Jeyakumar, M.; Lee, J.P.; Sibson, N.R.; Lowe, J.P.; Stuckey, D.J.; Tester, K.; Fu, G.; Newlin, R.; Smith, D.A.; Snyder, E.Y.; et al. Neural Stem Cell Transplantation Benefits a Monogenic Neurometabolic Disorder During the Symptomatic Phase of Disease. Stem Cells 2009, 27, 2362–2370. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ourednik, J.; Ourednik, V.; Lynch, W.P.; Schachner, M.; Snyder, E.Y. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat. Biotechnol. 2002, 20, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Redmond, D.E., Jr.; Bjugstad, K.B.; Teng, Y.D.; Ourednik, V.; Ourednik, J.; Wakeman, D.R.; Parsons, X.H.; Gonzalez, R.; Blanchard, B.C.; Kim, S.U.; et al. Behavioral improvement in a primate Parkinson’s model is associated with multiple homeostatic effects of human neural stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 12175–12180. [Google Scholar] [CrossRef] [PubMed]
- Sinden, J.D.; Vishnubhatla, I.; Muir, K.W. Prospects for stem cell-derived therapy in stroke. Prog. Brain Res. 2012, 201, 119–167. [Google Scholar] [CrossRef] [PubMed]
- Miljan, E.A.; Sinden, J.D. Stem cell treatment of ischemic brain injury. Curr. Opin. Mol. Ther. 2009, 11, 394–403. [Google Scholar] [PubMed]
- He, J.Q.; Sussman, E.S.; Steinberg, G.K. Revisiting Stem Cell-Based Clinical Trials for Ischemic Stroke. Front. Ag. Neurosci. 2020, 12, 575990. [Google Scholar] [CrossRef]
- Elder, G.A.; De Gasperi, R.; Sosa, M.A.G. Research update: Neurogenesis in adult brain and neuropsychiatric disorders. Mount. Sinai J. Med. 2006, 73, 931–940. [Google Scholar]
- Djavadian, R.L. Serotonin and neurogenesis in the hippocampal dentate gyrus of adult mammals. Acta Neurobiol. Exp. (Wars) 2004, 64, 189–200. [Google Scholar]
- Alvarez-Buylla, A.; Garcia-Verdugo, J.M. Neurogenesis in adult subventricular zone. J. Neurosci. 2002, 22, 629–634. [Google Scholar] [CrossRef]
- Alvarez-Buylla, A.; Lois, C. Neuronal stem cells in the brain of adult vertebrates. Stem. Cells 1995, 13, 263–272. [Google Scholar] [CrossRef]
- Lledo, P.M.; Alonso, M.; Grubb, M.S. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 2006, 7, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Iacovitti, L. Classic and novel stem cell niches in brain homeostasis and repair. Brain Res. 2015, 1628, 327–342. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Paredes, M.F.; Velmeshev, D.; Herranz-Perez, V.; Sandoval, K.; Mayer, S.; Chang, E.F.; Insausti, R.; Kriegstein, A.R.; Rubenstein, J.L.; et al. Immature excitatory neurons develop during adolescence in the human amygdala. Nat. Commun. 2019, 10, 2748. [Google Scholar] [CrossRef]
- Cheng, M.F. Hypothalamic neurogenesis in the adult brain. Front. Neuroendocrinol. 2013, 34, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Daniels, S.B.; Lennington, J.B.; Notti, R.Q.; Conover, J.C. The aging neurogenic subventricular zone. Aging Cell 2006, 5, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Silva-Vargas, V.; Maldonado-Soto, A.R.; Mizrak, D.; Codega, P.; Doetsch, F. Age-Dependent Niche Signals from the Choroid Plexus Regulate Adult Neural Stem Cells. Cell Stem Cell 2016, 19, 643–652. [Google Scholar] [CrossRef]
- Solano Fonseca, R.; Mahesula, S.; Apple, D.M.; Raghunathan, R.; Dugan, A.; Cardona, A.; O’Connor, J.; Kokovay, E. Neurogenic Niche Microglia Undergo Positional Remodeling and Progressive Activation Contributing to Age-Associated Reductions in Neurogenesis. Stem. Cells Dev. 2016, 25, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Y.; Wait, E.; Mankowski, W.; Bjornsson, C.S.; Cohen, A.R.; Zuloaga, K.L.; Temple, S. 3D image analysis of the complete ventricular-subventricular zone stem cell niche reveals significant vasculature changes and progenitor deficits in males versus females with aging. Stem. Cell Rep. 2021. [Google Scholar] [CrossRef]
- Quinones-Hinojosa, A.; Sanai, N.; Soriano-Navarro, M.; Gonzalez-Perez, O.; Mirzadeh, Z.; Gil-Perotin, S.; Romero-Rodriguez, R.; Berger, M.S.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and cytoarchitecture of the adult human subventricular zone: A niche of neural stem cells. J. Comp. Neurol. 2006, 494, 415–434. [Google Scholar] [CrossRef]
- Bond, A.M.; Ming, G.L.; Song, H. Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem. Cell 2015, 17, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Obernier, K.; Alvarez-Buylla, A. Neural stem cells: Origin, heterogeneity and regulation in the adult mammalian brain. Development 2019, 146. [Google Scholar] [CrossRef] [PubMed]
- Kernie, S.G.; Parent, J.M. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol. Dis. 2010, 37, 267–274. [Google Scholar] [CrossRef]
- Kokaia, Z.; Lindvall, O. Neurogenesis after ischaemic brain insults. Curr. Opin. Neurobiol. 2003, 13, 127–132. [Google Scholar] [CrossRef]
- Liu, Y.P.; Lang, B.T.; Baskaya, M.K.; Dempsey, R.J.; Vemuganti, R. The potential of neural stem cells to repair stroke-induced brain damage. Acta Neuropathol. 2009, 117, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Kokaia, Z. Neurogenesis following Stroke Affecting the Adult Brain. Cold Spring Harb Perspect. Biol. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Andres, R.H.; Choi, R.; Steinberg, G.K.; Guzman, R. Potential of adult neural stem cells in stroke therapy. Regen. Med. 2008, 3, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Guzman, R.; De Los Angeles, A.; Cheshier, S.; Choi, R.; Hoang, S.; Liauw, J.; Schaar, B.; Steinberg, G. Intracarotid injection of fluorescence activated cell-sorted CD49d-positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke 2008, 39, 1300–1306. [Google Scholar] [CrossRef]
- Steinberg, G.K.; Kondziolka, D.; Wechsler, L.R.; Lunsford, L.D.; Coburn, M.L.; Billigen, J.B.; Kim, A.S.; Johnson, J.N.; Bates, D.; King, B.; et al. Clinical Outcomes of Transplanted Modified Bone Marrow-Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2a Study. Stroke 2016, 47, 1817–1824. [Google Scholar] [CrossRef]
- Burns, T.C.; Steinberg, G.K. Stem cells and stroke: Opportunities, challenges and strategies. Exp. Opin Biol. Ther. 2011, 11, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.L.; Chopp, M.; Roberts, C.; Liu, X.; Wei, M.; Nejad-Davarani, S.P.; Wang, X.; Zhang, Z.G. Stroke increases neural stem cells and angiogenesis in the neurogenic niche of the adult mouse. PLoS ONE 2014, 9, e113972. [Google Scholar] [CrossRef]
- Zhang, R.L.; Zhang, Z.G.; Wang, L.; Wang, Y.; Gousev, A.; Zhang, L.; Ho, K.L.; Morshead, C.; Chopp, M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J. Cerebral Blood Flow Metabol. 2004, 24, 441–448. [Google Scholar] [CrossRef]
- Boese, A.C.; Le, Q.E.; Pham, D.; Hamblin, M.H.; Lee, J.P. Neural stem cell therapy for subacute and chronic ischemic stroke. Stem. Cell Res. Ther. 2018, 9, 154. [Google Scholar] [CrossRef]
- Lee, J.P.; McKercher, S.; Muller, F.J.; Snyder, E.Y. Neural stem cell transplantation in mouse brain. Curr. Protoc. Neurosci. 2008. [Google Scholar] [CrossRef] [PubMed]
- Reubinoff, B.E.; Itsykson, P.; Turetsky, T.; Pera, M.F.; Reinhartz, E.; Itzik, A.; Ben-Hur, T. Neural progenitors from human embryonic stem cells. Nat. Biotechnol. 2001, 19, 1134–1140. [Google Scholar] [CrossRef]
- Daadi, M.M.; Maag, A.L.; Steinberg, G.K. Adherent self-renewable human embryonic stem cell-derived neural stem cell line: Functional engraftment in experimental stroke model. PLoS ONE 2008, 3, e1644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.C.; Wernig, M.; Duncan, I.D.; Brustle, O.; Thomson, J.A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 2001, 19, 1129–1133. [Google Scholar] [CrossRef]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef]
- Liu, J. Induced pluripotent stem cell-derived neural stem cells: New hope for stroke? Stem. Cell Res Ther. 2013, 4, 115. [Google Scholar] [CrossRef]
- Aasen, T.; Raya, A.; Barrero, M.J.; Garreta, E.; Consiglio, A.; Gonzalez, F.; Vassena, R.; Bilic, J.; Pekarik, V.; Tiscornia, G.; et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 2008, 26, 1276–1284. [Google Scholar] [CrossRef]
- Staerk, J.; Dawlaty, M.M.; Gao, Q.; Maetzel, D.; Hanna, J.; Sommer, C.A.; Mostoslavsky, G.; Jaenisch, R. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem. Cell 2010, 7, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Petit, I.; Kesner, N.S.; Karry, R.; Robicsek, O.; Aberdam, E.; Muller, F.J.; Aberdam, D.; Ben-Shachar, D. Induced pluripotent stem cells from hair follicles as a cellular model for neurodevelopmental disorders. Stem. Cell Res. 2012, 8, 134–140. [Google Scholar] [CrossRef]
- Park, I.H.; Lerou, P.H.; Zhao, R.; Huo, H.; Daley, G.Q. Generation of human-induced pluripotent stem cells. Nat. Protocols 2008, 3, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.M.; Cooper, J.B. Lab-specific gene expression signatures in pluripotent stem cells. Cell Stem. Cell 2010, 7, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.H.; Mason, M.J.; Xie, W.; Volinia, S.; Singer, M.; Peterson, C.; Ambartsumyan, G.; Aimiuwu, O.; Richter, L.; Zhang, J.; et al. Induced Pluripotent Stem Cells and Embryonic Stem Cells Are Distinguished by Gene Expression Signatures. Cell Stem. Cell 2009, 5, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Efe, J.A.; Zhu, S.; Talantova, M.; Yuan, X.; Wang, S.; Lipton, S.A.; Zhang, K.; Ding, S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc. Natl. Acad. Sci. USA 2011, 108, 7838–7843. [Google Scholar] [CrossRef] [PubMed]
- Thier, M.; Worsdorfer, P.; Lakes, Y.B.; Gorris, R.; Herms, S.; Opitz, T.; Seiferling, D.; Quandel, T.; Hoffmann, P.; Nothen, M.M.; et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem. Cell 2012, 10, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Han, D.W.; Tapia, N.; Hermann, A.; Hemmer, K.; Hoing, S.; Arauzo-Bravo, M.J.; Zaehres, H.; Wu, G.; Frank, S.; Moritz, S.; et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem. Cell 2012, 10, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Lujan, E.; Chanda, S.; Ahlenius, H.; Sudhof, T.C.; Wernig, M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc. Natl. Acad. Sci. USA 2012, 109, 2527–2532. [Google Scholar] [CrossRef]
- Ring, K.L.; Tong, L.M.; Balestra, M.E.; Javier, R.; Andrews-Zwilling, Y.; Li, G.; Walker, D.; Zhang, W.R.; Kreitzer, A.C.; Huang, Y. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem. Cell 2012, 11, 100–109. [Google Scholar] [CrossRef]
- Shahbazi, E.; Moradi, S.; Nemati, S.; Satarian, L.; Basiri, M.; Gourabi, H.; Zare Mehrjardi, N.; Gunther, P.; Lampert, A.; Handler, K.; et al. Conversion of Human Fibroblasts to Stably Self-Renewing Neural Stem Cells with a Single Zinc-Finger Transcription Factor. Stem. Cell Rep. 2016, 6, 539–551. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, Y.H.; Sun, Y.J.; Zhu, S.; Zheng, J.; Liu, K.; Cao, N.; Li, K.; Huang, Y.; Ding, S. Pharmacological Reprogramming of Fibroblasts into Neural Stem Cells by Signaling-Directed Transcriptional Activation. Cell Stem Cell 2016, 18, 653–667. [Google Scholar] [CrossRef]
- Zhao, L.R.; Duan, W.M.; Reyes, M.; Keene, C.D.; Verfaillie, C.M.; Low, W.C. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp. Neurol. 2002, 174, 11–20. [Google Scholar] [CrossRef]
- Politi, L.S.; Bacigaluppi, M.; Brambilla, E.; Cadioli, M.; Falini, A.; Comi, G.; Scotti, G.; Martino, G.; Pluchino, S. Magnetic-resonance-based tracking and quantification of intravenously injected neural stem cell accumulation in the brains of mice with experimental multiple sclerosis. Stem. Cells 2007, 25, 2583–2592. [Google Scholar] [CrossRef]
- Zhang, M.; Methot, D.; Poppa, V.; Fujio, Y.; Walsh, K.; Murry, C.E. Cardiomyocyte grafting for cardiac repair: Graft cell death and anti-death strategies. J. Mol. Cell Cardiol. 2001, 33, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Imitola, J.; Raddassi, K.; Park, K.I.; Mueller, F.J.; Nieto, M.; Teng, Y.D.; Frenkel, D.; Li, J.; Sidman, R.L.; Walsh, C.A.; et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 18117–18122. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Acton, P.D.; Ferrari, V.A. Imaging stem cells implanted in infarcted myocardium. J. Am. Coll Cardiol. 2006, 48, 2094–2106. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.P.; Zhang, R.; Yan, M.; Duggineni, S.; Wakeman, D.R.; Niles, W.L.; Feng, Y.; Chen, J.; Hamblin, M.H.; Han, E.B.; et al. Chemical mutagenesis of a GPCR ligand: Detoxifying “inflammo-attraction” to direct therapeutic stem cell migration. Proc. Natl. Acad. Sci. USA 2020, 117, 31177–31188. [Google Scholar] [CrossRef]
- Boese, A.C.; Eckert, A.; Hamblin, M.H.; Lee, J.P. Human neural stem cells improve early stage stroke outcome in delayed tissue plasminogen activator-treated aged stroke brains. Exp. Neurol. 2020, 329, 113275. [Google Scholar] [CrossRef]
- Eckert, A.; Huang, L.; Gonzalez, R.; Kim, H.S.; Hamblin, M.H.; Lee, J.P. Bystander Effect Fuels Human Induced Pluripotent Stem Cell-Derived Neural Stem Cells to Quickly Attenuate Early Stage Neurological Deficits After Stroke. Stem. Cells Transl. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wong, S.; Snyder, E.Y.; Hamblin, M.H.; Lee, J.P. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury. Stem. Cell Res. Ther. 2014, 5, 129. [Google Scholar] [CrossRef] [PubMed]
- Imitola, J.; Comabella, M.; Chandraker, A.K.; Dangond, F.; Sayegh, M.H.; Snyder, E.Y.; Khoury, S.J. Neural stem/progenitor cells express costimulatory molecules that are differentially regulated by inflammatory and apoptotic stimuli. Am. J. Pathol. 2004, 164, 1615–1625. [Google Scholar] [CrossRef]
- Belmadani, A.; Tran, P.B.; Ren, D.; Miller, R.J. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J. Neurosci. 2006, 26, 3182–3191. [Google Scholar] [CrossRef]
- Li, M.; Hale, J.S.; Rich, J.N.; Ransohoff, R.M.; Lathia, J.D. Chemokine CXCL12 in neurodegenerative diseases: An SOS signal for stem cell-based repair. Trends Neurosci. 2012, 35, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Carbajal, K.S.; Schaumburg, C.; Strieter, R.; Kane, J.; Lane, T.E. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc. Natl. Acad Sci. USA 2010, 107, 11068–11073. [Google Scholar] [CrossRef]
- Tran, P.B.; Ren, D.; Veldhouse, T.J.; Miller, R.J. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J. Neurosci. Res. 2004, 76, 20–34. [Google Scholar] [CrossRef]
- Tran, P.B.; Banisadr, G.; Ren, D.; Chenn, A.; Miller, R.J. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J. Comp. Neurol. 2007, 500, 1007–1033. [Google Scholar] [CrossRef]
- Tran, P.B.; Miller, R.J. Chemokine receptors: Signposts to brain development and disease. Nat. Rev. Neurosci. 2003, 4, 444–455. [Google Scholar] [CrossRef]
- Itoh, T.; Satou, T.; Ishida, H.; Nishida, S.; Tsubaki, M.; Hashimoto, S.; Ito, H. The relationship between SDF-1alpha/CXCR4 and neural stem cells appearing in damaged area after traumatic brain injury in rats. Neurol. Res. 2009, 31, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Schober, A.; Karshovska, E.; Zernecke, A.; Weber, C. SDF-1alpha-mediated tissue repair by stem cells: A promising tool in cardiovascular medicine? Trends Cardiovasc. Med. 2006, 16, 103–108. [Google Scholar] [CrossRef]
- Togel, F.; Isaac, J.; Hu, Z.; Weiss, K.; Westenfelder, C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005, 67, 1772–1784. [Google Scholar] [CrossRef] [PubMed]
- Vasyutina, E.; Stebler, J.; Brand-Saberi, B.; Schulz, S.; Raz, E.; Birchmeier, C. CXCR4 and Gab1 cooperate to control the development of migrating muscle progenitor cells. Genes Devel. 2005, 19, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Wang, J.; Wang, W.; Yang, Z.; Hu, Z.; Hu, M.; Ding, P. The effect of stromal cell-derived factor 1 in the migration of neural stem cells. Cell Biochem. Biophys. 2014, 70, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.M.; Zhang, Z.G.; Wang, L.; Zhang, R.L.; Katakowski, M.; Zhang, L.; Wang, Y.; Zhang, C.; Chopp, M. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2006, 26, 125–134. [Google Scholar] [CrossRef]
- Smith, E.J.; Stroemer, R.P.; Gorenkova, N.; Nakajima, M.; Crum, W.R.; Tang, E.; Stevanato, L.; Sinden, J.D.; Modo, M. Implantation Site and Lesion Topology Determine Efficacy of a Human Neural Stem Cell Line in a Rat Model of Chronic Stroke. Stem Cells 2012, 30, 785–796. [Google Scholar] [CrossRef]
- Song, M.; Kim, Y.; Kim, Y.; Ryu, S.; Song, I.; Kim, S.U.; Yoon, B.W. MRI tracking of intravenously transplanted human neural stem cells in rat focal ischemia model. Neurosci. Res. 2009, 64, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.Y.; Pendharkar, A.V.; Wang, N.; Choi, R.; Andres, R.H.; Gaeta, X.; Zhang, J.; Moseley, M.E.; Guzman, R. Intra-arterial injection of neural stem cells using a microneedle technique does not cause microembolic strokes. J. Cereb. Blood Flow Metabolism 2011, 31, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Chu, K.; Jung, K.H.; Kim, S.J.; Kim, D.H.; Kang, K.M.; Hong, N.H.; Kim, J.H.; Ban, J.J.; Park, H.K.; et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain 2008, 131, 616–629. [Google Scholar] [CrossRef]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S., Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem. Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef]
- Hauger, O.; Frost, E.E.; van Heeswijk, R.; Deminiere, C.; Xue, R.; Delmas, Y.; Combe, C.; Moonen, C.T.; Grenier, N.; Bulte, J.W. MR evaluation of the glomerular homing of magnetically labeled mesenchymal stem cells in a rat model of nephropathy. Radiology 2006, 238, 200–210. [Google Scholar] [CrossRef]
- Kraitchman, D.L.; Tatsumi, M.; Gilson, W.D.; Ishimori, T.; Kedziorek, D.; Walczak, P.; Segars, W.P.; Chen, H.H.; Fritzges, D.; Izbudak, I.; et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation 2005, 112, 1451–1461. [Google Scholar] [CrossRef]
- Bliss, T.; Guzman, R.; Daadi, M.; Steinberg, G.K. Cell transplantation therapy for stroke. Stroke 2007, 38, 817–826. [Google Scholar] [CrossRef]
- Van Velthoven, C.T.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav. Immun. 2010, 24, 387–393. [Google Scholar] [CrossRef]
- Wei, N.; Yu, S.P.; Gu, X.; Taylor, T.M.; Song, D.; Liu, X.F.; Wei, L. Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplant 2013, 22, 977–991. [Google Scholar] [CrossRef] [PubMed]
- Begley, D.J.; Brightman, M.W. Structural and functional aspects of the blood-brain barrier. Prog. Drug Res. Fort. Arzneimittelf. Prog. Recher. Pharm. 2003, 61, 39–78. [Google Scholar]
- Abbott, N.J. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002, 200, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.R.; Dreifuss, J.J.; Dziegielewska, K.M.; Johansson, P.A.; Habgood, M.D.; Mollgard, K.; Bauer, H.C. The rights and wrongs of blood-brain barrier permeability studies: A walk through 100 years of history. Front. Neurosci. 2014, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Schoknecht, K.; David, Y.; Heinemann, U. The blood-brain barrier-gatekeeper to neuronal homeostasis: Clinical implications in the setting of stroke. Semin. Cell Devel. Biol. 2015, 38, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Ronnback, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Engelhardt, B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev. Immunol. 2012, 12, 623–635. [Google Scholar] [CrossRef]
- Shechter, R.; Schwartz, M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: No longer ‘if’ but ‘how’. J. Pathol. 2013, 229, 332–346. [Google Scholar] [CrossRef]
- White, B.C.; Sullivan, J.M.; DeGracia, D.J.; ’O’Neil, B.J.; Neumar, R.W.; Grossman, L.I.; Rafols, J.A.; Krause, G.S. Brain ischemia and reperfusion: Molecular mechanisms of neuronal injury. J. Neurol. Sci. 2000, 179, 1–33. [Google Scholar] [CrossRef]
- Kulik, T.; Kusano, Y.; Aronhime, S.; Sandler, A.L.; Winn, H.R. Regulation of cerebral vasculature in normal and ischemic brain. Neuropharmacology 2008, 55, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, L.; Pu, H.; Mao, L.; Hu, X.; Jiang, X.; Xu, N.; Stetler, R.A.; Zhang, F.; Liu, X.; et al. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat. Commun. 2016, 7, 10523. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rosenberg, G.A. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 2011, 42, 3323–3328. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Y.; Betz, A.L. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke 1994, 25, 1658–1664. [Google Scholar] [CrossRef]
- Yemisci, M.; Gursoy-Ozdemir, Y.; Vural, A.; Can, A.; Topalkara, K.; Dalkara, T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat. Med. 2009, 15, 1031–1037. [Google Scholar] [CrossRef]
- Daneman, R. The blood-brain barrier in health and disease. Ann. Neurol. 2012, 72, 648–672. [Google Scholar] [CrossRef]
- Pop, V.; Badaut, J. A neurovascular perspective for long-term changes after brain trauma. Transl. Stroke Res. 2011, 2, 533–545. [Google Scholar] [CrossRef]
- Jiao, H.; Wang, Z.; Liu, Y.; Wang, P.; Xue, Y. Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood-brain barrier in a focal cerebral ischemic insult. J. Mol. Neurosci. 2011, 44, 130–139. [Google Scholar] [CrossRef]
- Knowland, D.; Arac, A.; Sekiguchi, K.J.; Hsu, M.; Lutz, S.E.; Perrino, J.; Steinberg, G.K.; Barres, B.A.; Nimmerjahn, A.; Agalliu, D. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 2014, 82, 603–617. [Google Scholar] [CrossRef] [PubMed]
- del Zoppo, G.J. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience 2009, 158, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Lochter, A.; Sympson, C.J.; Huey, B.; Rougier, J.P.; Gray, J.W.; Pinkel, D.; Bissell, M.J.; Werb, Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 1999, 98, 137–146. [Google Scholar] [CrossRef]
- Chang, C.; Werb, Z. The many faces of metalloproteases: Cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001, 11, S37–S43. [Google Scholar] [CrossRef]
- Mannello, F.; Tonti, G.A.; Bagnara, G.P.; Papa, S. Role and function of matrix metalloproteinases in the differentiation and biological characterization of mesenchymal stem cells. Stem. Cells 2006, 24, 475–481. [Google Scholar] [CrossRef]
- Rosenberg, G.A.; Estrada, E.Y.; Dencoff, J.E. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke 1998, 29, 2189–2195. [Google Scholar] [CrossRef]
- Liu, W.; Hendren, J.; Qin, X.J.; Liu, K.J. Normobaric hyperoxia reduces the neurovascular complications associated with delayed tissue plasminogen activator treatment in a rat model of focal cerebral ischemia. Stroke 2009, 40, 2526–2531. [Google Scholar] [CrossRef]
- Gurney, K.J.; Estrada, E.Y.; Rosenberg, G.A. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol. Dis. 2006, 23, 87–96. [Google Scholar] [CrossRef]
- Mun-Bryce, S.; Rosenberg, G.A. Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. Am. J. Physiol. 1998, 274, R1203–R1211. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, H.Y.; Ahn, H.J.; Ju, B.G.; Yune, T.Y. Matrix metalloproteinase-3 promotes early blood-spinal cord barrier disruption and hemorrhage and impairs long-term neurological recovery after spinal cord injury. Am. J. Pathol. 2014, 184, 2985–3000. [Google Scholar] [CrossRef]
- Noble, L.J.; Donovan, F.; Igarashi, T.; Goussev, S.; Werb, Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J. Neurosci. 2002, 22, 7526–7535. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Yang, G.J.; Li, G.H. Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: Critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol. Dis. 2010, 38, 376–385. [Google Scholar] [CrossRef]
- Gu, Y.; Zheng, G.; Xu, M.; Li, Y.; Chen, X.; Zhu, W.; Tong, Y.; Chung, S.K.; Liu, K.J.; Shen, J. Caveolin-1 regulates nitric oxide-mediated matrix metalloproteinases activity and blood-brain barrier permeability in focal cerebral ischemia and reperfusion injury. J. Neurochem. 2012, 120, 147–156. [Google Scholar] [CrossRef]
- Del Zoppo, G.J. The neurovascular unit, matrix proteases, and innate inflammation. Ann. N. Y. Acad. Sci. 2010, 1207, 46–49. [Google Scholar] [CrossRef]
- Heo, J.H.; Lucero, J.; Abumiya, T.; Koziol, J.A.; Copeland, B.R.; del Zoppo, G.J. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1999, 19, 624–633. [Google Scholar] [CrossRef]
- Romanic, A.M.; White, R.F.; Arleth, A.J.; Ohlstein, E.H.; Barone, F.C. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: Inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke 1998, 29, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.T.; Bürgers, H.F.; Rabie, T.; Marti, H.H. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cerebral Blood Flow Metab. 2010, 30, 837–848. [Google Scholar] [CrossRef]
- Chang, D.I.; Hosomi, N.; Lucero, J.; Heo, J.H.; Abumiya, T.; Mazar, A.P.; del Zoppo, G.J. Activation systems for latent matrix metalloproteinase-2 are upregulated immediately after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2003, 23, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rosenberg, G.A. Matrix metalloproteinases as therapeutic targets for stroke. Brain Res. 2015, 1623, 30–38. [Google Scholar] [CrossRef]
- Cui, J.; Chen, S.; Zhang, C.; Meng, F.; Wu, W.; Hu, R.; Hadass, O.; Lehmidi, T.; Blair, G.J.; Lee, M.; et al. Inhibition of MMP-9 by a selective gelatinase inhibitor protects neurovasculature from embolic focal cerebral ischemia. Mol. Neurodegen. 2012, 7, 21. [Google Scholar] [CrossRef]
- Jin, X.; Sun, Y.; Xu, J.; Liu, W. Caveolin-1 mediates tissue plasminogen activator-induced MMP-9 up-regulation in cultured brain microvascular endothelial cells. J. Neurochem. 2015, 132, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, M.; Leira, R.; Serena, J.; Pumar, J.M.; Lizasoain, I.; Castillo, J.; Davalos, A. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke 2003, 34, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lee, S.R.; Arai, K.; Lee, S.R.; Tsuji, K.; Rebeck, G.W.; Lo, E.H. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat. Med. 2003, 9, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Mishiro, K.; Ishiguro, M.; Suzuki, Y.; Tsuruma, K.; Shimazawa, M.; Hara, H. A broad-spectrum matrix metalloproteinase inhibitor prevents hemorrhagic complications induced by tissue plasminogen activator in mice. Neuroscience 2012, 205, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Fanning, A.S.; Jameson, B.J.; Jesaitis, L.A.; Anderson, J.M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998, 273, 29745–29753. [Google Scholar] [CrossRef] [PubMed]
- Rosell, A.; Lo, E.H. Multiphasic roles for matrix metalloproteinases after stroke. Curr. Opin. Pharmacol. 2008, 8, 82–89. [Google Scholar] [CrossRef]
- Kuntz, M.; Mysiorek, C.; Petrault, O.; Petrault, M.; Uzbekov, R.; Bordet, R.; Fenart, L.; Cecchelli, R.; Berezowski, V. Stroke-induced brain parenchymal injury drives blood-brain barrier early leakage kinetics: A combined in vivo/in vitro study. J Cereb. Blood Flow Metab 2014, 34, 95–107. [Google Scholar] [CrossRef]
- Asahi, M.; Wang, X.Y.; Mori, T.; Sumii, T.; Jung, J.C.; Moskowitz, M.A.; Fini, M.E.; Lo, E.H. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J. Neurosci. 2001, 21, 7724–7732. [Google Scholar]
- Chin, J.R.; Murphy, G.; Werb, Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J. Biol. Chem. 1985, 260, 12367–12376. [Google Scholar] [CrossRef]
- Hahn-Dantona, E.; Ramos-DeSimone, N.; Sipley, J.; Nagase, H.; French, D.L.; Quigley, J.P. Activation of proMMP-9 by a plasmin/MMP-3 cascade in a tumor cell model. Regulation by tissue inhibitors of metalloproteinases. Ann. N. Y. Acad. Sci. 1999, 878, 372–387. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; Yang, Y.; Rosenberg, G.A. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience 2009, 158, 983–994. [Google Scholar] [CrossRef]
- Ramos-DeSimone, N.; Hahn-Dantona, E.; Sipley, J.; Nagase, H.; French, D.L.; Quigley, J.P. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J. Biol. Chem. 1999, 274, 13066–13076. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nagai, N.; Umemura, K.; Collen, D.; Lijnen, H.R. Stromelysin-1 (MMP-3) is critical for intracranial bleeding after t-PA treatment of stroke in mice. J. Thromb Haemost 2007, 5, 1732–1739. [Google Scholar] [CrossRef]
- Cuadrado, E.; Rosell, A.; Penalba, A.; Slevin, M.; Alvarez-Sabin, J.; Ortega-Aznar, A.; Montaner, J. Vascular MMP-9/TIMP-2 and neuronal MMP-10 up-regulation in human brain after stroke: A combined laser microdissection and protein array study. J. Proteome Res. 2009, 8, 3191–3197. [Google Scholar] [CrossRef]
- Herz, J.; Strickland, D.K. LRP: A multifunctional scavenger and signaling receptor. J. Clin. Invest. 2001, 108, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nagai, N.; Yamakawa, K.; Kawakami, J.; Lijnen, H.R.; Umemura, K. Tissue-type plasminogen activator (t-PA) induces stromelysin-1 (MMP-3) in endothelial cells through activation of lipoprotein receptor-related protein. Blood 2009, 114, 3352–3358. [Google Scholar] [CrossRef] [PubMed]
- Sole, S.; Petegnief, V.; Gorina, R.; Chamorro, A.; Planas, A.M. Activation of matrix metalloproteinase-3 and agrin cleavage in cerebral ischemia/reperfusion. J. Neuropathol. Exp. Neurol. 2004, 63, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Hafez, S.; Abdelsaid, M.; El-Shafey, S.; Johnson, M.H.; Fagan, S.C.; Ergul, A. Matrix Metalloprotease 3 Exacerbates Hemorrhagic Transformation and Worsens Functional Outcomes in Hyperglycemic Stroke. Stroke 2016, 47, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Mena, H.; Cadavid, D.; Rushing, E.J. Human cerebral infarct: A proposed histopathologic classification based on 137 cases. Acta Neuropathol. 2004, 108, 524–530. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Aktas, O.; Ullrich, O.; Infante-Duarte, C.; Nitsch, R.; Zipp, F. Neuronal damage in brain inflammation. Arch Neurol. 2007, 64, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Gendron, A.; Teitelbaum, J.; Cossette, C.; Nuara, S.; Dumont, M.; Geadah, D.; du Souich, P.; Kouassi, E. Temporal effects of left versus right middle cerebral artery occlusion on spleen lymphocyte subsets and mitogenic response in Wistar rats. Brain Res. 2002, 955, 85–97. [Google Scholar] [CrossRef]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.A.; Sansing, L.H. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin. Dev. Immunol. 2013, 2013, 746068. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Liu, C.; Zhao, Y.; Chen, Y. Activated microglia provide a neuroprotective role by balancing glial cell-line derived neurotrophic factor and tumor necrosis factor-alpha secretion after subacute cerebral ischemia. Int. J. Mol. Med. 2013, 31, 172–178. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Gate, D.; Town, T. CNS infiltration of peripheral immune cells: D-Day for neurodegenerative disease? J. Neuroimmun. Pharm. 2009, 4, 462–475. [Google Scholar] [CrossRef]
- Ritzel, R.M.; Patel, A.R.; Grenier, J.M.; Crapser, J.; Verma, R.; Jellison, E.R.; McCullough, L.D. Functional differences between microglia and monocytes after ischemic stroke. J. Neuroinflamm. 2015, 12, 106. [Google Scholar] [CrossRef]
- Kim, E.; Cho, S. Microglia and Monocyte-Derived Macrophages in Stroke. Neurother J. Am. Soc. Exp. NeuroTherapeutics 2016, 13, 702–718. [Google Scholar] [CrossRef]
- Xiong, X.Y.; Liu, L.; Yang, Q.W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog. Neurobiol. 2016, 142, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Vivien, D.; Ali, C. Transforming growth factor-beta signalling in brain disorders. Cytokine Growth Factor Rev. 2006, 17, 121–128. [Google Scholar] [CrossRef]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization-new prospects for brain repair. Nat. Rev. Neurol. 2015, 11, 56–64. [Google Scholar] [CrossRef]
- Cai, W.; Dai, X.; Chen, J.; Zhao, J.; Xu, M.; Zhang, L.; Yang, B.; Zhang, W.; Rocha, M.; Nakao, T.; et al. STAT6/Arg1 promotes microglia/macrophage efferocytosis and inflammation resolution in stroke mice. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Brunn, A.; Mihelcic, M.; Carstov, M.; Hummel, L.; Geier, F.; Schmidt, A.; Saupe, L.; Utermohlen, O.; Deckert, M. IL-10, IL-4, and STAT6 promote an M2 milieu required for termination of P0(106-125)-induced murine experimental autoimmune neuritis. Am. J. Pathol. 2014, 184, 2627–2640. [Google Scholar] [CrossRef]
- Qin, H.; Holdbrooks, A.T.; Liu, Y.; Reynolds, S.L.; Yanagisawa, L.L.; Benveniste, E.N. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J. Immunol. 2012, 189, 3439–3448. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Zhao, S.; Zhang, H.; Cai, W.; Cai, M.; Ji, X.; Leak, R.K.; Gao, Y.; Chen, J.; et al. Interleukin-4 Is Essential for Microglia/Macrophage M2 Polarization and Long-Term Recovery After Cerebral Ischemia. Stroke 2016, 47, 498–504. [Google Scholar] [CrossRef]
- Stevens, S.L.; Bao, J.; Hollis, J.; Lessov, N.S.; Clark, W.M.; Stenzel-Poore, M.P. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res. 2002, 932, 110–119. [Google Scholar] [CrossRef]
- Yilmaz, G.; Granger, D.N. Leukocyte recruitment and ischemic brain injury. Neuromol. Med. 2010, 12, 193–204. [Google Scholar] [CrossRef]
- Kunis, G.; Baruch, K.; Rosenzweig, N.; Kertser, A.; Miller, O.; Berkutzki, T.; Schwartz, M. IFN-gamma-dependent activation of the brain’s choroid plexus for CNS immune surveillance and repair. Brain 2013, 136, 3427–3440. [Google Scholar] [CrossRef]
- Niu, F.N.; Zhang, X.; Hu, X.M.; Chen, J.; Chang, L.L.; Li, J.W.; Liu, Z.; Cao, W.; Xu, Y. Targeted mutation of Fas ligand gene attenuates brain inflammation in experimental stroke. Brain Behav. Immun. 2012, 26, 61–71. [Google Scholar] [CrossRef]
- Schilling, M.; Besselmann, M.; Muller, M.; Strecker, J.K.; Ringelstein, E.B.; Kiefer, R. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: An investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp. Neurol. 2005, 196, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Besancon, E.; Guo, S.; Lok, J.; Tymianski, M.; Lo, E.H. Beyond NMDA and AMPA glutamate receptors: Emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol. Sci. 2008, 29, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 1999, 79, 1431–1568. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yenari, M.A.; Cheng, D.; Sapolsky, R.M.; Steinberg, G.K. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J. Neurochem. 2003, 85, 1026–1036. [Google Scholar] [CrossRef]
- Andreyev, A.Y.; Fahy, B.; Fiskum, G. Cytochrome c release from brain mitochondria is independent of the mitochondrial permeability transition. FEBS Lett. 1998, 439, 373–376. [Google Scholar] [CrossRef]
- Petrovic-Djergovic, D.; Goonewardena, S.N.; Pinsky, D.J. Inflammatory Disequilibrium in Stroke. Circ. Res. 2016, 119, 142–158. [Google Scholar] [CrossRef]
- Domercq, M.; Etxebarria, E.; Perez-Samartin, A.; Matute, C. Excitotoxic oligodendrocyte death and axonal damage induced by glutamate transporter inhibition. Glia 2005, 52, 36–46. [Google Scholar] [CrossRef]
- Schiene, K.; Bruehl, C.; Zilles, K.; Qu, M.; Hagemann, G.; Kraemer, M.; Witte, O.W. Neuronal hyperexcitability and reduction of GABAA-receptor expression in the surround of cerebral photothrombosis. J. Cereb Blood Flow Metab. 1996, 16, 906–914. [Google Scholar] [CrossRef]
- Jin, K.L.; Wang, X.M.; Xie, L.; Mao, X.O.; Zhu, W.; Wang, Y.; Shen, J.F.; Mao, Y.; Banwait, S.; Greenberg, D.A. Evidence for stroke-induced neurogenesis in the human brain. Proc. Nat. Acad. Sci. USA 2006, 103, 13198–13202. [Google Scholar] [CrossRef]
- Yu, T.S.; Washington, P.M.; Kernie, S.G. Injury-Induced Neurogenesis: Mechanisms and Relevance. Neuroscientist 2016, 22, 61–71. [Google Scholar] [CrossRef]
- Nakagomi, T.; Molnar, Z.; Nakano-Doi, A.; Taguchi, A.; Saino, O.; Kubo, S.; Clausen, M.; Yoshikawa, H.; Nakagomi, N.; Matsuyama, T. Ischemia-induced neural stem/progenitor cells in the pia mater following cortical infarction. Stem. Cells Dev. 2011, 20, 2037–2051. [Google Scholar] [CrossRef]
- Krupinski, J.; Kaluza, J.; Kumar, P.; Kumar, S.; Wang, J.M. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 1994, 25, 1794–1798. [Google Scholar] [CrossRef]
- Plate, K.H.; Beck, H.; Danner, S.; Allegrini, P.R.; Wiessner, C. Cell type specific upregulation of vascular endothelial growth factor in an MCA-occlusion model of cerebral infarct. J. Neuropathol. Exp. Neurol. 1999, 58, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Erinjeri, J.P.; Rovainen, C.M.; Woolsey, T.A. Collateral growth and angiogenesis around cortical stroke. Stroke 2001, 32, 2179–2184. [Google Scholar] [CrossRef]
- Yu, S.W.; Friedman, B.; Cheng, Q.; Lyden, P.D. Stroke-evoked angiogenesis results in a transient population of microvessels. J. Cereb. Blood Flow Metab. 2007, 27, 755–763. [Google Scholar] [CrossRef]
- Adamczak, J.M.; Schneider, G.; Nelles, M.; Que, I.; Suidgeest, E.; van der Weerd, L.; Lowik, C.; Hoehn, M. In vivo bioluminescence imaging of vascular remodeling after stroke. Front. Cell Neurosci. 2014, 8, 274. [Google Scholar] [CrossRef]
- Marti, H.J.; Bernaudin, M.; Bellail, A.; Schoch, H.; Euler, M.; Petit, E.; Risau, W. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am. J. Pathol. 2000, 156, 965–976. [Google Scholar] [CrossRef]
- Hayashi, T.; Noshita, N.; Sugawara, T.; Chan, P.H. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J. Cereb. Blood Flow Metab. 2003, 23, 166–180. [Google Scholar] [CrossRef]
- Liu, X.S.; Zhang, Z.G.; Zhang, R.L.; Gregg, S.; Morris, D.C.; Wang, Y.; Chopp, M. Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J. Cereb. Blood Flow Metab. 2007, 27, 564–574. [Google Scholar] [CrossRef]
- Hermann, D.M.; Zechariah, A. Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. J. Cereb. Blood Flow Metab. 2009, 29, 1620–1643. [Google Scholar] [CrossRef]
- Ma, Y.; Zechariah, A.; Qu, Y.; Hermann, D.M. Effects of vascular endothelial growth factor in ischemic stroke. J. Neurosci. Res. 2012, 90, 1873–1882. [Google Scholar] [CrossRef]
- Qiu, J.; Xu, J.; Zheng, Y.; Wei, Y.; Zhu, X.; Lo, E.H.; Moskowitz, M.A.; Sims, J.R. High-mobility group box 1 promotes metalloproteinase-9 upregulation through Toll-like receptor 4 after cerebral ischemia. Stroke 2010, 41, 2077–2082. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Katakowski, M.; Chen, X.; Wang, L.; Lu, D.; Lu, M.; Gautam, S.C.; Chopp, M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J. Neurosci. Res. 2003, 73, 778–786. [Google Scholar] [CrossRef]
- Krupinski, J.; Stroemer, P.; Slevin, M.; Marti, E.; Kumar, P.; Rubio, F. Three-dimensional structure and survival of newly formed blood vessels after focal cerebral ischemia. Neuroreport 2003, 14, 1171–1176. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, K.; Xie, L.; Childs, J.; Mao, X.O.; Logvinova, A.; Greenberg, D.A. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Invest. 2003, 111, 1843–1851. [Google Scholar] [CrossRef]
- Arenillas, J.F.; Sobrino, T.; Castillo, J.; Davalos, A. The role of angiogenesis in damage and recovery from ischemic stroke. Curr. Treat Options Cardiovasc. Med. 2007, 9, 205–212. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Cui, H.R.; Yang, S.Z.; Sun, H.P.; Qiu, M.H.; Feng, X.Y.; Sun, F.Y. VEGF enhance cortical newborn neurons and their neurite development in adult rat brain after cerebral ischemia. Neurochem. Int. 2009, 55, 629–636. [Google Scholar] [CrossRef]
- Petcu, E.B.; Smith, R.A.; Miroiu, R.I.; Opris, M.M. Angiogenesis in old-aged subjects after ischemic stroke: A cautionary note for investigators. J. Angiogen. Res. 2010, 2, 26. [Google Scholar] [CrossRef]
- Shen, Q.; Goderie, S.K.; Jin, L.; Karanth, N.; Sun, Y.; Abramova, N.; Vincent, P.; Pumiglia, K.; Temple, S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 2004, 304, 1338–1340. [Google Scholar] [CrossRef]
- Teng, H.; Zhang, Z.G.; Wang, L.; Zhang, R.L.; Zhang, L.; Morris, D.; Gregg, S.R.; Wu, Z.; Jiang, A.; Lu, M.; et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J. Cereb. Blood Flow Metab. 2008, 28, 764–771. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chang, C.; Cheung, W.M.; Lin, M.H.; Chen, J.J.; Hsu, C.Y.; Chen, J.H.; Lin, T.N. Dynamic changes in vascular permeability, cerebral blood volume, vascular density, and size after transient focal cerebral ischemia in rats: Evaluation with contrast-enhanced magnetic resonance imaging. J. Cereb. Blood Flow Metab 2008, 28, 1491–1501. [Google Scholar] [CrossRef]
- Thored, P.; Wood, J.; Arvidsson, A.; Cammenga, J.; Kokaia, Z.; Lindvall, O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke 2007, 38, 3032–3039. [Google Scholar] [CrossRef]
- Lacar, B.; Herman, P.; Platel, J.C.; Kubera, C.; Hyder, F.; Bordey, A. Neural progenitor cells regulate capillary blood flow in the postnatal subventricular zone. J. Neurosci. 2012, 32, 16435–16448. [Google Scholar] [CrossRef]
- Thored, P.; Arvidsson, A.; Cacci, E.; Ahlenius, H.; Kallur, T.; Darsalia, V.; Ekdahl, C.T.; Kokaia, Z.; Lindvall, O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem. Cells 2006, 24, 739–747. [Google Scholar] [CrossRef]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef]
- Goss, J.R.; O’Malley, M.E.; Zou, L.; Styren, S.D.; Kochanek, P.M.; DeKosky, S.T. Astrocytes are the major source of nerve growth factor upregulation following traumatic brain injury in the rat. Exp. Neurol. 1998, 149, 301–309. [Google Scholar] [CrossRef]
- Weidemann, A.; Krohne, T.U.; Aguilar, E.; Kurihara, T.; Takeda, N.; Dorrell, M.I.; Simon, M.C.; Haase, V.H.; Friedlander, M.; Johnson, R.S. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia 2010, 58, 1177–1185. [Google Scholar] [CrossRef]
- Venkat, P.; Chopp, M.; Chen, J. New insights into coupling and uncoupling of cerebral blood flow and metabolism in the brain. Croat. Med. J. 2016, 57, 223–228. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Z.B.; Zhuge, Q.; Zheng, W.; Shao, B.; Wang, B.; Sun, F.; Jin, K. Glial scar formation occurs in the human brain after ischemic stroke. Int. J. Med. Sci. 2014, 11, 344–348. [Google Scholar] [CrossRef]
- Roitbak, T.; Sykova, E. Diffusion barriers evoked in the rat cortex by reactive astrogliosis. Glia 1999, 28, 40–48. [Google Scholar] [CrossRef]
- Becerra-Calixto, A.; Cardona-Gomez, G.P. The Role of Astrocytes in Neuroprotection after Brain Stroke: Potential in Cell Therapy. Front. Mol. Neurosci. 2017, 10, 88. [Google Scholar] [CrossRef]
- Abeysinghe, H.C.; Phillips, E.L.; Chin-Cheng, H.; Beart, P.M.; Roulston, C.L. Modulating Astrocyte Transition after Stroke to Promote Brain Rescue and Functional Recovery: Emerging Targets Include Rho Kinase. Int. J. Mol. Sci. 2016, 17, 288. [Google Scholar] [CrossRef]
- De Feo, D.; Merlini, A.; Laterza, C.; Martino, G. Neural stem cell transplantation in central nervous system disorders: From cell replacement to neuroprotection. Curr. Opin Neurol. 2012, 25, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Andres, R.H.; Horie, N.; Slikker, W.; Keren-Gill, H.; Zhan, K.; Sun, G.; Manley, N.C.; Pereira, M.P.; Sheikh, L.A.; McMillan, E.L.; et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 2011, 134, 1777–1789. [Google Scholar] [CrossRef]
- Emanueli, C.; Schratzberger, P.; Kirchmair, R.; Madeddu, P. Paracrine control of vascularization and neurogenesis by neurotrophins. Br. J. Pharmacol. 2003, 140, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lim, I.J.; Lee, M.C.; Kim, S.U. Human Neural Stem Cells Genetically Modified To Overexpress Brain-Derived Neurotrophic Factor Promote Functional Recovery and Neuroprotection in a Mouse Stroke Model. J. Neurosci. Res. 2010, 88, 3282–3294. [Google Scholar] [CrossRef] [PubMed]
- Schabitz, W.R.; Steigleder, T.; Cooper-Kuhn, C.M.; Schwab, S.; Sommer, C.; Schneider, A.; Kuhn, H.G. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke 2007, 38, 2165–2172. [Google Scholar] [CrossRef]
- Lim, J.Y.; Park, S.I.; Oh, J.H.; Kim, S.M.; Jeong, C.H.; Jun, J.A.; Lee, K.S.; Oh, W.; Lee, J.K.; Jeun, S.S. Brain-derived neurotrophic factor stimulates the neural differentiation of human umbilical cord blood-derived mesenchymal stem cells and survival of differentiated cells through MAPK/ERK and PI3K/Akt-dependent signaling pathways. J. Neurosci. Res. 2008, 86, 2168–2178. [Google Scholar] [CrossRef]
- Abe, K. Therapeutic potential of neurotrophic factors and neural stem cells against ischemic brain injury. J. Cereb. Blood Flow Metab. 2000, 20, 1393–1408. [Google Scholar] [CrossRef]
- Sondell, M.; Sundler, F.; Kanje, M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur. J. Neurosci. 2000, 12, 4243–4254. [Google Scholar] [CrossRef]
- Leung, D.W.; Cachianes, G.; Kuang, W.J.; Goeddel, D.V.; Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [CrossRef]
- Gerber, H.P.; McMurtrey, A.; Kowalski, J.; Yan, M.; Keyt, B.A.; Dixit, V.; Ferrara, N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem. 1998, 273, 30336–30343. [Google Scholar] [CrossRef]
- Hayashi, T.; Abe, K.; Itoyama, Y. Reduction of ischemic damage by application of vascular endothelial growth factor in rat brain after transient ischemia. J. Cereb. Blood Flow Metab. 1998, 18, 887–895. [Google Scholar] [CrossRef]
- Kaya, D.; Gursoy-Ozdemir, Y.; Yemisci, M.; Tuncer, N.; Aktan, S.; Dalkara, T. VEGF protects brain against focal ischemia without increasing blood--brain permeability when administered intracerebroventricularly. J. Cereb. Blood. Flow Metab. 2005, 25, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Harms, K.M.; Li, L.; Cunningham, L.A. Murine Neural Stem/Progenitor Cells Protect Neurons against Ischemia by HIF-1 alpha-Regulated VEGF Signaling. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Ann. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Multiple sclerosis in 54 twinships: Concordance rate is independent of zygosity. French Research Group on Multiple Sclerosis. Ann. Neurol. 1992, 32, 724–727. [Google Scholar] [CrossRef]
- Kang, S.S.; Keasey, M.P.; Arnold, S.A.; Reid, R.; Geralds, J.; Hagg, T. Endogenous CNTF mediates stroke-induced adult CNS neurogenesis in mice. Neurobiol. Dis. 2013, 49, 68–78. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Howe, C.L.; Mobley, W.C. Nerve growth factor signaling, neuroprotection, and neural repair. Ann. Rev. Neurosci. 2001, 24, 1217–1281. [Google Scholar] [CrossRef]
- Cattaneo, E.; McKay, R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature 1990, 347, 762–765. [Google Scholar] [CrossRef]
- Yuan, M.; Wen, S.J.; Yang, C.X.; Pang, Y.G.; Gao, X.Q.; Liu, X.Q.; Huang, L.; Yuan, Q.L. Transplantation of neural stem cells overexpressing glial cell line-derived neurotrophic factor enhances Akt and Erk1/2 signaling and neurogenesis in rats after stroke. Chinese Med. J. 2013, 126, 1302–1309. [Google Scholar]
- Oki, K.; Tatarishvili, J.; Wood, J.; Koch, P.; Wattananit, S.; Mine, Y.; Monni, E.; Tornero, D.; Ahlenius, H.; Ladewig, J.; et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells 2012, 30, 1120–1133. [Google Scholar] [CrossRef]
- Lampl, Y.; Boaz, M.; Gilad, R.; Lorberboym, M.; Dabby, R.; Rapoport, A.; Anca-Hershkowitz, M.; Sadeh, M. Minocycline treatment in acute stroke: An open-label, evaluator-blinded study. Neurology 2007, 69, 1404–1410. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, L.; Wang, D.; Wang, Z.; Huang, Q.Y. Minocycline ameliorates hypoxia-induced blood-brain barrier damage by inhibition of HIF-1alpha through SIRT-3/PHD-2 degradation pathway. Neuroscience 2015, 304, 250–259. [Google Scholar] [CrossRef]
- Garrido-Mesa, N.; Camuesco, D.; Arribas, B.; Comalada, M.; Bailon, E.; Cueto-Sola, M.; Utrilla, P.; Nieto, A.; Zarzuelo, A.; Rodriguez-Cabezas, M.E.; et al. The intestinal anti-inflammatory effect of minocycline in experimental colitis involves both its immunomodulatory and antimicrobial properties. Pharmacol. Res. 2011, 63, 308–319. [Google Scholar] [CrossRef]
- Metz, L.M.; Li, D.K.B.; Traboulsee, A.L.; Duquette, P.; Eliasziw, M.; Cerchiaro, G.; Greenfield, J.; Riddehough, A.; Yeung, M.; Kremenchutzky, M.; et al. Trial of Minocycline in a Clinically Isolated Syndrome of Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 2122–2133. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, S.; Fujimoto, S.; Petrov, A.; Nakagami, H.; Haider, N.; Zhou, J.; Tahara, N.; Osako, M.K.; Fujimoto, A.; Zhu, J.; et al. Effect of an antimicrobial agent on atherosclerotic plaques: Assessment of metalloproteinase activity by molecular imaging. J. Am. Coll Cardiol. 2010, 55, 1240–1249. [Google Scholar] [CrossRef]
- Machado, L.S.; Kozak, A.; Ergul, A.; Hess, D.C.; Borlongan, C.V.; Fagan, S.C. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 2006, 7, 56. [Google Scholar] [CrossRef]
- Yang, Y.; Salayandia, V.M.; Thompson, J.F.; Yang, L.Y.; Estrada, E.Y.; Yang, Y. Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J. Neuroinflam. 2015, 12, 26. [Google Scholar] [CrossRef]
- Yrjanheikki, J.; Tikka, T.; Keinanen, R.; Goldsteins, G.; Chan, P.H.; Koistinaho, J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc. Natl. Acad. Sci. USA 1999, 96, 13496–13500. [Google Scholar] [CrossRef]
- Gasche, Y.; Fujimura, M.; Morita-Fujimura, Y.; Copin, J.C.; Kawase, M.; Massengale, J.; Chan, P.H. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: A possible role in blood-brain barrier dysfunction. J. Cereb. Blood Flow Meta. 1999, 19, 1020–1028. [Google Scholar] [CrossRef]
- Smith, H.K.; Gavins, F.N. The potential of stem cell therapy for stroke: Is PISCES the sign? FASEB J. 2012, 26, 2239–2252. [Google Scholar] [CrossRef][Green Version]
- Bacigaluppi, M.; Pluchino, S.; Peruzzotti-Jametti, L.; Kilic, E.; Kilic, U.; Salani, G.; Brambilla, E.; West, M.J.; Comi, G.; Martino, G.; et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain 2009, 132, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.J.; Lee, N.; Park, I.H.; Choi, C.; Jeon, I.; Kwon, J.; Oh, S.H.; Shin, D.A.; Do, J.T.; Lee, D.R.; et al. Therapeutic potential of human induced pluripotent stem cells in experimental stroke. Cell Transplant 2013, 22, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lee, S.H.; Kim, S.U.; Yoon, B.W. Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain. Neural Regen. Res. 2016, 11, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Sakata, H.; Niizuma, K.; Wakai, T.; Narasimhan, P.; Maier, C.M.; Chan, P.H. Neural Stem Cells Genetically Modified to Overexpress Cu/Zn-Superoxide Dismutase Enhance Amelioration of Ischemic Stroke in Mice. Stroke 2012, 43, 2423. [Google Scholar] [CrossRef]
- Mine, Y.; Tatarishvili, J.; Oki, K.; Monni, E.; Kokaia, Z.; Lindvall, O. Grafted human neural stem cells enhance several steps of endogenous neurogenesis and improve behavioral recovery after middle cerebral artery occlusion in rats. Neurobiol. Dis. 2013, 52, 191–203. [Google Scholar] [CrossRef]
- Chu, K.; Kim, M.; Park, K.I.; Jeong, S.W.; Park, H.K.; Jung, K.H.; Lee, S.T.; Kang, L.; Lee, K.; Park, D.K.; et al. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004, 1016, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Kim, M.; Jeong, S.W.; Kim, S.U.; Yoon, B.W. Human neural stem cells can migrate, differentiate, and integrate after intravenous transplantation in adult rats with transient forebrain ischemia. Neurosci. Lett. 2003, 343, 129–133. [Google Scholar] [CrossRef]
- Wu, W.F.; Chen, X.; Hu, C.L.; Li, J.F.; Yu, Z.; Cai, W.Q. Transplantation of neural stem cells expressing hypoxia-inducible factor-1 alpha (HIF-1 alpha) improves behavioral recovery in a rat stroke model. J. Clin. Neurosci. 2010, 17, 92–95. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zhu, J.J.; Hu, Y.B.; Xiang, G.H.; Deng, L.C.; Wu, F.Z.; Wei, X.J.; Wang, Y.H.; Sun, L.Y.; Lou, X.Q.; et al. Transplantation of bFGF-expressing neural stem cells promotes cell migration and functional recovery in rat brain after transient ischemic stroke. Oncotarget 2017, 8, 102067–102077. [Google Scholar] [CrossRef] [PubMed]
- Bouet, V.; Boulouard, M.; Toutain, J.; Divoux, D.; Bernaudin, M.; Schumann-Bard, P.; Freret, T. The adhesive removal test: A sensitive method to assess sensorimotor deficits in mice. Nature Protocols 2009, 4, 1560–1564. [Google Scholar] [CrossRef] [PubMed]
- Patkar, S.; Tate, R.; Modo, M.; Plevin, R.; Carswell, H.V. Conditionally immortalised neural stem cells promote functional recovery and brain plasticity after transient focal cerebral ischaemia in mice. Stem Cell Res 2012, 8, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Korshunova, I.; Rhein, S.; Garcia-Gonzalez, D.; Stolting, I.; Pfisterer, U.; Barta, A.; Dmytriyeva, O.; Kirkeby, A.; Schwaninger, M.; Khodosevich, K. Genetic modification increases the survival and the neuroregenerative properties of transplanted neural stem cells. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Ergul, A.; Kelly-Cobbs, A.; Abdalla, M.; Fagan, S.C. Cerebrovascular complications of diabetes: Focus on stroke. Endocrine Metab.Immune Disord. Drug Targets 2012, 12, 148–158. [Google Scholar] [CrossRef]
- Ergul, A.; Li, W.; Elgebaly, M.M.; Bruno, A.; Fagan, S.C. Hyperglycemia, diabetes and stroke: Focus on the cerebrovasculature. Vasc. Pharmacol. 2009, 51, 44–49. [Google Scholar] [CrossRef]
- Tureyen, K.; Bowen, K.; Liang, J.; Dempsey, R.J.; Vemuganti, R. Exacerbated brain damage, edema and inflammation in type-2 diabetic mice subjected to focal ischemia. J. Neurochem. 2011, 116, 499–507. [Google Scholar] [CrossRef]
- Kalladka, D.; Sinden, J.; Pollock, K.; Haig, C.; McLean, J.; Smith, W.; McConnachie, A.; Santosh, C.; Bath, P.M.; Dunn, L.; et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet 2016, 388, 787–796. [Google Scholar] [CrossRef]
- Muir, K.W. Clinical trial design for stem cell therapies in stroke: What have we learned? Neurochem. Int. 2016. [Google Scholar] [CrossRef]
- Muir, K.W.; Bulters, D.; Willmot, M.; Sprigg, N.; Dixit, A.; Ward, N.; Tyrrell, P.; Majid, A.; Dunn, L.; Bath, P.; et al. Intracerebral implantation of human neural stem cells and motor recovery after stroke: Multicentre prospective single-arm study (PISCES-2). J. Neurol Neurosurg. Psychiatry 2020, 91, 396–401. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamblin, M.H.; Lee, J.-P. Neural Stem Cells for Early Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 7703. https://doi.org/10.3390/ijms22147703
Hamblin MH, Lee J-P. Neural Stem Cells for Early Ischemic Stroke. International Journal of Molecular Sciences. 2021; 22(14):7703. https://doi.org/10.3390/ijms22147703
Chicago/Turabian StyleHamblin, Milton H., and Jean-Pyo Lee. 2021. "Neural Stem Cells for Early Ischemic Stroke" International Journal of Molecular Sciences 22, no. 14: 7703. https://doi.org/10.3390/ijms22147703
APA StyleHamblin, M. H., & Lee, J.-P. (2021). Neural Stem Cells for Early Ischemic Stroke. International Journal of Molecular Sciences, 22(14), 7703. https://doi.org/10.3390/ijms22147703




