Emerging Role of Transient Receptor Potential Vanilloid 4 (TRPV4) Ion Channel in Acute and Chronic Itch
Abstract
:1. Introduction
2. TRPV4 in Pruritogen-Evoked Acute Itch
3. TRPV4 in Experimental Chronic Itch
4. TRPV4 in Itch: Evidence from Human Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ikoma, A.; Steinhoff, M.; Ständer, S.; Yosipovitch, G.; Schmelz, M. The neurobiology of itch. Nat. Rev. Neurosci. 2006, 7, 535–547. [Google Scholar] [CrossRef]
- Ständer, S.; Weisshaar, E.; Mettang, T.; Szepietowski, J.; Carstens, E.; Ikoma, A.; Bergasa, N.V.; Gieler, U.; Misery, L.; Wallengren, J.; et al. Clinical Classification of Itch: A Position Paper of the International Forum for the Study of Itch. Acta Derm. Venereol. 2007, 87, 291–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, T.; Carstens, E. Neural processing of itch. Neuroscience 2013, 250, 697–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Ji, R.R. New insights into the mechanisms of itch: Are pain and itch controlled by distinct mechanisms? Pflügers Arch. Eur. J. Physiol. 2013, 465, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R. Neuroimmune interactions in itch: Do chronic itch, chronic pain, and chronic cough share similar mechanisms? Pulm. Pharmacol. Ther. 2015, 35, 81–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmelz, M. Itch and Pain Differences and Commonalities. In Pain Control; Schaible, H.-G., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 285–301. [Google Scholar]
- Ross, S.E. Pain and itch: Insights into the neural circuits of aversive somatosensation in health and disease. Curr. Opin. Neurobiol. 2011, 21, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Wilson, S.R.; Hoon, M.A. Why we scratch an itch: The molecules, cells and circuits of itch. Nat. Neurosci. 2014, 17, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Gupta, R.; Jordt, S.-E.; Chen, Y.; Liedtke, W.B. Regulation of Pain and Itch by TRP Channels. Neurosci. Bull. 2018, 34, 120–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Dong, X. Trp channels and itch. Semin. Immunopathol. 2016, 38, 293–307. [Google Scholar] [CrossRef] [Green Version]
- Bíró, T.; Tóth, B.I.; Marincsák, R.; Dobrosi, N.; Géczy, T.; Paus, R. TRP channels as novel players in the pathogenesis and therapy of itch. Biochim. Biophys. Acta 2007, 1772, 1004–1021. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.R.; Bautista, D.M. Role of Transient Receptor Potential Channels in Acute and Chronic Itch. In Itch: Mechanisms and Treatment; Carstens, E., Akiyama, T., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2014. [Google Scholar]
- Kittaka, H.; Tominaga, M. The molecular and cellular mechanisms of itch and the involvement of TRP channels in the pe-ripheral sensory nervous system and skin. Allergol. Int. 2017, 66, 22–30. [Google Scholar] [CrossRef]
- Xie, Z.; Hu, H. TRP Channels as Drug Targets to Relieve Itch. Pharmaceuticals 2018, 11, 100. [Google Scholar] [CrossRef] [Green Version]
- Mascarenhas, N.L.; Wang, Z.; Chang, Y.-L.; Di Nardo, A. TRPV4 Mediates Mast Cell Activation in Cathelicidin-Induced Rosacea Inflammation. J. Investig. Dermatol. 2017, 137, 972–975. [Google Scholar] [CrossRef] [Green Version]
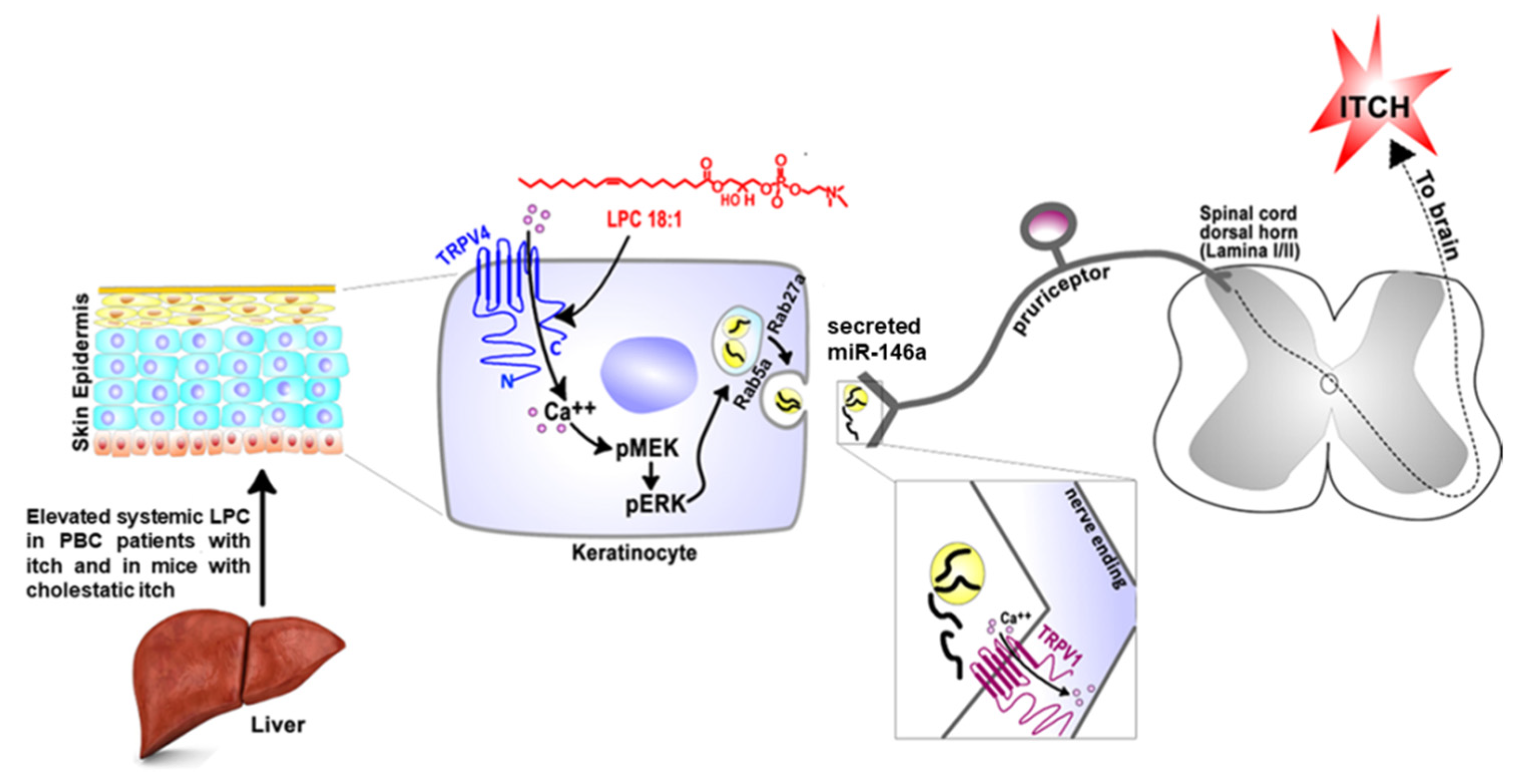
- Chen, Y.; Wang, Z.L.; Yeo, M.; Zhang, Q.J.; López-Romero, A.E.; Ding, H.P.; Zhang, X.; Zeng, Q.; Morales-Lázaro, S.L.; Moore, C.; et al. Epithe-lia-Sensory Neuron Cross Talk Underlies Cholestatic Itch Induced by Lysophosphatidylcholine. Gastroenterology 2021, 161, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fang, Q.; Wang, Z.; Zhang, J.Y.; MacLeod, A.S.; Hall, R.P.; Liedtke, W.B. Transient Receptor Potential Vanilloid 4 Ion Channel Functions as a Pruriceptor in Epidermal Keratinocytes to Evoke Histaminergic Itch. J. Biol. Chem. 2016, 291, 10252–10262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Williams, S.H.; McNulty, A.L.; Hong, J.H.; Lee, S.H.; Rothfusz, N.E.; Parekh, P.K.; Moore, C.; Gereau, R.W., IV; Taylor, A.B.; et al. Temporomandibular joint pain: A critical role for Trpv4 in the trigeminal gan-glion. PAIN 2013, 154, 1295–1304. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Feng, J.; Yu, G.; Yang, P.; Mack, M.R.; Du, J.; Yu, W.; Qian, A.; Zhang, Y.; Liu, S.; et al. Transient receptor potential vanilloid 4–expressing macrophages and keratinocytes contribute differentially to allergic and nonallergic chronic itch. J. Allergy Clin. Immunol. 2018, 141, 608–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokabe, T.; Fukumi-Tominaga, T.; Yonemura, S.; Mizuno, A.; Tominaga, M. The TRPV4 Channel Contributes to Intercellular Junction Formation in Keratinocytes. J. Biol. Chem. 2010, 285, 18749–18758. [Google Scholar] [CrossRef] [Green Version]
- Kida, N.; Sokabe, T.; Kashio, M.; Haruna, K.; Mizuno, Y.; Suga, Y.; Nishikawa, K.; Kanamaru, A.; Hongo, M.; Oba, A.; et al. Importance of transient receptor potential vanilloid 4 (TRPV4) in epidermal barrier function in human skin keratinocytes. Pflügers Arch. Eur. J. Physiol. 2012, 463, 715–725. [Google Scholar] [CrossRef]
- Moore, C.; Cevikbas, F.; Pasolli, H.A.; Chen, Y.; Kong, W.; Kempkes, C.; Parekh, P.; Lee, S.H.; Kontchou, N.-A.; Yeh, I.; et al. UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc. Natl. Acad. Sci. USA 2013, 110, E3225–E3234. [Google Scholar] [CrossRef] [Green Version]
- Trentin, P.G.; Fernandes, M.B.; D’Orléans-Juste, P.; Rae, G.A. Endothelin-1 causes pruritus in mice. Exp. Biol. Med. 2006, 231, 1146–1151. [Google Scholar]
- Akiyama, T.; Nagamine, M.; Davoodi, A.; Carstens, M.I.; Cevikbas, F.; Steinhoff, M.; Carstens, E. Intradermal endothelin-1 excites bombesin-responsive superficial dorsal horn neurons in the mouse. J. Neurophysiol. 2015, 114, 2528–2534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kido-Nakahara, M.; Buddenkotte, J.; Kempkes, C.; Ikoma, A.; Cevikbas, F.; Akiyama, T.; Nunes, F.; Seeliger, S.; Hasdemir, B.; Mess, C.; et al. Neural peptidase endothelin-converting enzyme 1 regulates endothelin 1–induced pruritus. J. Clin. Investig. 2014, 124, 2683–2695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, T.; Ivanov, M.; Nagamine, M.; Davoodi, A.; Carstens, M.I.; Ikoma, A.; Cevikbas, F.; Kempkes, C.; Buddenkotte, J.; Steinhoff, M.; et al. Involvement of TRPV4 in Serotonin-Evoked Scratching. J. Investig. Dermatol. 2016, 136, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Barry, D.; Liu, X.; Yin, S.; Munanairi, A.; Meng, Q.-T.; Cheng, W.; Mo, P.; Wan, L.; Liu, S.-B.; et al. Facilitation of TRPV4 by TRPV1 is required for itch transmission in some sensory neuron populations. Sci. Signal. 2016, 9, ra71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Q.; Wang, Z.; Suttle, A.; Chen, Y. Involvement of sensory neuron-TRPV4 in acute and chronic itch behaviors. bioRxiv 2021. [Google Scholar] [CrossRef]
- Watanabe, H.; Vriens, J.; Suh, S.H.; Benham, C.D.; Droogmans, G.; Nilius, B. Heat-evoked Activation of TRPV4 Channels in a HEK293 Cell Expression System and in Native Mouse Aorta Endothelial Cells. J. Biol. Chem. 2002, 277, 47044–47051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, K.M.; Hashimoto, T.; Sakai, K.; Akiyama, T. Modulation of Itch by Localized Skin Warming and Cooling. Acta Derm. Venereol. 2018, 98, 855–861. [Google Scholar] [CrossRef] [Green Version]
- Højland, C.; Andersen, H.; Poulsen, J.; Arendt-Nielsen, L.; Gazerani, P. A Human Surrogate Model of Itch Utilizing the TRPA1 Agonist Trans-cinnamaldehyde. Acta Derm. Venereol. 2014, 95, 798–803. [Google Scholar] [CrossRef] [Green Version]
- Domocos, D.; Follansbee, T.; Nguyen, A.; Nguyen, T.; Carstens, M.I.; Carstens, E. Cinnamaldehyde elicits itch behavior via TRPV1 and TRPV4 but not TRPA1. Itch 2020, 5, e36. [Google Scholar] [CrossRef]
- Düll, M.M.; Kremer, A.E. Treatment of Pruritus Secondary to Liver Disease. Curr. Gastroenterol. Rep. 2019, 21, 48. [Google Scholar] [CrossRef]
- Kremer, A.E.; Martens, J.J.; Kulik, W.; Rueff, F.; Kuiper, E.M.; van Buuren, H.R.; van Erpecum, K.J.; Kondrackiene, J.; Prieto, J.; Rust, C.; et al. Lysophosphatidic acid is a po-tential mediator of cholestatic pruritus. Gastroenterology 2010, 139, 1008–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieu, T.; Jayaweera, G.; Zhao, P.; Poole, D.P.; Jensen, D.; Grace, M.; McIntyre, P.; Bron, R.; Wilson, Y.M.; Krappitz, M.; et al. The bile acid re-ceptor TGR5 activates the TRPA1 channel to induce itch in mice. Gastroenterology 2014, 147, 1417–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Hayyeh, S.; Ovadia, C.; Lieu, T.; Jensen, D.D.; Chambers, J.; Dixon, P.H.; Lövgren-Sandblom, A.; Bolier, R.; Tolenaars, D.; Kremer, A.; et al. Prognostic and mechanistic potential of progesterone sulfates in intrahepatic cholestasis of pregnancy and pruritus gravidarum. Hepatology 2016, 63, 1287–1298. [Google Scholar] [CrossRef] [Green Version]
- Meixiong, J.; Vasavda, C.; Snyder, S.H.; Dong, X. MRGPRX4 is a G protein-coupled receptor activated by bile acids that may contribute to cholestatic pruritus. Proc. Natl. Acad. Sci. USA 2019, 116, 10525–10530. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Zhao, T.; Liu, S.; Wu, Q.; Johnson, O.; Wu, Z.; Zhuang, Z.; Shi, Y.; Peng, L.; He, R.; et al. MRGPRX4 is a bile acid receptor for human cholestatic itch. Elife 2019, 8, 48431. [Google Scholar] [CrossRef] [PubMed]
- Kittaka, H.; Uchida, K.; Fukuta, N.; Tominaga, M. Lysophosphatidic acid-induced itch is mediated by signalling of LPA5receptor, phospholipase D and TRPA1/TRPV1. J. Physiol. 2017, 595, 2681–2698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguchi, K.; Herr, D.; Mutoh, T.; Chun, J. Lysophosphatidic acid (LPA) and its receptors. Curr. Opin. Pharmacol. 2009, 9, 15–23. [Google Scholar] [CrossRef]
- Zeng, C.; Wen, B.; Hou, G.; Lei, L.; Mei, Z.; Jia, X.; Chen, X.; Zhu, W.; Li, J.; Kuang, Y.; et al. Lipidomics profiling reveals the role of glycerophospholipid metabolism in psoriasis. GigaScience 2017, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryborg, A.; Grøn, B.; Kragballe, K.; Ryborg, A.; Grøn, B.; Kragballe, K. Increased lysophosphatidylcholine content in lesional psoriatic skin. Br. J. Dermatol. 1995, 133, 398–402. [Google Scholar] [CrossRef]
- Berdyshev, E.; Goleva, E.; Bronova, I.; Dyjack, N.; Rios, C.; Jung, J.; Taylor, P.; Jeong, M.; Hall, C.F.; Richers, B.N.; et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight 2018, 3, e98006. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Zhang, H.; Ding, J.-R.; Hong, Z.-Y.; Wu, H.; Zhu, Z.-Y.; Guo, Z.-Y.; Chai, Y.-F. UPLC-QTOF MS-Based Serum Metabolomic Profiling Analysis Reveals the Molecular Perturbations Underlying Uremic Pruritus. BioMed Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, C.; Marks, R. Stratum corneum changes in patients with senile pruritus. J. Am. Acad. Dermatol. 1992, 27, 560–564. [Google Scholar] [CrossRef]
- Morton, C.A.; Lafferty, M.; Hau, C.; Henderson, I.; Jones, M.; Lowe, J.G. Pruritus and skin hydration during dialysis. Nephrol. Dial. Transplant. 1996, 11, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Denda, M.; Sokabe, T.; Fukumi-Tominaga, T.; Tominaga, M. Effects of Skin Surface Temperature on Epidermal Permeability Barrier Homeostasis. J. Investig. Dermatol. 2007, 127, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akazawa, Y.; Yuki, T.; Yoshida, H.; Sugiyama, Y.; Inoue, S. Activation of TRPV4 Strengthens the Tight-Junction Barrier in Human Epidermal Keratinocytes. Ski. Pharmacol. Physiol. 2013, 26, 15–21. [Google Scholar] [CrossRef]
- Kostner, L.; Anzengruber, F.; Guillod, C.; Recher, M.; Schmid-Grendelmeier, P.; Navarini, A.A. Allergic Contact Dermatitis. Immunol. Allergy Clin. N. Am. 2017, 37, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Xiong, H.; Liu, S.; Liu, Y.; Yin, L.; Tu, C.; Wang, H.; Zhao, Z.; Chen, W.; Mei, Z. Qingpeng Ointment Ameliorates Inflammatory Responses and Dysregulation of Itch-Related Molecules for Its Antipruritic Effects in Experimental Allergic Contact Dermatitis. Front. Pharmacol. 2019, 10, 354. [Google Scholar] [CrossRef] [Green Version]
- Gordon, W.C.; López, V.G.; Bhattacharjee, S.; Gil, D.R.; Díaz, J.A.; De La Losa, F.P.; Pelaez, R.P.; Ferrer, C.T.; Bacchini, G.S.; Jun, B.; et al. A Nonsteroidal Novel Formulation Targeting Inflammatory and Pruritus-Related Mediators Modulates Experimental Allergic Contact Dermatitis. Dermatol. Ther. 2018, 8, 111–126. [Google Scholar] [CrossRef] [Green Version]
- Yosipovitch, G.; Goon, A.; Wee, J.; Chan, Y.H.; Goh, C.L. The prevalence and clinical characteristics of pruritus among pa-tients with extensive psoriasis. Br. J. Dermatol. 2000, 143, 969–973. [Google Scholar] [CrossRef]
- Yan, J.; Ye, F.; Ju, Y.; Wang, D.; Chen, J.; Zhang, X.; Yin, Z.; Wang, C.; Yang, Y.; Zhu, C.; et al. Cimifugin relieves pruritus in psoriasis by inhibiting TRPV4. Cell Calcium 2021, 97, 102429. [Google Scholar] [CrossRef]
- Sakai, K.; Sanders, K.; Youssef, M.R.; Yanushefski, K.M.; Jensen, L.; Yosipovitch, G.; Akiyama, T. Mouse model of imiquimod-induced psoriatic itch. Pain 2016, 157, 2536–2543. [Google Scholar] [CrossRef]
- Van Loey, N.; Bremer, M.; Faber, A.; Middelkoop, E.; Nieuwenhuis, M. The Research Group Itching following burns: Epidemiology and predictors. Br. J. Dermatol. 2007, 158, 95–100. [Google Scholar] [CrossRef]
- Yang, Y.; Cho, S.; Choi, M.; Choi, Y.; Kwak, I.; Park, C.; Kim, H. Increased Expression of Three Types of Transient Receptor Potential Channels (TRPA1, TRPV4 and TRPV3) in Burn Scars with Post-burn Pruritus. Acta Derm. Venereol. 2015, 95, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Nattkemper, L.A.; Tey, H.L.; Valdes-Rodriguez, R.; Lee, H.; Mollanazar, N.K.; Albornoz, C.; Sanders, K.M.; Yosipovitch, G. The Genetics of Chronic Itch: Gene Expression in the Skin of Patients with Atopic Dermatitis and Psoriasis with Severe Itch. J. Investig. Dermatol. 2018, 138, 1311–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ständer, S.; Pogatzki-Zahn, E.; Stumpf, A.; Fritz, F.; Pfleiderer, B.; Ritzkat, A.; Bruland, P.; Lotts, T.; Müller-Tidow, C.; Heuft, G.; et al. Facing the challenges of chronic pruritus: A report from a multi-disciplinary medical itch centre in Germany. Acta Derm. Venereol. 2015, 95, 266–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, G.; Pogatzki-Zahn, E.; Marziniak, M.; Stumpf, A.; Ständer, S. Cutaneous sensory function is not related to depres-sion and anxiety in patients with chronic pruritus with dysesthetic subqualities. Acta Derm. Venereol. 2015, 95, 289–293. [Google Scholar] [CrossRef] [Green Version]
- Ott, S.; Maihöfner, C. Signs and Symptoms in 1,043 Patients with Complex Regional Pain Syndrome. J. Pain 2018, 19, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Hidding, J.; Agelopoulos, K.; Pereira, M.P.; Conrad, H.; Hatt, H.; Lotts, T.; Osada, N.; Pogatzki-Zahn, E.; Schmelz, M.; Ständer, S. Sensory Qualities Point to Different Structural and Functional Skin Patterns in Chronic Pruritus Patients. A Translational Explorative Study. Acta Derm. Venereol. 2019, 99, 668–674. [Google Scholar] [CrossRef] [Green Version]
- Kanju, P.; Liedtke, W. Pleiotropic function of TRPV4 ion channels in the central nervous system. Exp. Physiol. 2016, 101, 1472–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.-R.; Donnelly, C.; Nedergaard, M. Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 2019, 20, 667–685. [Google Scholar] [CrossRef] [PubMed]
| Pruritogen-Evoked Acute Itch | Trpv4 KO | Keratinocyte-Trpv4 cKO | Sensory Neuron-Trpv4 cKO | Macrophage-Trpv4 cKO |
|---|---|---|---|---|
| Histamine | Unchanged [26] or attenuated [17,27] | Attenuated [17] | Attenuated [28] | Unknown |
| Compound 48/80 | Attenuated [17] | Attenuated [17] | Attenuated [28] | Unknown |
| ET-1 | Attenuated [17] | Attenuated [17] | Unknown | Unknown |
| 5-HT | Attenuated [26] | Unknown | Attenuated [28] | Unknown |
| SLIGRL | Unchanged [26] | Unknown | Unchanged [28] | Unknown |
| Chloroquine | Increased [26], unchanged [17] or attenuated [27] | Unchanged [17] | Unchanged [28] | Unknown |
| Chronic Itch Model | Trpv4 KO | Keratinocyte-Trpv4 cKO | Sensory Neuron-Trpv4 cKO | Macrophage-Trpv4 cKO |
|---|---|---|---|---|
| Dry skin model (AEW) | Attenuated [19] | Attenuated [19] | Attenuated [28] | Unchanged [19] |
| Allergic contact dermatitis models (SADBE or DNFB) | Attenuated [19] | Unchanged [19] | Unchanged [28] | Attenuated [19] |
| Cholestatic itch model (ANIT) | Unknown | Attenuated [16] | Unknown | Unknown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Henry, G.; Chen, Y. Emerging Role of Transient Receptor Potential Vanilloid 4 (TRPV4) Ion Channel in Acute and Chronic Itch. Int. J. Mol. Sci. 2021, 22, 7591. https://doi.org/10.3390/ijms22147591
Zhang Q, Henry G, Chen Y. Emerging Role of Transient Receptor Potential Vanilloid 4 (TRPV4) Ion Channel in Acute and Chronic Itch. International Journal of Molecular Sciences. 2021; 22(14):7591. https://doi.org/10.3390/ijms22147591
Chicago/Turabian StyleZhang, Qiaojuan, Gwendolyn Henry, and Yong Chen. 2021. "Emerging Role of Transient Receptor Potential Vanilloid 4 (TRPV4) Ion Channel in Acute and Chronic Itch" International Journal of Molecular Sciences 22, no. 14: 7591. https://doi.org/10.3390/ijms22147591
APA StyleZhang, Q., Henry, G., & Chen, Y. (2021). Emerging Role of Transient Receptor Potential Vanilloid 4 (TRPV4) Ion Channel in Acute and Chronic Itch. International Journal of Molecular Sciences, 22(14), 7591. https://doi.org/10.3390/ijms22147591






