Knockdown of Mitogen-Activated Protein Kinase Kinase 3 Negatively Regulates Hepatitis A Virus Replication
Abstract
1. Introduction
2. Results
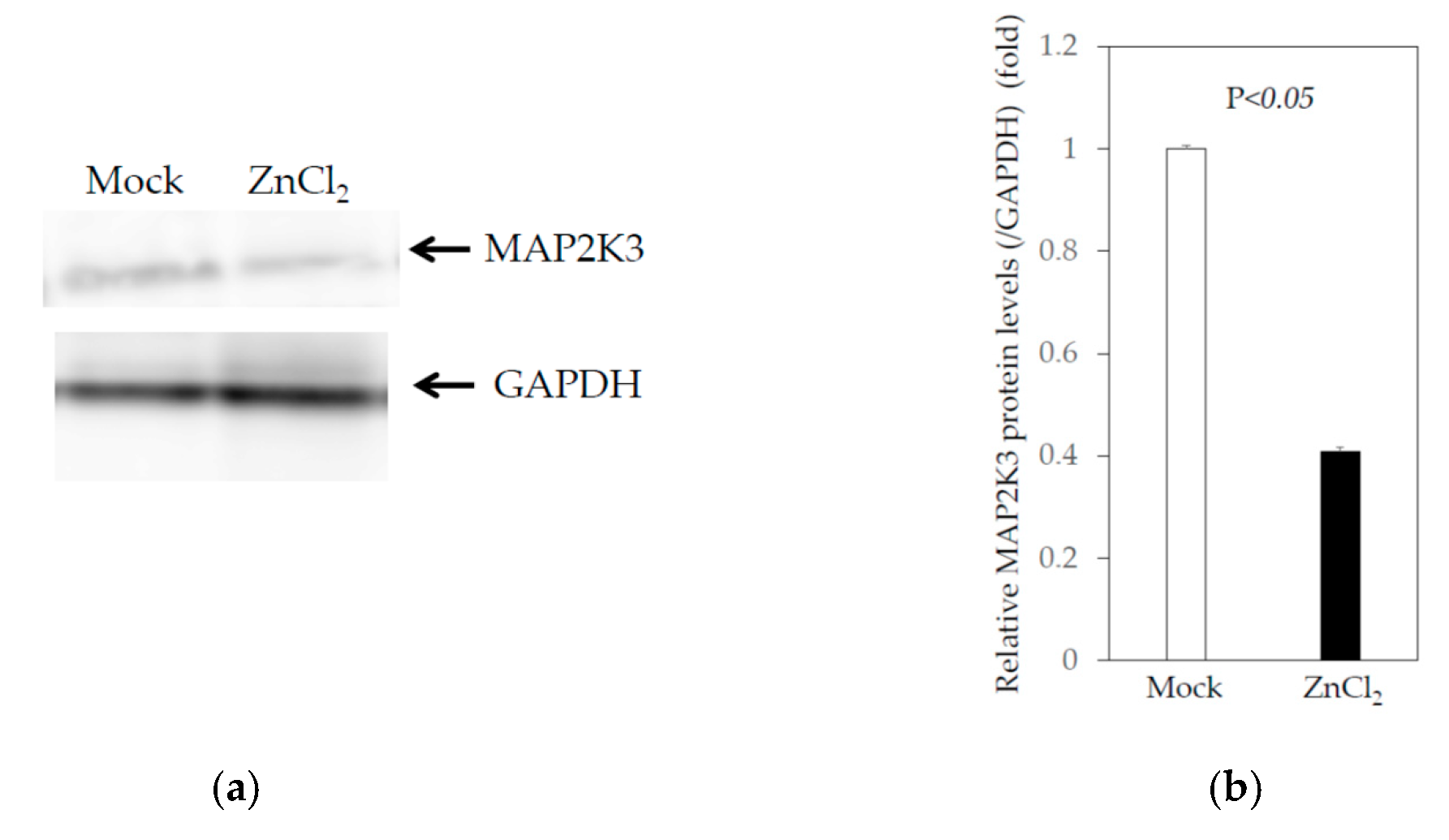
2.1. Effects of Zinc Chloride on the TLR Signaling Pathways in Huh7 Cells
2.2. Effects of Zinc Chloride on TLR Signaling Pathways in PLC/PRF/5 Cells
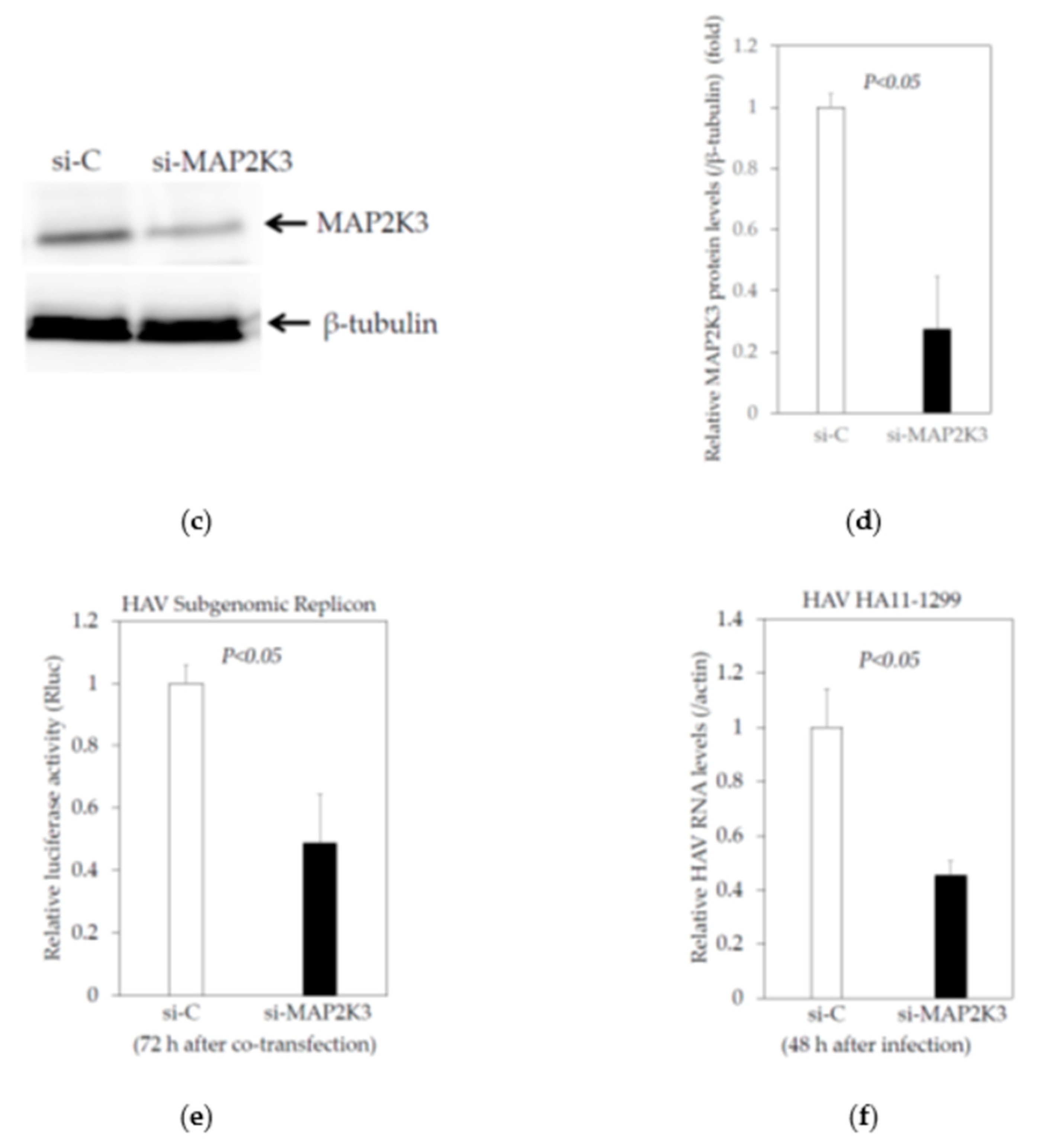
2.3. Knockdown of MAP2K3 Negatively Regulates HAV Replication
2.3.1. Knockdown of MAP2K3 by siRNA Did Not Reduce the Viability of Huh7 Cells
2.3.2. Effects of the Knockdown of MAP2K3 by Specific Small Interfering RNA (siRNA) on the HAV Subgenomic Replicon Replication in HuhT7 Cells
2.3.3. Effects of the Knockdown of MAP2K3 by Specific siRNA on the HAV Genotype IIIA HA11-1299 Replication in Huh7 cells
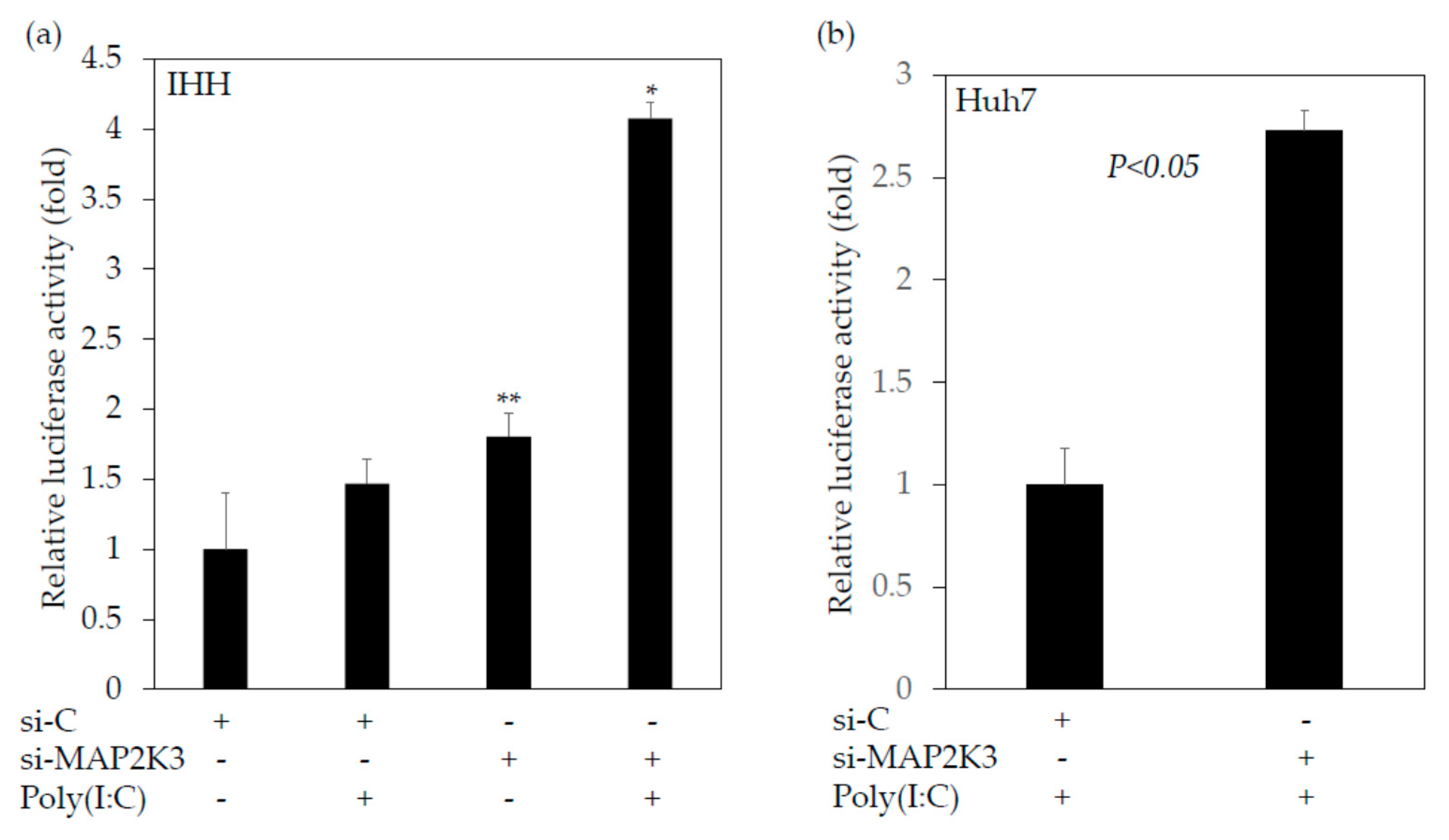
2.4. Inhibition of MAP2K3 Enhances the Interferon-β Promoter Activities Stimulated by Polyinosinic–Polycytidylic Acid (poly(I:C)) and Suppresses HAV Replication
2.4.1. Knockdown of MAP2K3 Enhances the Interferon-β Promoter Activities Stimulated by Poly(I:C)
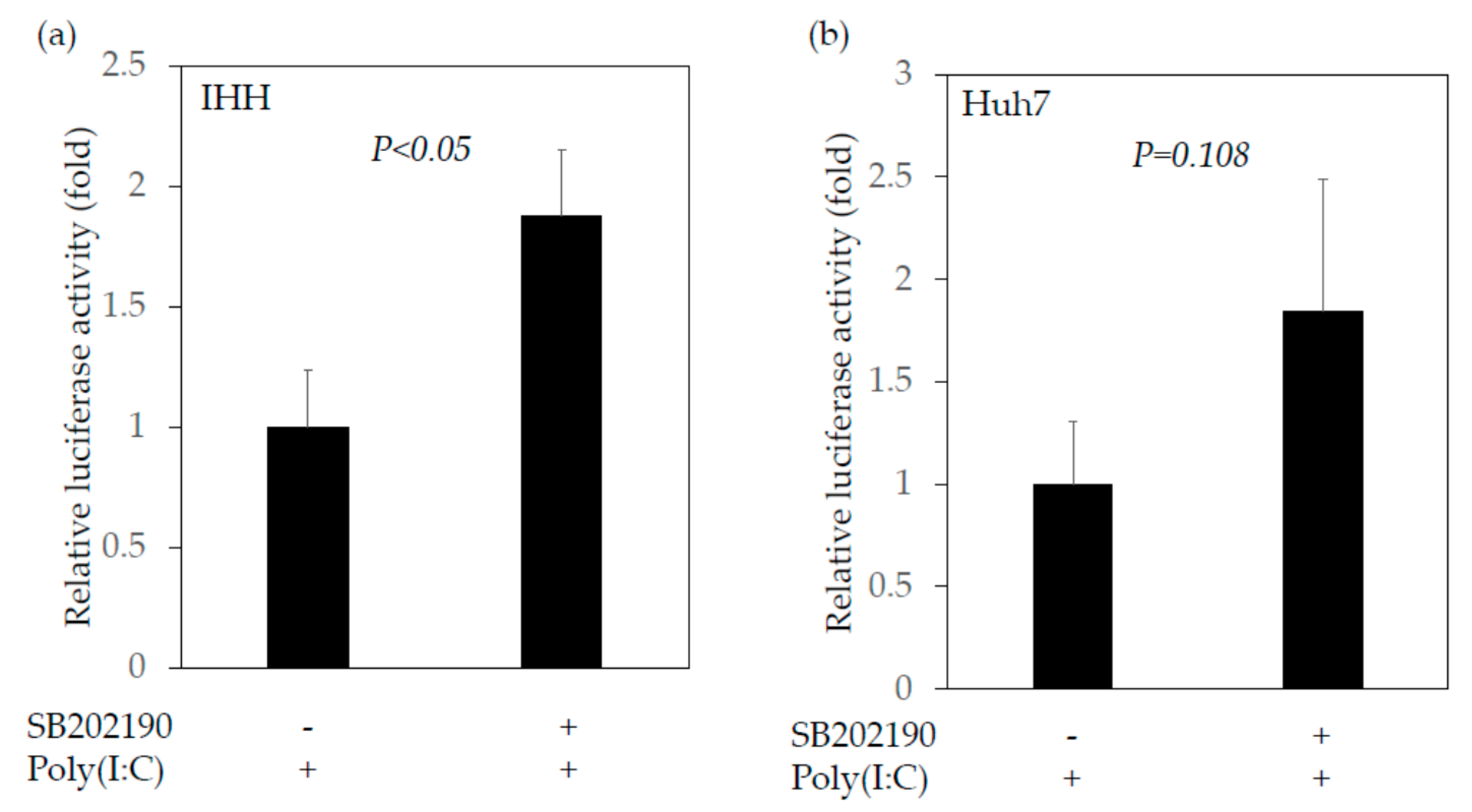
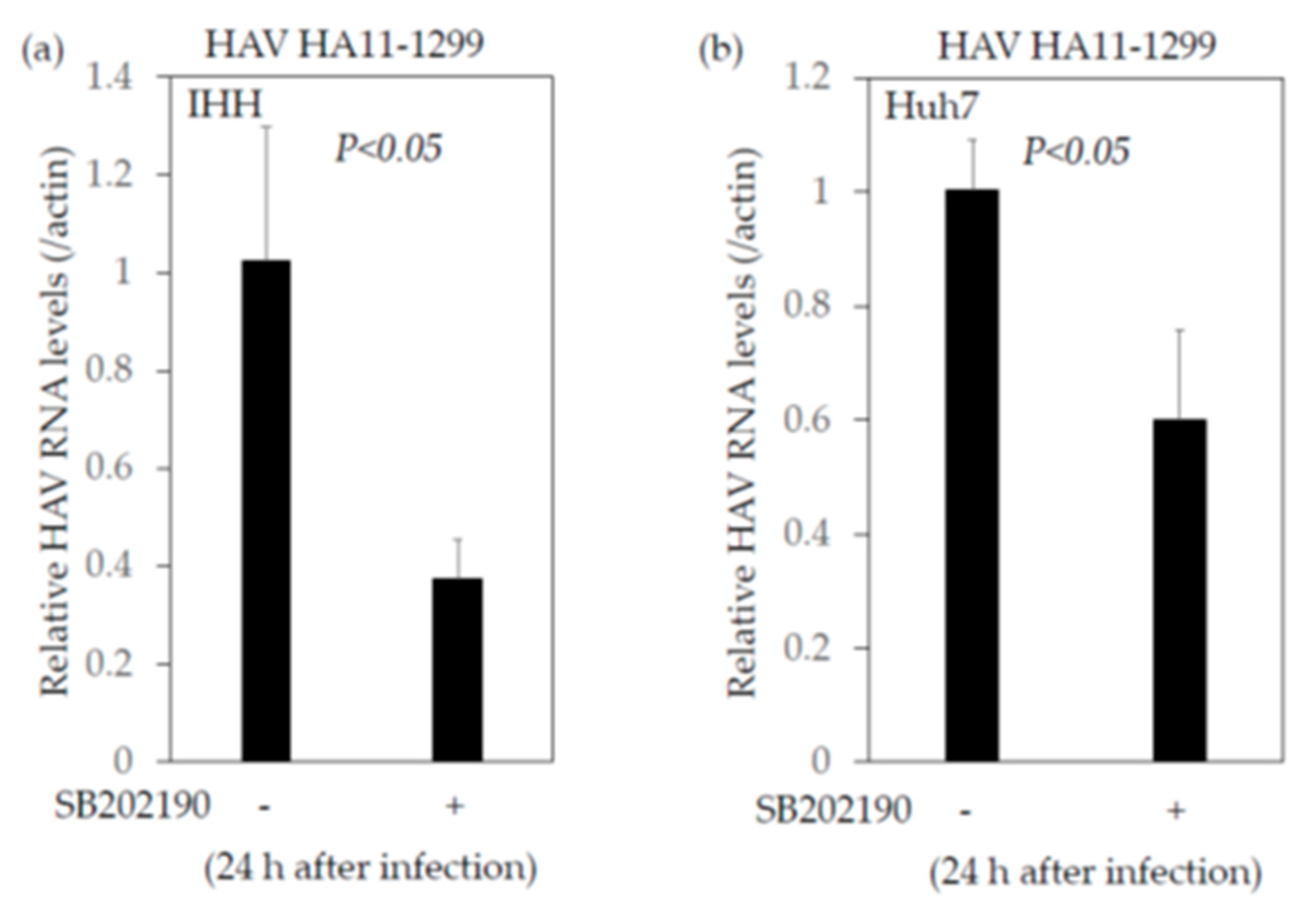
2.4.2. SB202190, a Potent Inhibitor of the MAP2K3–p38 MAPK Signaling Pathway, Enhances the Interferon-β Promoter Activities Stimulated by Poly(I:C)
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. HAV Subgenomic Replicon, its Transfection and Reporter Assay
4.3. HAV Infection
4.4. Cell Viability Assay
4.5. Western Blotting
4.6. RNA Extraction, cDNA Synthesis and Human TLR Signaling Targets PCR Array
4.7. Reporter Assay for the Activation of the Interferon-β Promoter
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, D.Y.; Chae, S.J.; Cho, S.R.; Choi, W.; Kim, C.K.; Han, M.G. Nationwide seroprevalence of hepatitis A in South Korea from 2009 to 2019. PLoS ONE. 2021, 16, e0245162. [Google Scholar] [CrossRef]
- Honda, M.; Asakura, H.; Kanda, T.; Somura, Y.; Ishii, T.; Yamana, Y.; Kaneko, T.; Mizutani, T.; Takahashi, H.; Kumagawa, M.; et al. Male-Dominant Hepatitis A Outbreak Observed among Non-HIV-Infected Persons in the Northern Part of Tokyo, Japan. Viruses. 2021, 13, 207. [Google Scholar] [CrossRef]
- Samala, N.; Abdallah, W.; Poole, A.; Shamseddeen, H.S.; Are, V.S.; Orman, E.; Patidar, K.R.; Vuppalanchi, R. Insight into an acute hepatitis A outbreak in Indiana. J. Viral Hepat. 2021, 28, 964–971. [Google Scholar] [CrossRef]
- Kanda, T.; Sasaki, R.; Masuzaki, R.; Takahashi, H.; Mizutani, T.; Matsumoto, N.; Nirei, K.; Moriyama, M. Co-Occurrence of Hepatitis a Infection and Chronic Liver Disease. Int. J. Mol. Sci. 2020, 21, 6384. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, B.; Andani, A.; Kolhapure, S.; Agrawal, A.; Mazumdar, J. Endemicity change of hepatitis A infection necessitates vaccination in food handlers: An Indian perspective. Hum. Vaccin Immunother. 2021, 17, 1–8. [Google Scholar] [CrossRef]
- Nagashima, S.; Ko, K.; Yamamoto, C.; Bunthen, E.; Ouoba, S.; Chuon, C.; Ohisa, M.; Sugiyama, A.; Akita, T.; Hossain, M.S.; et al. Prevalence of total hepatitis A antibody among 5 to 7 years old children and their mothers in Cambodia. Sci. Rep. 2021, 11, 4778. [Google Scholar] [CrossRef]
- Yan, J.; Kanda, T.; Wu, S.; Imazeki, F.; Yokosuka, O. Hepatitis A, B, C and E virus markers in Chinese residing in Tokyo, Japan. Hepatol. Res. 2012, 42, 974–981. [Google Scholar] [CrossRef]
- Yamamoto, C.; Ko, K.; Nagashima, S.; Harakawa, T.; Fujii, T.; Ohisa, M.; Katayama, K.; Takahashi, K.; Okamoto, H.; Tanaka, J. Very low prevalence of anti-HAV in Japan: High potential for future outbreak. Sci. Rep. 2019, 9, 1493. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Kanda, T.; Suganami, A.; Nakamoto, S.; Win, N.N.; Tamura, Y.; Nakamura, M.; Matsuoka, S.; Yokosuka, O.; Kato, N.; et al. Antiviral activity of zinc sulfate against hepatitis A virus replication. Future Virol. 2019, 14, 399–406. [Google Scholar] [CrossRef]
- Kanda, T.; Sasaki, R.; Masuzaki, R.; Takahashi, H.; Fujisawa, M.; Matsumoto, N.; Okamoto, H.; Moriyama, M. Additive Effects of Zinc Chloride on the Suppression of Hepatitis A Virus Replication by Interferon in Human Hepatoma Huh7 Cells. In Vivo 2020, 34, 3301–3308. [Google Scholar] [CrossRef] [PubMed]
- Attia, G.H.; Moemen, Y.S.; Youns, M.; Ibrahim, A.M.; Abdou, R.; El Raey, M.A. Antiviral zinc oxide nanoparticles mediated by hesperidin and in silico comparison study between antiviral phenolics as anti-SARS-CoV-2. Colloids Surf. B. Biointerfaces. 2021, 203, 111724. [Google Scholar] [CrossRef]
- Nwe Win, N.; Kanda, T.; Nakamura, M.; Nakamoto, S.; Okamoto, H.; Yokosuka, O.; Shirasawa, H. Free fatty acids or high-concentration glucose enhances hepatitis A virus replication in association with a reduction in glucose-regulated protein 78 expression. Biochem. Biophys. Res. Commun. 2017, 483, 694–699. [Google Scholar] [CrossRef]
- Jiang, X.; Kanda, T.; Haga, Y.; Sasaki, R.; Nakamura, M.; Wu, S.; Nakamoto, S.; Shirasawa, H.; Okamoto, H.; Yokosuka, O. Glucose-regulated protein 78 is an antiviral against hepatitis A virus replication. Exp. Ther. Med. 2017, 13, 3305–3308. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Kanda, T.; Imazeki, F.; Wu, S.; Nakamoto, S.; Tanaka, T.; Arai, M.; Fujiwara, K.; Saito, K.; Roger, T.; et al. Hepatitis C Virus nonstructural 5A protein inhibits lipopolysaccharide-mediated apoptosis of hepatocytes by decreasing expression of Toll-like receptor 4. J. Infect. Dis. 2011, 204, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Brieger, A.; Rink, L.; Haase, H. Differential regulation of TLR-dependent MyD88 and TRIF signaling pathways by free zinc ions. J. Immunol. 2013, 191, 1808–1817. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, H.; Cho, J.H. Molecular cloning and functional characterization of TRAF6 and TAK1 in rainbow trout, Oncorhynchus mykiss. Fish. Shellfish Immunol. 2019, 84, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Lamothe, B.; Campos, A.D.; Webster, W.K.; Gopinathan, A.; Hur, L.; Darnay, B.G. The RING domain and first zinc finger of TRAF6 coordinate signaling by interleukin-1, lipopolysaccharide, and RANKL. J. Biol. Chem. 2008, 283, 24871–24880. [Google Scholar] [CrossRef]
- Menikdiwela, K.R.; Ramalingam, L.; Abbas, M.M.; Bensmail, H.; Scoggin, S.; Kalupahana, N.S.; Palat, A.; Gunaratne, P.; Moustaid-Moussa, N. Role of microRNA 690 in Mediating Angiotensin II Effects on Inflammation and Endoplasmic Reticulum Stress. Cells 2020, 9, 1327. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, M.; Gotoh, Y.; Katagiri, H.; Sakoda, H.; Ogihara, T.; Anai, M.; Onishi, Y.; Ono, H.; Funaki, M.; Inukai, K.; et al. MKK6/3 and p38 MAPK pathway activation is not necessary for insulin-induced glucose uptake but regulates glucose transporter expression. J. Biol. Chem. 2001, 276, 19800–19806. [Google Scholar] [CrossRef]
- Kanda, T.; Steele, R.; Ray, R.; Ray, R.B. Hepatitis C virus infection induces the beta interferon signaling pathway in immortalized human hepatocytes. J. Virol. 2007, 81, 12375–12381. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, W.; Chen, Y.; Lin, Y.; Yang, X.; Wang, H.; Liu, Z. The miR-19b-3p-MAP2K3-STAT3 feedback loop regulates cell proliferation and invasion in esophageal squamous cell carcinoma. Mol. Oncol. 2021, 15, 1566–1583. [Google Scholar] [CrossRef] [PubMed]
- Gauss-Müller, V.; Kusov, Y.Y. Replication of a hepatitis A virus replicon detected by genetic recombination in vivo. J. Gen. Virol. 2002, 83 Pt. 9, 2183–2192. [Google Scholar] [CrossRef]
- Arentz, S.; Hunter, J.; Yang, G.; Goldenberg, J.; Beardsley, J.; Myers, S.P.; Mertz, D.; Leeder, S. Zinc for the prevention and treatment of SARS-CoV-2 and other acute viral respiratory infections: A rapid review. Adv. Integr. Med. 2020, 7, 252–260. [Google Scholar] [CrossRef]
- Lee, H.; Komano, J.; Saitoh, Y.; Yamaoka, S.; Kozaki, T.; Misawa, T.; Takahama, M.; Satoh, T.; Takeuchi, O.; Yamamoto, N.; et al. Zinc-finger antiviral protein mediates retinoic acid inducible gene I-like receptor-independent antiviral response to murine leukemia virus. Proc. Natl. Acad. Sci. USA 2013, 110, 12379–12384. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liang, Y.; Qu, L.; Chen, Z.; Yi, M.; Li, K.; Lemon, S.M. Disruption of innate immunity due to mitochondrial targeting of a picornaviral protease precursor. Proc. Natl. Acad. Sci. USA. 2007, 104, 7253–7258. [Google Scholar] [CrossRef]
- Paulmann, D.; Magulski, T.; Schwarz, R.; Heitmann, L.; Flehmig, B.; Vallbracht, A.; Dotzauer, A. Hepatitis A virus protein 2B suppresses beta interferon (IFN) gene transcription by interfering with IFN regulatory factor 3 activation. J. Gen. Virol. 2008, 89(Pt. 7), 1593–1604. [Google Scholar] [CrossRef]
- Qu, L.; Feng, Z.; Yamane, D.; Liang, Y.; Lanford, R.E.; Li, K.; Lemon, S.M. Disruption of TLR3 signaling due to cleavage of TRIF by the hepatitis A virus protease-polymerase processing intermediate, 3CD. PLoS Pathog. 2011, 7, e1002169. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Yokosuka, O.; Kato, N.; Imazeki, F.; Fujiwara, K.; Kawai, S.; Saisho, H.; Omata, M. Hepatitis A virus VP3 may activate serum response element associated transcription. Scand. J. Gastroenterol. 2003, 38, 307–313. [Google Scholar] [CrossRef]
- Paulmann, D.; Bortmann, S.; Grimm, F.; Berk, I.; Kraemer, L.; Vallbracht, A.; Dotzauer, A. NF-kappaB activation induced by hepatitis A virus and Newcastle disease virus occurs by different pathways depending on the structural pattern of viral nucleic acids. Arch. Virol. 2014, 159, 1723–1733. [Google Scholar] [CrossRef]
- Mo, L.; Zeng, Z.; Deng, R.; Li, Z.; Sun, J.; Hu, N.; Shi, J.; Hu, Y. Hepatitis A virus-induced hsa-miR-146a-5p attenuates IFN-beta signaling by targeting adaptor protein TRAF6. Arch. Virol. 2021, 166, 789–799. [Google Scholar] [CrossRef]
- Lebeau, P.F.; Wassef, H.; Byun, J.H.; Platko, K.; Ason, B.; Jackson, S.; Dobroff, J.; Shetterly, S.; Richards, W.G.; Al-Hashimi, A.A.; et al. The loss-of-function PCSK9Q152H variant increases ER chaperones GRP78 and GRP94 and protects against liver injury. J. Clin. Investig. 2021, 131, e128650. [Google Scholar] [CrossRef]
- Jiang, X.; Kanda, T.; Tanaka, T.; Wu, S.; Nakamoto, S.; Imazeki, F.; Yokosuka, O. Lipopolysaccharide blocks induction of unfolded protein response in human hepatoma cell lines. Immunol. Lett. 2013, 152, 8–15. [Google Scholar] [CrossRef]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef]
- Johnson, R.A.; Huong, S.M.; Huang, E.S. Activation of the mitogen-activated protein kinase p38 by human cytomegalovirus infection through two distinct pathways: A novel mechanism for activation of p38. J. Virol. 2000, 74, 1158–1167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, F.; Xiao, Z.; Yao, H.; Li, S.; Feng, M.; Wang, W.; Liu, Z.; Liu, Z.; Wu, J. The protective role of microRNA-21 against coxsackievirus B3 infection through targeting the MAP2K3/P38 MAPK signaling pathway. J. Transl. Med. 2019, 17, 335. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.; Wang, H.; Shi, J.; Wu, K.; Liu, S.; Liu, Y.; Wu, J. HCV-induced miR-21 contributes to evasion of host immune system by targeting MyD88 and IRAK1. PLoS Pathog. 2013, 9, e1003248. [Google Scholar] [CrossRef]
- Kodama, H.; Tanaka, M.; Naito, Y.; Katayama, K.; Moriyama, M. Japan’s Practical Guidelines for Zinc Deficiency with a Particular Focus on Taste Disorders, Inflammatory Bowel Disease, and Liver Cirrhosis. Int J. Mol. Sci. 2020, 21, 2941. [Google Scholar] [CrossRef]
- Cooksley, W.G. What did we learn from the Shanghai hepatitis A epidemic? J. Viral Hepat. 2000, 7 (Suppl. 1), 1–3. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.C.; Martinez, O.; Honko, A.N.; Hensley, L.E.; Olinger, G.G.; Basler, C.F. Pyridinyl imidazole inhibitors of p38 MAP kinase impair viral entry and reduce cytokine induction by Zaire ebolavirus in human dendritic cells. Antivir. Res. 2014, 107, 102–109. [Google Scholar] [CrossRef]
- Wei, L.; Zhu, Z.; Wang, J.; Liu, J. JNK and p38 mitogen-activated protein kinase pathways contribute to porcine circovirus type 2 infection. J. Virol. 2009, 83, 6039–6047. [Google Scholar] [CrossRef][Green Version]
- Kanda, T.; Sasaki, R.; Masuzaki, R.; Matsumoto, N.; Ogawa, M.; Moriyama, M. Cell Culture Systems and Drug Targets for Hepatitis A Virus Infection. Viruses 2020, 12, 533. [Google Scholar] [CrossRef] [PubMed]
- Stramucci, L.; Pranteda, A.; Stravato, A.; Amoreo, C.A.; Pennetti, A.; Diodoro, M.G.; Bartolazzi, A.; Milella, M.; Bossi, G. MKK3 sustains cell proliferation and survival through p38DELTA MAPK activation in colorectal cancer. Cell Death Dis. 2019, 10, 842. [Google Scholar] [CrossRef] [PubMed]
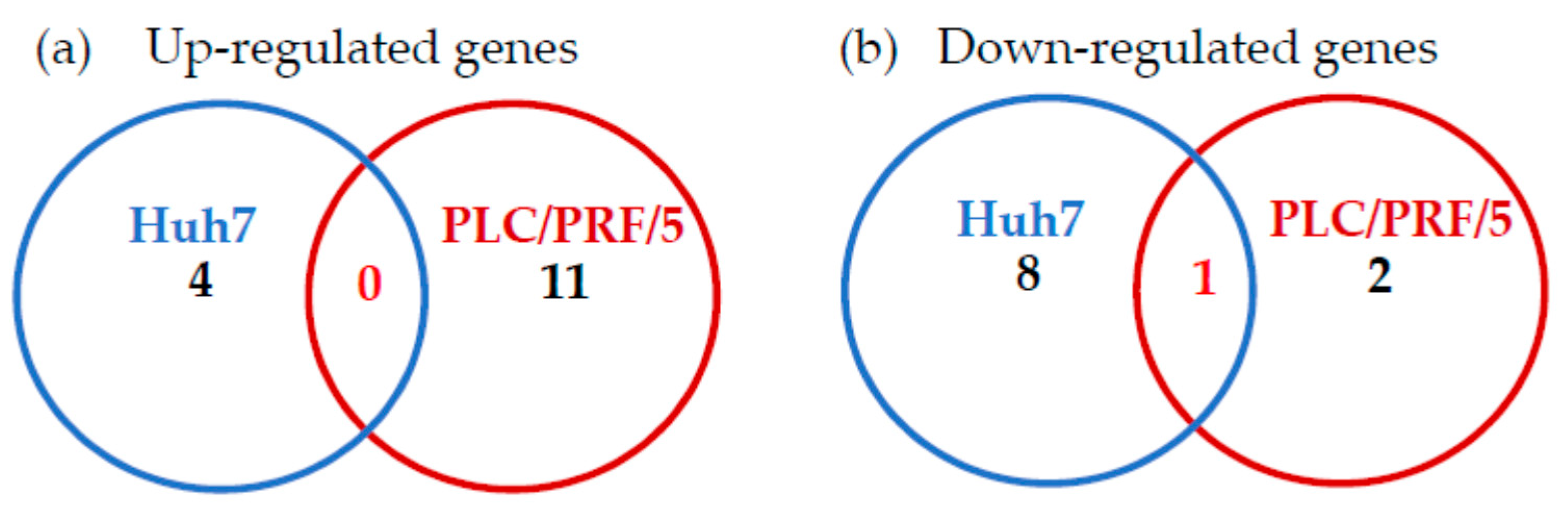
| Gene Symbol | Roles of Genes | Fold Change 1 | p-Values |
|---|---|---|---|
| (a) Upregulated Genes | |||
| NFKB2 | Signaling downstream of TLRs (NFκB signaling) | 1.07 | 0.009920 |
| MAPK8IP3 | Signaling downstream of TLRs (JNK/p38 signaling); TLR interacting proteins and adaptors | 1.11 | 0.007072 |
| IL8 | Pathogen-specific responses (bacterial response; fungal/parasitic response) | 1.09 | 0.008839 |
| FOS | Pathogen-specific responses (bacterial response); signaling downstream of TLRs (JNK/p38 signaling) | 1.45 | 0.001211 |
| (b) Downregulated genes | |||
| IL6 | Pathogen-specific responses (bacterial response; viral response); signaling downstream of TLRs (JAK/STAT signaling; cytokine signaling) | 0.53 | 0.000928 |
| TLR5 | TLRs; TLR signaling (MYD88-dependent signaling) | 0.57 | 0.044855 |
| IRF3 | Pathogen-specific responses (viral response); TLR signaling [TICAM1 (TRIF)-dependent (MYD88-independent) signaling] | 0.77 | 0.001549 |
| RIPK2 | Pathogen-specific responses (bacterial response); TLR-interacting proteins and adaptors | 0.80 | 0.015430 |
| TICAM1 | Pathogen-specific responses (bacterial response; viral response); TLR signaling [TICAM1 (TRIF)-dependent (MYD88-independent)]; TLR-interacting proteins and adaptors | 0.82 | 0.003990 |
| PPARA | Signaling downstream of TLRs (NFκB signaling); downstream effectors of TLR signaling | 0.84 | 0.019047 |
| TICAM2 | TLR signaling [TICAM1 (TRIF)-dependent (MYD88-independent)]; TLR-interacting proteins and adaptors | 0.89 | 0.001470 |
| HRAS | Pathogen-specific responses (bacterial response; fungal/parasitic response); TLR-interacting proteins and adaptors | 0.89 | 0.021661 |
| MAP2K3 | Signaling downstream of TLRs (JNK/p38 signaling) | 0.90 | 0.034805 |
| Gene Symbol | Roles of Genes | Fold Change 1 | p-Values |
|---|---|---|---|
| (a) Upregulated Genes | |||
| EIF2AK2 | Pathogen-specific responses (viral response); downstream effectors of TLR Signaling | 1.07 | 0.030044 |
| PPARA | Signaling downstream of TLRs (NFκB signaling); downstream effectors of TLR signaling | 1.08 | 0.028574 |
| HMGB1 | Pathogen-specific responses (bacterial response); TLR-interacting proteins and adaptors | 1.12 | 0.028282 |
| SARM1 | TLR signaling (negative regulation of TLR signaling); TLR-interacting proteins and adaptors | 1.12 | 0.007063 |
| MAP2K4 | Signaling downstream of TLRs (JNK/p38 signaling) | 1.13 | 0.019787 |
| REL | Signaling downstream of TLRs (NFκB signaling) | 1.15 | 0.019521 |
| HRAS | Pathogen-specific responses (bacterial response; fungal/parasitic response); TLR-interacting proteins and adaptors | 1.15 | 0.007300 |
| MAPK8 | Signaling downstream of TLRs (JNK/p38 signaling) | 1.19 | 0.000817 |
| IRAK1 | Pathogen-specific responses (bacterial response); TLR signaling (MYD88-dependent signaling); signaling downstream of TLRs (NFκB signaling; cytokine signaling); downstream effectors of TLR signaling | 1.29 | 0.004801 |
| TIRAP | Pathogen-specific responses (fungal/parasitic response); TLR signaling (MYD88-dependent signaling); TLR-interacting proteins and adaptors | 1.32 | 0.035440 |
| IL1A | Signaling downstream of TLRs (cytokine signaling) | 1.45 | 0.024464 |
| (b) Downregulated genes | |||
| SIGIRR | TLRs; TLR signaling (negative regulation of TLR signaling); signaling downstream of TLRs (cytokine signaling) | 0.62 | 0.034271 |
| MAP2K3 | Signaling downstream of TLRs (JNK/p38 signaling) | 0.86 | 0.000931 |
| MYD88 | TLR signaling (MYD88-dependent); TLR-interacting proteins and adaptors | 0.93 | 0.028868 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanda, T.; Sasaki-Tanaka, R.; Masuzaki, R.; Matsumoto, N.; Okamoto, H.; Moriyama, M. Knockdown of Mitogen-Activated Protein Kinase Kinase 3 Negatively Regulates Hepatitis A Virus Replication. Int. J. Mol. Sci. 2021, 22, 7420. https://doi.org/10.3390/ijms22147420
Kanda T, Sasaki-Tanaka R, Masuzaki R, Matsumoto N, Okamoto H, Moriyama M. Knockdown of Mitogen-Activated Protein Kinase Kinase 3 Negatively Regulates Hepatitis A Virus Replication. International Journal of Molecular Sciences. 2021; 22(14):7420. https://doi.org/10.3390/ijms22147420
Chicago/Turabian StyleKanda, Tatsuo, Reina Sasaki-Tanaka, Ryota Masuzaki, Naoki Matsumoto, Hiroaki Okamoto, and Mitsuhiko Moriyama. 2021. "Knockdown of Mitogen-Activated Protein Kinase Kinase 3 Negatively Regulates Hepatitis A Virus Replication" International Journal of Molecular Sciences 22, no. 14: 7420. https://doi.org/10.3390/ijms22147420
APA StyleKanda, T., Sasaki-Tanaka, R., Masuzaki, R., Matsumoto, N., Okamoto, H., & Moriyama, M. (2021). Knockdown of Mitogen-Activated Protein Kinase Kinase 3 Negatively Regulates Hepatitis A Virus Replication. International Journal of Molecular Sciences, 22(14), 7420. https://doi.org/10.3390/ijms22147420







