Megalin, Proton Pump Inhibitors and the Renin–Angiotensin System in Healthy and Pre-Eclamptic Placentas
Abstract
1. Introduction
2. Results
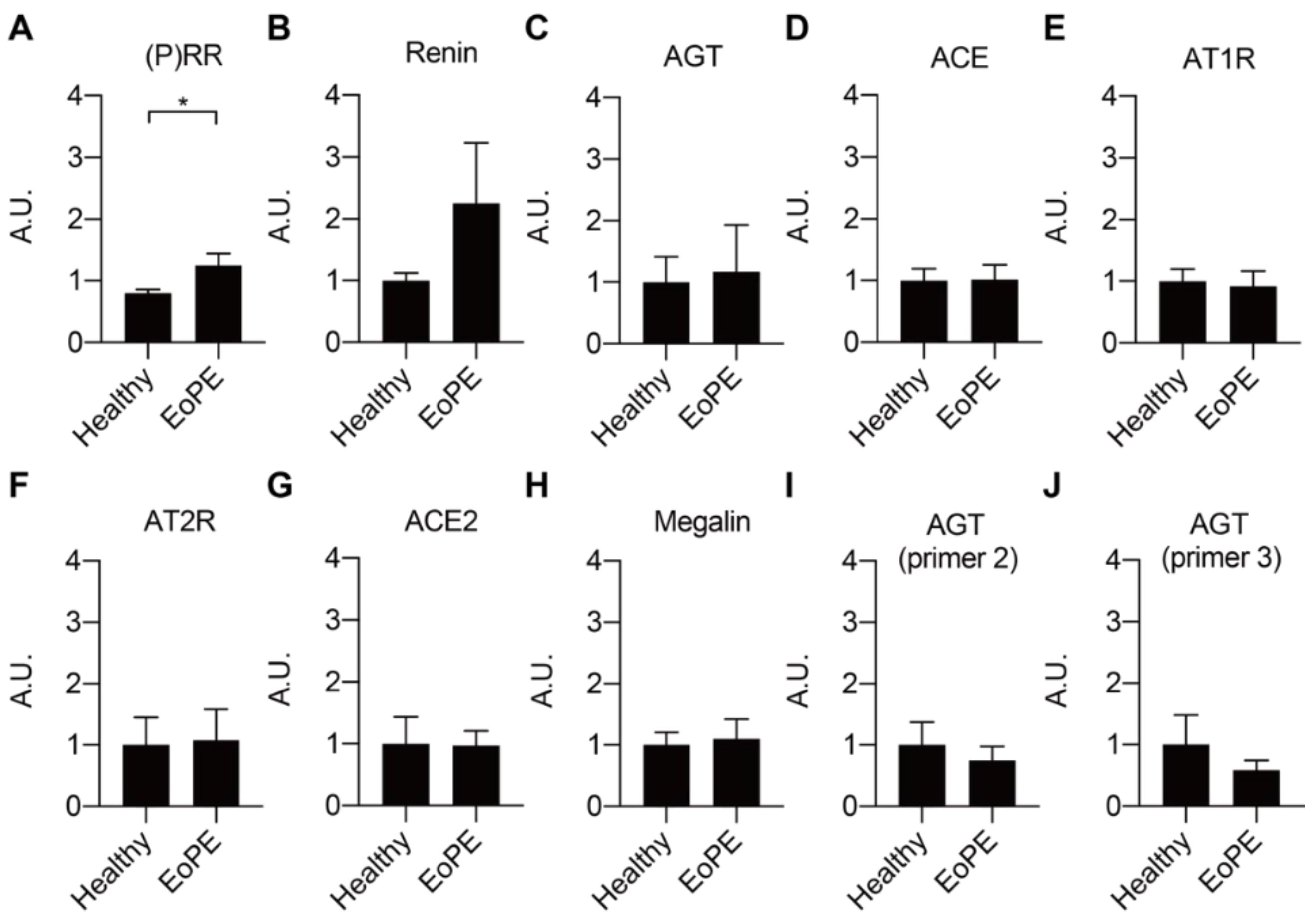
2.1. Expression of RAS Components in Healthy and Pre-Eclamptic Placentas
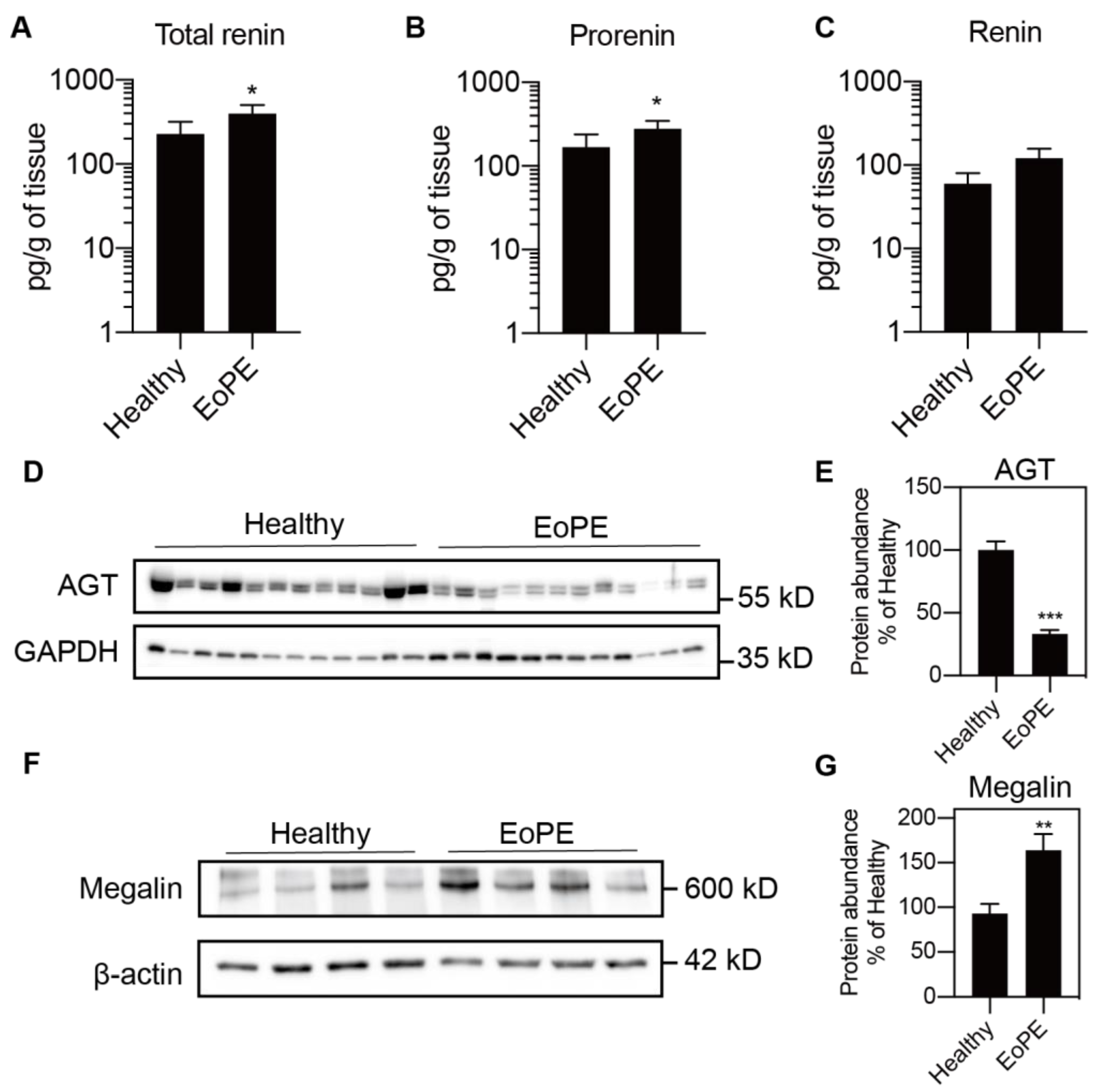
2.2. Placental Release of (Pro)renin and AGT
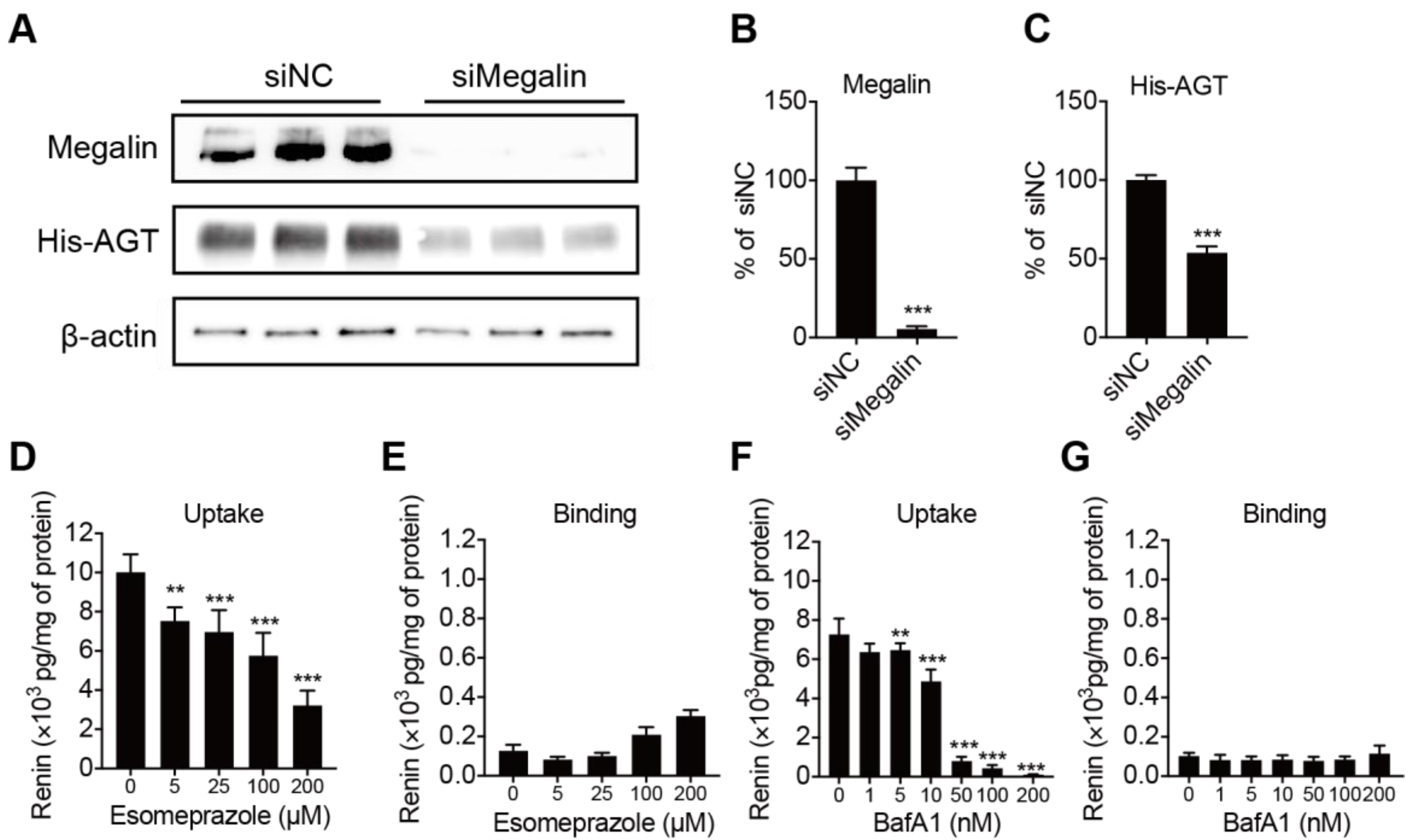
2.3. Megalin Internalizes AGT and PPI Decreases Renin Internalization but Not Binding in BN16 Cells
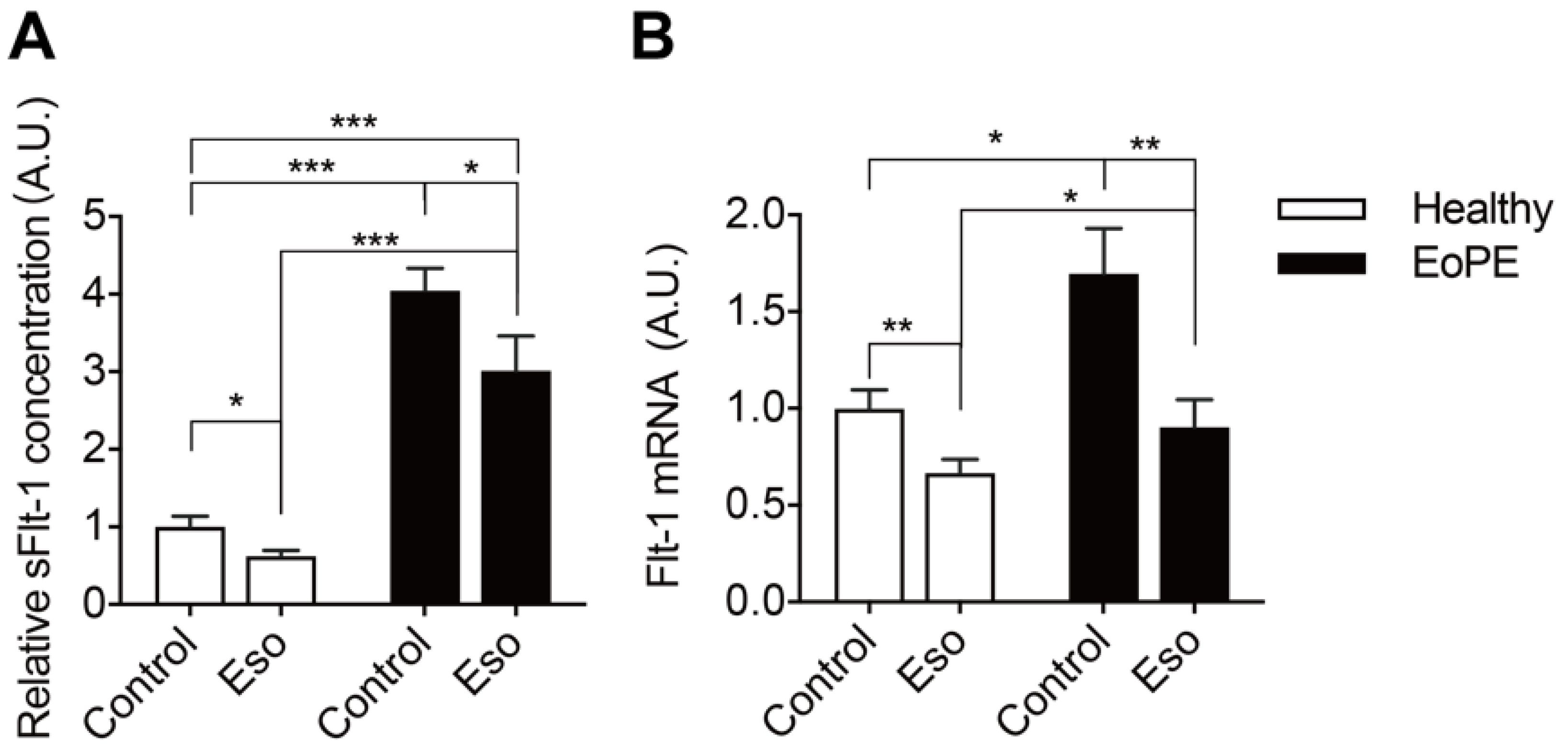
2.4. PPI Reduces sFlt-1 Secretion in Human Placental Villous Explants
3. Discussion
4. Materials and Methods
4.1. Tissue Collection
4.2. Placenta Perfusion Experiments
4.3. Placental Villous Explants
4.4. Placental Lysates
4.5. Studies in Brown Norway Rat Yolk Sac Epithelial Cells
4.6. Measurement of (Pro)renin and Angiotensinogen
4.7. Immunoblotting
4.8. RNA Isolation and qPCR Analysis
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mol, B.W.J.; Roberts, C.T.; Thangaratinam, S.; Magee, L.A.; de Groot, C.J.M.; Hofmeyr, G.J. Pre-eclampsia. Lancet 2016, 387, 999–1011. [Google Scholar] [CrossRef]
- Miklus, R.M.; Elliott, C.; Snow, N. Surgical cricothyrotomy in the field: Experience of a helicopter transport team. J. Trauma 1989, 29, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef]
- Verdonk, K.; Visser, W.; Van Den Meiracker, A.H.; Danser, A.H. The renin-angiotensin-aldosterone system in pre-eclampsia: The delicate balance between good and bad. Clin. Sci. 2014, 126, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Powe, C.E.; Salahuddin, S.; Verlohren, S.; Perschel, F.H.; Levine, R.J.; Lim, K.H.; Wenger, J.B.; Thadhani, R.; Karumanchi, S.A. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012, 125, 911–919. [Google Scholar] [CrossRef]
- Derkx, F.H.; Alberda, A.T.; de Jong, F.H.; Zeilmaker, F.H.; Makovitz, J.W.; Schalekamp, M.A. Source of plasma prorenin in early and late pregnancy: Observations in a patient with primary ovarian failure. J. Clin. Endocrinol. Metab. 1987, 65, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, W.A.; Luetscher, J.A.; Carlson, E.J.; Grislis, G.; Fraze, E.; McHargue, A. Changes in active and inactive renin throughout pregnancy. J. Clin. Endocrinol. Metab. 1982, 54, 1010–1016. [Google Scholar] [CrossRef]
- Irani, R.A.; Xia, Y. Renin angiotensin signaling in normal pregnancy and preeclampsia. Semin. Nephrol. 2011, 31, 47–58. [Google Scholar] [CrossRef]
- Sealey, J.E.; McCord, D.; Taufield, P.A.; Ales, K.A.; Druzin, M.L.; Atlas, S.A.; Laragh, J.H. Plasma prorenin in first-trimester pregnancy: Relationship to changes in human chorionic gonadotropin. Am. J. Obstet. Gynecol. 1985, 153, 514–519. [Google Scholar] [CrossRef]
- Krop, M.; Danser, A.H. Circulating versus tissue renin-angiotensin system: On the origin of (pro)renin. Curr. Hypertens. Rep. 2008, 10, 112–118. [Google Scholar] [CrossRef]
- Irani, R.A.; Xia, Y. The functional role of the renin-angiotensin system in pregnancy and preeclampsia. Placenta 2008, 29, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, K.; Saleh, L.; Lankhorst, S.; Smilde, J.E.; van Ingen, M.M.; Garrelds, I.M.; Friesema, E.C.; Russcher, H.; van den Meiracker, A.H.; Visser, W.; et al. Association studies suggest a key role for endothelin-1 in the pathogenesis of preeclampsia and the accompanying renin-angiotensin-aldosterone system suppression. Hypertension 2015, 65, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Brar, H.S.; Kjos, S.L.; Dougherty, W.; Do, Y.S.; Tam, H.B.; Hsueh, W.A. Increased fetoplacental active renin production in pregnancy-induced hypertension. Am. J. Obstet. Gynecol. 1987, 157, 363–367. [Google Scholar] [CrossRef]
- Kalenga, M.K.; Thomas, K.; de Gasparo, M.; De Hertogh, R. Determination of renin, angiotensin converting enzyme and angiotensin II levels in human placenta, chorion and amnion from women with pregnancy induced hypertension. Clin. Endocrinol. 1996, 44, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Herse, F.; Dechend, R.; Harsem, N.K.; Wallukat, G.; Janke, J.; Qadri, F.; Hering, L.; Muller, D.N.; Luft, F.C.; Staff, A.C. Dysregulation of the circulating and tissue-based renin-angiotensin system in preeclampsia. Hypertension 2007, 49, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Pringle, K.G.; Wang, Y.; Lumbers, E.R. The synthesis, secretion and uptake of prorenin in human amnion. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef]
- Koizumi, M.; Ueda, K.; Niimura, F.; Nishiyama, A.; Yanagita, M.; Saito, A.; Pastan, I.; Fujita, T.; Fukagawa, M.; Matsusaka, T. Podocyte Injury Augments Intrarenal Angiotensin II Generation and Sodium Retention in a Megalin-Dependent Manner. Hypertension 2019, 74, 509–517. [Google Scholar] [CrossRef]
- Storm, T.; Christensen, E.I.; Christensen, J.N.; Kjaergaard, T.; Uldbjerg, N.; Larsen, A.; Honore, B.; Madsen, M. Megalin Is Predominantly Observed in Vesicular Structures in First and Third Trimester Cytotrophoblasts of the Human Placenta. J. Histochem. Cytochem. 2016, 64, 769–784. [Google Scholar] [CrossRef]
- Gleixner, E.M.; Canaud, G.; Hermle, T.; Guida, M.C.; Kretz, O.; Helmstadter, M.; Huber, T.B.; Eimer, S.; Terzi, F.; Simons, M. V-ATPase/mTOR signaling regulates megalin-mediated apical endocytosis. Cell Rep. 2014, 8, 10–19. [Google Scholar] [CrossRef]
- Hastie, R.; Bergman, L.; Cluver, C.A.; Wikman, A.; Hannan, N.J.; Walker, S.P.; Wikstrom, A.K.; Tong, S.; Hesselman, S. Proton Pump Inhibitors and Preeclampsia Risk Among 157,720 Women. Hypertension 2019, 73, 1097–1103. [Google Scholar] [CrossRef]
- Saleh, L.; Samantar, R.; Garrelds, I.M.; van den Meiracker, A.H.; Visser, W.; Danser, A.H.J. Low Soluble Fms-Like Tyrosine Kinase-1, Endoglin, and Endothelin-1 Levels in Women With Confirmed or Suspected Preeclampsia Using Proton Pump Inhibitors. Hypertension 2017, 70, 594–600. [Google Scholar] [CrossRef]
- Zhou, C.C.; Ahmad, S.; Mi, T.; Xia, L.; Abbasi, S.; Hewett, P.W.; Sun, C.; Ahmed, A.; Kellems, R.E.; Xia, Y. Angiotensin II induces soluble fms-Like tyrosine kinase-1 release via calcineurin signaling pathway in pregnancy. Circ. Res. 2007, 100, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Spugnini, E.P.; Citro, G.; Fais, S. Proton pump inhibitors as anti vacuolar-ATPases drugs: A novel anticancer strategy. J. Exp. Clin. Cancer Res. 2010, 29, 44. [Google Scholar] [CrossRef] [PubMed]
- Sabolic, I.; Brown, D.; Verbavatz, J.M.; Kleinman, J. H(+)-ATPases of renal cortical and medullary endosomes are differentially sensitive to Sch-28080 and omeprazole. Am. J. Physiol. 1994, 266, F868–F877. [Google Scholar] [CrossRef] [PubMed]
- Onda, K.; Tong, S.; Beard, S.; Binder, N.; Muto, M.; Senadheera, S.N.; Parry, L.; Dilworth, M.; Renshall, L.; Brownfoot, F.; et al. Proton Pump Inhibitors Decrease Soluble fms-Like Tyrosine Kinase-1 and Soluble Endoglin Secretion, Decrease Hypertension, and Rescue Endothelial Dysfunction. Hypertension 2017, 69, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Sun, Y.; Goes Martini, A.; Janssen, M.J.; Garrelds, I.M.; Masereeuw, R.; Lu, X.; Danser, A.H.J. Megalin: A Novel Endocytic Receptor for Prorenin and Renin. Hypertension 2020, 75, 1242–1250. [Google Scholar] [CrossRef]
- Poisner, A.M. Regulation of utero-placental prorenin. Adv. Exp. Med. Biol. 1995, 377, 411–426. [Google Scholar] [CrossRef]
- Shah, D.M.; Banu, J.M.; Chirgwin, J.M.; Tekmal, R.R. Reproductive tissue renin gene expression in preeclampsia. Hypertens. Pregnancy 2000, 19, 341–351. [Google Scholar] [CrossRef]
- Anton, L.; Brosnihan, K.B. Systemic and uteroplacental renin--angiotensin system in normal and pre-eclamptic pregnancies. Ther. Adv. Cardiovasc. Dis. 2008, 2, 349–362. [Google Scholar] [CrossRef]
- Anton, L.; Merrill, D.C.; Neves, L.A.; Stovall, K.; Gallagher, P.E.; Diz, D.I.; Moorefield, C.; Gruver, C.; Ferrario, C.M.; Brosnihan, K.B. Activation of local chorionic villi angiotensin II levels but not angiotensin (1-7) in preeclampsia. Hypertension 2008, 51, 1066–1072. [Google Scholar] [CrossRef]
- Anton, L.; Merrill, D.C.; Neves, L.A.; Diz, D.I.; Corthorn, J.; Valdes, G.; Stovall, K.; Gallagher, P.E.; Moorefield, C.; Gruver, C.; et al. The uterine placental bed Renin-Angiotensin system in normal and preeclamptic pregnancy. Endocrinology 2009, 150, 4316–4325. [Google Scholar] [CrossRef]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ansari, R.; Yu, Z.; Shah, D. Definitive molecular evidence of renin-angiotensin system in human uterine decidual cells. Hypertension 2000, 36, 159–164. [Google Scholar] [CrossRef]
- Nonn, O.; Fischer, C.; Geisberger, S.; El-Heliebi, A.; Kroneis, T.; Forstner, D.; Desoye, G.; Staff, A.C.; Sugulle, M.; Dechend, R.; et al. Maternal Angiotensin Increases Placental Leptin in Early Gestation via an Alternative Renin-Angiotensin System Pathway: Suggesting a Link to Preeclampsia. Hypertension 2021, 77, 1723–1736. [Google Scholar] [CrossRef]
- Takimoto, E.; Ishida, J.; Sugiyama, F.; Horiguchi, H.; Murakami, K.; Fukamizu, A. Hypertension induced in pregnant mice by placental renin and maternal angiotensinogen. Science 1996, 274, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Haase, N.; Foster, D.J.; Cunningham, M.W.; Bercher, J.; Nguyen, T.; Shulga-Morskaya, S.; Milstein, S.; Shaikh, S.; Rollins, J.; Golic, M.; et al. RNA interference therapeutics targeting angiotensinogen ameliorate preeclamptic phenotype in rodent models. J. Clin. Invest. 2020, 130, 2928–2942. [Google Scholar] [CrossRef]
- Lenz, T.; James, G.D.; Laragh, J.H.; Sealey, J.E. Prorenin secretion from human placenta perfused in vitro. Am. J. Physiol. 1991, 260, E876–E882. [Google Scholar] [CrossRef] [PubMed]
- Lenz, T. Release of prorenin and placental hormones from superfused minced chorion laeve. Acta Obstet. Gynecol. Scand. 1997, 76, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Wang, Y.; Wu, C.; Howatt, D.A.; Wu, C.H.; Balakrishnan, A.; Mullick, A.E.; Graham, M.J.; Danser, A.H.J.; Wang, J.; et al. Angiotensinogen and Megalin Interactions Contribute to Atherosclerosis-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, M.; McKinnon, T.; Herr, F.; Lala, P.K.; Han, V.K. HCG increases trophoblast migration in vitro via the insulin-like growth factor-II/mannose-6 phosphate receptor. Mol. Hum. Reprod. 2005, 11, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.D.; Zsengeller, Z.K.; Khankin, E.V.; Lo, A.S.; Rajakumar, A.; DuPont, J.J.; McCurley, A.; Moss, M.E.; Zhang, D.; Clark, C.D.; et al. Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia. J. Clin. Invest. 2016, 126, 2561–2574. [Google Scholar] [CrossRef] [PubMed]
- Hitzerd, E.; Neuman, R.I.; Broekhuizen, M.; Simons, S.H.P.; Schoenmakers, S.; Reiss, I.K.M.; Koch, B.C.P.; van den Meiracker, A.H.; Versmissen, J.; Visser, W.; et al. Transfer and Vascular Effect of Endothelin Receptor Antagonists in the Human Placenta. Hypertension 2020, 75, 877–884. [Google Scholar] [CrossRef]
- Hitzerd, E.; Broekhuizen, M.; Mirabito Colafella, K.M.; Glisic, M.; de Vries, R.; Koch, B.C.P.; de Raaf, M.A.; Merkus, D.; Schoenmakers, S.; Reiss, I.K.M.; et al. Placental effects and transfer of sildenafil in healthy and preeclamptic conditions. EBioMedicine 2019, 45, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Batenburg, W.W.; de Bruin, R.J.; van Gool, J.M.; Muller, D.N.; Bader, M.; Nguyen, G.; Danser, A.H. Aliskiren-binding increases the half life of renin and prorenin in rat aortic vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1151–1157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van den Heuvel, M.; Batenburg, W.W.; Jainandunsing, S.; Garrelds, I.M.; van Gool, J.M.; Feelders, R.A.; van den Meiracker, A.H.; Danser, A.H. Urinary renin, but not angiotensinogen or aldosterone, reflects the renal renin-angiotensin-aldosterone system activity and the efficacy of renin-angiotensin-aldosterone system blockade in the kidney. J. Hypertens. 2011, 29, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Krop, M.; Garrelds, I.M.; de Bruin, R.J.; van Gool, J.M.; Fisher, N.D.; Hollenberg, N.K.; Jan Danser, A.H. Aliskiren accumulates in Renin secretory granules and binds plasma prorenin. Hypertension 2008, 52, 1076–1083. [Google Scholar] [CrossRef][Green Version]

 , inhibition; PPI, proton pump inhibitor; (P)RR, (pro)renin receptor; Ang I, angiotensin I; ACE, angiotensin-converting enzyme; Ang II, angiotensin II; AT1R, angiotensin II type 1 receptor; PE, pre-eclampsia; sFlt-1, soluble Fms-like tyrosine kinase-1; +, normal sensitivity to Ang II; +++, increased sensitivity to Ang II.
, inhibition; PPI, proton pump inhibitor; (P)RR, (pro)renin receptor; Ang I, angiotensin I; ACE, angiotensin-converting enzyme; Ang II, angiotensin II; AT1R, angiotensin II type 1 receptor; PE, pre-eclampsia; sFlt-1, soluble Fms-like tyrosine kinase-1; +, normal sensitivity to Ang II; +++, increased sensitivity to Ang II.
 , inhibition; PPI, proton pump inhibitor; (P)RR, (pro)renin receptor; Ang I, angiotensin I; ACE, angiotensin-converting enzyme; Ang II, angiotensin II; AT1R, angiotensin II type 1 receptor; PE, pre-eclampsia; sFlt-1, soluble Fms-like tyrosine kinase-1; +, normal sensitivity to Ang II; +++, increased sensitivity to Ang II.
, inhibition; PPI, proton pump inhibitor; (P)RR, (pro)renin receptor; Ang I, angiotensin I; ACE, angiotensin-converting enzyme; Ang II, angiotensin II; AT1R, angiotensin II type 1 receptor; PE, pre-eclampsia; sFlt-1, soluble Fms-like tyrosine kinase-1; +, normal sensitivity to Ang II; +++, increased sensitivity to Ang II.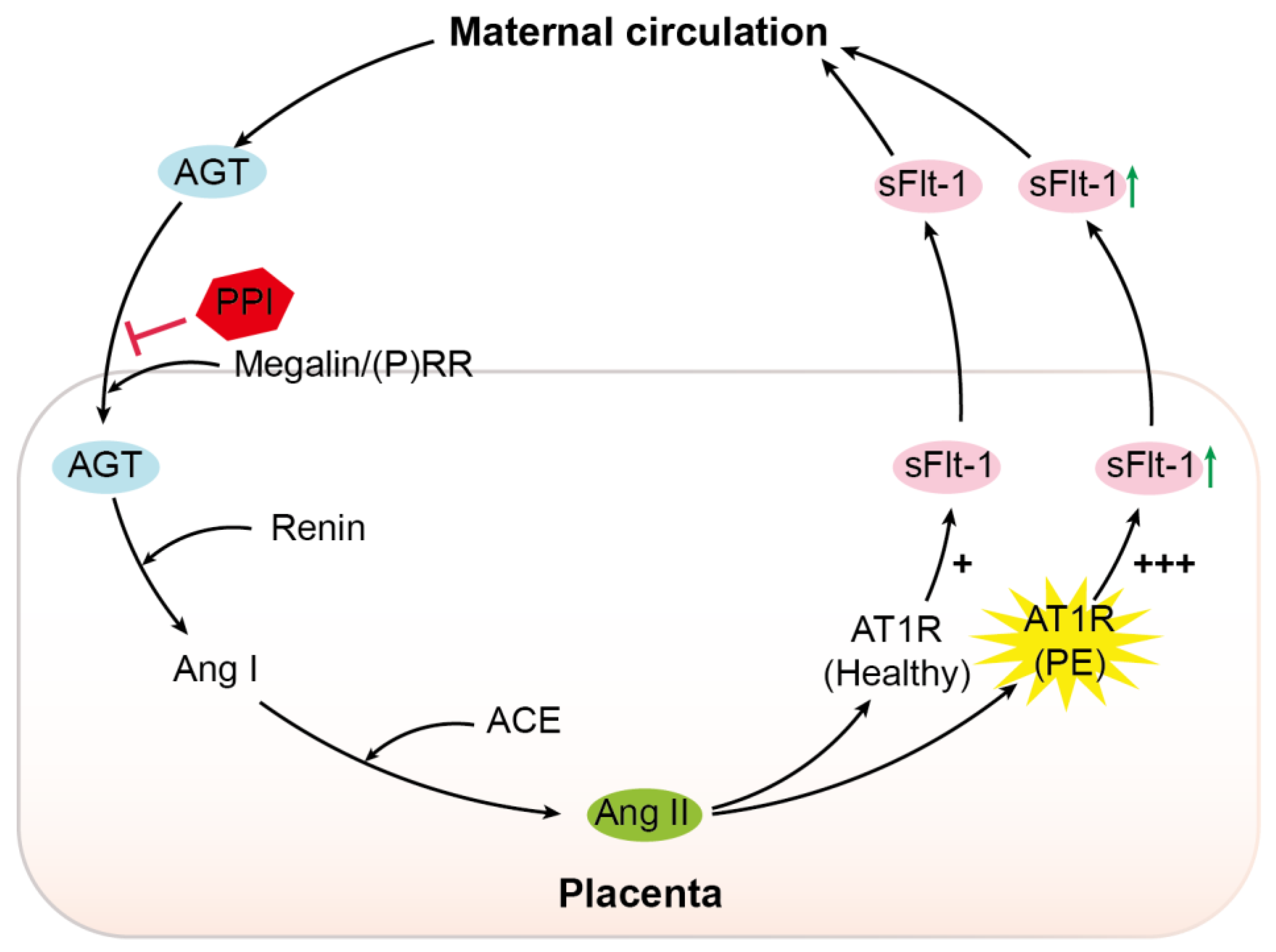
| Characteristic | Healthy (n = 25) | EoPE (n = 17) |
|---|---|---|
| Maternal age (years) | 31 (28–36) | 30 (26–32) |
| Nulliparity/multiparity (n) | 6/19 | 11/6 * |
| Ethnicity (n = Caucasian/other) | 15/10 | 12/5 |
| Highest diastolic blood pressure (mmHg) | 80 (74–82) | 109 (100–116) * |
| Urinary protein-to-creatinine ratio | ND | 351 (92–861) * |
| Mode of delivery (n = caesarean/spontaneous) | 25/0 | 16/1 |
| Gestational age (weeks) | 39.0 (38.7–39.1) | 29.9 (28.8–31.8) * |
| Sex (n = female/male) | 7/18 | 9/8 |
| Birth weight (g) | 3465 (3175–3763) | 1125 (920–1300) * |
| Birth weight centile < 10th (n) | 0 | 9 |
| Placental weight (g) | 623 (568–729) | 295 (232–347) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Tan, L.; Neuman, R.I.; Broekhuizen, M.; Schoenmakers, S.; Lu, X.; Danser, A.H.J. Megalin, Proton Pump Inhibitors and the Renin–Angiotensin System in Healthy and Pre-Eclamptic Placentas. Int. J. Mol. Sci. 2021, 22, 7407. https://doi.org/10.3390/ijms22147407
Sun Y, Tan L, Neuman RI, Broekhuizen M, Schoenmakers S, Lu X, Danser AHJ. Megalin, Proton Pump Inhibitors and the Renin–Angiotensin System in Healthy and Pre-Eclamptic Placentas. International Journal of Molecular Sciences. 2021; 22(14):7407. https://doi.org/10.3390/ijms22147407
Chicago/Turabian StyleSun, Yuan, Lunbo Tan, Rugina I. Neuman, Michelle Broekhuizen, Sam Schoenmakers, Xifeng Lu, and A. H. Jan Danser. 2021. "Megalin, Proton Pump Inhibitors and the Renin–Angiotensin System in Healthy and Pre-Eclamptic Placentas" International Journal of Molecular Sciences 22, no. 14: 7407. https://doi.org/10.3390/ijms22147407
APA StyleSun, Y., Tan, L., Neuman, R. I., Broekhuizen, M., Schoenmakers, S., Lu, X., & Danser, A. H. J. (2021). Megalin, Proton Pump Inhibitors and the Renin–Angiotensin System in Healthy and Pre-Eclamptic Placentas. International Journal of Molecular Sciences, 22(14), 7407. https://doi.org/10.3390/ijms22147407







