Pulsed Electromagnetic Fields in Bone Healing: Molecular Pathways and Clinical Applications
Abstract
1. Introduction
2. Materials and Methods
- All studies were written in the English language
- All studies were an available full text
- All studies were published in peer-reviewed journals
- bone physiology AND fracture healing.
- electromagnetic field AND fracture healing.
- electromagnetic field AND bone pathway.
- To evaluate the clinical application of PEMFs the following strings in PubMed and in Embase were used. Fracture healing and magnetic field
- Magnetic field AND delayed union
- Electromagnetic field AND bone healing
3. Physical Stimulations in Bone Healing
- Mechanical Forces, which direct cellular activities influencing the tissue-level processes of growth, modeling, remodeling, and repair.
- Ultrasound, that usually refers to a longitudinal wave propagation, a special type of sonic wave with a frequency greater than 20 kHz (this is the upper limit of human audibility), that causes local oscillation of particles. Ultrasound with a frequency around 3–10 MHz is widely used in clinical settings for bone healing.
- Shock wave, that is a kind of short-duration and acoustic pressure wave consisting of two phases, the positive phase evoking compressive stress (peak pressure: 30–100 MPa) and the negative phase arousing tensile and shear stress (negative pressure). After propagating into tissue, shock waves may lead to microbubble formation of liquid molecules on the focal area, as to increase cell membrane permeability and facilitate the delivery of macromolecules into cells.
- Scaffold stimulation, which should provide a good environment to guarantee secure attachment, survival, and differentiation of stem cells grown into scaffolds, due to their good osteoconductive and osteogenic ability in bone tissue engineering.
- Electrical stimulation (EF), which can control and regulate physiologically the cellular and tissue homeostasis. The human body generates a biological EF ranging between 10 and 60 mV at various locations. Furthermore, bioelectricity is very important in the wound healing process. When tissue gets damaged, an EF is created. This endogenous EF causes cell migration to the wound. Indeed, wound healing is compromised when the EF is inhibited. However, the exact mechanism underlying the intracellular signal transduction of Electrical Stimulation in bone repair is still unclear.
- Electromagnetic stimulation with Pulsed Electromagnetic Fields (PEMFs), focus of this review.
4. Pulsed Electromagnetic Fields (PEMFs)
- intensity: ranging from 0.1 mT to 2 mT;
- frequency: ranging from 15 Hz to 75 Hz;
- duration: in vitro, the treatment duration ranges from 8 min to 24 h for many days (from 1 to 28 days). In vivo, the treatment duration ranges from 1 h to 8 h for many weeks (from 1 to 12 weeks).
5. PEMFs Molecular Pathways on Bone Healing
- Fracture and inflammatory phase.
- Angio-mesenchymal phase.
- Bone formation.
- Bone remodeling.
5.1. Inflammatory Phase and Wnt/β-Catenin Signaling
5.2. Angio-Mesenchymal Phase and VEGF Pathways
5.3. Bone Formation
- Bone Morphogenetic Protein Signaling Pathway (BMPs) and Tumor Growth Factor β Signaling Pathway (TGF-β)
- Phosphoinositide 3-Kinases/Akt/mammalian Target of Rapamycin Signaling Pathway (PI3K/Akt/mTOR)
- Notch Signaling Pathway (NSP)
- Mitogen-Activated Protein Kinase (MAPK).
5.3.1. TGF-β/BMPs Pathways
TGF-β Signaling Pathway
BMPs Signaling Pathway
Crosstalk between WNT & BMPs Pathways
Extracellular Regulation
Intracellular Regulation
Nuclear Regulation
5.3.2. PI3K/Akt/mTOR Signaling
5.3.3. Notch Signaling
5.3.4. ERK/MAPK Signaling
5.4. Bone Remodeling
5.4.1. GH
5.4.2. IGF
6. PEMFs Clinical Effects on Bone Healing
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A2A | adenosine receptor |
| A3 | adenosine receptor |
| ADSCs | adipose-derived stem cells |
| ALP/alp | alkaline phosphatase protein/gene |
| Alk2 | activin receptor-like kinase-2 |
| Alk3 | activin receptor-like kinase-3 |
| Alk6 | activin receptor-like kinase-6 |
| APC | adenomatous polyposis coli |
| BM-MSCs | bone marrow mesenchymal stem cells |
| BMPs | bone morphogenetic proteins |
| BMP RI | bone morphogenetic protein receptor I |
| BPS | bone sialoprotein |
| CD31 | cluster of differentiation 31 |
| CK1 | casein kinase 1 |
| c-fms | colony-stimulating factor-1 receptor |
| CNS | central nervous system |
| COL1/col1 | collagen type 1 protein/gene |
| DSH | disheveled |
| EF | electrical stimulation |
| ELF-PEMF | extremely low-frequency pulsed electromagnetic field |
| ERK | extracellular signal-regulated kinase 1/2 |
| FGF | fibroblast growth factor |
| FGF-2 | fibroblast growth factor 2 |
| Fz | frizzled |
| GH | growth hormone |
| GHR | growth hormone receptor |
| GSK3 | glycogen synthase kinase 3 |
| hBMSCs | human bone marrow stromal cells |
| HUVECs | human umbilical vein endothelial cells |
| IGF | insulin-like growth factor |
| IGFBP | Insulin-like growth factor binding protein |
| JAK | janus kinase |
| KDR/Flk-1 | phosphorylated vegf receptor 2 |
| LAP | latency-associated propeptide |
| LEF | lymphoid enhancer factor family |
| LIPUS | low-intensity pulsed ultrasound |
| LRP | low-density lipoprotein receptor-related protein |
| LTBP | latent TGF-β binding protein |
| MAPK | mitogen-activated protein kinase |
| MCSF | monocyte/macrophage colony-stimulating factor |
| MSCs | mesenchymal stem cells |
| mTOR | mammalian/mechanistic target of rapamycin |
| NF-kB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NICD | notch intracellular domain |
| NSP | notch signaling pathway |
| OCN/ocn | osteocalcin protein/gene |
| OPN/opn | osteopontin protein/gene |
| OSX/osx | osterix protein/gene |
| PCP | planar cell polarity |
| PEMFs | pulsed electromagnetic fields |
| PI3K/Akt/mTOR | phosphoinositide 3-kinases/akt/mammalian target of rapamycin |
| PKA | protein kinase A |
| PP2A | protein phosphatase 2 A |
| PTH | parathyroid hormone |
| RANK | receptor activator of nuclear factor κ B |
| RANK-L | nuclear factor kappa B ligand |
| RUNX-2/runx-2 | runt-related transcription factor 2 protein/gene |
| SAPK/JNK | stress-activated protein kinase/c-Jun NH2-terminal kinase |
| SMAD | small mothers against decapentaplegic |
| SOCS | suppression of cytokine signaling |
| SOST | sclerostin |
| STAT | signal transducer activating the transcription |
| TCF | T cell factor |
| TGF-β | transforming growth factor-β |
| TGF-β R I | transforming growth factor-β receptor I |
| TGF-β R II | transforming growth factor-β receptor II |
| VEGF | vascular endothelial growth factor |
| VEGFR-2 | vascular endothelial growth factor receptor 2 |
References
- Massari, L.; Benazzo, F.; Falez, F.; Perugia, D.; Pietrogrande, L.; Setti, S.; Osti, R.; Vaienti, E.; Ruosi, C.; Cadossi, R. Biophysical Stimulation of Bone and Cartilage: State of the Art and Future Perspectives. Int. Orthop. 2019, 43, 539–551. [Google Scholar] [CrossRef]
- Fukada, E.; Yasuda, I. On the Piezoelectric Effect of Bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Huang, X.; Das, R.; Patel, A.; Nguyen, T.D. Physical Stimulations for Bone and Cartilage Regeneration. Regen. Eng. Transl. Med. 2018, 4, 216–237. [Google Scholar] [CrossRef] [PubMed]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular Biology of Fracture Healing. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2019, 37, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Xin, F.; Jiang, W. Underlying Signaling Pathways and Therapeutic Applications of Pulsed Electromagnetic Fields in Bone Repair. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 46, 1581–1594. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yu, Q.; Sun, Y.; Tian, J. Synergistic Effect of a LPEMF and SPIONs on BMMSC Proliferation, Directional Migration, and Osteoblastogenesis. Am. J. Transl. Res. 2018, 10, 1431–1443. [Google Scholar]
- Chen, Y.; Alman, B.A. Wnt Pathway, an Essential Role in Bone Regeneration. J. Cell. Biochem. 2009, 106, 353–362. [Google Scholar] [CrossRef]
- Komiya, Y.; Habas, R. Wnt Signal Transduction Pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef]
- Rodríguez-Merchán, E.C. A Review of Recent Developments in the Molecular Mechanisms of Bone Healing. Int. J. Mol. Sci. 2021, 22, 767. [Google Scholar] [CrossRef]
- Yun, Y.-R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.-H.; Shin, U.S.; Kim, H.-W. Fibroblast Growth Factors: Biology, Function, and Application for Tissue Regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef]
- Callaghan, M.J.; Chang, E.I.; Seiser, N.; Aarabi, S.; Ghali, S.; Kinnucan, E.R.; Simon, B.J.; Gurtner, G.C. Pulsed Electromagnetic Fields Accelerate Normal and Diabetic Wound Healing by Increasing Endogenous FGF-2 Release. Plast. Reconstr. Surg. 2008, 121, 130–141. [Google Scholar] [CrossRef]
- Delle Monache, S.; Alessandro, R.; Iorio, R.; Gualtieri, G.; Colonna, R. Extremely Low Frequency Electromagnetic Fields (ELF-EMFs) Induce in Vitro Angiogenesis Process in Human Endothelial Cells. Bioelectromagnetics 2008, 29, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Tepper, O.M.; Callaghan, M.J.; Chang, E.I.; Galiano, R.D.; Bhatt, K.A.; Baharestani, S.; Gan, J.; Simon, B.; Hopper, R.A.; Levine, J.P.; et al. Electromagnetic Fields Increase in Vitro and in Vivo Angiogenesis through Endothelial Release of FGF-2. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004, 18, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The Roles of Signaling Pathways in Bone Repair and Regeneration. J. Cell. Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, Z.; Chen, Y. Regulation of TGF-Beta Signaling by Smad7. Acta Biochim. Biophys. Sin. 2009, 41, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.Q.; Andersson, J.; Wang, R.; Ramsey, H.; Unutmaz, D.; Shevach, E.M. GARP (LRRC32) Is Essential for the Surface Expression of Latent TGF-Beta on Platelets and Activated FOXP3+ Regulatory T Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 13445–13450. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L. BMP Signaling and Stem Cell Regulation. Dev. Biol. 2005, 284, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, P.R.; Schemitsch, E.H. The Science of Electrical Stimulation Therapy for Fracture Healing. Indian J. Orthop. 2009, 43, 127–131. [Google Scholar] [CrossRef]
- Selvamurugan, N.; He, Z.; Rifkin, D.; Dabovic, B.; Partridge, N.C. Pulsed Electromagnetic Field Regulates MicroRNA 21 Expression to Activate TGF-β Signaling in Human Bone Marrow Stromal Cells to Enhance Osteoblast Differentiation. Stem Cells Int. 2017, 2017, 2450327. [Google Scholar] [CrossRef]
- Streit, A.; Watson, B.C.; Granata, J.D.; Philbin, T.M.; Lin, H.-N.; O’Connor, J.P.; Lin, S. Effect on Clinical Outcome and Growth Factor Synthesis With Adjunctive Use of Pulsed Electromagnetic Fields for Fifth Metatarsal Nonunion Fracture: A Double-Blind Randomized Study. Foot Ankle Int. 2016, 37, 919–923. [Google Scholar] [CrossRef]
- Itasaki, N.; Hoppler, S. Crosstalk between Wnt and Bone Morphogenic Protein Signaling: A Turbulent Relationship. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2010, 239, 16–33. [Google Scholar] [CrossRef]
- Chen, X.-J.; Shen, Y.-S.; He, M.-C.; Yang, F.; Yang, P.; Pang, F.-X.; He, W.; Cao, Y.-M.; Wei, Q.-S. Polydatin Promotes the Osteogenic Differentiation of Human Bone Mesenchymal Stem Cells by Activating the BMP2-Wnt/β-Catenin Signaling Pathway. Biomed. Pharmacother. Biomedecine Pharmacother. 2019, 112, 108746. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Gou, X.; Deng, J.; Dong, Z.; Ye, P.; Hu, Z. FAK and BMP-9 Synergistically Trigger Osteogenic Differentiation and Bone Formation of Adipose Derived Stem Cells through Enhancing Wnt-β-Catenin Signaling. Biomed. Pharmacother. Biomedecine Pharmacother. 2018, 105, 753–757. [Google Scholar] [CrossRef]
- Liang, K.; Du, Y.; Chen, L.; Wang, L.; Li, R.; Yan, Z.; Liu, Y. Contrary Roles of Wnt/β-Catenin Signaling in BMP9-Induced Osteogenic and Adipogenic Differentiation of 3T3-L1 Preadipocytes. Cell Biochem. Biophys. 2020, 78, 347–356. [Google Scholar] [CrossRef]
- Kim, M.-B.; Song, Y.; Hwang, J.-K. Kirenol Stimulates Osteoblast Differentiation through Activation of the BMP and Wnt/β-Catenin Signaling Pathways in MC3T3-E1 Cells. Fitoterapia 2014, 98, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Kim, H.J.; Won, H.Y.; Min, Y.K.; Hwang, E.S. Acceleration of Osteoblast Differentiation by a Novel Osteogenic Compound, DMP-PYT, through Activation of Both the BMP and Wnt Pathways. Sci. Rep. 2017, 7, 8455. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; Gardin, C.; Dolkart, O.; Salai, M.; Barak, S.; Piattelli, A.; Amir-Barak, H.; Zavan, B. Pulsed Electromagnetic Fields Increase Osteogenetic Commitment of MSCs via the MTOR Pathway in TNF-α Mediated Inflammatory Conditions: An in-Vitro Study. Sci. Rep. 2018, 8, 5108. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, Y.; Ni, Z.; Chen, L. Effects and Mechanisms of Exogenous Electromagnetic Field on Bone Cells: A Review. Bioelectromagnetics 2020, 41, 263–278. [Google Scholar] [CrossRef]
- Poh, P.S.P.; Seeliger, C.; Unger, M.; Falldorf, K.; Balmayor, E.R.; van Griensven, M. Osteogenic Effect and Cell Signaling Activation of Extremely Low-Frequency Pulsed Electromagnetic Fields in Adipose-Derived Mesenchymal Stromal Cells. Stem Cells Int. 2018, 2018, 5402853. [Google Scholar] [CrossRef]
- Borggrefe, T.; Oswald, F. The Notch Signaling Pathway: Transcriptional Regulation at Notch Target Genes. Cell. Mol. Life Sci. CMLS 2009, 66, 1631–1646. [Google Scholar] [CrossRef] [PubMed]
- Ehnert, S.; Falldorf, K.; Fentz, A.-K.; Ziegler, P.; Schröter, S.; Freude, T.; Ochs, B.G.; Stacke, C.; Ronniger, M.; Sachtleben, J.; et al. Primary Human Osteoblasts with Reduced Alkaline Phosphatase and Matrix Mineralization Baseline Capacity Are Responsive to Extremely Low Frequency Pulsed Electromagnetic Field Exposure-Clinical Implication Possible. Bone Rep. 2015, 3, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.M.; Kang, K.S.; Yi, H.-G.; Kim, S.-Y.; Cho, D.-W. Electromagnetically Controllable Osteoclast Activity. Bone 2014, 62, 99–107. [Google Scholar] [CrossRef]
- Olney, R.C. Regulation of Bone Mass by Growth Hormone. Med. Pediatr. Oncol. 2003, 41, 228–234. [Google Scholar] [CrossRef]
- Locatelli, V.; Bianchi, V.E. Effect of GH/IGF-1 on Bone Metabolism and Osteoporsosis. Int. J. Endocrinol. 2014, 2014, e235060. [Google Scholar] [CrossRef] [PubMed]
- Xian, L.; Wu, X.; Pang, L.; Lou, M.; Rosen, C.J.; Qiu, T.; Crane, J.; Frassica, F.; Zhang, L.; Rodriguez, J.P.; et al. Matrix IGF-1 Maintains Bone Mass by Activation of MTOR in Mesenchymal Stem Cells. Nat. Med. 2012, 18, 1095–1101. [Google Scholar] [CrossRef]
- Wang, Y.; Nishida, S.; Elalieh, H.Z.; Long, R.K.; Halloran, B.P.; Bikle, D.D. Role of IGF-I Signaling in Regulating Osteoclastogenesis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2006, 21, 1350–1358. [Google Scholar] [CrossRef]
- Peng, L.; Fu, C.; Xiong, F.; Zhang, Q.; Liang, Z.; Chen, L.; He, C.; Wei, Q. Effectiveness of Pulsed Electromagnetic Fields on Bone Healing: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Bioelectromagnetics 2020, 41, 323–337. [Google Scholar] [CrossRef]
- Wennergren, D.; Ekholm, C.; Sandelin, A.; Möller, M. The Swedish Fracture Register: 103,000 Fractures Registered. BMC Musculoskelet. Disord. 2015, 16, 338. [Google Scholar] [CrossRef] [PubMed]
- Ekegren, C.L.; Edwards, E.R.; de Steiger, R.; Gabbe, B.J. Incidence, Costs and Predictors of Non-Union, Delayed Union and Mal-Union Following Long Bone Fracture. Int. J. Environ. Res. Public. Health 2018, 15, 2845. [Google Scholar] [CrossRef]
- Fisher, J.S.; Kazam, J.J.; Fufa, D.; Bartolotta, R.J. Radiologic Evaluation of Fracture Healing. Skeletal Radiol. 2019, 48, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Griffin, X.L.; Costa, M.L.; Parsons, N.; Smith, N. Electromagnetic Field Stimulation for Treating Delayed Union or Non-Union of Long Bone Fractures in Adults. Cochrane Database Syst. Rev. 2011, CD008471. [Google Scholar] [CrossRef]
- Hannemann, P.F.W.; Mommers, E.H.H.; Schots, J.P.M.; Brink, P.R.G.; Poeze, M. The Effects of Low-Intensity Pulsed Ultrasound and Pulsed Electromagnetic Fields Bone Growth Stimulation in Acute Fractures: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Arch. Orthop. Trauma Surg. 2014, 134, 1093–1106. [Google Scholar] [CrossRef]
- Hannemann, P.F.W.; Göttgens, K.W.A.; van Wely, B.J.; Kolkman, K.A.; Werre, A.J.; Poeze, M.; Brink, P.R.G. The Clinical and Radiological Outcome of Pulsed Electromagnetic Field Treatment for Acute Scaphoid Fractures: A Randomised Double-Blind Placebo-Controlled Multicentre Trial. J. Bone Joint Surg. Br. 2012, 94, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, P.F.W.; van Wezenbeek, M.R.; Kolkman, K.A.; Twiss, E.L.L.; Berghmans, C.H.J.; Dirven, P.A.M.G.M.; Brink, P.R.G.; Poeze, M. CT Scan-Evaluated Outcome of Pulsed Electromagnetic Fields in the Treatment of Acute Scaphoid Fractures: A Randomised, Multicentre, Double-Blind, Placebo-Controlled Trial. Bone Jt. J. 2014, 96-B, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Adie, S.; Harris, I.A.; Naylor, J.M.; Rae, H.; Dao, A.; Yong, S.; Ying, V. Pulsed Electromagnetic Field Stimulation for Acute Tibial Shaft Fractures: A Multicenter, Double-Blind, Randomized Trial. J. Bone Jt. Surg. 2011, 93, 1569–1576. [Google Scholar] [CrossRef]
- Faldini, C.; Cadossi, M.; Luciani, D.; Betti, E.; Chiarello, E.; Giannini, S. Electromagnetic Bone Growth Stimulation in Patients with Femoral Neck Fractures Treated with Screws: Prospective Randomized Double-Blind Study. Curr. Orthop. Pract. 2010, 21, 282–287. [Google Scholar] [CrossRef]
- Martinez-Rondanelli, A.; Martinez, J.P.; Moncada, M.E.; Manzi, E.; Pinedo, C.R.; Cadavid, H. Electromagnetic Stimulation as Coadjuvant in the Healing of Diaphyseal Femoral Fractures: A Randomized Controlled Trial. Colomb. Medica Cali Colomb. 2014, 45, 67–71. [Google Scholar] [CrossRef]
- Massari, L.; Benazzo, F.; Falez, F.; Cadossi, R.; Perugia, D.; Pietrogrande, L.; Aloj, D.C.; Capone, A.; D’Arienzo, M.; Cadossi, M.; et al. Can Clinical and Surgical Parameters Be Combined to Predict How Long It Will Take a Tibia Fracture to Heal? A Prospective Multicentre Observational Study: The FRACTING Study. BioMed Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Cadossi, R.; Massari, L.; Racine-Avila, J.; Aaron, R.K. Pulsed Electromagnetic Field Stimulation of Bone Healing and Joint Preservation: Cellular Mechanisms of Skeletal Response. JAAOS Glob. Res. Rev. 2020, 4, e19.00155. [Google Scholar] [CrossRef]
- Assiotis, A.; Sachinis, N.P.; Chalidis, B.E. Pulsed Electromagnetic Fields for the Treatment of Tibial Delayed Unions and Nonunions. A Prospective Clinical Study and Review of the Literature. J. Orthop. Surg. 2012, 7, 24. [Google Scholar] [CrossRef]
- Cebrián, J.L.; Gallego, P.; Francés, A.; Sánchez, P.; Manrique, E.; Marco, F.; López-Durán, L. Comparative Study of the Use of Electromagnetic Fields in Patients with Pseudoarthrosis of Tibia Treated by Intramedullary Nailing. Int. Orthop. 2010, 34, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Xiong, J.; Chen, Y.; Wang, J.; Qiu, X.; Wang, Y.; Qiu, Y. Early Application of Pulsed Electromagnetic Field in the Treatment of Postoperative Delayed Union of Long-Bone Fractures: A Prospective Randomized Controlled Study. BMC Musculoskelet. Disord. 2013, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, L.; Pellati, A.; Rizzo, P.; Aquila, G.; Massari, L.; De Mattei, M.; Ongaro, A. Notch Pathway Is Active during Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells Induced by Pulsed Electromagnetic Fields. J. Tissue Eng. Regen. Med. 2018, 12, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Shao, X.; Yang, Q.; Yang, Y.; Yan, Z.; Luo, E.; Feng, X.; Jing, D. Pulsed Electromagnetic Fields Modify the Adverse Effects of Glucocorticoids on Bone Architecture, Bone Strength and Porous Implant Osseointegration by Rescuing Bone-Anabolic Actions. Bone 2020, 133, 115266. [Google Scholar] [CrossRef]
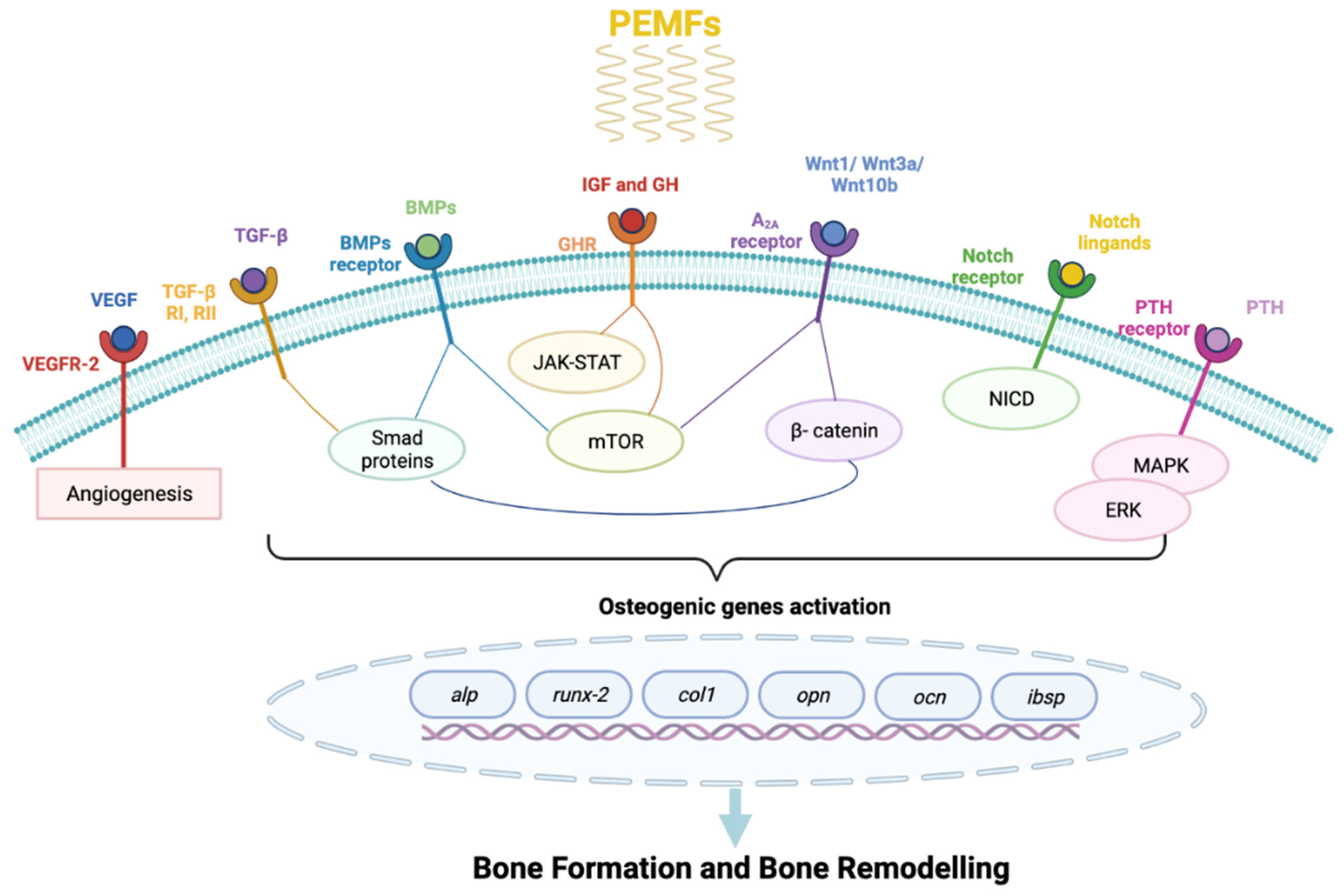
| Pathway Activated | Cell Response | Bone Healing Phase |
|---|---|---|
| β-Catenin/Wnt | NF-kβ inhibition col1 and opn increase | phase 1- inflammatory phase; phase 4- bone remodeling |
| FGF and VEGF | endothelial cells and osteoblastic stimulation | phase 2- angio-mesenchymal phase |
| TGF-β/BMPs | runx-2 increase | phase 3- bone formation |
| PI3K/Akt/mTOR | osteoblastic genes activation | phase 3- bone formation |
| Notch | osteoblastic genes activation | phase 3- bone formation |
| ERK/MAPK | osteoblastic genes activation | phase 3- bone formation |
| GH/IGF | JAK-STAT activation | phase 4- bone remodeling |
| Study | Field of Application | PEMFs (Device) | Frequency, Dose, Duration |
|---|---|---|---|
| Adie et al. | Adjuvant in surgery (tibial shaft) | EBI Bone Healing System (Biomet, New Jersey) | 10 h/day 12 weeks |
| Faldini et al. | Adjuvant in surgery (femoral neck fractures) | Biostim (Igea, Carpi) | 75 Hz, 2 mT 8 h/day 90 days |
| Hanneman et al. (2012) | Acute scaphoid fractures | Ossatec (Uden) | 24 h/day 6/12 weeks |
| Hanneman et al. (2014) | Acute scaphoid fractures | Ossatec (Uden) | 24 h/day 6 weeks |
| Martinez-Rondanelli et al. | Adjuvant in surgery (Diaphiseal femoral fractures) | Authors provided | 5–105 Hz, 0.5–2.0 mT 1 h/day 8 weeks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caliogna, L.; Medetti, M.; Bina, V.; Brancato, A.M.; Castelli, A.; Jannelli, E.; Ivone, A.; Gastaldi, G.; Annunziata, S.; Mosconi, M.; et al. Pulsed Electromagnetic Fields in Bone Healing: Molecular Pathways and Clinical Applications. Int. J. Mol. Sci. 2021, 22, 7403. https://doi.org/10.3390/ijms22147403
Caliogna L, Medetti M, Bina V, Brancato AM, Castelli A, Jannelli E, Ivone A, Gastaldi G, Annunziata S, Mosconi M, et al. Pulsed Electromagnetic Fields in Bone Healing: Molecular Pathways and Clinical Applications. International Journal of Molecular Sciences. 2021; 22(14):7403. https://doi.org/10.3390/ijms22147403
Chicago/Turabian StyleCaliogna, Laura, Marta Medetti, Valentina Bina, Alice Maria Brancato, Alberto Castelli, Eugenio Jannelli, Alessandro Ivone, Giulia Gastaldi, Salvatore Annunziata, Mario Mosconi, and et al. 2021. "Pulsed Electromagnetic Fields in Bone Healing: Molecular Pathways and Clinical Applications" International Journal of Molecular Sciences 22, no. 14: 7403. https://doi.org/10.3390/ijms22147403
APA StyleCaliogna, L., Medetti, M., Bina, V., Brancato, A. M., Castelli, A., Jannelli, E., Ivone, A., Gastaldi, G., Annunziata, S., Mosconi, M., & Pasta, G. (2021). Pulsed Electromagnetic Fields in Bone Healing: Molecular Pathways and Clinical Applications. International Journal of Molecular Sciences, 22(14), 7403. https://doi.org/10.3390/ijms22147403









