Functional Analyses of Four CYP1A1 Missense Mutations Present in Patients with Atypical Femoral Fractures
Abstract
1. Introduction
2. Results and Discussion
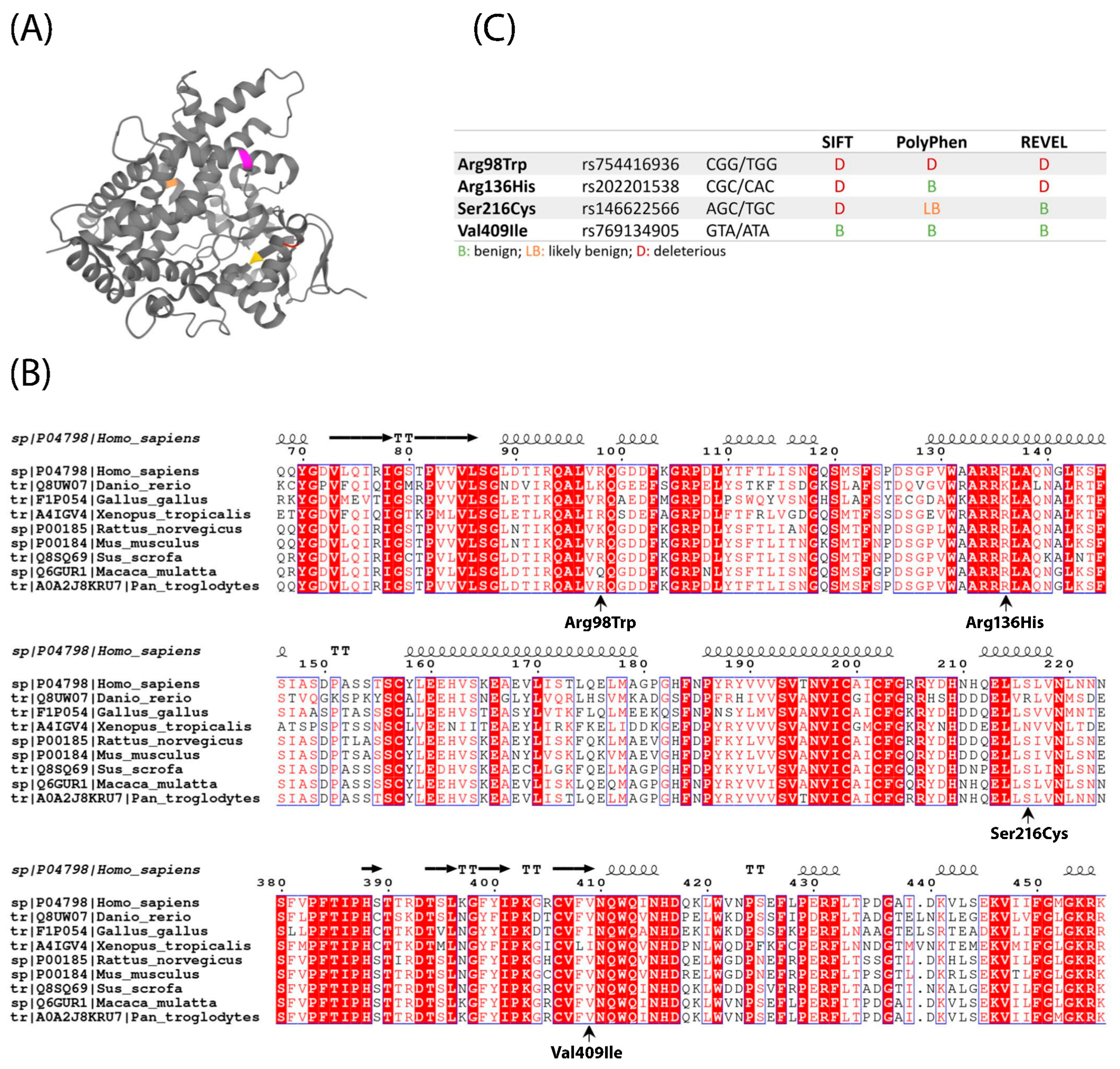
2.1. Location, Conservation and Pathogenicity Predictions of Four CYP1A1 Variants
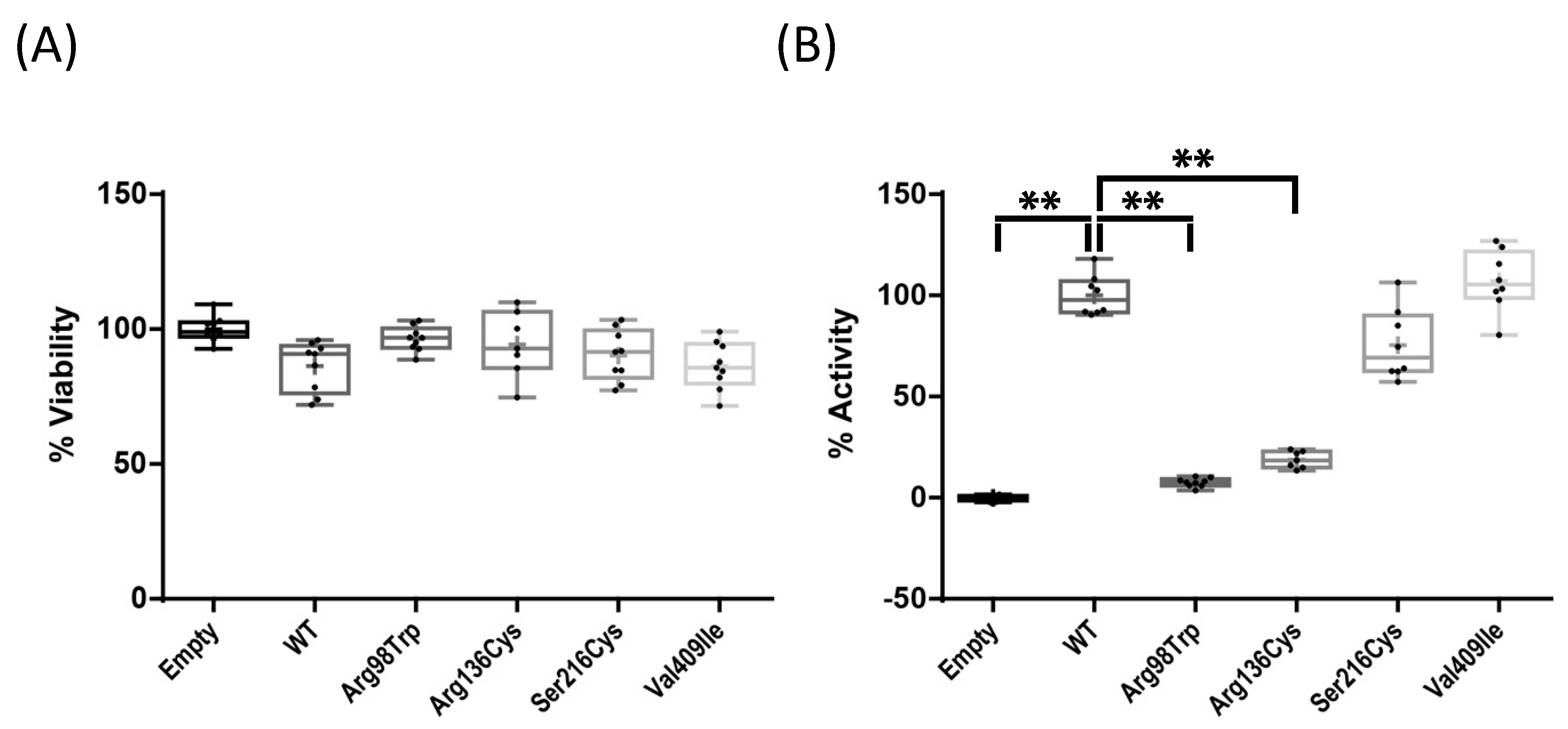
2.2. Enzyme Activity Assays of Four CYP1A1 Variants
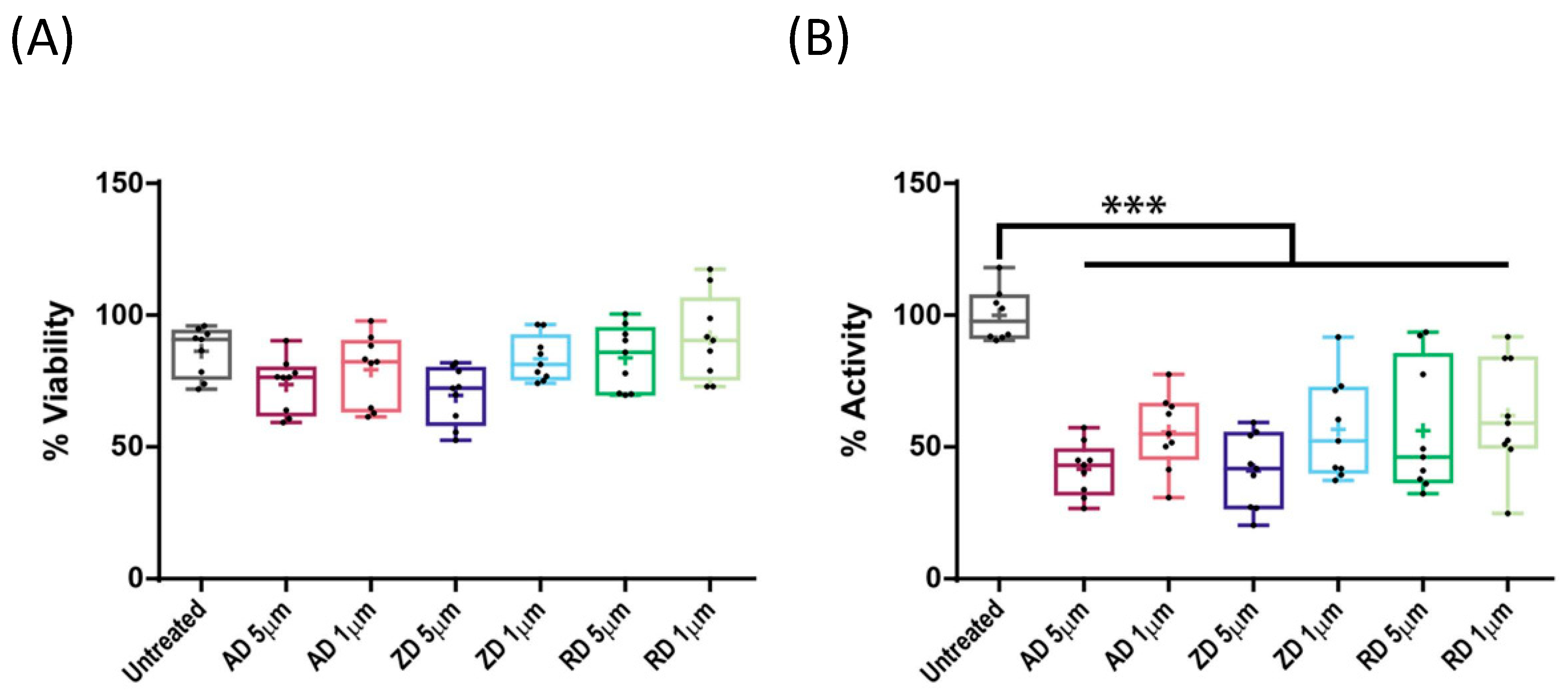
2.3. Effect of BP Treatment on Wild-Type CYP1A1 Enzyme Activity
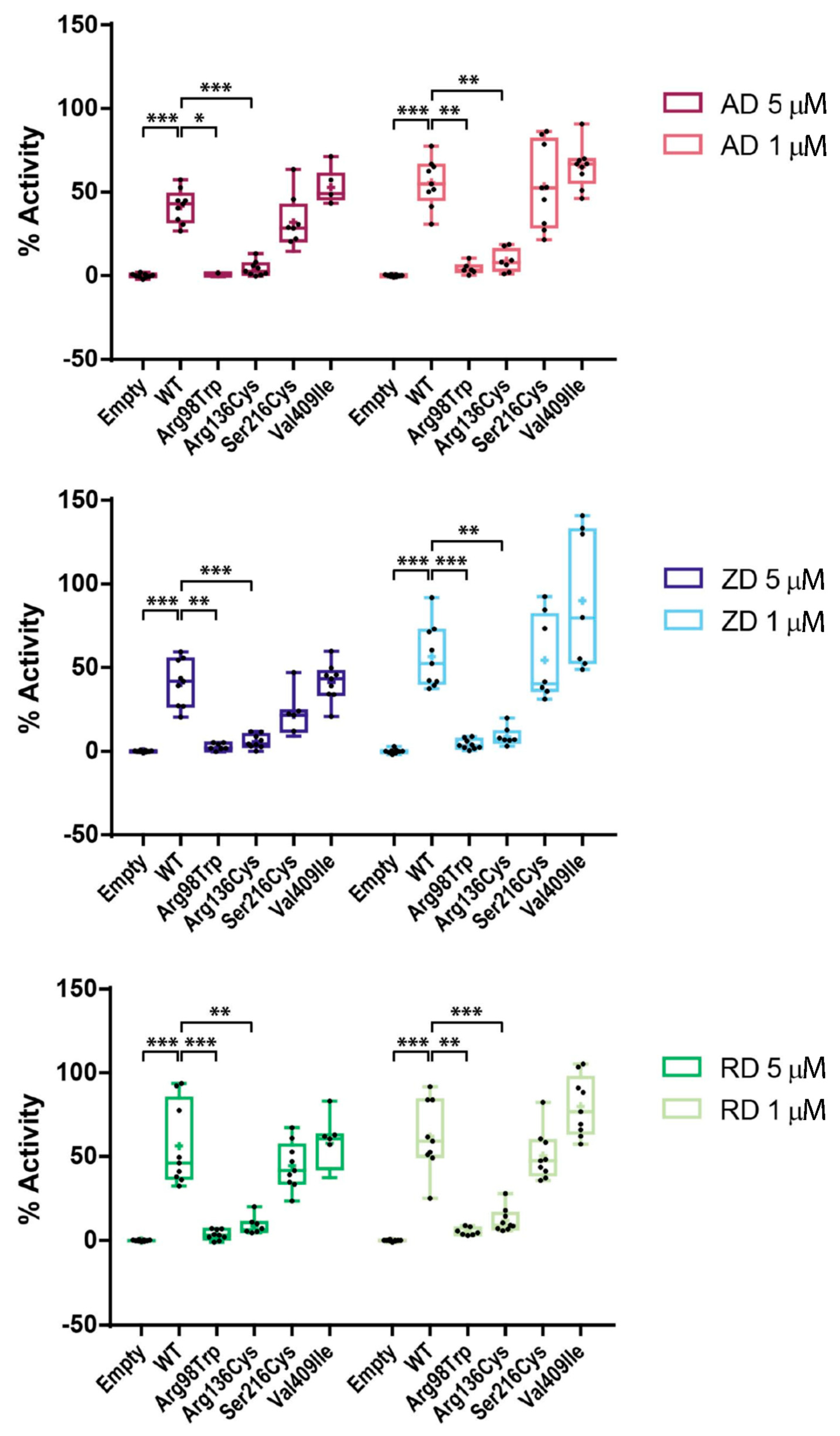
2.4. Effect of BP Treatment on Enzyme Activity of Four CYP1A1 Variants
2.5. AFF as an Oligogenic Phenotype; a Role for CYP1A1 Variants in Combination with BPs
3. Materials and Methods
3.1. In Silico Prediction of Protein Structure and Pathogenicity
3.2. Site-Directed Mutagenesis
3.3. Cell Culture and Transfection
3.4. RT-qPCR
3.5. Bisphosphonate Treatment and Enzymatic and Viability Assays
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, R.G.G.; Xia, Z.; Dunford, J.E.; Oppermann, U.; Kwaasi, A.; Hulley, P.A.; Kavanagh, K.L.; Triffitt, J.T.; Lundy, M.W.; Phipps, R.J.; et al. Bisphosphonates: An update on mechanisms of action and how these relate to clinical efficacy. Ann. N. Y. Acad. Sci. 2007, 1117, 209–257. [Google Scholar] [CrossRef] [PubMed]
- Chapurlat, R.D.; Delmas, P.D. Drug insight: Bisphosphonates for postmenopausal osteoporosis. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 211–219. [Google Scholar] [CrossRef]
- Freemantle, N.; Cooper, C.; Diez-Perez, A.; Gitlin, M.; Radcliffe, H.; Shepherd, S.; Roux, C. Results of indirect and mixed treatment comparison of fracture efficacy for osteoporosis treatments: A meta-analysis. Osteoporos. Int. 2013, 24, 209–217. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, X.; Wang, T.; Zhai, S. Comparative efficacy of bisphosphonates in short-term fracture prevention for primary osteoporosis: A systematic review with network meta-analyses. Osteoporos. Int. 2016, 27, 3289–3300. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, Y.; Chen, L.; Peng, K.; Xu, Z.; Zhang, D.; Xiang, Z. Bisphosphonates can prevent recurrent hip fracture and reduce the mortality in osteoporotic patient with hip fracture: A meta-analysis. Pak. J. Med. Sci. 2016, 32, 499–504. [Google Scholar] [CrossRef]
- Kennel, K.A.; Drake, M.T. Adverse effects of bisphosphonates: Implications for osteoporosis management. Mayo Clin. Proc. 2009, 84, 632–637. [Google Scholar] [CrossRef]
- Black, D.M.; Geiger, E.J.; Eastell, R.; Vittinghoff, E.; Li, B.H.; Ryan, D.S.; Dell, R.M.; Adams, A.L. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N. Engl. J. Med. 2020, 383, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Shane, E.; Burr, D.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; Dempster, D.W. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2014, 29, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; van Rooij, J.G.J.; Ebeling, P.R.; Verkerk, A.J.M.H.; Zillikens, M.C. The genetics of atypical femur fractures—A systematic review. Curr. Osteoporos. Rep. 2021, 19, 123–130. [Google Scholar] [CrossRef]
- Shin, W.C.; Moon, N.H.; Jang, J.H.; Park, K.Y.; Suh, K.T. Anterolateral femoral bowing and loss of thigh muscle are associated with occurrence of atypical femoral fracture: Effect of failed tension band mechanism in mid-thigh. J. Orthop. Sci. 2017, 22, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Saita, Y.; Ishijima, M.; Mogami, A.; Kubota, M.; Baba, T.; Kaketa, T.; Nagao, M.; Sakamoto, Y.; Sakai, K.; Kato, R.; et al. The fracture sites of atypical femoral fractures are associated with the weight-bearing lower limb alignment. Bone 2014, 66, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Saita, Y.; Ishijima, M.; Mogami, A.; Kubota, M.; Baba, T.; Kaketa, T.; Nagao, M.; Sakamoto, Y.; Sakai, K.; Homma, Y.; et al. The incidence of and risk factors for developing atypical femoral fractures in Japan. J. Bone Miner. Metab. 2015, 33, 311–318. [Google Scholar] [CrossRef]
- Kim, D.; Sung, Y.K.; Cho, S.K.; Han, M.; Kim, Y.-S. Factors associated with atypical femoral fracture. Rheumatol. Int. 2016, 36, 65–71. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lakhani, A.; Shore-Lorenti, C.; Zebaze, R.; Vincent, A.J.; Milat, F.; Ebeling, P.R. Asian ethnicity is associated with atypical femur fractures in an Australian population study. Bone 2020, 135, 115319. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; van de Laarschot, D.M.; Verkerk, A.J.M.H.; Milat, F.; Zillikens, M.C.; Ebeling, P.R. Genetic risk factors for atypical femoral fractures (AFFs): A Systematic Review. JBMR Plus 2018, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Roca-Ayats, N.; Ying, P.; Garcia-Giralt, N.; Falcó-Mascaró, M.; Cozar, M.; Abril, J.F.; Quesada-Gómez, J.M.; Prieto-Alhambra, D.; Nogués, X.; Dunford, J.E.; et al. Functional characterization of a GGPPS variant identified in atypical femoral fracture patients and delineation of the role of GGPPS in bone-relevant cell types. J. Bone Miner. Res. 2018, 33, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Peris, P.; González-Roca, E.; Rodríguez-García, S.C.; López-Cobo, M.M.; Monegal, A.; Guañabens, N. Incidence of mutations in the ALPL, GGPS1, and CYP1A1 genes in patients with atypical femoral fractures. JBMR Plus 2019, 3, 29–36. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Rendic, S.P.; Guengerich, F.P. Human family 1-4 cytochrome P450 enzymes involved in the metabolic activation of xenobiotic and physiological chemicals: An update. Arch. Toxicol. 2021, 95, 395–472. [Google Scholar] [CrossRef]
- Kisselev, P.; Schunck, W.H.; Roots, I.; Schwarz, D. Association of CYP1A1 polymorphisms with differential metabolic activation of 17β-estradiol and estrone. Cancer Res. 2005, 65, 2972–2978. [Google Scholar] [CrossRef]
- Leelawattana, R.; Ziambaras, K.; Roodman-Weiss, J.; Lyss, C.; Wagner, D.; Klug, T.; Armamento-Villareal, R.; Civitelli, R. The oxidative metabolism of estradiol conditions postmenopausal bone density and bone loss. J. Bone Miner. Res. 2000, 15, 2513–2520. [Google Scholar] [CrossRef]
- Napoli, N.; Villareal, D.T.; Mumm, S.; Halstead, L.; Sheikh, S.; Cagaanan, M.; Rini, G.B.; Armamento-Villareal, R. Effect of CYP1A1 gene polymorphisms on estrogen metabolism and bone density. J. Bone Miner. Res. 2005, 20, 232–239. [Google Scholar] [CrossRef]
- Rogers, M.J.; Mönkkonen, J.; Muñoz, M.A. Molecular mechanisms of action of bisphosphonates and new insights into their effects outside the skeleton. Bone 2020, 139, 115493. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.T.; Cao, R.; Liang, P.H.; Ko, T.-P.; Chang, T.-H.; Hudock, M.P.; Jeng, W.-Y.; Chen, C.K.-M.; Zhang, Y.; Song, Y.; et al. Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases. Proc. Natl. Acad. Sci. USA 2007, 104, 10022–10027. [Google Scholar] [CrossRef] [PubMed]
- Diab, D.L.; Watts, N.B.; Miller, P.D. Bisphosphonates pharmacology and use in the treatment of osteoporosis. In Marcus and Feldman’s Osteoporosis, 5th ed.; Dempster, D.W., Cauley, J.A., Bouxsein, M.L., Cosman, F., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 1721–1736. [Google Scholar]
- Nancollas, G.H.; Tang, R.; Phipps, R.J.; Henneman, Z.; Gulde, S.; Wu, W.; Mangood, A.; Russell, R.G.G.; Ebetino, F.H. Novel insights into actions of bisphosphonates on bone: Differences in interactions with hydroxyapatite. Bone 2006, 38, 617–627. [Google Scholar] [CrossRef]
- Li, P.; Zhao, Z.; Wang, L.; Jin, X.; Shen, Y.; Nan, C.; Liu, H. Minimally effective concentration of zoledronic acid to suppress osteoclasts. Exp. Ther. Med. 2018, 15, 5330–5336. [Google Scholar] [PubMed]
- Bellido, T.; Plotkin, L.I. Novel actions of bisphosphonates in bone: Preservation of osteoblast and osteocyte viability. Bone 2011, 49, 50–55. [Google Scholar] [CrossRef]
- Such, E.; Cervera, J.; Terpos, E.; Bagán, J.V.; Avaria, A.; Gómez, I.; Margaix, M.; Ibañez, M.; Luna, I.; Cordón, L.; et al. CYP2C8 gene polymorphism and bisphosphonate-related osteonecrosis of the jaw in patients with multiple myeloma. Haematologica 2011, 96, 1557–1559. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sarasquete, M.E.; García-Sanz, R.; Marin, L.; Alcoceba, M.; Chillón, M.C.; Balanzategui, A.; Santamaria, C.; Rosiñol, L.; de la Rubia, J.; Hernandez, M.T.; et al. Bisphosphonate-related osteonecrosis of the jaw is associated with polymorphisms of the cytochrome P450 CYP2C8 in multiple myeloma: A genome-wide single nucleotide polymorphism analysis. Blood 2008, 112, 2709–2712. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.A.; Szklarz, G.D.; Scott, E.E. Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism. J. Biol. Chem. 2013, 288, 12932–12943. [Google Scholar] [CrossRef]
- Dutkiewicz, Z.; Mikstacka, R. Structure-based drug design for cytochrome P450 family 1 inhibitors. Bioinorg. Chem. Appl. 2018, 2018. [Google Scholar] [CrossRef]
- Bart, A.G.; Takahashi, R.H.; Wang, X.; Scott, E.E. Human cytochrome P450 1A1 adapts active site for atypical nonplanar substrate. Drug Metab. Dispos. 2020, 48, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Scheper, M.A.; Badros, A.; Salama, A.R. A novel bioassay model to determine clinically significant bisphosphonate levels. Support. Care Cancer 2009, 17, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, N.M.; Rothstein, J.H.; Pejaver, V.; Middha, S.; McDonnell, S.K.; Baheti, S.; Musolf, A.; Li, Q.; Holzinger, E.; Karyadi, D.; et al. REVEL: An ensemble method for predicting the pathogenicity of rare missense variants. Am. J. Hum. Genet. 2016, 99, 877–885. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugartondo, N.; Martínez-Gil, N.; Esteve, M.; Garcia-Giralt, N.; Roca-Ayats, N.; Ovejero, D.; Nogués, X.; Díez-Pérez, A.; Rabionet, R.; Grinberg, D.; et al. Functional Analyses of Four CYP1A1 Missense Mutations Present in Patients with Atypical Femoral Fractures. Int. J. Mol. Sci. 2021, 22, 7395. https://doi.org/10.3390/ijms22147395
Ugartondo N, Martínez-Gil N, Esteve M, Garcia-Giralt N, Roca-Ayats N, Ovejero D, Nogués X, Díez-Pérez A, Rabionet R, Grinberg D, et al. Functional Analyses of Four CYP1A1 Missense Mutations Present in Patients with Atypical Femoral Fractures. International Journal of Molecular Sciences. 2021; 22(14):7395. https://doi.org/10.3390/ijms22147395
Chicago/Turabian StyleUgartondo, Nerea, Núria Martínez-Gil, Mònica Esteve, Natàlia Garcia-Giralt, Neus Roca-Ayats, Diana Ovejero, Xavier Nogués, Adolfo Díez-Pérez, Raquel Rabionet, Daniel Grinberg, and et al. 2021. "Functional Analyses of Four CYP1A1 Missense Mutations Present in Patients with Atypical Femoral Fractures" International Journal of Molecular Sciences 22, no. 14: 7395. https://doi.org/10.3390/ijms22147395
APA StyleUgartondo, N., Martínez-Gil, N., Esteve, M., Garcia-Giralt, N., Roca-Ayats, N., Ovejero, D., Nogués, X., Díez-Pérez, A., Rabionet, R., Grinberg, D., & Balcells, S. (2021). Functional Analyses of Four CYP1A1 Missense Mutations Present in Patients with Atypical Femoral Fractures. International Journal of Molecular Sciences, 22(14), 7395. https://doi.org/10.3390/ijms22147395





