Raman Study on Lipid Droplets in Hepatic Cells Co-Cultured with Fatty Acids
Abstract
:1. Introduction
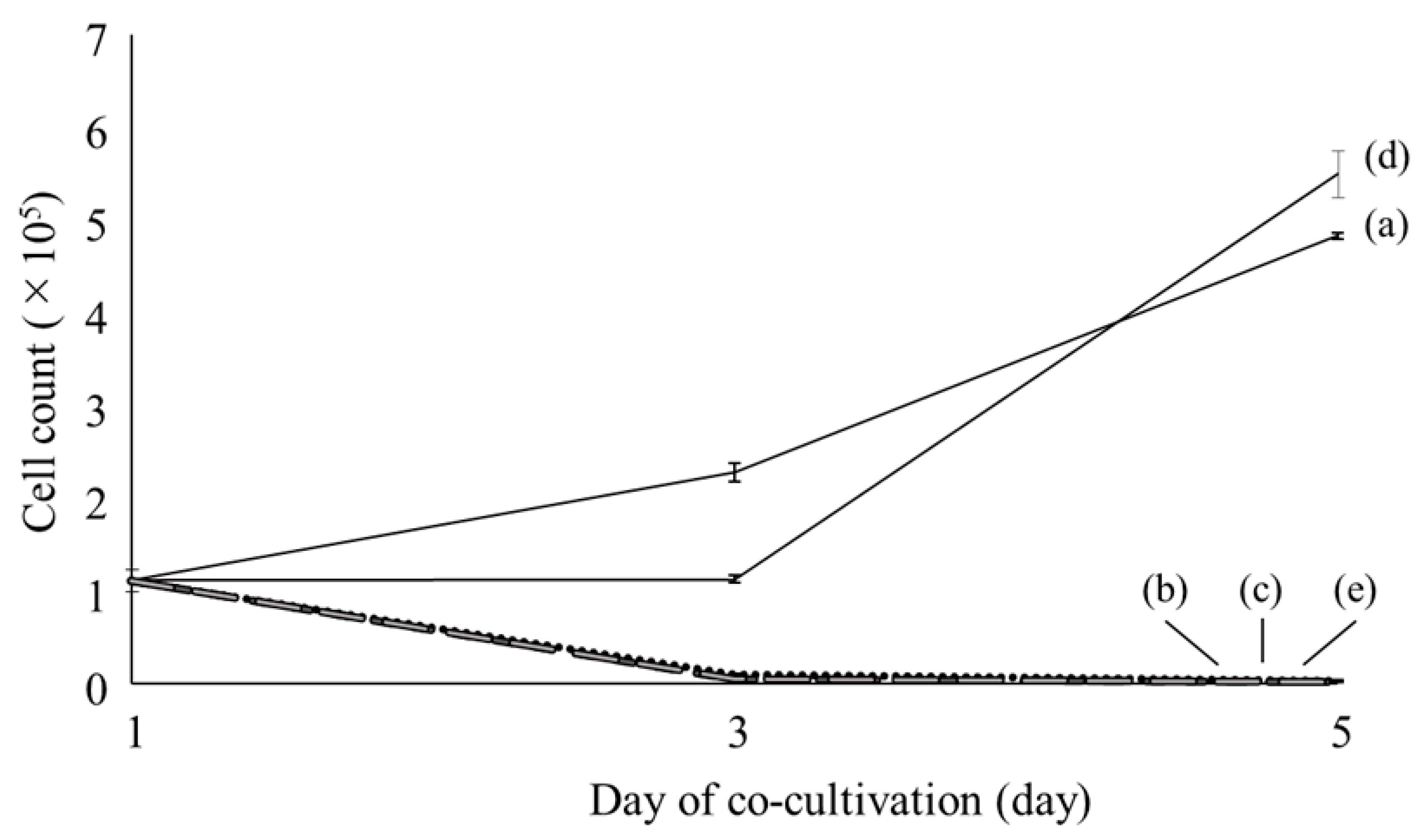
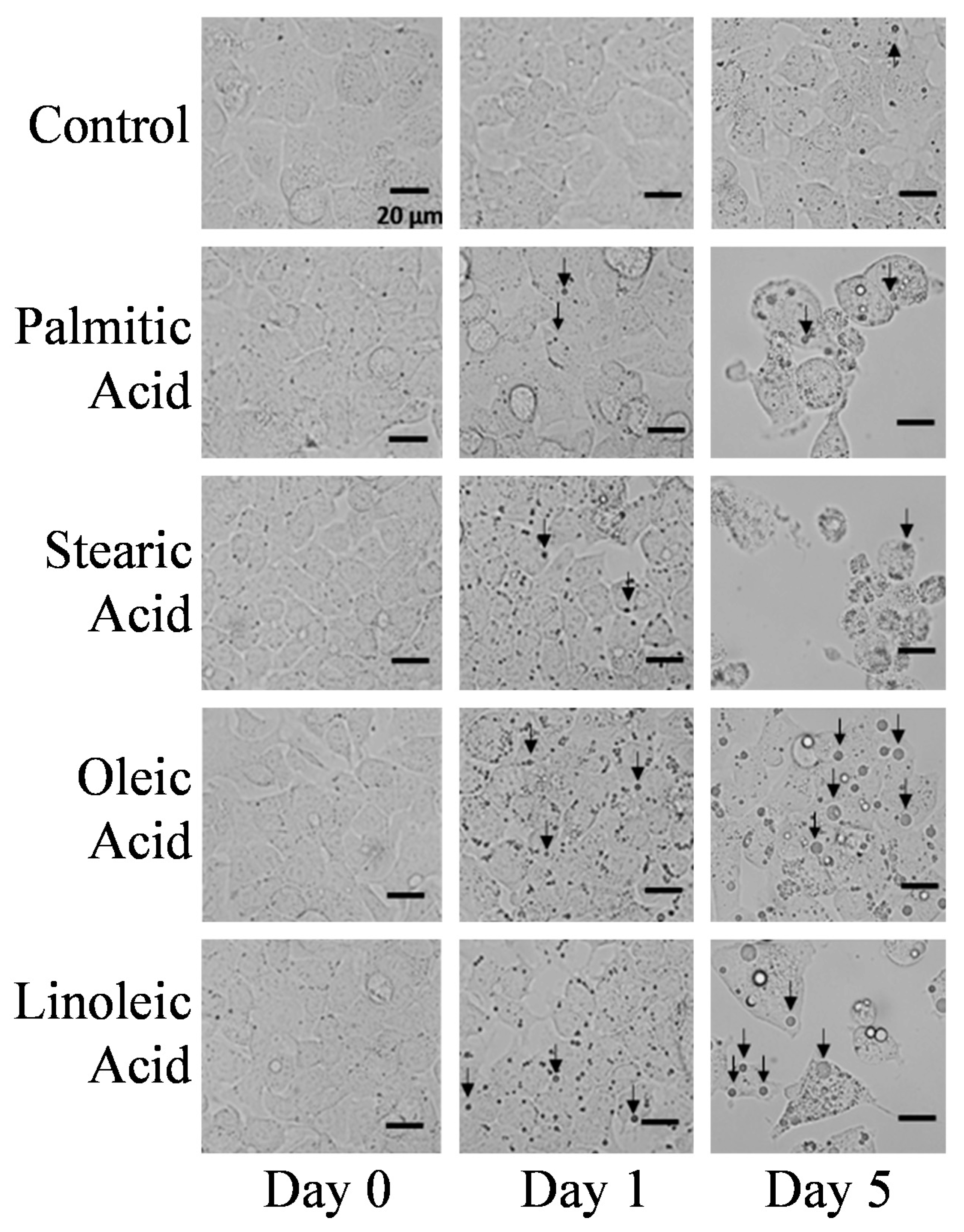
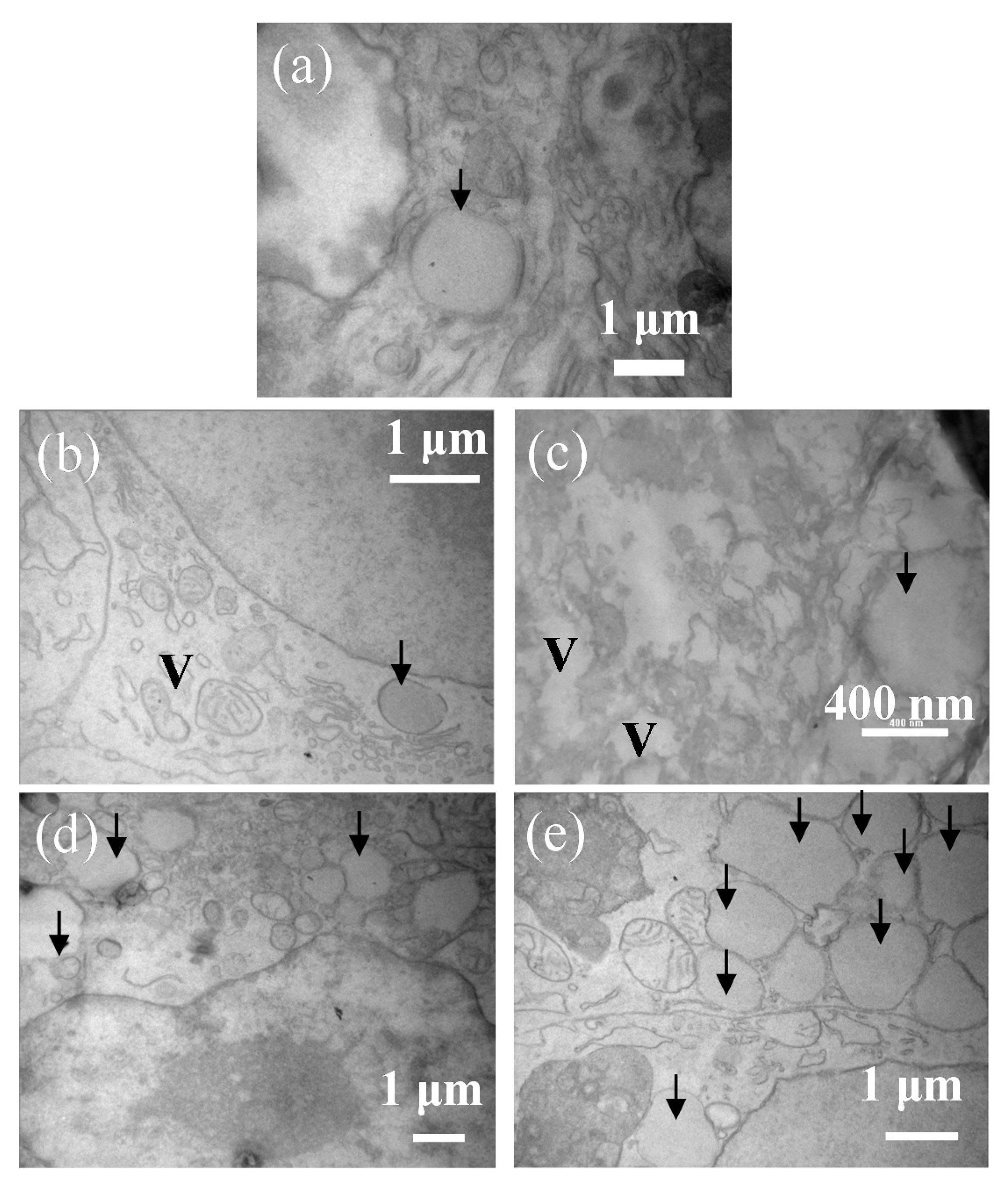
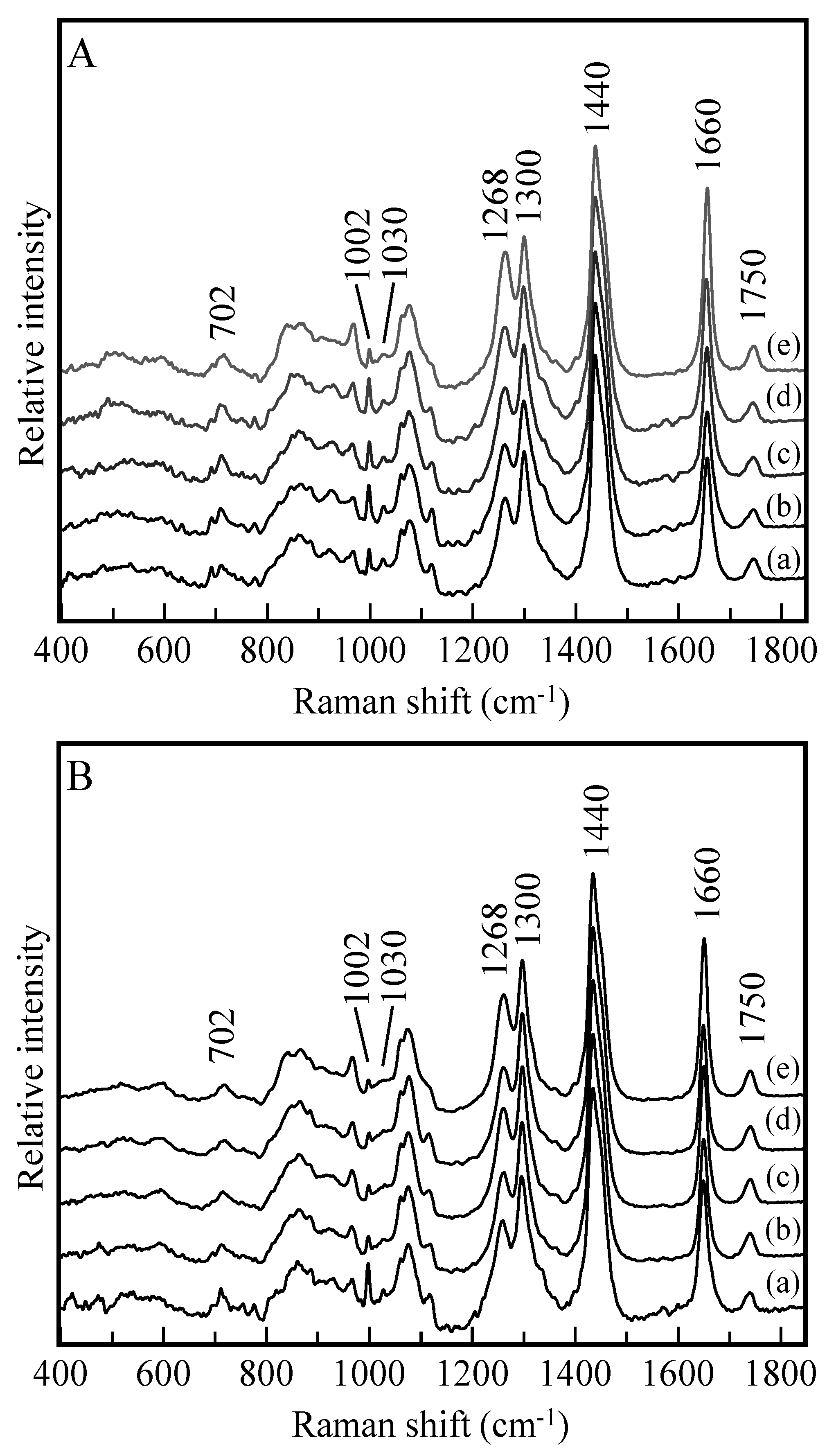
2. Results and Discussion
2.1. Microscopic Observations
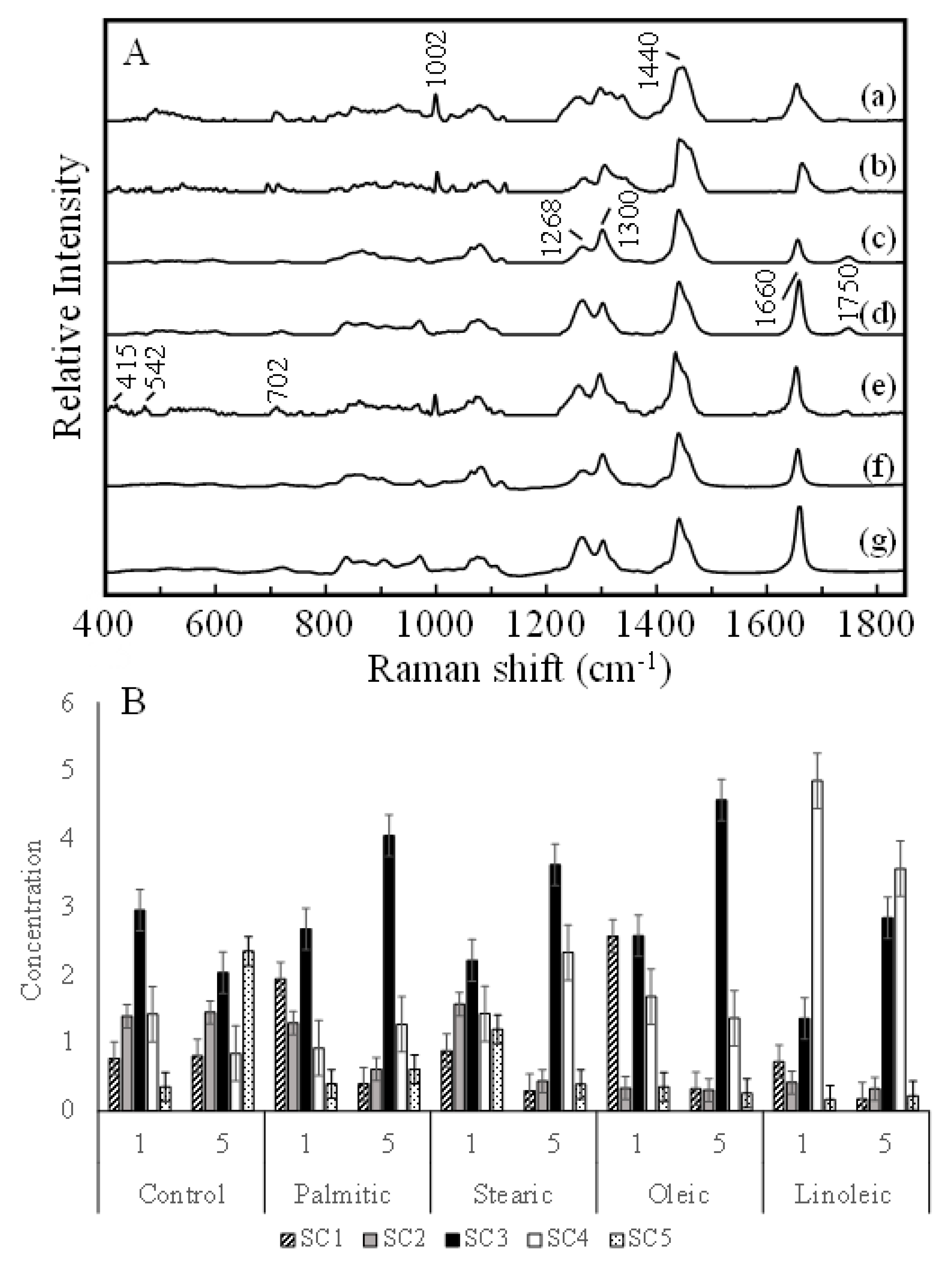
2.2. Raman Spectroscopy Analysis
3. Materials and Methods
3.1. Cell Culture
3.2. Confocal Raman Micro-Spectroscopy
3.3. Data Analysis
3.4. Transmission Electron Microscopy (TEM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 656–665. [Google Scholar] [CrossRef]
- Kanda, T.; Goto, T.; Hirotsu, Y.; Masuzaki, R.; Moriyama, M.; Omata, M. Molecular Mechanisms: Connections between Nonalcoholic Fatty Liver Disease, Steatohepatitis and Hepatocellular Carcinoma. Int. J. Mol. Sci. 2020, 21, 1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geisler, C.E.; Renquist, B.J. Hepatic lipid accumulation: Cause and consequence of dysregulated glucoregulatory hormones. J. Endocrinol. 2017, 234, R1–R21. [Google Scholar] [CrossRef]
- Parker, R. The role of adipose tissue in fatty liver diseases. Liver Res. 2018, 2, 35–42. [Google Scholar] [CrossRef]
- Schäffler, A.; Schölmerich, J.; Büchler, C. Mechanisms of disease: Adipocytokines and visceral adipose tissue--emerging role in nonalcoholic fatty liver disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Wree, A.; Kahraman, A.; Gerken, G.; Canbay, A. Obesity affects the liver—The link between adipocytes and hepatocytes. Digestion 2011, 83, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Rosso, C.; Kazankov, K.; Younes, R.; Esmaili, S.; Marietti, M.; Sacco, M.; Carli, F.; Gaggini, M.; Salomone, F.; Møller, H.J.; et al. Crosstalk between adipose tissue insulin resistance and liver macrophages in non-alcoholic fatty liver disease. J. Hepatol. 2019, 71, 1012–1021. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, D.; Topczewski, F.; Pagliassotti, M.J. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E275–E281. [Google Scholar] [CrossRef]
- Ricchi, M.; Odoardi, M.R.; Carulli, L.; Anzivino, C.; Ballestri, S.; Pinetti, A.; Fantoni, L.I.; Marra, F.; Bertolotti, M.; Banni, S.; et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J. Gastroenterol. Hepatol. 2009, 24, 830–840. [Google Scholar] [CrossRef]
- Mei, S.; Ni, H.-M.; Manley, S.; Bockus, A.; Kassel, K.M.; Luyendyk, J.P.; Copple, B.L.; Ding, W.-X. Differential Roles of Unsaturated and Saturated Fatty Acids on Autophagy and Apoptosis in Hepatocytes. J. Pharmacol. Exp. Ther. 2011, 339, 487–498. [Google Scholar] [CrossRef] [Green Version]
- Najt, C.P.; Khan, S.A.; Heden, T.D.; Witthuhn, B.A.; Perez, M.; Heier, J.L.; Mead, L.E.; Franklin, M.P.; Karanja, K.K.; Graham, M.J.; et al. Lipid Droplet-Derived Monounsaturated Fatty Acids Traffic via PLIN5 to Allosterically Activate SIRT1. Molecular Cell 2020, 77, 810–824. [Google Scholar] [CrossRef]
- Puri, P.; Baillie, R.A.; Wiest, M.M.; Mirshahi, F.; Choudhury, J.; Cheung, O.; Sargeant, C.; Contos, M.J.; Sanyal, A.J. A Lipidomic Analysis of Nonalcoholic Fatty Liver Disease. Hepatology 2007, 46, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xue, R.; Zhang, Z.; Yang, X.; Shi, H. Palmitic and linoleic acids induce ER stress and apoptosis in hepatoma cells. Lipids Health Dis. 2012, 11, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meksiarun, P.; Andriana, B.B.; Matsuyoshi, H.; Sato, H. Non-invasive Quantitative Analysis of Specific Fat Accumulation in Subcutaneous Adipose Tissues using Raman Spectroscopy. Sci. Rep. 2016, 6, 37068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kochan, K.; Maslak, E.; Krafft, C.; Kostogrys, R.; Chlopicki, S.; Baranska, M. Raman spectroscopy analysis of lipid droplets content, distribution and saturation level in Non-Alcoholic Fatty Liver Disease in mice. J. Biophotonics 2015, 8, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, E.; Kus, E.; Wislocka, A.; Kukla, B.; Sierka, E.; Untereiner, V.; Sockalingum, G.D.; Chlopicki, S.; Baranska, M. Raman spectroscopy–based insight into lipid droplets presence and contents in liver sinusoidal endothelial cells and hepatocytes. J. Biophotonics. 2019, 12, e201800290. [Google Scholar] [CrossRef]
- Emmison, N.; Agius, L. Fatty acid uptake and metabolism to ketone bodies and triacyglycerol in rat and human hepatocyte cultures is dependent on chain-length and degree of saturation. Effects of carnitine and glucagon. FEBS Lett. 1988, 236, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Matuszyk, E.; Baranska, M. Primary murine hepatocytes exposed to fatty acids analyzed by Raman and infrared microscopy. Clin. Spectrosc. 2021, 3, 100007. [Google Scholar] [CrossRef]
- Schie, I.W.; Nolte, L.; Pedersen, T.L.; Smith, Z.; Wu, J.; Yahiatène, I.; Newman, J.W.; Huser, T. Direct comparison of fatty acid ratios in single cellular lipid droplets as determined by comparative Raman spectroscopy and gas chromatography. Analyst 2013, 138, 6662–6670. [Google Scholar] [CrossRef] [Green Version]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods- a review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.; Faber, K.N.; de Meijer, V.E.; Blokzijl, H.; Moshage, H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol. Int. 2021, 15, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.; Varga, Á.; Kovács, A.L.; Takáts, S.; Juhász, G. How and why to study autophagy in Drosophila: It’s more than just a garbage chute. Methods 2015, 75, 151–161. [Google Scholar] [CrossRef]
- Schmidt, W.F.; Mookherji, S.; Crawford, M.A. Unit cell volume and liquid-phase immiscibility in oleate–stearate lipid mixtures. Chem. Phys. Lipids 2009, 158, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Afseth, N.K.; Segtnan, V.H.; Marquardt, B.J.; Wold, J.P. Raman and near-infrared spectroscopy for quantification of fat composition in a complex food model system. Appl. Spectrosc. 2005, 59, 1324–1332. [Google Scholar] [CrossRef]
- Nedeljković, A.; Rösch, P.; Popp, J.; Miočinović, J.; Radovanović, M.; Pudja, P. Raman Spectroscopy as a Rapid Tool for Quantitative Analysis of Butter Adulterated with Margarine. Food Anal. Methods 2016, 9, 1315–1320. [Google Scholar] [CrossRef]
- Karacaglar, N.N.Y.; Bulat, T.; Boyaci, I.H.; Topcu, A. Raman spectroscopy coupled with chemometric methods for the discrimination of foreign fats and oils in cream and yogurt. J. Food Drug Anal. 2019, 27, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsabeeh, N.; Chausse, B.; Kakimoto, P.A.; Kowaltowski, A.J.; Shirihai, O. Cell Culture Models of Fatty Acid Overload: Problems and Solutions. Biochim. Biophys. Acta 2018, 2, 143–151. [Google Scholar] [CrossRef]
- Notingher, I.; Verrier, S.; Romanska, H.; Bishop, A.E.; Polak, J.M.; Hench, L.L. In situ Characterisation of Living Cells by Raman Spectroscopy. J. Spectrosc. 2002, 16, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Ashton, L.; Wright, K.; Smith, R. Raman Spectroscopy: An evolving technique for live cell studies. Analyst 2016, 141, 3590–3600. [Google Scholar] [CrossRef] [Green Version]
- Noothalapati, H.; Iwasaki, K.; Yamamoto, T. Biological and Medical Applications of Multivariate Curve Resolution Assisted Raman Spectroscopy. Anal. Sci. 2017, 33, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Treatment Time (day) | Average Number of Lipid Droplets/Cell | Average Size of Lipid Droplets | |
|---|---|---|---|
| Control | 1 | 0.20 | 1.88 ± 0.30 |
| 5 | 0.27 | 2.10 ± 0.43 | |
| Palmitic acid | 1 | 0.55 | 2.36 ± 0.51 |
| 5 | 0.87 | 2.62 ± 1.43 | |
| Stearic acid | 1 | 0.72 | 2.09 ± 0.72 |
| 5 | 1.69 | 3.63 ± 2.20 | |
| Oleic acid | 1 | 0.78 | 2.14 ± 0.48 |
| 5 | 5.45 | 3.01 ± 1.52 | |
| Linoleic acid | 1 | 1.09 | 2.51 ± 1.10 |
| 5 | 3.63 | 4.47 ± 2.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paramitha, P.N.; Zakaria, R.; Maryani, A.; Kusaka, Y.; Andriana, B.B.; Hashimoto, K.; Nakazawa, H.; Kato, S.; Sato, H. Raman Study on Lipid Droplets in Hepatic Cells Co-Cultured with Fatty Acids. Int. J. Mol. Sci. 2021, 22, 7378. https://doi.org/10.3390/ijms22147378
Paramitha PN, Zakaria R, Maryani A, Kusaka Y, Andriana BB, Hashimoto K, Nakazawa H, Kato S, Sato H. Raman Study on Lipid Droplets in Hepatic Cells Co-Cultured with Fatty Acids. International Journal of Molecular Sciences. 2021; 22(14):7378. https://doi.org/10.3390/ijms22147378
Chicago/Turabian StyleParamitha, Pradjna N., Riki Zakaria, Anisa Maryani, Yukako Kusaka, Bibin B. Andriana, Kosuke Hashimoto, Hiromitsu Nakazawa, Satoru Kato, and Hidetoshi Sato. 2021. "Raman Study on Lipid Droplets in Hepatic Cells Co-Cultured with Fatty Acids" International Journal of Molecular Sciences 22, no. 14: 7378. https://doi.org/10.3390/ijms22147378
APA StyleParamitha, P. N., Zakaria, R., Maryani, A., Kusaka, Y., Andriana, B. B., Hashimoto, K., Nakazawa, H., Kato, S., & Sato, H. (2021). Raman Study on Lipid Droplets in Hepatic Cells Co-Cultured with Fatty Acids. International Journal of Molecular Sciences, 22(14), 7378. https://doi.org/10.3390/ijms22147378






