Platelet and Erythrocyte Extravasation across Inflamed Corneal Venules Depend on CD18, Neutrophils, and Mast Cell Degranulation
Abstract
1. Introduction
2. Results
2.1. CD18 Is Important for Platelet and Erythrocyte (RBC) Extravasation across Inflamed Venules in the Abraded Cornea
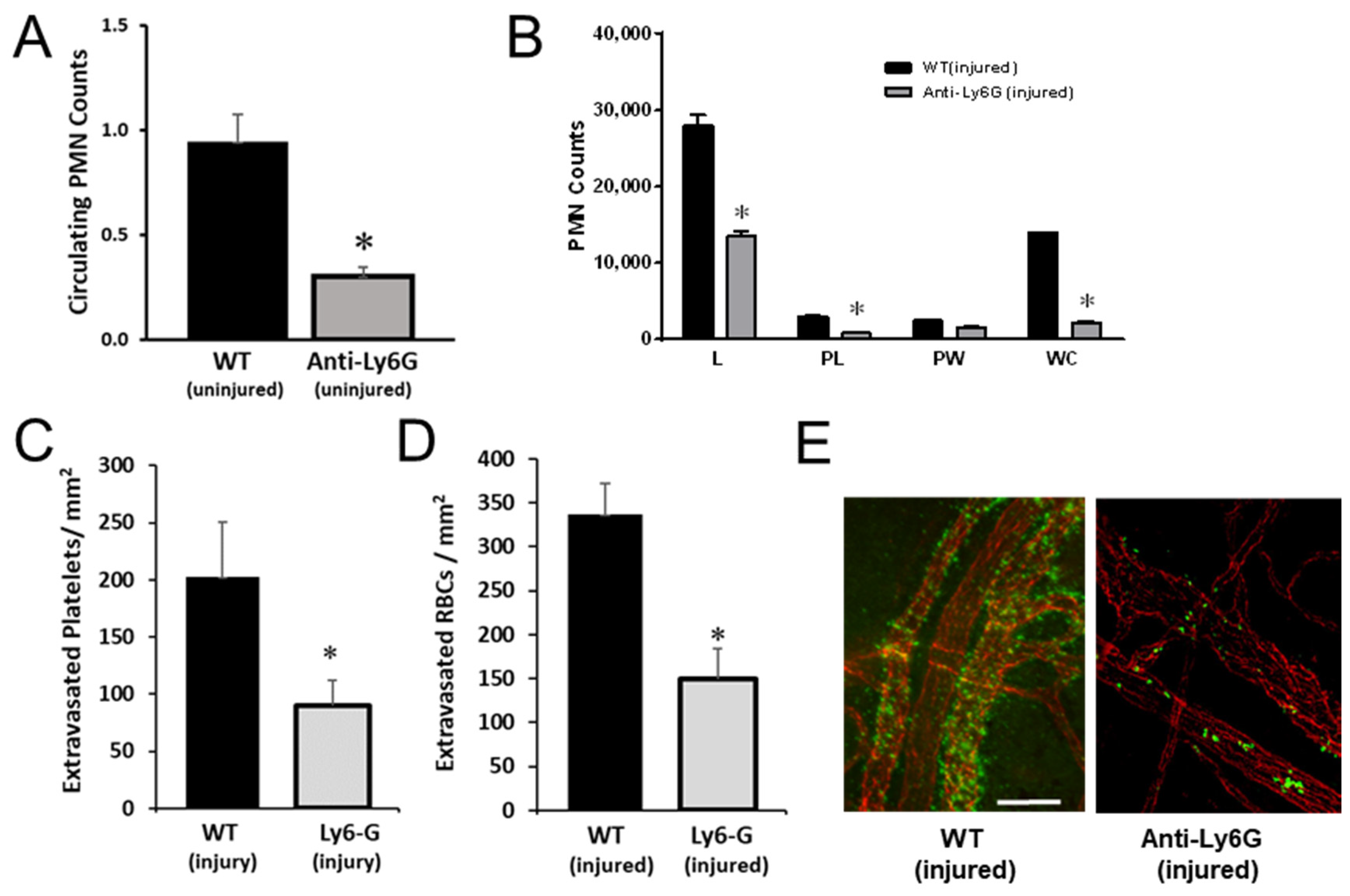
2.2. PMNs Are Important for Platelet and RBC Extravasation across Inflamed Venules in the Abraded Cornea
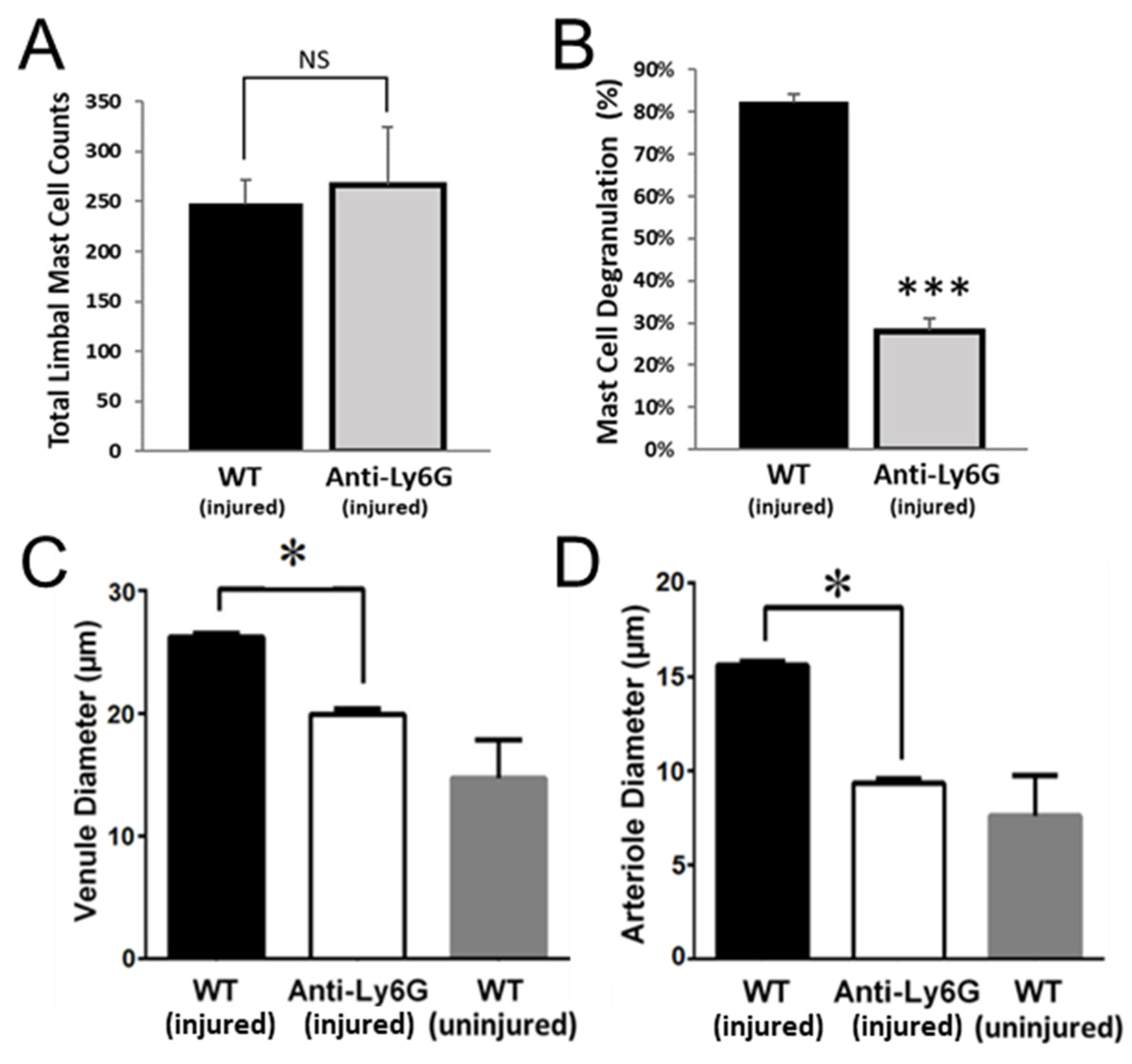
2.3. Mast Cell Degranulation Is Critical for Platelet and RBC Extravasation in the Abraded Cornea
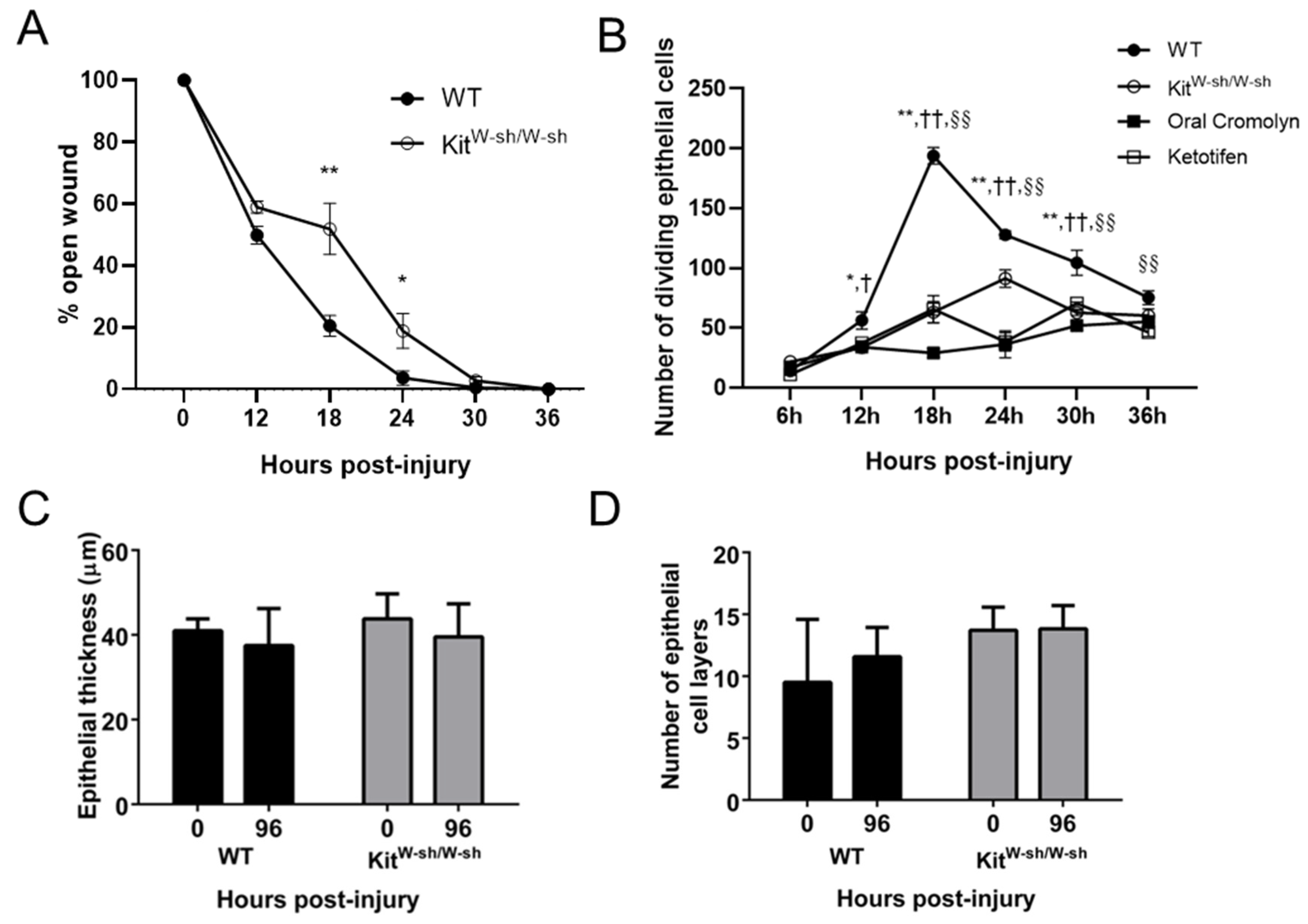
2.4. Mast Cells Are Important for Recovery of the Epithelium in the Abraded Cornea
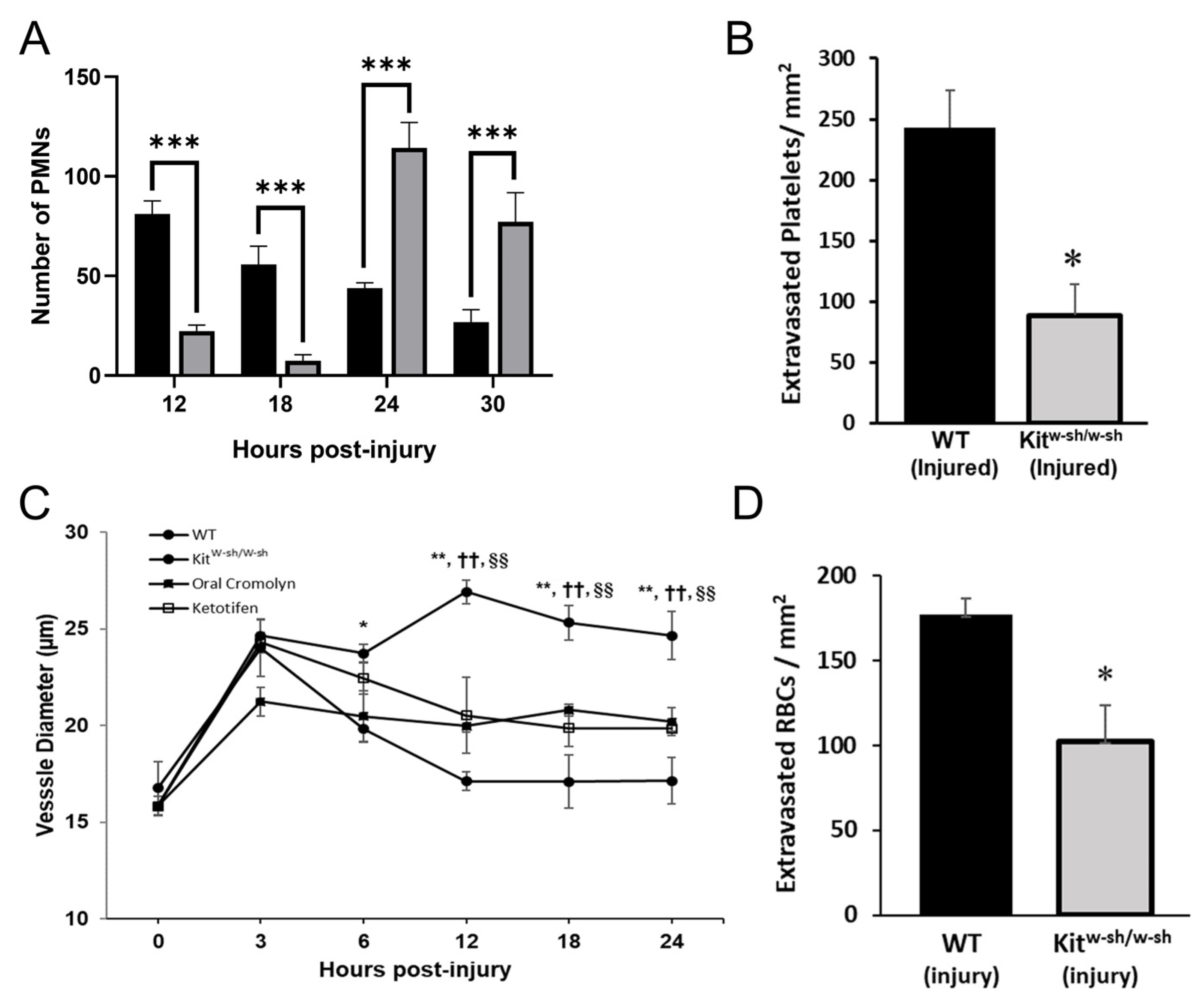
2.5. PMNs Are Important for Mast Cell Degranulation and Venule Engorgement
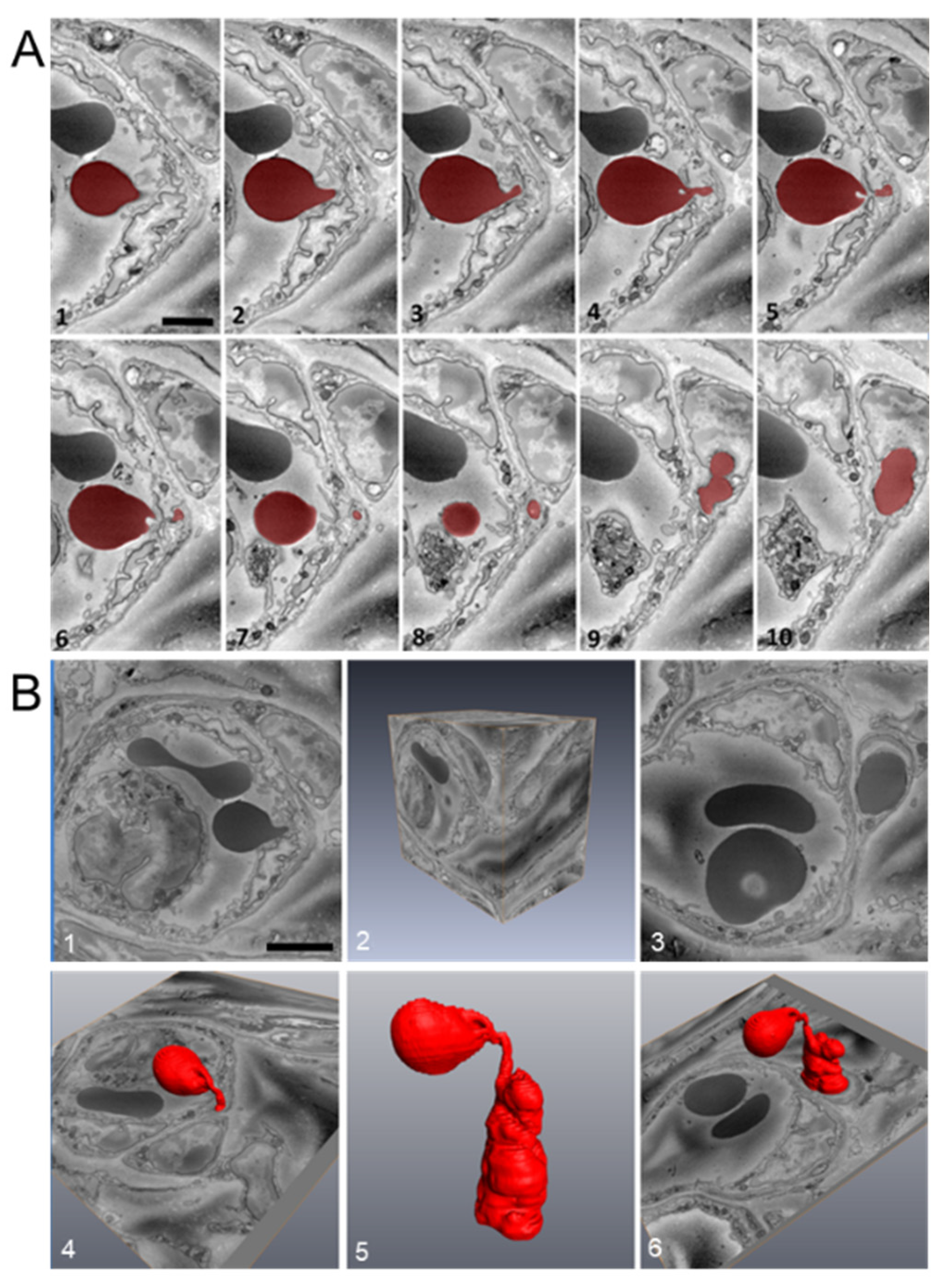
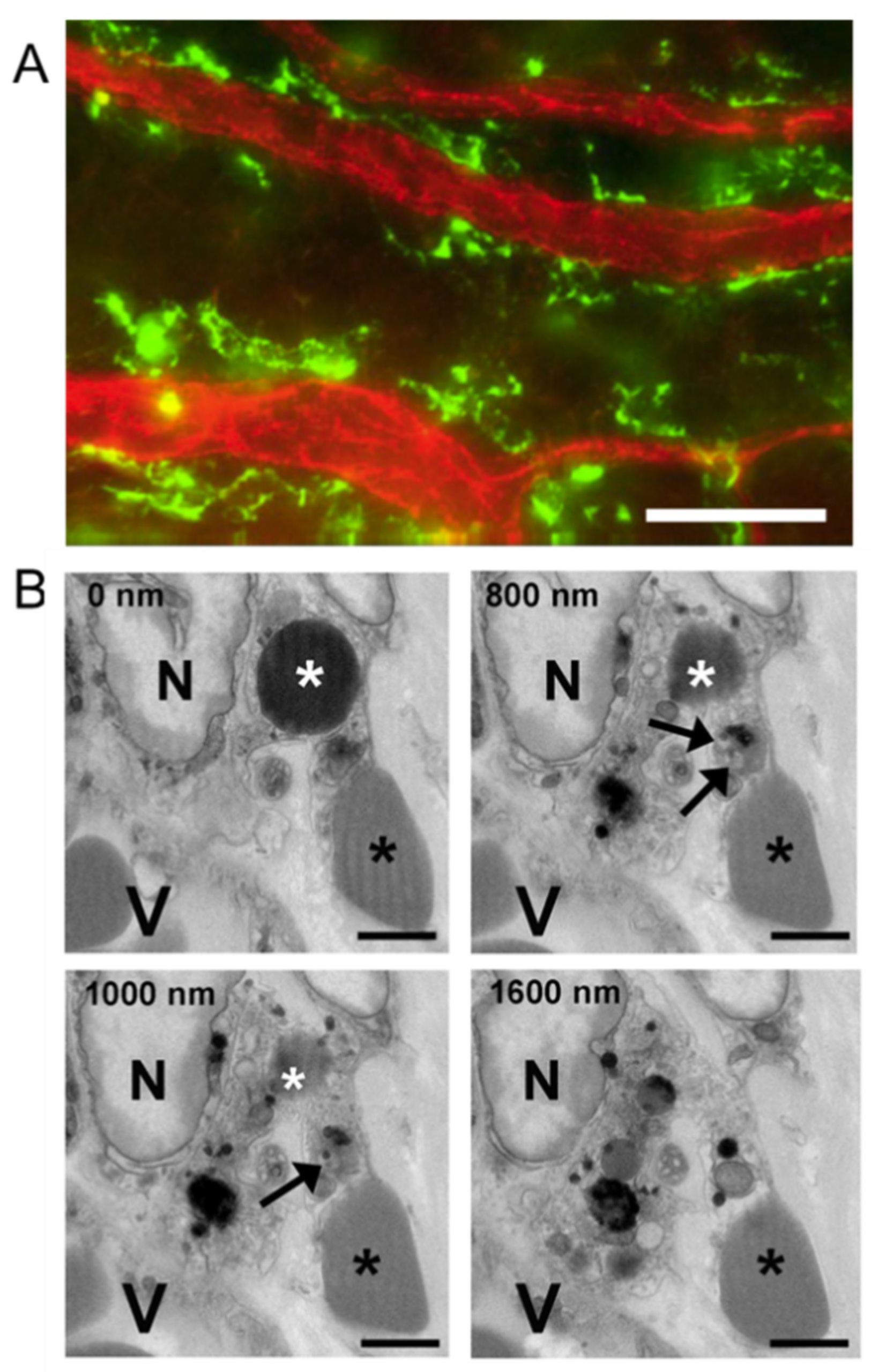
2.6. Platelet Extravasation across Inflamed Venules Is Accompanied by RBC Extravasation: Role of Disruption of Microvascular Wall Integrity
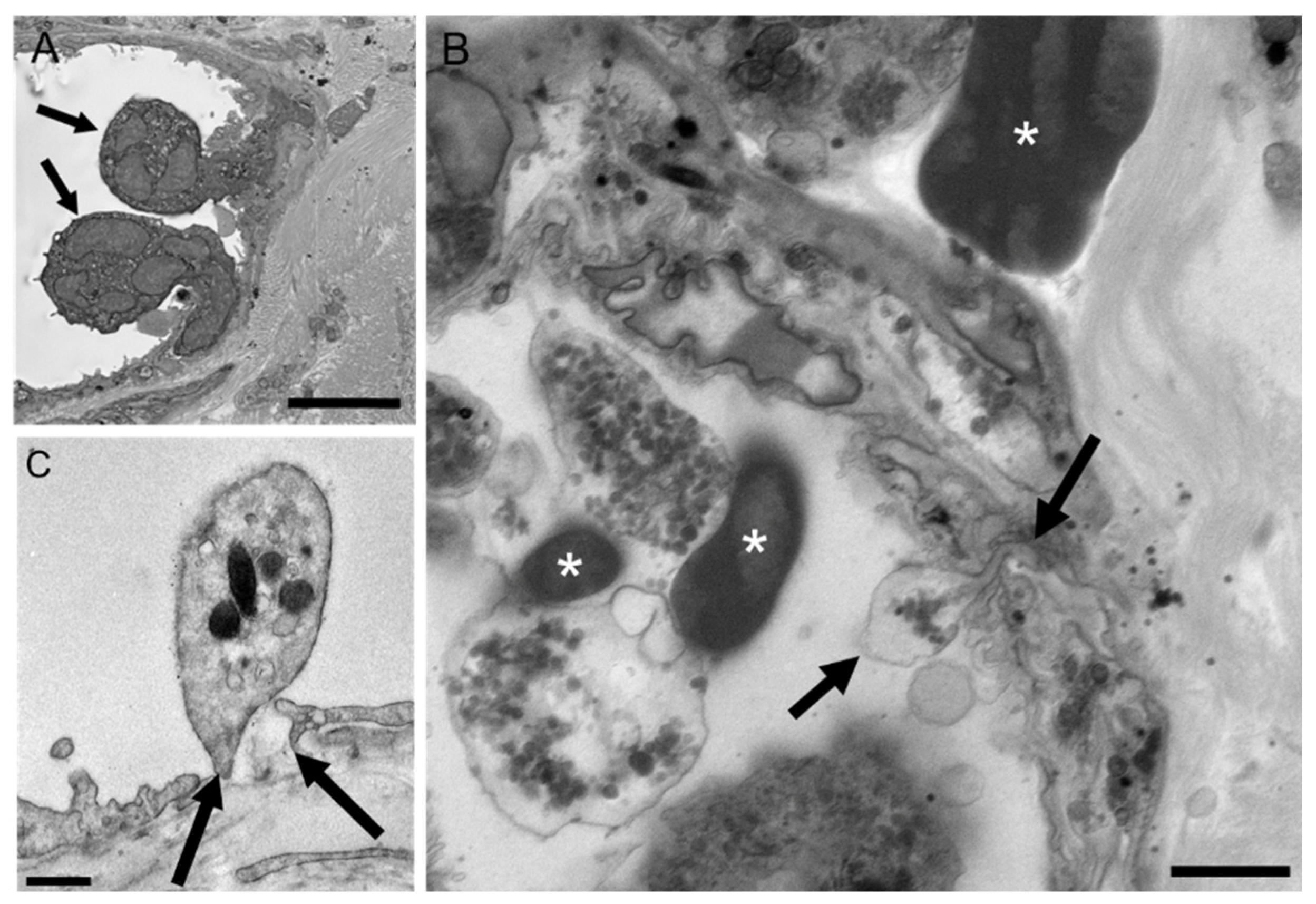
2.7. Extravasated RBCs and Platelets Are Phagocytosed by Perivascular Macrophages
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ethics Statement
4.3. Wound Protocol
4.4. Treatment Protocol
4.5. Immunofluorescence Staining
4.6. Electron Microscopy
4.7. Morphometric Analysis of Epithelial Thickness
4.8. Morphometric Analysis of Platelet and RBC Recruitment and Blood Vessel Diameter
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allison, F.; Smith, M.R.; Wood, W.B. Studies on the pathogenesis of acute inflammation. I. The inflammatory reaction to thermal injury observed in the rabbit ear chamber. J. Exp. Med. 1955, 102, 655–668. [Google Scholar]
- Sreeramkumar, V.; Adrover, J.M.; Ballesteros, I.; Cuartero, M.I.; Rossaint, J.; Bilbao, I.; Nacher, M.; Pitaval, C.; Radovanovic, I.; Fukui, Y.; et al. Neutrophils scan for activated platelets to initiate inflammation. Science 2014, 346, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Singbartl, K.; Ley, K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J. Clin. Investig. 2006, 116, 3211–3219. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rumbaut, R.E.; Burns, A.R.; Smith, C.W. Platelet Response to Corneal Abrasion Is Necessary for Acute Inflammation and Efficient Re-epithelialization. Invest Ophthalmol. Vis. Sci. 2006, 47, 4794–4802. [Google Scholar]
- Walker, M.E.; Hatfield, J.K.; Brown, M.A. New insights into the role of mast cells in autoimmunity: Evidence for a common mechanism of action? Biochim. Biophys. Acta 2012, 1822, 57–65. [Google Scholar] [CrossRef]
- Li, Z.; Burns, A.R.; Han, L.; Rumbaut, R.E.; Smith, C.W. IL-17 and VEGF are necessary for efficient corneal nerve regeneration. Am. J. Pathol. 2011, 178, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Burns, A.R.; Smith, C.W. Lymphocyte function-associated antigen-1-dependent inhibition of corneal wound healing. Am. J. Pathol. 2006, 169, 1590–1600. [Google Scholar] [CrossRef]
- Li, Z.; Burns, A.R.; Smith, C.W. Two waves of neutrophil emigration in response to corneal epithelial abrasion: Distinct adhesion molecule requirements. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1947–1955. [Google Scholar] [CrossRef]
- Feng, D.; Nagy, J.A.; Pyne, K.; Dvorak, H.F.; Dvorak, A.M. Platelets exit venules by a transcellular pathway at sites of F-met peptide-induced acute inflammation in guinea pigs. Int. Arch. Allergy Immunol. 1998, 116, 188–195. [Google Scholar] [CrossRef]
- Lellouch-Tubiana, A.; Lefort, J.; Pirotzky, E.; Vargaftig, B.B.; Pfister, A. Ultrastructural evidence for extravascular platelet recruitment in the lung upon intravenous injection of platelet-activating factor (PAF-acether) to guinea-pigs. Br. J. Exp. Pathol. 1985, 66, 345–355. [Google Scholar]
- Pitchford, S.C.; Momi, S.; Baglioni, S.; Casali, L.; Giannini, S.; Rossi, R.; Page, C.P.; Gresele, P. Allergen induces the migration of platelets to lung tissue in allergic asthma. Am. J. Respir. Crit. Care Med. 2008, 177, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.W.; Vijayan, K.V.; Rumbaut, R.E. Platelets and Their Interactions with Other Immune Cells. Compr. Physiol. 2015, 5, 1265–1280. [Google Scholar] [CrossRef] [PubMed]
- Leslie, M. Cell biology. Beyond clotting: The powers of platelets. Science 2010, 328, 562–564. [Google Scholar] [CrossRef]
- Lam, F.W.; Burns, A.R.; Smith, C.W.; Rumbaut, R.E. Platelets enhance neutrophil transendothelial migration via P-selectin glycoprotein ligand-1. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H468–H475. [Google Scholar] [CrossRef]
- Lam, F.W.; Phillips, J.; Landry, P.; Magadi, S.; Smith, C.W.; Rumbaut, R.E.; Burns, A.R. Platelet Recruitment Promotes Keratocyte Repopulation following Corneal Epithelial Abrasion in the Mouse. PLoS ONE 2015, 10, e0118950. [Google Scholar] [CrossRef]
- Li, Z.; Burns, A.R.; Rumbaut, R.E.; Smith, C.W. gamma delta T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epithelial repair after corneal abrasion. Am. J. Pathol. 2007, 171, 838–845. [Google Scholar] [CrossRef]
- Zhang, W.; Magadi, S.; Li, Z.; Smith, C.W.; Burns, A.R. IL-20 promotes epithelial healing of the injured mouse cornea. Exp. Eye Res. 2017, 154, 22–29. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Ivetic, A.; Hoskins Green, H.L.; Hart, S.J. L-selectin: A Major Regulator of Leukocyte Adhesion, Migration and Signaling. Front. Immunol. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.D. Neutrophil transendothelial migration: Updates and new perspectives. Blood 2019, 133, 2149–2158. [Google Scholar] [CrossRef]
- Wilson, R.W.; Ballantyne, C.M.; Smith, C.W.; Montgomery, C.; Bradley, A.; O’Brien, W.E.; Beaudet, A.L. Gene targeting yields a CD18-mutant mouse for study of inflammation. J. Immunol. 1993, 151, 1571–1578. [Google Scholar]
- Piguet, P.F.; Vesin, C.; Rochat, A. β2 integrin modulates platelet caspase activation and life span in mice. Eur. J. Cell Biol. 2001, 80, 171–177. [Google Scholar] [CrossRef]
- Philippeaux, M.M.; Vesin, C.; Tacchini-Cottier, F.; Piguet, P.F. Activated human platelets express beta2 integrin. Eur. J. Haematol. 1996, 56, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Grommes, J.; Alard, J.E.; Drechsler, M.; Wantha, S.; Mörgelin, M.; Kuebler, W.M.; Jacobs, M.; von Hundelshausen, P.; Markart, P.; Wygrecka, M.; et al. Disruption of platelet-derived chemokine heteromers prevents neutrophil extravasation in acute lung injury. Am. J. Respir. Crit. Care Med. 2012, 185, 628–636. [Google Scholar] [CrossRef]
- Finsterbusch, M.; Norman, M.U.; Hall, P.; Kitching, A.R.; Hickey, M.J. Platelet retention in inflamed glomeruli occurs via selective prolongation of interactions with immune cells. Kidney Int. 2019, 95, 363–374. [Google Scholar] [CrossRef]
- Tachimoto, H.; Bochner, B.S. The surface phenotype of human eosinophils. Chem. Immunol. 2000, 76, 45–62. [Google Scholar] [PubMed]
- Fernandez, N.J.; West, K.H.; Jackson, M.L.; Kidney, B.A. Immunohistochemical and histochemical stains for differentiating canine cutaneous round cell tumors. Vet. Pathol. 2005, 42, 437–445. [Google Scholar] [CrossRef]
- Teodosio, C.; Mayado, A.; Sánchez-Muñoz, L.; Morgado, J.M.; Jara-Acevedo, M.; Álvarez-Twose, I.; García-Montero, A.C.; Matito, A.; Caldas, C.; Escribano, L.; et al. The immunophenotype of mast cells and its utility in the diagnostic work-up of systemic mastocytosis. J. Leukoc. Biol. 2015, 97, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Hargrave, A.; Courson, J.A.; Pham, V.; Landry, P.; Magadi, S.; Shankar, P.; Hanlon, S.; Das, A.; Rumbaut, R.E.; Smith, C.W.; et al. Corneal dysfunction precedes the onset of hyperglycemia in a mouse model of diet-induced obesity. PLoS ONE 2020, 15, e0238750. [Google Scholar] [CrossRef]
- Li, Z.; Burns, A.R.; Byeseda, M.S.; Smith, C.W. CCL20, γδ T cells, and IL-22 in corneal epithelial healing. FASEB J 2011, 25, 2659–2668. [Google Scholar] [CrossRef] [PubMed]
- Fantozzi, R.; Brunelleschi, S.; Giuliattini, L.; Blandina, P.; Masini, E.; Cavallo, G.; Mannaioni, P.F. Mast cell and neutrophil interactions: A role for superoxide anion and histamine. Agents Actions 1985, 16, 260–264. [Google Scholar] [CrossRef]
- Buttari, B.; Profumo, E.; Rigano, R. Crosstalk between red blood cells and the immune system and its impact on atherosclerosis. Biomed Res. Int. 2015, 2015, 616834. [Google Scholar] [CrossRef]
- Rosenkranz, A.R.; Coxon, A.; Maurer, M.; Gurish, M.F.; Austen, K.F.; Friend, D.S.; Galli, S.J.; Mayadas, T.N. Impaired mast cell development and innate immunity in Mac-1 (CD11b/CD18, CR3)-deficient mice. J. Immunol. 1998, 161, 6463–6467. [Google Scholar] [PubMed]
- Huang, D.; Shi, F.D.; Jung, S.; Pien, G.C.; Wang, J.; Salazar-Mather, T.P.; He, T.T.; Weaver, J.T.; Ljunggren, H.G.; Biron, C.A.; et al. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006, 20, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Issekutz, A.C.; Rowter, D.; Springer, T.A. Role of ICAM-1 and ICAM-2 and alternate CD11/CD18 ligands in neutrophil transendothelial migration. J. Leukoc. Biol. 1999, 65, 117–126. [Google Scholar] [CrossRef]
- Simon, D.I.; Chen, Z.; Xu, H.; Li, C.Q.; Dong, J.; McIntire, L.V.; Ballantyne, C.M.; Zhang, L.; Furman, M.I.; Berndt, M.C.; et al. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J. Exp. Med. 2000, 192, 193–204. [Google Scholar] [CrossRef]
- Wu, X.; Helfrich, M.H.; Horton, M.A.; Feigen, L.P.; Lefkowith, J.B. Fibrinogen mediates platelet-polymorphonuclear leukocyte cooperation during immune-complex glomerulonephritis in rats. J. Clin. Investig. 1994, 94, 928–936. [Google Scholar] [CrossRef][Green Version]
- Diacovo, T.G.; deFougerolles, A.R.; Bainton, D.F.; Springer, T.A. A functional integrin ligand on the surface of platelets: Intercellular adhesion molecule-2. J. Clin. Investig. 1994, 94, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Kuijper, P.H.; Gallardo Tores, H.I.; Lammers, J.W.; Sixma, J.J.; Koenderman, L.; Zwaginga, J.J. Platelet associated fibrinogen and ICAM-2 induce firm adhesion of neutrophils under flow conditions. Thromb. Haemost. 1998, 80, 443–448. [Google Scholar] [CrossRef]
- Weber, C.; Springer, T.A. Neutrophil accumulation on activated, surface-adherent platelets in flow is mediated by interaction of Mac-1 with fibrinogen bound to alphaIIbbeta3 and stimulated by platelet-activating factor. J. Clin. Investig. 1997, 100, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sakuma, M.; Chen, Z.; Ustinov, V.; Shi, C.; Croce, K.; Zago, A.C.; Lopez, J.; Andre, P.; Plow, E.; et al. Leukocyte engagement of platelet glycoprotein Ibalpha via the integrin Mac-1 is critical for the biological response to vascular injury. Circulation 2005, 112, 2993–3000. [Google Scholar] [CrossRef]
- Evangelista, V.; Manarini, S.; Sideri, R.; Rotondo, S.; Martelli, N.; Piccoli, A.; Totani, L.; Piccardoni, P.; Vestweber, D.; de Gaetano, G.; et al. Platelet/polymorphonuclear leukocyte interaction: P-selectin triggers protein-tyrosine phosphorylation-dependent CD11b/CD18 adhesion: Role of PSGL-1 as a signaling molecule. Blood 1999, 93, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Ihanus, E.; Uotila, L.M.; Toivanen, A.; Varis, M.; Gahmberg, C.G. Red-cell ICAM-4 is a ligand for the monocyte/macrophage integrin CD11c/CD18: Characterization of the binding sites on ICAM-4. Blood 2007, 109, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, L.; Schiefelbein, K.; Lipsky, S.; Leunig, A.; Hoffknecht, M.; Pekayvaz, K.; Raude, B.; Marx, C.; Ehrlich, A.; Pircher, J.; et al. Vascular surveillance by haptotactic blood platelets in inflammation and infection. Nat. Commun. 2020, 11, 5778. [Google Scholar] [CrossRef]
- Witte, A.; Rohlfing, A.K.; Dannenmann, B.; Dicenta, V.; Nasri, M.; Kolb, K.; Sudmann, J.; Castor, T.; Rath, D.; Borst, O.; et al. The chemokine CXCL14 mediates platelet function and migration via direct interaction with CXCR4. Cardiovasc. Res. 2021, 117, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Czapiga, M.; Gao, J.L.; Kirk, A.; Lekstrom-Himes, J. Human platelets exhibit chemotaxis using functional N-formyl peptide receptors. Exp. Hematol. 2005, 33, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Waugh, R.E.; Sassi, M. An in vitro model of erythroid egress in bone marrow. Blood 1986, 68, 250–257. [Google Scholar] [CrossRef]
- Shoukas, A.A.; Bohlen, H.G. Rat venular pressure-diameter relationships are regulated by sympathetic activity. Am. J. Physiol. Heart Circ. Physiol. 1990, 259, H674–H680. [Google Scholar] [CrossRef] [PubMed]
- White, J.G.; Burris, S.M.; Tukey, D.; Smith, C., 2nd; Clawson, C.C. Micropipette aspiration of human platelets: Influence of microtubules and actin filaments on deformability. Blood 1984, 64, 210–214. [Google Scholar] [PubMed]
- Haga, J.H.; Beaudoin, A.J.; White, J.G.; Strony, J. Quantification of the passive mechanical properties of the resting platelet. Ann. Biomed. Eng. 1998, 26, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.A. Structure and deformation properties of red blood cells: Concepts and quantitative methods. Methods Enzym. 1989, 173, 3–35. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Ieni, A.; Barresi, V.; Branca, G.; Alberto Caruso, R.; Tuccari, G. Mast Cell Interaction with Neutrophils in Human Gastric Carcinomas: Ultrastructural Observations. Anal. Cell. Pathol. 2016, 2016, 6891971. [Google Scholar] [CrossRef]
- Jung, J.Y.; Yasoshima, A.; Saegusa, J.; Nakayama, H.; Doi, K. Ultrastructural features of mast cells in picryl chloride (PCL)-induced contact dermatitis in IQI/Jic mice. Exp. Toxicol. Pathol. 2003, 54, 265–271. [Google Scholar] [CrossRef]
- Inamura, N.; Mekori, Y.A.; Bhattacharyya, S.P.; Bianchine, P.J.; Metcalfe, D.D. Induction and enhancement of Fc(epsilon)RI-dependent mast cell degranulation following coculture with activated T cells: Dependency on ICAM-1- and leukocyte function-associated antigen (LFA)-1-mediated heterotypic aggregation. J. Immunol. 1998, 160, 4026–4033. [Google Scholar] [PubMed]
- Karhausen, J.; Choi, H.W.; Maddipati, K.R.; Mathew, J.P.; Ma, Q.; Boulaftali, Y.; Lee, R.H.; Bergmeier, W.; Abraham, S.N. Platelets trigger perivascular mast cell degranulation to cause inflammatory responses and tissue injury. Sci. Adv. 2020, 6, eaay6314. [Google Scholar] [CrossRef]
- Singhal, R.; Chawla, S.; Batra, H.; Gupta, S.; Ojha, A.; Rathore, D.K.; Seth, T.; Guchhait, P. Engulfment of Hb-activated platelets differentiates monocytes into pro-inflammatory macrophages in PNH patients. Eur. J. Immunol. 2018, 48, 1285–1294. [Google Scholar] [CrossRef]
- Katz, H.R.; Austen, K.F. Mast cell deficiency, a game of kit and mouse. Immunity 2011, 35, 668–670. [Google Scholar] [CrossRef]
- Miyamoto, K.; Kobayashi, T.; Hayashi, Y.; Zhang, Y.; Hara, Y.; Higashine, M.; Shiraishi, A.; Ohashi, Y. Involvement of stem cell factor and c-kit in corneal wound healing in mice. Mol. Vis. 2012, 18, 1505–1515. [Google Scholar] [PubMed]
- Rodewald, H.R.; Feyerabend, T.B. Widespread immunological functions of mast cells: Fact or fiction? Immunity 2012, 37, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Smith, C.W.; Zhang, W.; Burns, A.R.; Li, Z. NK cells modulate the inflammatory response to corneal epithelial abrasion and thereby support wound healing. Am. J. Pathol. 2012, 181, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Skalak, R.; Branemark, P.I.; Ekholm, R. Erythrocyte adherence and diapedesis. Some aspects of a possible mechanism based on vital and electron microscopic observations. Angiology 1970, 21, 224–239. [Google Scholar] [CrossRef]
- Ng, M.F. The role of mast cells in wound healing. Int. Wound J. 2010, 7, 55–61. [Google Scholar] [CrossRef]
- Tharp, M.D.; Seelig, L.L., Jr.; Tigelaar, R.E.; Bergstresser, P.R. Conjugated avidin binds to mast cell granules. J. Histochem. Cytochem. 1985, 33, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Ho-Tin-Noé, B.; Boulaftali, Y.; Camerer, E. Platelets and vascular integrity: How platelets prevent bleeding in inflammation. Blood 2018, 131, 277–288. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De La Cruz, A.; Hargrave, A.; Magadi, S.; Courson, J.A.; Landry, P.T.; Zhang, W.; Lam, F.W.; Bray, M.A.; Smith, C.W.; Burns, A.R.; et al. Platelet and Erythrocyte Extravasation across Inflamed Corneal Venules Depend on CD18, Neutrophils, and Mast Cell Degranulation. Int. J. Mol. Sci. 2021, 22, 7360. https://doi.org/10.3390/ijms22147360
De La Cruz A, Hargrave A, Magadi S, Courson JA, Landry PT, Zhang W, Lam FW, Bray MA, Smith CW, Burns AR, et al. Platelet and Erythrocyte Extravasation across Inflamed Corneal Venules Depend on CD18, Neutrophils, and Mast Cell Degranulation. International Journal of Molecular Sciences. 2021; 22(14):7360. https://doi.org/10.3390/ijms22147360
Chicago/Turabian StyleDe La Cruz, Angie, Aubrey Hargrave, Sri Magadi, Justin A. Courson, Paul T. Landry, Wanyu Zhang, Fong W. Lam, Monica A. Bray, C. Wayne Smith, Alan R. Burns, and et al. 2021. "Platelet and Erythrocyte Extravasation across Inflamed Corneal Venules Depend on CD18, Neutrophils, and Mast Cell Degranulation" International Journal of Molecular Sciences 22, no. 14: 7360. https://doi.org/10.3390/ijms22147360
APA StyleDe La Cruz, A., Hargrave, A., Magadi, S., Courson, J. A., Landry, P. T., Zhang, W., Lam, F. W., Bray, M. A., Smith, C. W., Burns, A. R., & Rumbaut, R. E. (2021). Platelet and Erythrocyte Extravasation across Inflamed Corneal Venules Depend on CD18, Neutrophils, and Mast Cell Degranulation. International Journal of Molecular Sciences, 22(14), 7360. https://doi.org/10.3390/ijms22147360





