Genetic Modeling of the Neurodegenerative Disease Spinocerebellar Ataxia Type 1 in Zebrafish
Abstract
:1. Introduction
2. Results
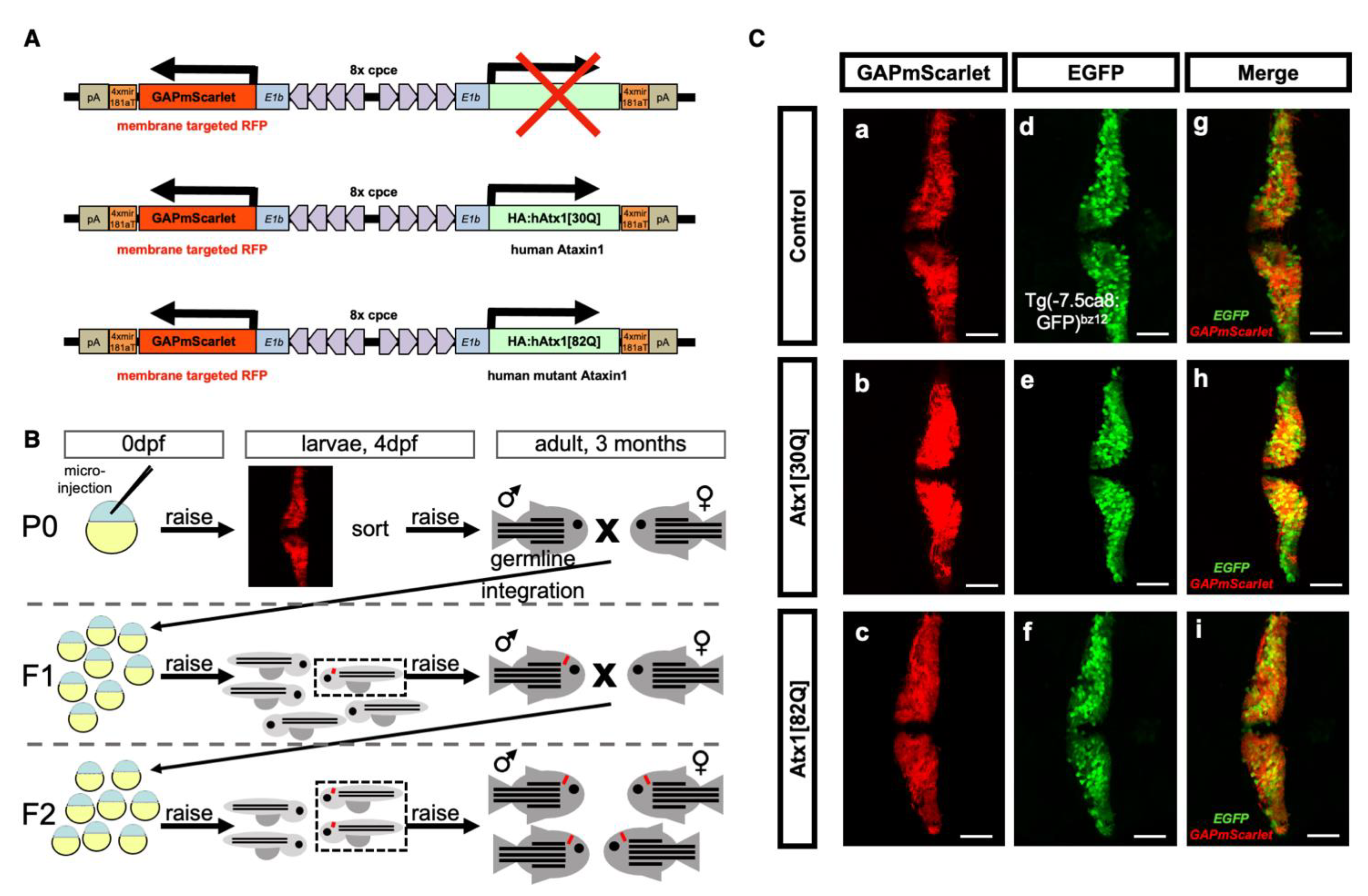
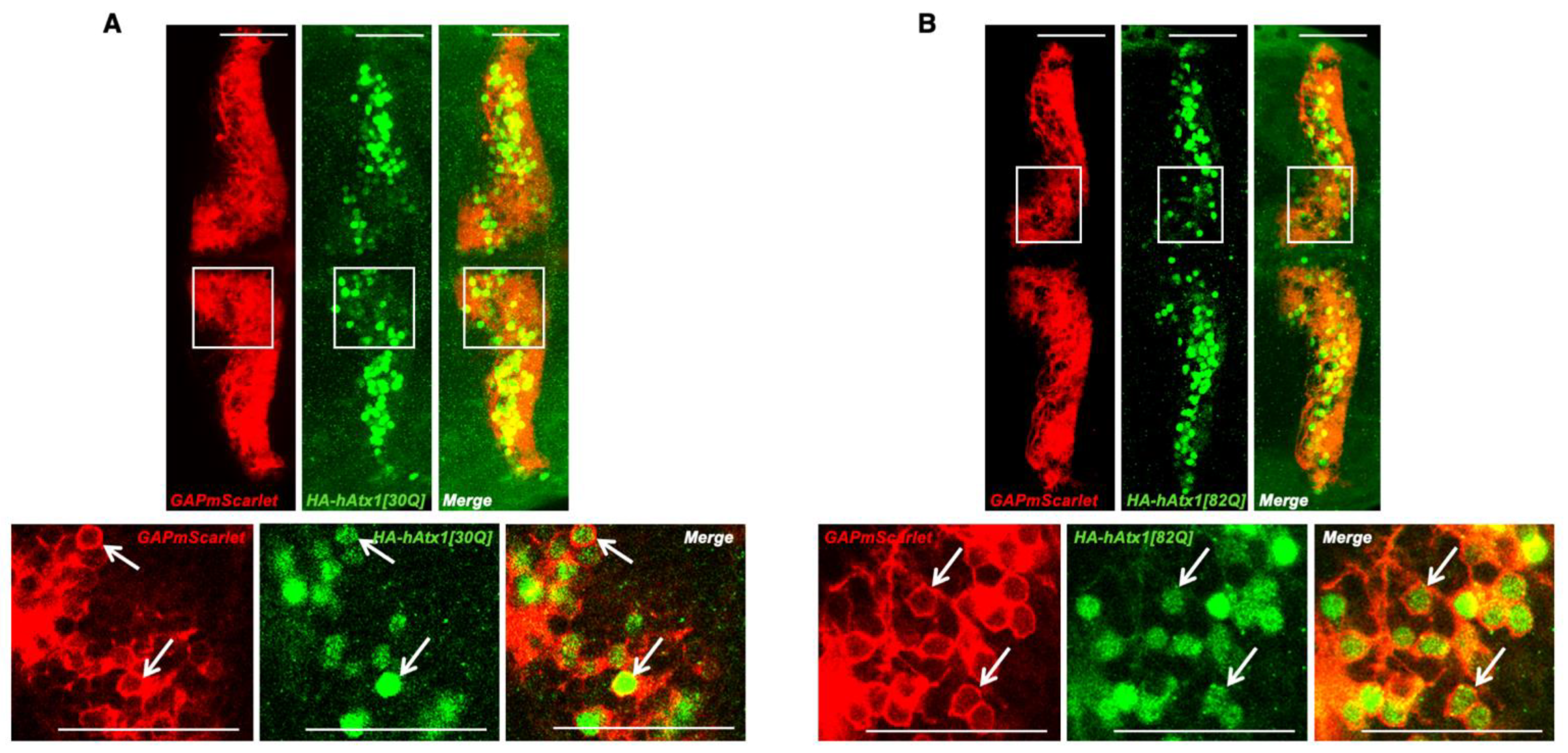
2.1. Genetic Modeling of SCA1 in Zebrafish
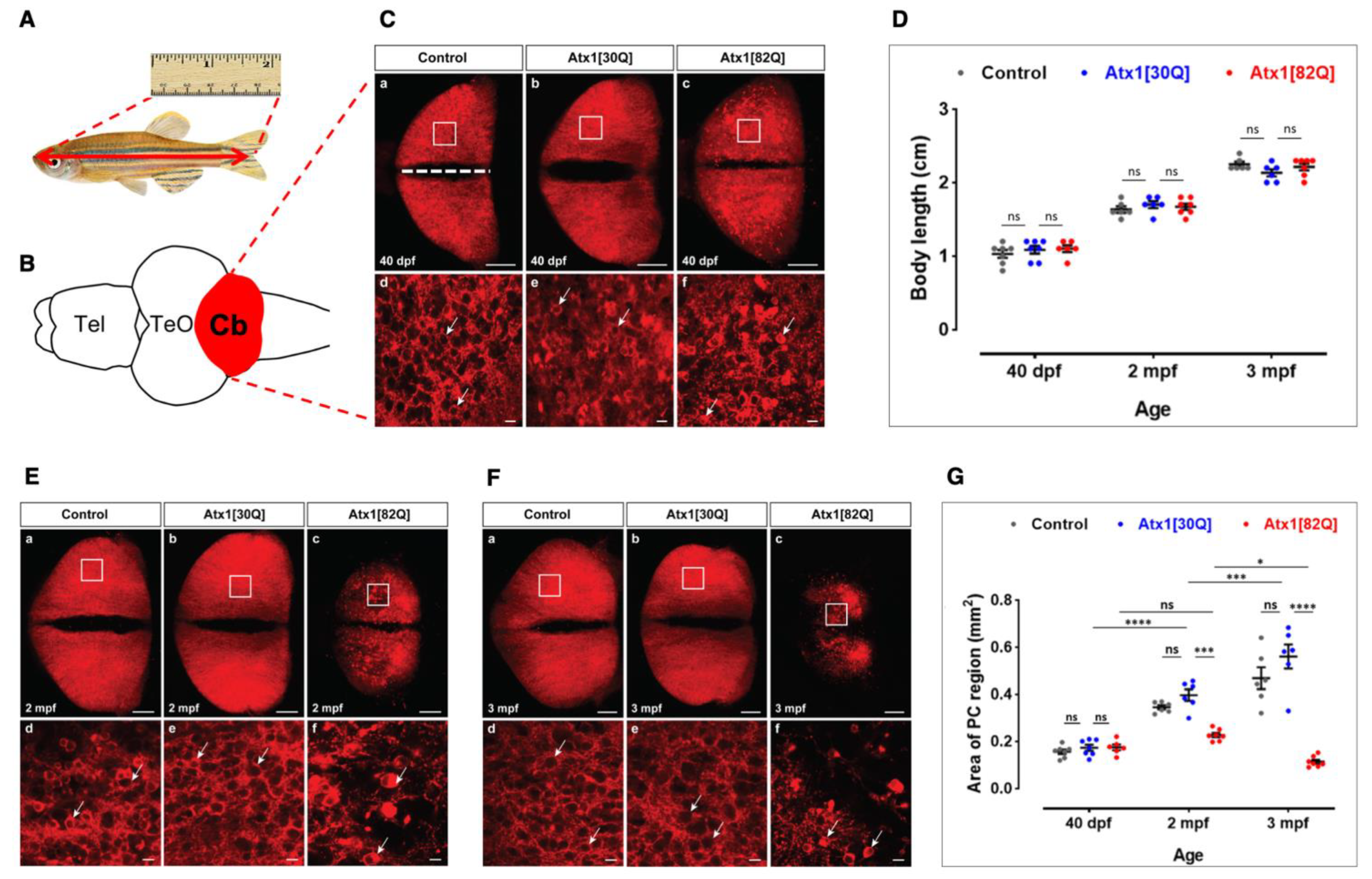
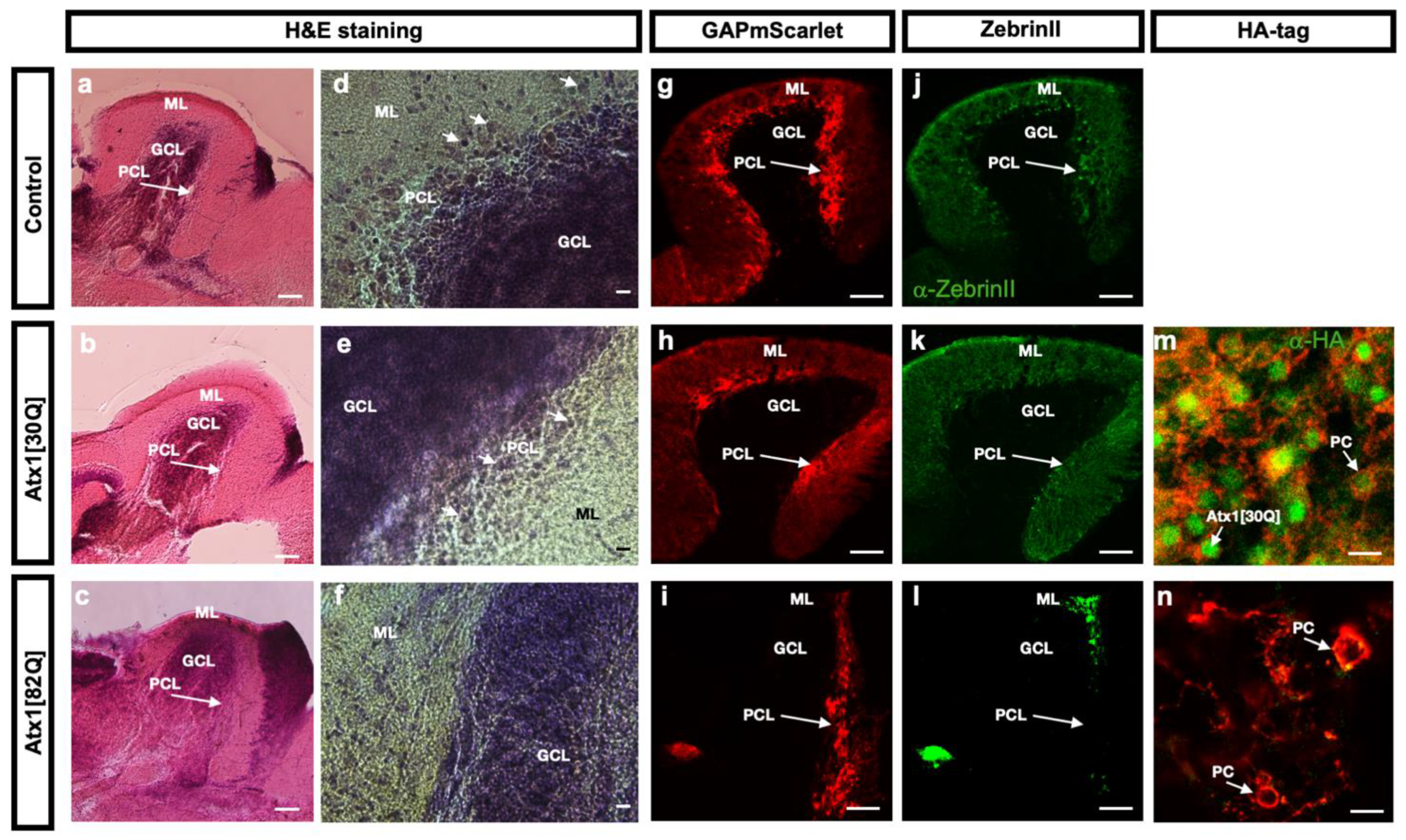
2.2. Age-Related Progressive Disturbances of Purkinje Cell Layer Integrity in Zebrafish Genetic Model of SCA1
2.3. Progredient PC Degeneration in Zebrafish Genetic Model of SCA1
2.4. Behavioural Performance of Zebrafish SCA1 Model
3. Discussion
4. Materials and Methods
4.1. Animal Husbandry and Maintenance
4.2. Plasmid Construction
4.3. Generation of Transgenic Zebrafish
4.4. Histology and Immunohistochemistry
4.5. Behavioural Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zoghbi, H.Y.; Orr, H.T. Pathogenic mechanisms of a polyglutamine-mediated neurodegenerative disease, spinocerebellar ataxia type 1. J. Biol. Chem. 2009, 284, 7425–7429. [Google Scholar] [CrossRef] [Green Version]
- Schmitz-Hübsch, T.; Coudert, M.; Giunti, P.; Globas, C.; Baliko, L.; Fancellu, R.; Mariotti, C.; Filla, A.; Rakowicz, M.; Charles, P.; et al. Self-rated health status in spinocerebellar ataxia—Results from a European multicenter study. Mov. Disord. 2010, 25, 587–595. [Google Scholar] [CrossRef]
- Fancellu, R.; Paridi, D.; Tomasello, C.; Panzeri, M.; Castaldo, A.; Genitrini, S.; Soliveri, P.; Girotti, F. Longitudinal study of cognitive and psychiatric functions in spinocerebellar ataxia types 1 and 2. J. Neurol. 2013, 260, 3134–3143. [Google Scholar] [CrossRef]
- Paulson, H.L.; Shakkottai, V.G.; Clark, H.B.; Orr, H.T. Polyglutamine spinocerebellar ataxias—From genes to potential treatments. Nat. Rev. Neurosci. 2017, 18, 613–626. [Google Scholar] [CrossRef]
- Perez, J.M.O.; Orr, H. Spinocerebellar Ataxia Type 1: Molecular Mechanisms of Neurodegeneration and Preclinical Studies. Adv. Exp. Med. Biol. 2018, 1049, 135–145. [Google Scholar]
- Rousseaux, M.W.C.; Tschumperlin, T.; Lu, H.-C.; Lackey, E.P.; Bondar, V.V.; Wan, Y.-W.; Tan, Q.; Adamski, C.J.; Friedrich, J.; Twaroski, K.; et al. ATXN1-CIC Complex Is the Primary Driver of Cerebellar Pathology in Spinocerebellar Ataxia Type 1 through a Gain-of-Function Mechanism. Neuron 2018, 97, 1235–1243.e5. [Google Scholar] [CrossRef] [Green Version]
- Kraus-Perrotta, C.; Lagalwar, S. Expansion, mosaicism and interruption: Mechanisms of the CAG repeat mutation in spinocerebellar ataxia type 1. Cerebellum Ataxias 2016, 3, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nethisinghe, S.; Pigazzini, M.L.; Pemble, S.; Sweeney, M.G.; Labrum, R.; Manso, K.; Moore, D.; Warner, J.; Davis, M.B.; Giunti, P. PolyQ Tract Toxicity in SCA1 is Length Dependent in the Absence of CAG Repeat Interruption. Front. Cell. Neurosci. 2018, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, H.Y.; Orr, H.T. Glutamine Repeats and Neurodegeneration. Annu. Rev. Neurosci. 2000, 23, 217–247. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-M.; Correia, K.; Loupe, J.; Kim, K.-H.; Barker, D.; Hong, E.P.; Chao, M.J.; Long, J.D.; Lucente, D.; Vonsattel, J.P.G.; et al. CAG Repeat Not Polyglutamine Length Determines Timing of Huntington’s Disease Onset. Cell 2019, 178, 887–900.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaraman, M.; Kodali, R.; Wetzel, R. The impact of ataxin-1-like histidine insertions on polyglutamine aggregation. Protein Eng. Des. Sel. 2009, 22, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Emamian, E.S.; Kaytor, M.D.; Duvick, L.A.; Zu, T.; Tousey, S.K.; Zoghbi, H.Y.; Clark, H.B.; Orr, H.T. Serine 776 of Ataxin-1 Is Critical for Polyglutamine-Induced Disease in SCA1 Transgenic Mice. Neuron 2003, 38, 375–387. [Google Scholar] [CrossRef] [Green Version]
- Klement, I.A.; Skinner, P.J.; Kaytor, M.D.; Yi, H.; Hersch, S.M.; Clark, H.B.; Zoghbi, H.Y.; Orr, H.T. Ataxin-1 Nuclear Localization and Aggregation: Role in Polyglutamine-Induced Disease in SCA1 Transgenic Mice. Cell 1998, 95, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Al-Ramahi, I.; Tan, Q.; Mollema, N.; Diaz-Garcia, J.R.; Gallego-Flores, T.; Lu, H.-C.; Lagalwar, S.; Duvick, L.; Kang, H.; et al. RAS–MAPK–MSK1 pathway modulates ataxin 1 protein levels and toxicity in SCA1. Nature 2013, 498, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuda, H.; Jafar-Nejad, H.; Patel, A.J.; Sun, Y.; Chen, H.-K.; Rose, M.F.; Venken, K.J.T.; Botas, J.; Orr, H.T.; Bellen, H.J.; et al. The AXH Domain of Ataxin-1 Mediates Neurodegeneration through Its Interaction with Gfi-1/Senseless Proteins. Cell 2005, 122, 633–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Funez, P.; Nino-Rosales, M.L.; de Gouyon, B.; She, W.-C.; Luchak, J.M.; Martinez, P.; Turiegano, E.; Benito, J.; Capovilla, M.; Skinner, P.J.; et al. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature 2000, 408, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Feany, M.B. Comparison of pathways controlling toxicity in the eye and brain in Drosophila models of human neurodegenerative diseases. Hum. Mol. Genet. 2004, 13, 2011–2018. [Google Scholar] [CrossRef]
- Burright, E.N.; Clark, H.B.; Servadio, A.; Matilla-Dueñas, A.; Feddersen, R.M.; Yunis, W.S.; Duvick, L.A.; Zoghbi, H.; Orr, H. SCA1 Transgenic Mice: A Model for Neurodegeneration Caused by an Expanded CAG Trinucleotide Repeat. Cell 1995, 82, 937–948. [Google Scholar] [CrossRef] [Green Version]
- Distel, M.; Hocking, J.C.; Volkmann, K.; Köster, R.W. The centrosome neither persistently leads migration nor determines the site of axonogenesis in migrating neurons in vivo. J. Cell Biol. 2010, 191, 875–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, H.; Namikawa, K.; Babaryka, A.; Köster, R.W. Functional regionalization of the teleost cerebellum analyzed in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 11846–11851. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, M.; Thirumalai, V. AMPA receptor mediated synaptic excitation drives state-dependent bursting in Purkinje neurons of zebrafish larvae. eLife 2015, 4, e09158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, T.; Namikawa, K.; Winter, B.; Müller-Brown, K.; Kühn, R.; Wurst, W.; Köster, R.W. Caspase-mediated apoptosis induction in zebrafish cerebellar Purkinje neurons. Development 2016, 143, 4279–4287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibi, M.; Shimizu, T. Development of the cerebellum and cerebellar neural circuits. Dev. Neurobiol. 2012, 72, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Carlson, K.M.; Melcher, L.; Lai, S.; Zoghbi, H.Y.; Clark, H.B.; Orr, H.T. Characterization of the zebrafish atxn1/axh gene family. J. Neurogenet. 2009, 23, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Namikawa, K.; Dorigo, A.; Zagrebelsky, M.; Russo, G.; Kirmann, T.; Fahr, W.; Dübel, S.; Korte, M.; Köster, R.W. Modeling Neurodegenerative Spinocerebellar Ataxia Type 13 in Zebrafish Using a Purkinje Neuron Specific Tunable Coexpression System. J. Neurosci. 2019, 39, 3948–3969. [Google Scholar] [CrossRef] [Green Version]
- Koga, A.; Cheah, F.S.H.; Hamaguchi, S.; Yeo, G.H.; Chong, S.S. Germline transgenesis of zebrafish using the medaka Tol1 transposon system. Dev. Dyn. 2008, 237, 2466–2474. [Google Scholar] [CrossRef]
- Zoghbi, H.Y. Spinocerebellar ataxia type 1. J. Clin. Neurosci. 1995, 3, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Maximino, C.; de Brito, T.M.; da Silva Batista, A.W.; Herculano, A.M.; Morato, S.; Gouveia, A. Measuring anxiety in zebrafish: A critical review. Behav. Brain Res. 2010, 214, 157–171. [Google Scholar] [CrossRef]
- Mastammanavar, V.S.; Kamble, N.; Yadav, R.; Netravathi, M.; Jain, S.; Kumar, K.; Pal, P.K. Non-motor symptoms in patients with autosomal dominant spinocerebellar ataxia. Acta Neurol. Scand. 2020, 142, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Quelle-Regaldie, A.; Sobrido-Cameán, D.; Barreiro-Iglesias, A.; Sobrido, M.; Sánchez, L. Zebrafish Models of Autosomal Recessive Ataxias. Cells 2021, 10, 836. [Google Scholar] [CrossRef]
- Tanabe, K.; Kani, S.; Shimizu, T.; Bae, Y.-K.; Abe, T.; Hibi, M. Atypical Protein Kinase C Regulates Primary Dendrite Specification of Cerebellar Purkinje Cells by Localizing Golgi Apparatus. J. Neurosci. 2010, 30, 16983–16992. [Google Scholar] [CrossRef]
- Quelle-Regaldie, A.; Sobrido-Cameán, D.; Barreiro-Iglesias, A.; Sobrido, M.; Sánchez, L. Zebrafish Models of Autosomal Dominant Ataxias. Cells 2021, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Watchon, M.; Yuan, K.C.; Mackovski, N.; Svahn, A.J.; Cole, N.J.; Goldsbury, C.; Rinkwitz, S.; Becker, T.S.; Nicholson, G.A.; Laird, A.S. Calpain Inhibition Is Protective in Machado–Joseph Disease Zebrafish Due to Induction of Autophagy. J. Neurosci. 2017, 37, 7782–7794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamling, K.R.; Tobias, Z.J.C.; Weissman, T.A. Mapping the development of cerebellar Purkinje cells in zebrafish. Dev. Neurobiol. 2015, 75, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Koyama, W.; Hosomi, R.; Matsuda, K.; Kawakami, K.; Hibi, M.; Shimizu, T. Involvement of cerebellar neural circuits in active avoidance conditioning in zebrafish. Eneuro 2021, 8, 507. [Google Scholar] [CrossRef]
- Knogler, L.D.; Kist, A.M.; Portugues, R. Motor context dominates output from purkinje cell functional regions during reflexive visuomotor behaviours. eLife 2019, 8, e42138. [Google Scholar] [CrossRef]
- Sarna, J.R.; Hawkes, R. Patterned Purkinje cell death in the cerebellum. Prog. Neurobiol. 2003, 70, 473–507. [Google Scholar] [CrossRef]
- Gilman, S. The Spinocerebellar Ataxias. Clin. Neuropharmacol. 2000, 23, 296–303. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio Rerio), 5th ed.; University of Orgeon: Eugene, OR, USA, 2007. [Google Scholar]
- Aleström, P.; D’Angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and husbandry recommendations. Lab. Anim. 2019, 54, 213–224. [Google Scholar] [CrossRef] [Green Version]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Kani, S.; Bae, Y.-K.; Shimizu, T.; Tanabe, K.; Satou, C.; Parsons, M.J.; Scott, E.; Higashijima, S.; Hibi, M. Proneural gene-linked neurogenesis in zebrafish cerebellum. Dev. Biol. 2010, 343, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cachat, J.; Stewart, A.; Grossman, L.; Gaikwad, S.; Kadri, F.; Chung, K.M.; Wu, N.; Wong, K.; Roy, S.; Suciu, C.; et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc. 2010, 5, 1786–1799. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsaey, M.A.; Namikawa, K.; Köster, R.W. Genetic Modeling of the Neurodegenerative Disease Spinocerebellar Ataxia Type 1 in Zebrafish. Int. J. Mol. Sci. 2021, 22, 7351. https://doi.org/10.3390/ijms22147351
Elsaey MA, Namikawa K, Köster RW. Genetic Modeling of the Neurodegenerative Disease Spinocerebellar Ataxia Type 1 in Zebrafish. International Journal of Molecular Sciences. 2021; 22(14):7351. https://doi.org/10.3390/ijms22147351
Chicago/Turabian StyleElsaey, Mohamed A., Kazuhiko Namikawa, and Reinhard W. Köster. 2021. "Genetic Modeling of the Neurodegenerative Disease Spinocerebellar Ataxia Type 1 in Zebrafish" International Journal of Molecular Sciences 22, no. 14: 7351. https://doi.org/10.3390/ijms22147351
APA StyleElsaey, M. A., Namikawa, K., & Köster, R. W. (2021). Genetic Modeling of the Neurodegenerative Disease Spinocerebellar Ataxia Type 1 in Zebrafish. International Journal of Molecular Sciences, 22(14), 7351. https://doi.org/10.3390/ijms22147351







