Tissue Regeneration: The Dark Side of Opioids
Abstract
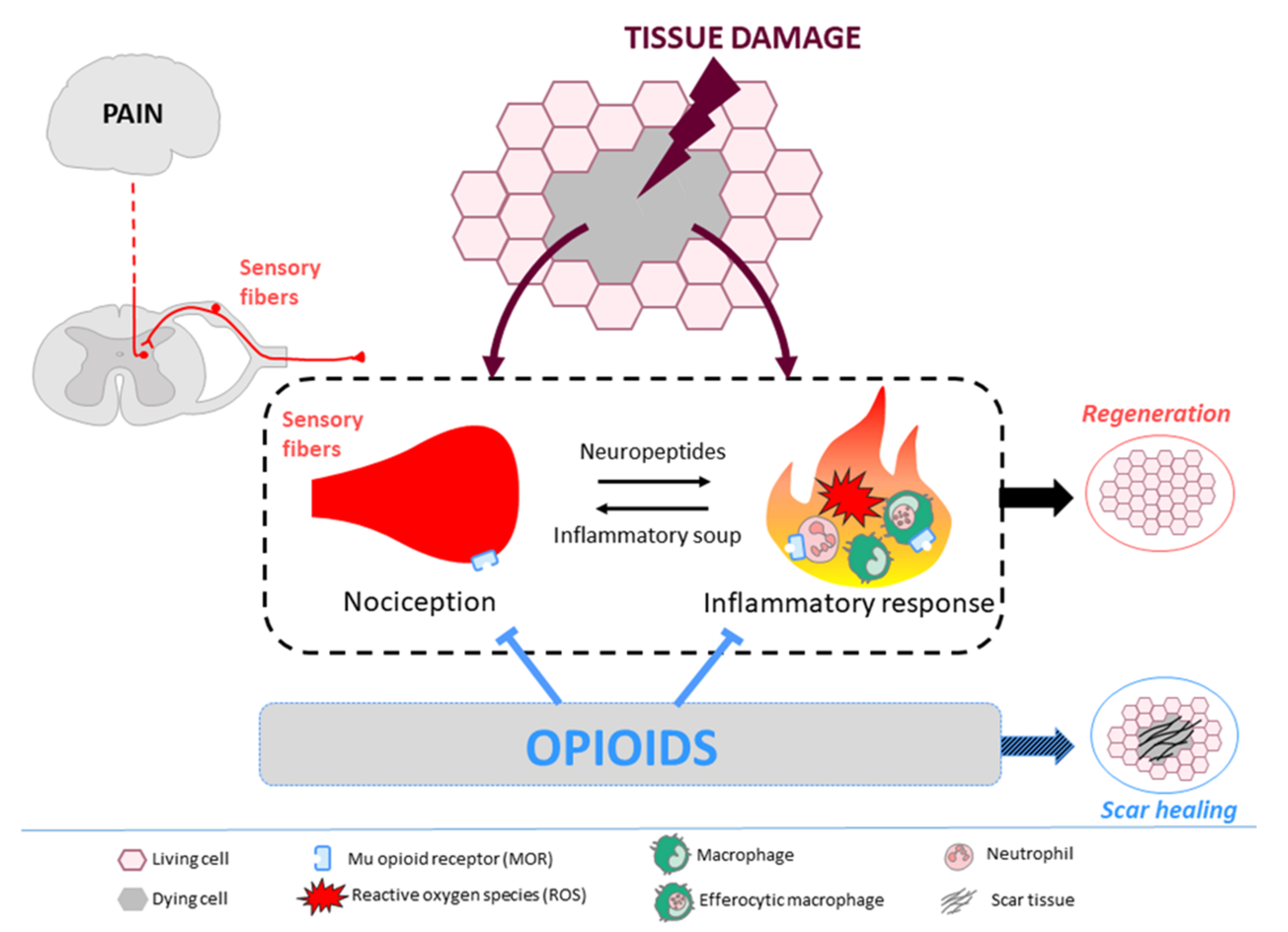
:1. Tissue Repair: Scar Healing Versus Regeneration
2. Opioids, Immune System, and Tissue Regeneration
2.1. Endogenous Opioids and Their Receptors
2.2. Opioid Effects on the Immune System
2.3. Opioid Effects on Regeneration via Immune Cells
3. Opioids, Nervous System, and Tissue Regeneration
3.1. Nervous System Organization and Tissue Injury
3.2. Innervation and Regeneration
3.3. Opioids and Nociceptors
3.4. Nociceptors and Regeneration
3.5. Nociception and Opioid System Development
4. What Is Known in Human-Being?
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Erickson, J.R.; Echeverri, K. Learning from regeneration research organisms: The circuitous road to scar free wound healing. Dev. Biol. 2018, 433, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.C.G. Why polyps regenerate and we don’t: Towards a cellular and molecular framework for Hydra regeneration. Dev. Biol. 2007, 303, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Agata, K. Regeneration and gene regulation in planarians. Curr. Opin. Genet. Dev. 2003, 13, 492–496. [Google Scholar] [CrossRef] [PubMed]
- TODD, T. On the process of reproduction of the members of the aquatic salamander. Q. J. Sci. Arts Lib. 1823, 16, 84–86. [Google Scholar]
- Mahmoud, A.I.; O’Meara, C.C.; Gemberling, M.; Zhao, L.; Bryant, D.M.; Zheng, R.; Gannon, J.B.; Cai, L.; Choi, W.-Y.; Egnaczyk, G.F.; et al. Nerves Regulate Cardiomyocyte Proliferation and Heart Regeneration. Dev. Cell 2015, 34, 387–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauron, C.; Rampon, C.; Bouzaffour, M.; Ipendey, E.; Teillon, J.; Volovitch, M.; Vriz, S. Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Sci. Rep. 2013, 3, 2084. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Godwin, J.W.; Gates, P.B.; Garza-Garcia, A.A.; Brockes, J.P. Molecular Basis for the Nerve Dependence of Limb Regeneration in an Adult Vertebrate. Science 2007, 318, 772–777. [Google Scholar] [CrossRef] [Green Version]
- Brockes, J.P.; Kumar, A. Comparative Aspects of Animal Regeneration. Annu. Rev. Cell Dev. Biol. 2008, 24, 525–549. [Google Scholar] [CrossRef] [Green Version]
- Illingworth, C.M. Trapped fingers and amputated finger tips in children. J. Pediatr. Surg. 1974, 9, 853–858. [Google Scholar] [CrossRef]
- Reginelli, A.D.; Wang, Y.-Q.; Sassoon, D.; Muneoka, K. Digit tip regeneration correlates with regions of Msx1 (Hox 7) expression in fetal and newborn mice. 12. Development 1995, 121, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Han, M. Digit regeneration is regulated by Msx1 and BMP4 in fetal mice. Development 2003, 130, 5123–5132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Han, M.; Yan, M.; Lee, E.-C.; Lee, J.; Muneoka, K. BMP signaling induces digit regeneration in neonatal mice. Development 2010, 137, 551–559. [Google Scholar] [CrossRef] [Green Version]
- Drenckhahn, J.-D.; Schwarz, Q.P.; Gray, S.; Laskowski, A.; Kiriazis, H.; Ming, Z.; Harvey, R.P.; Du, X.-J.; Thorburn, D.R.; Cox, T.C. Compensatory Growth of Healthy Cardiac Cells in the Presence of Diseased Cells Restores Tissue Homeostasis during Heart Development. Dev. Cell 2008, 15, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Aurora, A.B.; Porrello, E.R.; Tan, W.; Mahmoud, A.I.; Hill, J.A.; Bassel-Duby, R.; Sadek, H.A.; Olson, E.N. Macrophages are required for neonatal heart regeneration. J. Clin. Invest. 2014, 124, 1382–1392. [Google Scholar] [CrossRef] [Green Version]
- Stein, C.; Küchler, S. Targeting inflammation and wound healing by opioids. Trends Pharmacol. Sci. 2013, 34, 303–312. [Google Scholar] [CrossRef]
- Ondrovics, M.; Hoelbl-Kovacic, A.; Fux, D.A. Opioids: Modulators of angiogenesis in wound healing and cancer. Oncotarget 2017, 8, 25783–25796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.A.; Hui, F.W. Inhibition of neurotrophic activity in salamanders treated with opioids. Exp. Neurol. 1973, 39, 36–43. [Google Scholar] [CrossRef]
- Barlass, U.; Dutta, R.; Cheema, H.; George, J.; Sareen, A.; Dixit, A.; Yuan, Z.; Giri, B.; Meng, J.; Banerjee, S.; et al. Morphine worsens the severity and prevents pancreatic regeneration in mouse models of acute pancreatitis. Gut 2017. [Google Scholar] [CrossRef]
- Labit, E.; Rabiller, L.; Rampon, C.; Guissard, C.; André, M.; Barreau, C.; Cousin, B.; Carrière, A.; Eddine, M.A.; Pipy, B.; et al. Opioids prevent regeneration in adult mammals through inhibition of ROS production. Sci. Rep. 2018, 8, 12170. [Google Scholar] [CrossRef] [Green Version]
- Clark, L.D.; Clark, R.K.; Heber-Katz, E. A New Murine Model for Mammalian Wound Repair and Regeneration. Clin. Immunol. Immunopathol. 1998, 88, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, G.; Metcalfe, A.D.; Ferguson, M.W.J. Peripheral nerve regeneration in the MRL/MpJ ear wound model: Peripheral nerve regeneration in the mouse ear. J. Anat. 2011, 218, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Koneru, A.; Satyanarayana, S.; Rizwan, S. Endogenous Opioids: Their Physiological Role and Receptors. Glob. J. Pharmacol. 2009, 3, 149–153. [Google Scholar]
- Kaur, J.; Kumar, V.; Sharma, K.; Kaur, S.; Gat, Y.; Goyal, A.; Tanwar, B. Opioid Peptides: An Overview of Functional Significance. Int. J. Pept. Res. Ther. 2020, 26, 33–41. [Google Scholar] [CrossRef]
- Kapitzke, D.; Vetter, I.; Cabot, P.J. Endogenous opioid analgesia in peripheral tissues and the clinical implications for pain control. Ther. Clin. Risk Manag. 2005, 1, 279–297. [Google Scholar]
- Rittner, H.L.; Brack, A.; Machelska, H.; Mousa, S.A.; Bauer, M.; Schäfer, M.; Stein, C. Opioid Peptide–expressing Leukocytes: Identification, Recruitment, and Simultaneously Increasing Inhibition of Inflammatory Pain. Anesthesiology 2001, 95, 500–508. [Google Scholar] [CrossRef]
- Benarroch, E.E. Endogenous opioid systems: Current concepts and clinical correlations. Neurology 2012, 79, 807–814. [Google Scholar] [CrossRef]
- Pasternak, G.W.; Pan, Y.-X. Mu opioids and their receptors: Evolution of a concept. Pharmacol. Rev. 2013, 65, 1257–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hasani, R.; Bruchas, M.R. Molecular Mechanisms of Opioid Receptor-Dependent Signaling and Behavior. Anesthesiology 2011, 115, 1363–1381. [Google Scholar] [CrossRef] [Green Version]
- Kieffer, B.L.; Evans, C.J. Opioid receptors: From binding sites to visible molecules in vivo. Neuropharmacology 2009, 56, 205–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, M.C.; Ben-Baruch, A.; Taub, D.D.; Howard, O.M.Z.; Wang, J.M.; Oppenheim, J.J. Opiate Inhibition of Chemokine-Induced Chemotaxis. Ann. N. Y. Acad. Sci. 1998, 840, 9–20. [Google Scholar] [CrossRef]
- Stefano, G.B.; Leung, M.K.; Bilfinger, T.V.; Scharrer, B. Effect of prolonged exposure to morphine on responsiveness of human and invertebrate immunocytes to stimulatory molecules. J. Neuroimmunol. 1995, 63, 175–181. [Google Scholar] [CrossRef]
- Eisenstein, T.K. The Role of Opioid Receptors in Immune System Function. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Wybran, J.; Appelboom, T.; Famaey, J.P.; Govaerts, A. Suggestive evidence for receptors for morphine and methionine-enkephalin on normal human blood T lymphocytes. J. Immunol. Baltim. Md. 1950 1979, 123, 1068–1070. [Google Scholar]
- Tubaro, E.; Borelli, G.; Croce, C.; Cavallo, G.; Santiangeli, C. Effect of morphine on resistance to infection. J. Infect. Dis. 1983, 148, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Rojavin, M.; Szabo, I.; Bussiere, J.L.; Rogers, T.J.; Adler, M.W.; Eisenstein, T.K. Morphine treatment in vitro or in vivo decreases phagocytic functions of murine macrophages. Life Sci. 1993, 53, 997–1006. [Google Scholar] [CrossRef]
- Ninković, J.; Roy, S. Role of the mu opioid receptor in opioid modulation of immune function. Amino Acids 2013, 45, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Simpkins, C.O.; Alailima, S.T.; Tate, E.A. Inhibition by naloxone of neutrophil superoxide release: A potentially useful antiinflammatory effect. Circ. Shock 1986, 20, 181–191. [Google Scholar] [PubMed]
- Peterson, P.K.; Sharp, B.; Gekker, G.; Brummitt, C.; Keane, W.F. Opioid-mediated Suppression of Interferon-γ Production by Cultured Peripheral Blood Mononuclear Cells. J. Clin. Invest. 1987, 80, 824–831. [Google Scholar] [CrossRef] [Green Version]
- Ni, X.; Gritman, K.R.; Eisenstein, T.K.; Adler, M.W.; Arfors, K.E.; Tuma, R.F. Morphine Attenuates Leukocyte/Endothelial Interactions. Microvasc. Res. 2000, 60, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni-Narla, A.; Walcheck, B.; Brown, D.R. Opioid receptors on bone marrow neutrophils modulate chemotaxis and CD11b/CD18 expression. Eur. J. Pharmacol. 2001, 414, 289–294. [Google Scholar] [CrossRef]
- Ferreira, F.; Luxardi, G.; Reid, B.; Zhao, M. Early bioelectric activities mediate redox-modulated regeneration. Development 2016, 143, 4582–4594. [Google Scholar] [CrossRef] [Green Version]
- Wenger, Y.; Buzgariu, W.; Reiter, S.; Galliot, B. Injury-induced immune responses in Hydra. Semin. Immunol. 2014, 26, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.; Man, L.; Zhu, Z.; Bai, X.; Wei, S.; Liu, Y.; Liu, M.; Wang, X.; Gu, X.; et al. Reactive oxygen species generated from skeletal muscles are required for gecko tail regeneration. Sci. Rep. 2016, 6, 20752. [Google Scholar] [CrossRef] [Green Version]
- Godwin, J.W.; Pinto, A.R.; Rosenthal, N.A. Macrophages are required for adult salamander limb regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 9415–9420. [Google Scholar] [CrossRef] [Green Version]
- Nguyen-Chi, M.; Laplace-Builhé, B.; Travnickova, J.; Luz-Crawford, P.; Tejedor, G.; Lutfalla, G.; Kissa, K.; Jorgensen, C.; Djouad, F. TNF signaling and macrophages govern fin regeneration in zebrafish larvae. Cell Death Dis. 2017, 8, e2979. [Google Scholar] [CrossRef]
- Petrie, T.A.; Strand, N.S.; Yang, C.-T.; Rabinowitz, J.S.; Moon, R.T. Macrophages modulate adult zebrafish tail fin regeneration. Development 2015, 142, 406. [Google Scholar] [CrossRef]
- Godwin, J.W.; Debuque, R.; Salimova, E.; Rosenthal, N.A. Heart regeneration in the salamander relies on macrophage-mediated control of fibroblast activation and the extracellular landscape. NPJ Regen. Med. 2017, 2, 22. [Google Scholar] [CrossRef]
- Simkin, J.; Sammarco, M.C.; Marrero, L.; Dawson, L.A.; Yan, M.; Tucker, C.; Cammack, A.; Muneoka, K. Macrophages are required to coordinate mouse digit tip regeneration. Development 2017, 144, 3907–3916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simkin, J.; Gawriluk, T.R.; Gensel, J.C.; Seifert, A.W. Macrophages are necessary for epimorphic regeneration in African spiny mice. eLife 2017, 6, e24623. [Google Scholar] [CrossRef]
- Tsarouchas, T.M.; Wehner, D.; Cavone, L.; Munir, T.; Keatinge, M.; Lambertus, M.; Underhill, A.; Barrett, T.; Kassapis, E.; Ogryzko, N.; et al. Dynamic control of proinflammatory cytokines Il-1β and Tnf-α by macrophages in zebrafish spinal cord regeneration. Nat. Commun. 2018, 9, 4670. [Google Scholar] [CrossRef] [Green Version]
- Aztekin, C.; Hiscock, T.W.; Butler, R.; de Jesús Andino, F.; Robert, J.; Gurdon, J.B.; Jullien, J. The myeloid lineage is required for the emergence of a regeneration-permissive environment following Xenopus tail amputation. Development 2020, 147, dev185496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, G.; Wong, J.; Metcalfe, A.D.; Ferguson, M.W.J. Denervation affects regenerative responses in MRL/MpJ and repair in C57BL/6 ear wounds: Denervation influences regeneration and repair in mouse ear wounds. J. Anat. 2012, 220, 3–12. [Google Scholar] [CrossRef]
- Miljkovic-Licina, M.; Chera, S.; Ghila, L.; Galliot, B. Head regeneration in wild-type hydra requires de novo neurogenesis. Development 2007, 134, 1191–1201. [Google Scholar] [CrossRef] [Green Version]
- Marcum, B.A.; Campbell, R.D. Developmental roles of epithelial and interstitial cell lineages in hydra: Analysis of chimeras. J. Cell Sci. 1978, 32, 233–247. [Google Scholar] [CrossRef]
- Simões, M.G.; Bensimon-Brito, A.; Fonseca, M.; Farinho, A.; Valério, F.; Sousa, S.; Afonso, N.; Kumar, A.; Jacinto, A. Denervation impairs regeneration of amputated zebrafish fins. BMC Dev. Biol. 2014, 14, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Brockes, J.P. Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci. 2012, 35, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Oviedo, N.J.; Morokuma, J.; Walentek, P.; Kema, I.P.; Gu, M.B.; Ahn, J.-M.; Hwang, J.S.; Gojobori, T.; Levin, M. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev. Biol. 2010, 339, 188–199. [Google Scholar] [CrossRef] [Green Version]
- Meda, F.; Gauron, C.; Rampon, C.; Teillon, J.; Volovitch, M.; Vriz, S. Nerves Control Redox Levels in Mature Tissues Through Schwann Cells and Hedgehog Signaling. Antioxid. Redox Signal. 2016, 24, 299–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.W.; Oh, U. Current concepts of nociception: Nociceptive molecular sensors in sensory neurons. Curr. Opin. Anaesthesiol. 2007, 20, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boddeke, E.W. Involvement of chemokines in pain. Eur. J. Pharmacol. 2001, 429, 115–119. [Google Scholar] [CrossRef]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [Green Version]
- Mambretti, E.M.; Kistner, K.; Mayer, S.; Massotte, D.; Kieffer, B.L.; Hoffmann, C.; Reeh, P.W.; Brack, A.; Asan, E.; Rittner, H.L. Functional and structural characterization of axonal opioid receptors as targets for analgesia. Mol. Pain 2016, 12, 1744806916628734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leenders, A.G.M.; Sheng, Z.-H. Modulation of neurotransmitter release by the second messenger-activated protein kinases: Implications for presynaptic plasticity. Pharmacol. Ther. 2005, 105, 69–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tumati, S.; Roeske, W.R.; Vanderah, T.W.; Varga, E.V. Sustained morphine treatment augments prostaglandin E2-evoked Calcitonin Gene-Related Peptide release from primary sensory neurons in a PKA- dependent manner. Eur. J. Pharmacol. 2010, 648, 95–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodin, E.; Gazelius, B.; Panopoulos, P.; Olgart, L. Morphine inhibits substance P release from peripheral sensory nerve endings. Acta Physiol. Scand. 1983, 117, 567–570. [Google Scholar] [CrossRef]
- Stein, C.; Lang, L.J. Peripheral mechanisms of opioid analgesia. Curr. Opin. Pharmacol. 2009, 9, 3–8. [Google Scholar] [CrossRef]
- Baillie, L.D.; Schmidhammer, H.; Mulligan, S.J. Peripheral μ-opioid receptor mediated inhibition of calcium signaling and action potential-evoked calcium fluorescent transients in primary afferent CGRP nociceptive terminals. Neuropharmacology 2015, 93, 267–273. [Google Scholar] [CrossRef]
- Wei, J.J.; Kim, H.S.; Spencer, C.A.; Brennan-Crispi, D.; Zheng, Y.; Johnson, N.M.; Rosenbach, M.; Miller, C.; Leung, D.H.; Cotsarelis, G.; et al. Activation of TRPA1 nociceptor promotes systemic adult mammalian skin regeneration. Sci. Immunol. 2020, 5, eaba5683. [Google Scholar] [CrossRef]
- Le Pichon, C.E.; Chesler, A.T. The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Front. Neuroanat. 2014, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, C.H.; Zarebidaki, E.; Ehlen, J.C.; Bartness, T.J. Analysis and Measurement of the Sympathetic and Sensory Innervation of White and Brown Adipose Tissue. Methods Enzymol. 2014, 537, 199–225. [Google Scholar] [PubMed] [Green Version]
- Benemei, S.; Nicoletti, P.; Capone, J.G.; Geppetti, P. CGRP receptors in the control of pain and inflammation. Curr. Opin. Pharmacol. 2009, 9, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Suvas, S. Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J. Immunol. 2017, 199, 1543–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Song, C.K.; Giordano, A.; Cinti, S.; Bartness, T.J. Sensory or sympathetic white adipose tissue denervation differentially affects depot growth and cellularity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1028–R1037. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Zhang, P.; Liu, T.; Xu, J.; Fan, Z.; Shen, Y.; Li, W.; Zhang, H. Calcitonin gene-related peptide is a key factor in the homing of transplanted human MSCs to sites of spinal cord injury. Sci. Rep. 2016, 6, 27724. [Google Scholar] [CrossRef] [Green Version]
- Laharrague, P.; Casteilla, L. The emergence of adipocytes. Endocr. Dev. 2010, 19, 21–30. [Google Scholar]
- Bossaller, C.; Reither, K.; Hehlert-Friedrich, C.; Auch-Schwelk, W.; Graf, K.; Gräfe, M.; Fleck, E. In vivo measurement of endothelium-dependent vasodilation with substance P in man. Herz 1992, 17, 284–290. [Google Scholar]
- Hong, H.S.; Lee, J.; Lee, E.; Kwon, Y.S.; Lee, E.; Ahn, W.; Jiang, M.H.; Kim, J.C.; Son, Y. A new role of substance P as an injury-inducible messenger for mobilization of CD29 + stromal-like cells. Nat. Med. 2009, 15, 425–435. [Google Scholar] [CrossRef]
- Rook, J.M.; McCarson, K.E. Delay of cutaneous wound closure by morphine via local blockade of peripheral tachykinin release. Biochem. Pharmacol. 2007, 74, 752–757. [Google Scholar] [CrossRef] [Green Version]
- Rook, J.M.; Hasan, W.; McCarson, K.E. Morphine-induced early delays in wound closure: Involvement of sensory neuropeptides and modification of neurokinin receptor expression. Biochem. Pharmacol. 2009, 77, 1747–1755. [Google Scholar] [CrossRef] [Green Version]
- Eming, S.A.; Hammerschmidt, M.; Krieg, T.; Roers, A. Interrelation of immunity and tissue repair or regeneration. Semin. Cell Dev. Biol. 2009, 20, 517–527. [Google Scholar] [CrossRef]
- Mescher, A.L.; Neff, A.W. Regenerative Capacity and the Developing Immune System. In Regenerative Medicine I; Yannas, I.V., Ed.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2005; Volume 93, pp. 39–66. ISBN 978-3-540-22871-4. [Google Scholar]
- Aurora, A.B.; Olson, E.N. Immune Modulation of Stem Cells and Regeneration. Cell Stem Cell 2014, 15, 14–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koltzenburg, M.; Stucky, C.L.; Lewin, G.R. Receptive properties of mouse sensory neurons innervating hairy skin. J. Neurophysiol. 1997, 78, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.J.; Abbott, F.V. The formalin test: A dose-response analysis at three developmental stages. Pain 1998, 76, 337–347. [Google Scholar] [CrossRef]
- Yi, D.K.; Barr, G.A. The induction of Fos-like immunoreactivity by noxious thermal, mechanical and chemical stimuli in the lumbar spinal cord of infant rats. Pain 1995, 60, 257–265. [Google Scholar] [CrossRef]
- Fitzgerald, M. The post-natal development of cutaneous afferent fibre input and receptive field organization in the rat dorsal horn. J. Physiol. 1985, 364, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Falcon, M.; Guendellman, D.; Stolberg, A.; Frenk, H.; Urca, G. Development of thermal nociception in rats. Pain 1996, 67, 203–208. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Jennings, E. The postnatal development of spinal sensory processing. Proc. Natl. Acad. Sci. USA 1999, 96, 7719–7722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, M. The development of nociceptive circuits. Nat. Rev. Neurosci. 2005, 6, 507–520. [Google Scholar] [CrossRef]
- Koch, S.C.; Fitzgerald, M. Activity-dependent development of tactile and nociceptive spinal cord circuits. Ann. N. Y. Acad. Sci. 2013, 1279, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Hathway, G.J.; Vega-Avelaira, D.; Fitzgerald, M. A critical period in the supraspinal control of pain: Opioid-dependent changes in brainstem rostroventral medulla function in preadolescence. Pain 2012, 153, 775–783. [Google Scholar] [CrossRef] [Green Version]
- Kwok, C.H.T.; Devonshire, I.M.; Bennett, A.J.; Hathway, G.J. Postnatal maturation of endogenous opioid systems within the periaqueductal grey and spinal dorsal horn of the rat. Pain 2014, 155, 168–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hathway, G.J.; Koch, S.; Low, L.; Fitzgerald, M. The changing balance of brainstem–spinal cord modulation of pain processing over the first weeks of rat postnatal life. J. Physiol. 2009, 587, 2927–2935. [Google Scholar] [CrossRef]
- Wang, H.; Cuzon, V.C.; Pickel, V.M. Postnatal development of μ-opioid receptors in the rat caudate-putamen nucleus parallels asymmetric synapse formation. Neuroscience 2003, 118, 695–708. [Google Scholar] [CrossRef]
- Kivell, B.M.; Day, D.J.; McDonald, F.J.; Miller, J.H. Developmental expression of μ and δ opioid receptors in the rat brainstem: Evidence for a postnatal switch in μ isoform expression. Dev. Brain Res. 2004, 148, 185–196. [Google Scholar] [CrossRef]
- Beland, B.; Fitzgerald, M. Mu- and delta-opioid receptors are downregulated in the largest diameter primary sensory neurons during postnatal development in rats. Pain 2001, 90, 143–150. [Google Scholar] [CrossRef]
- Rahman, W.; Dashwood, M.R.; Fitzgerald, M.; Aynsley-Green, A.; Dickenson, A.H. Postnatal development of multiple opioid receptors in the spinal cord and development of spinal morphine analgesia. Dev. Brain Res. 1998, 108, 239–254. [Google Scholar] [CrossRef]
- Talbot, J.N.; Happe, H.K.; Murrin, L.C. μ Opioid Receptor Coupling to Gi/o Proteins Increases during Postnatal Development in Rat Brain. J. Pharmacol. Exp. Ther. 2005, 314, 596–602. [Google Scholar] [CrossRef] [Green Version]
- Thornton, S.R.; Compton, D.R.; Smith, F.L. Ontogeny of mu opioid agonist anti-nociception in postnatal rats. Dev. Brain Res. 1998, 105, 269–276. [Google Scholar] [CrossRef]
- Nandi, R.; Beacham, D.; Middleton, J.; Koltzenburg, M.; Howard, R.F.; Fitzgerald, M. The functional expression of mu opioid receptors on sensory neurons is developmentally regulated; morphine analgesia is less selective in the neonate. Pain 2004, 111, 38–50. [Google Scholar] [CrossRef]
- George, M.D.; Baker, J.F.; Leonard, C.E.; Mehta, S.; Miano, T.A.; Hennessy, S. Risk of Nonunion with Nonselective NSAIDs, COX-2 Inhibitors, and Opioids. J. Bone Joint Surg. Am. 2020, 102, 1230–1238. [Google Scholar] [CrossRef]
- Coluzzi, F.; Scerpa, M.S.; Centanni, M. The Effect of Opiates on Bone Formation and Bone Healing. Curr. Osteoporos. Rep. 2020, 18, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, V.K.; Couch, K.S.; McNish, S.; Amdur, R.L. Relationship between opioid treatment and rate of healing in chronic wounds: Opioids in chronic wounds. Wound Repair Regen. 2017, 25, 120–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twillman, R.K.; Long, T.D.; Cathers, T.A.; Mueller, D.W. Treatment of painful skin ulcers with topical opioids. J. Pain Symptom Manag. 1999, 17, 288–292. [Google Scholar] [CrossRef]
- Zaslansky, R.; Ben-Nun, O.; Ben-Shitrit, S.; Ullmann, Y.; Kopf, A.; Stein, C. A randomized, controlled, clinical pilot study assessing the analgesic effect of morphine applied topically onto split-thickness skin wounds. J. Pharm. Pharmacol. 2014, 66, 1559–1566. [Google Scholar] [CrossRef]
- Plein, L.M.; Rittner, H.L. Opioids and the immune system—Friend or foe. Br. J. Pharmacol. 2018, 175, 2717–2725. [Google Scholar] [CrossRef]
- Shanthanna, H.; Ladha, K.S.; Kehlet, H.; Joshi, G.P. Perioperative Opioid Administration. Anesthesiology 2021, 134, 645–659. [Google Scholar] [CrossRef]
- Lisowska, B.; Jakubiak, J.; Siewruk, K.; Sady, M.; Kosson, D. Which idea is better with regard to immune response? Opioid anesthesia or opioid free anesthesia. J. Inflamm. Res. 2020, 13, 859–869. [Google Scholar] [CrossRef]
- Thota, R.S.; Ramkiran, S.; Garg, R.; Goswami, J.; Baxi, V.; Thomas, M. Opioid free onco-anesthesia: Is it time to convict opioids? A systematic review of literature. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, 441–452. [Google Scholar] [CrossRef]
- Koepke, E.J.; Manning, E.L.; Miller, T.E.; Ganesh, A.; Williams, D.G.A.; Manning, M.W. The rising tide of opioid use and abuse: The role of the anesthesiologist. Perioper. Med. Lond. Engl. 2018, 7, 16. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berthézène, C.D.; Rabiller, L.; Jourdan, G.; Cousin, B.; Pénicaud, L.; Casteilla, L.; Lorsignol, A. Tissue Regeneration: The Dark Side of Opioids. Int. J. Mol. Sci. 2021, 22, 7336. https://doi.org/10.3390/ijms22147336
Berthézène CD, Rabiller L, Jourdan G, Cousin B, Pénicaud L, Casteilla L, Lorsignol A. Tissue Regeneration: The Dark Side of Opioids. International Journal of Molecular Sciences. 2021; 22(14):7336. https://doi.org/10.3390/ijms22147336
Chicago/Turabian StyleBerthézène, Cécile Dromard, Lise Rabiller, Géraldine Jourdan, Béatrice Cousin, Luc Pénicaud, Louis Casteilla, and Anne Lorsignol. 2021. "Tissue Regeneration: The Dark Side of Opioids" International Journal of Molecular Sciences 22, no. 14: 7336. https://doi.org/10.3390/ijms22147336
APA StyleBerthézène, C. D., Rabiller, L., Jourdan, G., Cousin, B., Pénicaud, L., Casteilla, L., & Lorsignol, A. (2021). Tissue Regeneration: The Dark Side of Opioids. International Journal of Molecular Sciences, 22(14), 7336. https://doi.org/10.3390/ijms22147336





