Coumarin Interferes with Polar Auxin Transport Altering Microtubule Cortical Array Organization in Arabidopsis thaliana (L.) Heynh. Root Apical Meristem
Abstract
1. Introduction
2. Results
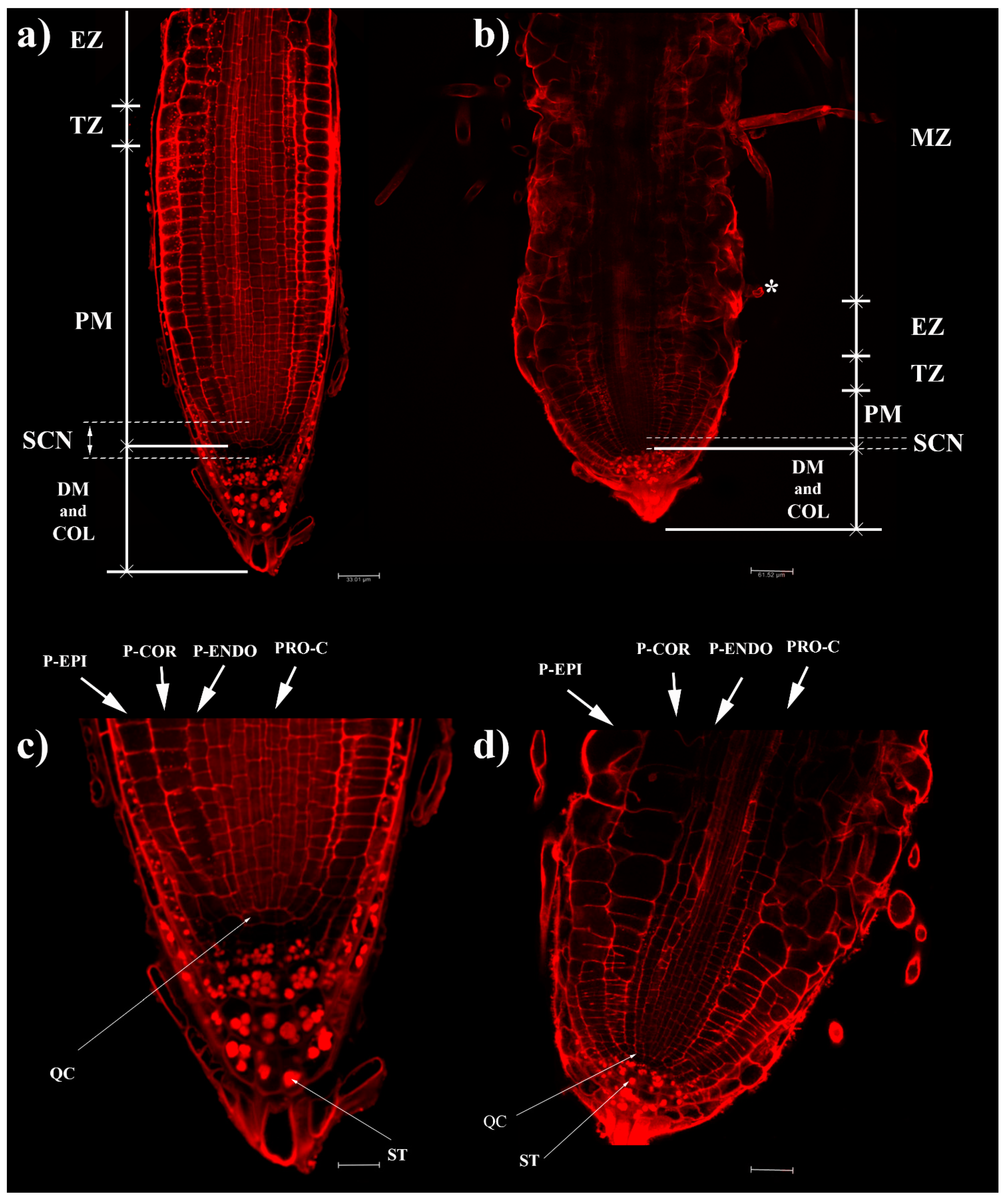
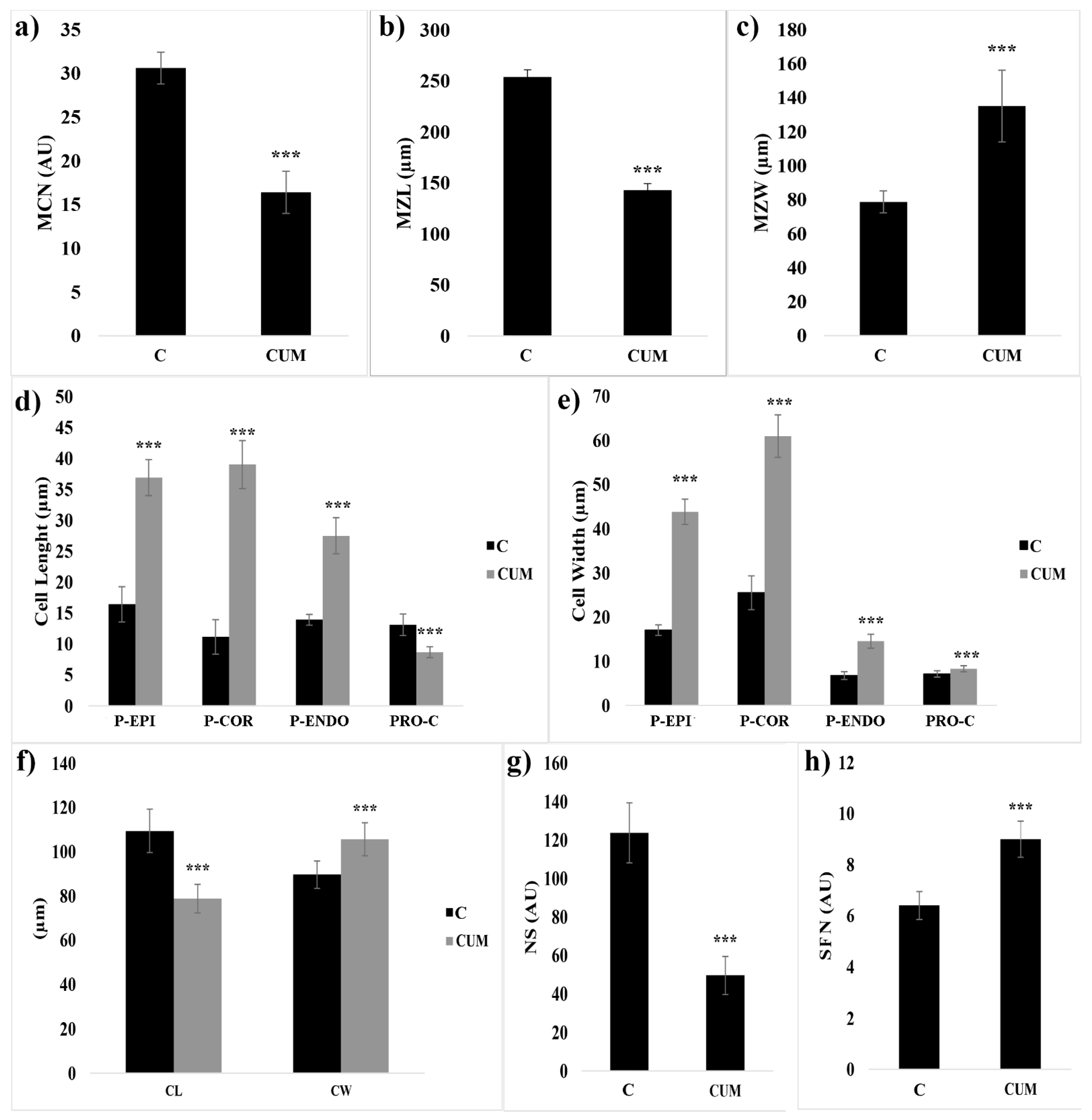
2.1. Effects of Coumarin on RAM
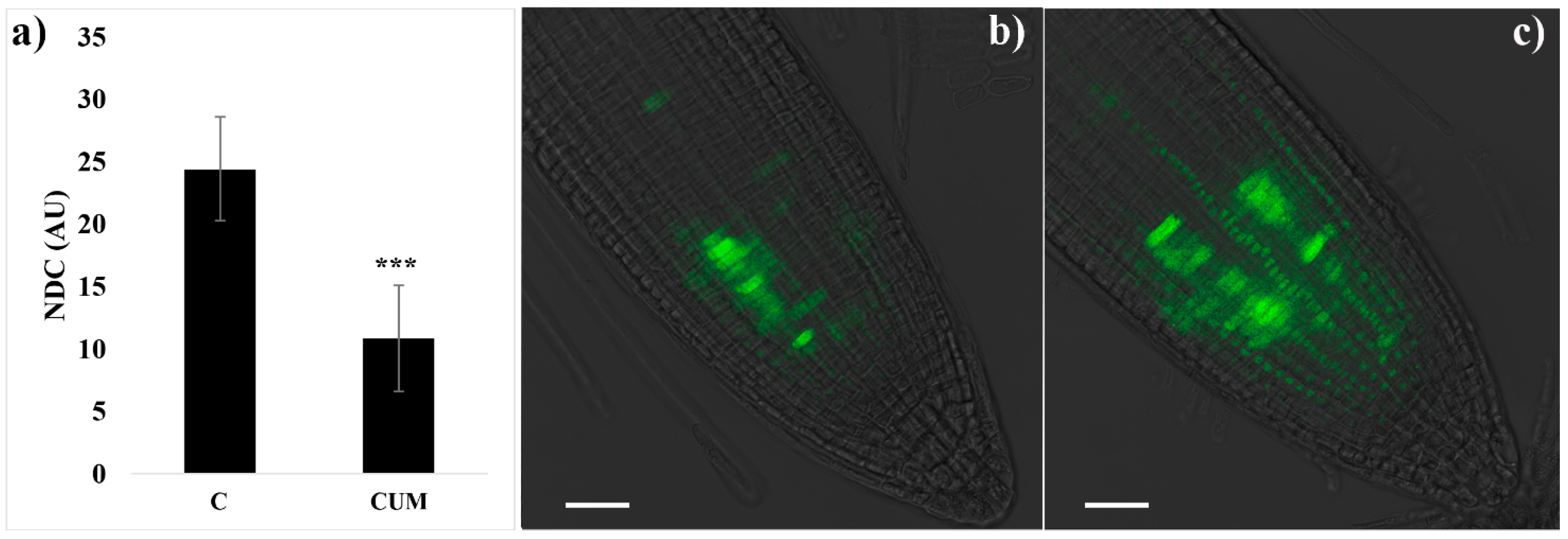
2.2. Effects of Coumarin on Cell Division
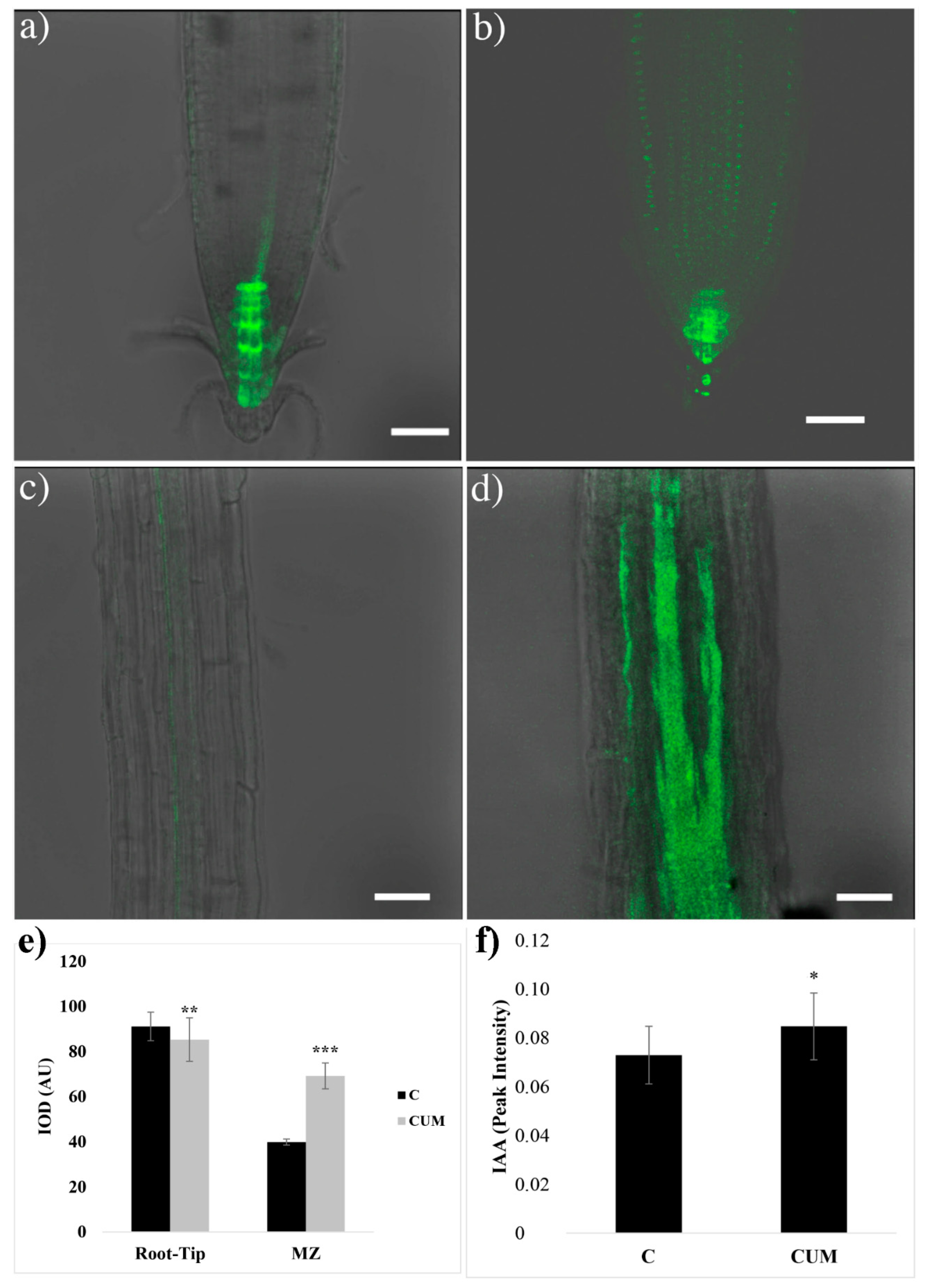
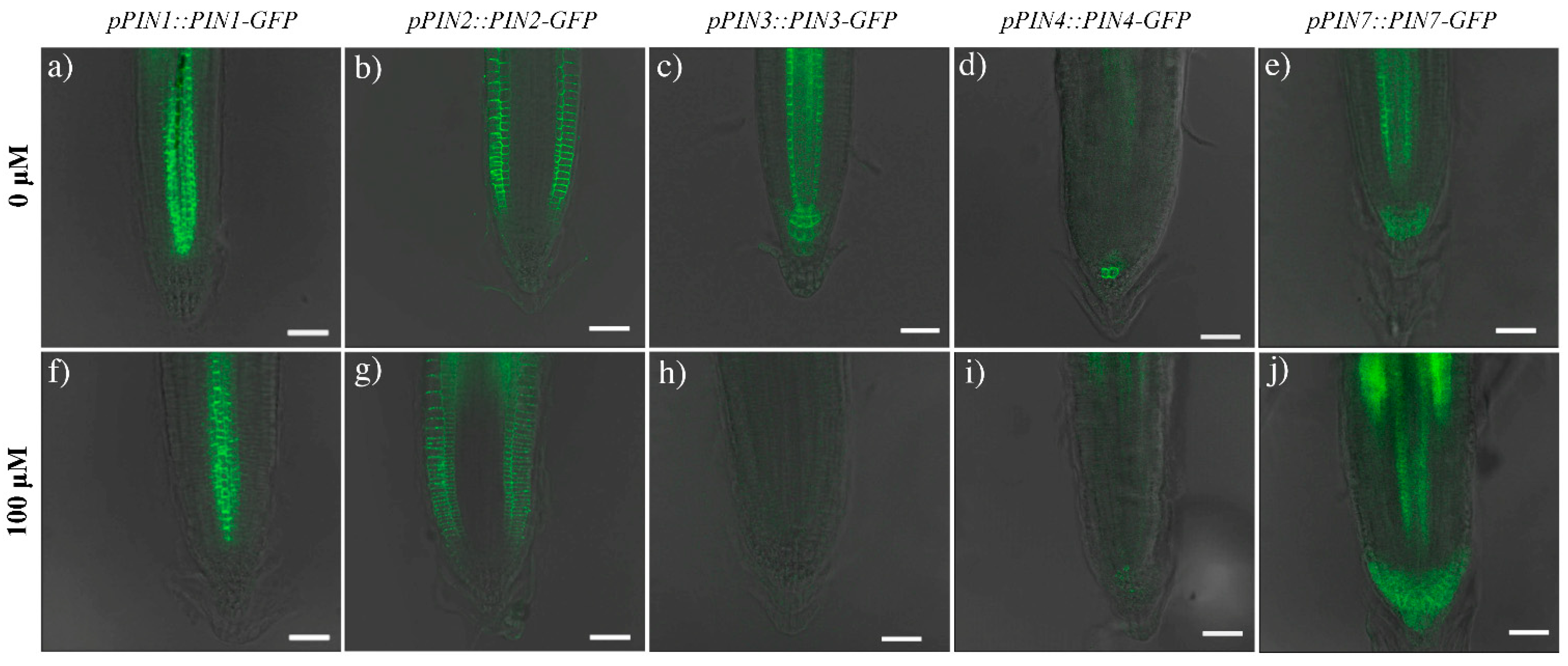
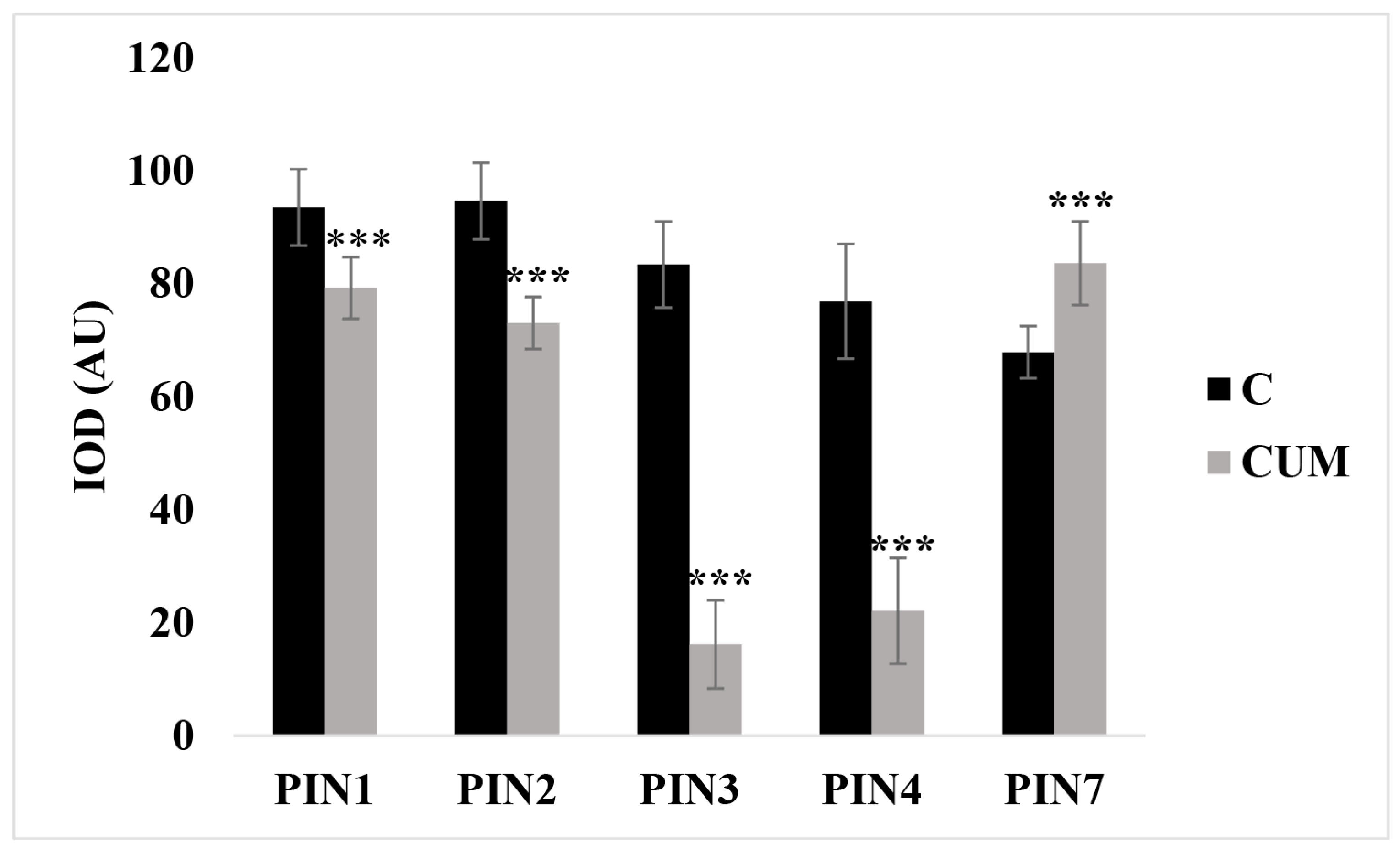
2.3. Effects of Coumarin on Auxin Content and Polar Transport
3. Discussion
4. Materials and Methods
4.1. Reagents Used
4.2. Plant Material and Growth Conditions
4.3. Long-Term Experiments
4.3.1. Root Anatomy: Meristem Size Analysis
4.3.2. Microtubules Immunolabeling
4.4. Short-Term Experiments
4.4.1. Mitotic Indices and Cyclin B1;1::GFP Localization
4.4.2. Localization of GFP Signal in Arabidosis’s Primary Roots
4.4.3. Auxin Quantification
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delgoda, R.; Murray, J. Evolutionary perspectives on the role of plant secondary metabolites. In Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 93–100. [Google Scholar]
- Theis, N.; Lerdau, M. The evolution of function in plant secondary metabolites. Int. J. Plant Sci. 2003, 164, S93–S102. [Google Scholar] [CrossRef]
- Kessler, A.; Kalske, A. Plant secondary metabolite diversity and species interactions. Annu. Rev. Ecol. Evol. System. 2018, 49, 115–138. [Google Scholar] [CrossRef]
- Duke, S.O.; Pan, Z.; Bajsa-Hirschel, J. Proving the mode of action of phytotoxic phytochemicals. Plants 2020, 9, 1756. [Google Scholar] [CrossRef]
- Duke, S.O. Ecophysiological aspects of allelopathy. Planta 2003, 217, 529–539. [Google Scholar]
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef]
- Graña, E.; Costas-Gil, A.; Longueira, S.; Celeiro, M.; Teijeira, M.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Auxin-like effects of the natural coumarin scopoletin on Arabidopsis cell structure and morphology. J. Plant Physiol. 2017, 218, 45–55. [Google Scholar] [CrossRef]
- Bourgaud, F.; Hehn, A.; Larbat, R.; Doerper, S.; Gontier, E.; Kellner, S.; Matern, U. Biosynthesis of coumarins in plants: A major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem. Rev. 2006, 5, 293–308. [Google Scholar] [CrossRef]
- Razavi, S.M. Plant Counnarins as Allelopathic Agents. Int. J. Biol. Chem. 2011, 5, 86–90. [Google Scholar] [CrossRef]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An important class of phytochemicals. Phytochem. Isol. Characterisation Role Hum. Health 2015, 25, 533–538. [Google Scholar]
- Costa, T.M.; Tavares, L.B.B.; de Oliveira, D. Fungi as a source of natural coumarins production. Appl. Microbiol. Biotech. 2016, 100, 6571–6584. [Google Scholar] [CrossRef] [PubMed]
- Niro, E.; Marzaioli, R.; De Crescenzo, S.; D’Abrosca, B.; Castaldi, S.; Esposito, A.; Fiorentino, A.; Rutigliano, F. Effects of the allelochemical coumarin on plants and soil microbial community. Soil Biol. Biochem. 2016, 95, 30–39. [Google Scholar] [CrossRef]
- Robe, K.; Conejero, G.; Gao, F.; Lefebvre-Legendre, L.; Sylvestre-Gonon, E.; Rofidal, V.; Hem, S.; Rouhier, N.; Barberon, M.; Hecker, A. Coumarin accumulation and trafficking in Arabidopsis thaliana: A complex and dynamic process. New Phytol. 2021, 229, 2062–2079. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Weber, M. The essential role of coumarin secretion for Fe acquisition from alkaline soil. Plant Signal. Behav. 2016, 11, e1114197. [Google Scholar] [CrossRef]
- Tsai, H.H.; Schmidt, W. Mobilization of iron by plant-borne coumarins. Trends Plant Sci. 2017, 22, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Robe, K.; Izquierdo, E.; Vignols, F.; Rouached, H.; Dubos, C. The coumarins: Secondary metabolites playing a primary role in plant nutrition and health. Trends Plant Sci. 2020, 26, 248–259. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Teixeira, P.J. Root-exuded coumarin shapes the root microbiome. Proc. Natl. Acad. Sci. USA 2018, 115, 5629–5631. [Google Scholar] [CrossRef]
- Lupini, A.; Sorgonà, A.; Miller, A.J.; Abenavoli, M.R. Short-term effects of coumarin along the maize primary root axis. Plant Signal. Behav. 2010, 5, 1395–1400. [Google Scholar] [CrossRef]
- Abenavoli, M.; Nicolò, A.; Lupini, A.; Oliva, S.; Sorgonà, A. Effects of different allelochemicals on root morphology of Arabidopsis thaliana. Allelopath. J. 2008, 22, 245–252. [Google Scholar]
- Neumann, J. An auxin-like action of coumarin. Science 1959, 129, 1675–1676. [Google Scholar] [CrossRef]
- Jansson, E.; Svensson, S.-B. Coumarin effects on Glycine max hypocotyl explants. Physiol. Plant 1980, 48, 486–490. [Google Scholar] [CrossRef]
- Lupini, A.; Araniti, F.; Sunseri, F.; Abenavoli, M.R. Coumarin interacts with auxin polar transport to modify root system architecture in Arabidopsis thaliana. Plant Growth Regul. 2014, 74, 23–31. [Google Scholar] [CrossRef]
- Pop, T.I.; Pamfil, D.; Bellini, C. Auxin control in the formation of adventitious roots. Not. Bot. Horti Agrobot. Cluj Napoca 2011, 39, 307–316. [Google Scholar] [CrossRef]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A. Stress-induced morphogenic responses: Growing out of trouble? Trends Plant Sci. 2007, 12, 98–105. [Google Scholar] [CrossRef]
- Zažímalová, E.; Murphy, A.S.; Yang, H.; Hoyerová, K.; Hošek, P. Auxin transporters—Why so many? Cold Spring Harb. Perspect. Biol. 2010, 2, a001552. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, I.; Beeckman, T.; Graham, N.; Bhalerao, R.; Zhang, H.; Casero, P.; Sandberg, G.; Bennett, M.J. Dissecting Arabidopsis lateral root development. Trends Plant Sci. 2003, 8, 165–171. [Google Scholar] [CrossRef]
- Singh, S.; Yadav, S.; Singh, A.; Mahima, M.; Singh, A.; Gautam, V.; Sarkar, A.K. Auxin signaling modulates LATERAL ROOT PRIMORDIUM 1 (LRP 1) expression during lateral root development in Arabidopsis. Plant J. 2020, 101, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef]
- Laskowski, M.; Biller, S.; Stanley, K.; Kajstura, T.; Prusty, R. Expression profiling of auxin-treated Arabidopsis roots: Toward a molecular analysis of lateral root emergence. Plant Cell Physiol. 2006, 47, 788–792. [Google Scholar] [CrossRef]
- Dubrovsky, J.G.; Napsucialy-Mendivil, S.; Duclercq, J.; Cheng, Y.; Shishkova, S.; Ivanchenko, M.G.; Friml, J.; Murphy, A.S.; Benková, E. Auxin minimum defines a developmental window for lateral root initiation. New Phytol. 2011, 191, 970–983. [Google Scholar] [CrossRef]
- Lehman, T.A.; Smertenko, A.; Sanguinet, K.A. Auxin, microtubules, and vesicle trafficking: Conspirators behind the cell wall. J. Exp. Bot. 2017, 68, 3321–3329. [Google Scholar] [CrossRef]
- Kleine-Vehn, J.; Łangowski, Ł.; Wiśniewska, J.; Dhonukshe, P.; Brewer, P.B.; Friml, J. Cellular and molecular requirements for polar PIN targeting and transcytosis in plants. Mol. Plant 2008, 1, 1056–1066. [Google Scholar] [CrossRef]
- Na, X.; Hu, Y.; Yue, K.; Lu, H.; Jia, P.; Wang, H.; Wang, X.; Bi, Y. Narciclasine modulates polar auxin transport in Arabidopsis roots. J. Plant Physiol. 2011, 168, 1149–1156. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, L.; Na, X.; You, J.; Hu, W.; Liang, X.; Liu, J.; Mao, L.; Wang, X.; Wang, H. Narciclasine inhibits the responses of Arabidopsis roots to auxin. Planta 2012, 236, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Graña, E.; Sotelo, T.; Díaz-Tielas, C.; Araniti, F.; Krasuska, U.; Bogatek, R.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Citral induces auxin and ethylene-mediated malformations and arrests cell division in Arabidopsis thaliana roots. J. Chem. Ecol. 2013, 39, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Na, X.; Li, J.; Yang, L.; You, J.; Liang, X.; Wang, J.; Peng, L.; Bi, Y. Narciclasine, a potential allelochemical, affects subcellular trafficking of auxin transporter proteins and actin cytoskeleton dynamics in Arabidopsis roots. Planta 2015, 242, 1349–1360. [Google Scholar] [CrossRef]
- Araniti, F.; Grana, E.; Krasuska, U.; Bogatek, R.; Reigosa, M.J.; Abenavoli, M.R.; Sanchez-Moreiras, A.M. Loss of gravitropism in farnesene-treated arabidopsis is due to microtubule malformations related to hormonal and ROS unbalance. PLoS ONE 2016, 11, e0160202. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ding, L.; Zhang, L.; He, J.; Huan, Z. Weisiensin B inhibits primary and lateral root development by interfering with polar auxin transport in Arabidopsis thaliana. Plant Physiol. Biochem. 2019, 139, 738–745. [Google Scholar] [CrossRef] [PubMed]
- López-González, D.; Costas-Gil, A.; Reigosa, M.J.; Araniti, F.; Sánchez-Moreiras, A.M. A natural indole alkaloid, norharmane, affects PIN expression patterns and compromises root growth in Arabidopsis thaliana. Plant Physiol. Biochem. 2020, 151, 378–390. [Google Scholar] [CrossRef]
- Araniti, F.; Bruno, L.; Sunseri, F.; Pacenza, M.; Forgione, I.; Bitonti, M.B.; Abenavoli, M.R. The allelochemical farnesene affects Arabidopsis thaliana root meristem altering auxin distribution. Plant Physiol. Biochem. 2017, 121, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Chaimovitsh, D.; Abu-Abied, M.; Belausov, E.; Rubin, B.; Dudai, N.; Sadot, E. Microtubules are an intracellular target of the plant terpene citral. Plant J. 2010, 61, 399–408. [Google Scholar] [CrossRef]
- Graña, E.; Díaz-Tielas, C.; López-González, D.; Martínez-Peñalver, A.; Reigosa, M.; Sánchez-Moreiras, A. The plant secondary metabolite citral alters water status and prevents seed formation in Arabidopsis thaliana. Plant Biol. 2016, 18, 423–432. [Google Scholar] [CrossRef]
- Svensson, S.B. The effect of coumarin on root growth and root histology. Physiol. Plant 1971, 24, 446–470. [Google Scholar] [CrossRef]
- Mizukami, Y. A matter of size: Developmental control of organ size in plants. Curr. Opin. Plant Biol. 2001, 4, 533–539. [Google Scholar] [CrossRef]
- Ioio, R.D.; Linhares, F.S.; Scacchi, E.; Casamitjana-Martinez, E.; Heidstra, R.; Costantino, P.; Sabatini, S. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 2007, 17, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Ioio, R.D.; Nakamura, K.; Moubayidin, L.; Perilli, S.; Taniguchi, M.; Morita, M.T.; Aoyama, T.; Costantino, P.; Sabatini, S. A genetic framework for the control of cell division and differentiation in the root meristem. Science 2008, 322, 1380–1384. [Google Scholar] [CrossRef]
- Doerner, P.; Jørgensen, J.-E.; You, R.; Steppuhn, J.; Lamb, C. Control of root growth and development by cyclin expression. Nature 1996, 380, 520–523. [Google Scholar] [CrossRef]
- Criqui, M.C.; Parmentier, Y.; Derevier, A.; Shen, W.H.; Dong, A.; Genschik, P. Cell cycle-dependent proteolysis and ectopic overexpression of cyclin B1 in tobacco BY2 cells. Plant J. 2000, 24, 763–773. [Google Scholar] [CrossRef]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, M.R.; Sorgonà, A.; Albano, S.; Cacco, G. Coumarin differentially affects the morphology of different root types of maize seedlings. J. Chem. Ecol. 2004, 30, 1871–1883. [Google Scholar] [CrossRef] [PubMed]
- Jingyi, W.; Dandan, Y.; Jun, X.; Cai-xia, W.; Guo-qi, Z.; Chen-lan, H. Effect of coumarin on Soughum sudanense seed germination and seedling growth. Pratac. Sci. 2017, 34, 2279–2288. [Google Scholar]
- Chen, B.-X.; Peng, Y.-X.; Gao, J.-D.; Zhang, Q.; Liu, Q.-J.; Fu, H.; Liu, J. Coumarin-induced delay of rice seed germination is mediated by suppression of abscisic acid catabolism and reactive oxygen species production. Front. Plant Sci. 2019, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Laplaze, L.; Benkova, E.; Casimiro, I.; Maes, L.; Vanneste, S.; Swarup, R.; Weijers, D.; Calvo, V.; Parizot, B.; Herrera-Rodriguez, M.B. Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 2007, 19, 3889–3900. [Google Scholar] [CrossRef] [PubMed]
- Bruno, L.; Pacenza, M.; Forgione, I.; Lamerton, L.R.; Greco, M.; Chiappetta, A.; Bitonti, M.B. In Arabidopsis thaliana cadmium impact on the growth of primary root by altering SCR expression and auxin-cytokinin cross-talk. Front. Plant Sci. 2017, 8, 1323. [Google Scholar] [CrossRef] [PubMed]
- Na, X.; Hu, Y.; Yue, K.; Lu, H.; Jia, P.; Wang, H.; Wang, X.; Bi, Y. Concentration-dependent effects of narciclasine on cell cycle progression in Arabidopsis root tips. BMC Plant Biol. 2011, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cornman, I. The responses of onion and lily mitosis to coumarin and parasorbic acid. J. Exp. Biol. 1947, 23, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, B.; Aksoy, O. Cytological effects of coumarin on the mitosis of Lens culinaris Medik. Fresenius Environ. Bull. 2017, 26, 6400–6407. [Google Scholar]
- Culligan, K.; Tissier, A.; Britt, A. ATR regulates a G2-phase cell-cycle checkpoint in Arabidopsis thaliana. Plant Cell 2004, 16, 1091–1104. [Google Scholar] [CrossRef]
- Wu, S.; Scheible, W.-R.; Schindelasch, D.; Van Den Daele, H.; De Veylder, L.; Baskin, T.I. A conditional mutation in Arabidopsis thaliana separase induces chromosome non-disjunction, aberrant morphogenesis and cyclin B1; 1 stability. Development 2010, 137, 953–961. [Google Scholar] [CrossRef][Green Version]
- De Schutter, K.; Joubès, J.; Cools, T.; Verkest, A.; Corellou, F.; Babiychuk, E.; Van Der Schueren, E.; Beeckman, T.; Kushnir, S.; Inze, D. Arabidopsis WEE1 kinase controls cell cycle arrest in response to activation of the DNA integrity checkpoint. Plant Cell 2007, 19, 211–225. [Google Scholar] [CrossRef]
- Hefner, E.; Huefner, N.; Britt, A.B. Tissue-specific regulation of cell-cycle responses to DNA damage in Arabidopsis seedlings. DNA Repair 2006, 5, 102–110. [Google Scholar] [CrossRef]
- Weingartner, M.; Criqui, M.-C.; Mészáros, T.; Binarova, P.; Schmit, A.-C.; Helfer, A.; Derevier, A.; Erhardt, M.; Bögre, L.; Genschik, P. Expression of a nondegradable cyclin B1 affects plant development and leads to endomitosis by inhibiting the formation of a phragmoplast. Plant Cell 2004, 16, 643–657. [Google Scholar] [CrossRef]
- Serralbo, O.; Pérez-Pérez, J.M.; Heidstra, R.; Scheres, B. Non-cell-autonomous rescue of anaphase-promoting complex function revealed by mosaic analysis of HOBBIT, an Arabidopsis CDC27 homolog. Proc. Natl. Acad. Sci. USA 2006, 103, 13250–13255. [Google Scholar] [CrossRef]
- Baskin, T.I.; Wilson, J.E.; Cork, A.; Williamson, R.E. Morphology and microtubule organization in Arabidopsis roots exposed to oryzalin or taxol. Plant Cell Physiol. 1994, 35, 935–942. [Google Scholar] [PubMed]
- Baskin, T.I.; Beemster, G.T.; Judy-March, J.E.; Marga, F. Disorganization of cortical microtubules stimulates tangential expansion and reduces the uniformity of cellulose microfibril alignment among cells in the root of Arabidopsis. Plant Physiol. 2004, 135, 2279–2290. [Google Scholar] [CrossRef]
- Fanale, D.; Bronte, G.; Passiglia, F.; Calò, V.; Castiglia, M.; Di Piazza, F.; Barraco, N.; Cangemi, A.; Catarella, M.T.; Insalaco, L. Stabilizing versus destabilizing the microtubules: A double-edge sword for an effective cancer treatment option? Anal. Cell. Pathol. 2015, 2015, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Morejohn, L.C.; Fosket, D.E. The biochemistry of compounds with anti-microtubule activity in plant cells. Pharmacol. Ther. 1991, 51, 217–230. [Google Scholar] [CrossRef]
- Hugdahl, J.D.; Morejohn, L.C. Rapid and reversible high-affinity binding of the dinitroaniline herbicide oryzalin to tubulin from Zea mays L. Plant Physiol. 1993, 102, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Pitts, R.J.; Cernac, A.; Estelle, M. Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J. 1998, 16, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Hosokawa, S.; Oono, Y.; Amakawa, T.; Goto, N.; Tsurumi, S. Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiol. 2002, 130, 1908–1917. [Google Scholar] [CrossRef]
- Campanoni, P.; Nick, P. Auxin-dependent cell division and cell elongation. 1-Naphthaleneacetic acid and 2, 4-dichlorophenoxyacetic acid activate different pathways. Plant Physiol. 2005, 137, 939–948. [Google Scholar] [CrossRef]
- Li, X.; Gruber, M.Y.; Hegedus, D.D.; Lydiate, D.J.; Gao, M.-J. Effects of a coumarin derivative, 4-methylumbelliferone, on seed germination and seedling establishment in Arabidopsis. J. Chem. Ecol. 2011, 37, 880–890. [Google Scholar] [CrossRef]
- Tian, H.; Niu, T.; Yu, Q.; Quan, T.; Ding, Z. Auxin gradient is crucial for the maintenance of root distal stem cell identity in Arabidopsis. Plant Sign. Behav. 2013, 8, e26429. [Google Scholar] [CrossRef]
- Goren, R.; Tomer, E. Effects of seselin and coumarin on growth, indoleacetic acid oxidase, and peroxidase, with special reference to cucumber (Cucumis sativa L.) radicles. Plant Physiol. 1971, 47, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Andreae, W. Effect of scopoletin on indoleacetic acid metabolism. Nature 1952, 170, 83–84. [Google Scholar] [CrossRef]
- Casimiro, I.; Marchant, A.; Bhalerao, R.P.; Beeckman, T.; Dhooge, S.; Swarup, R.; Graham, N.; Inzé, D.; Sandberg, G.; Casero, P.J. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 2001, 13, 843–852. [Google Scholar] [CrossRef] [PubMed]
- De Rybel, B.; Audenaert, D.; Xuan, W.; Overvoorde, P.; Strader, L.C.; Kepinski, S.; Hoye, R.; Brisbois, R.; Parizot, B.; Vanneste, S. A role for the root cap in root branching revealed by the non-auxin probe naxillin. Nat. Chem. Biol. 2012, 8, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Himanen, K.; Boucheron, E.; Vanneste, S.; de Almeida Engler, J.; Inzé, D.; Beeckman, T. Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 2002, 14, 2339–2351. [Google Scholar] [CrossRef]
- Vanneste, S.; De Rybel, B.; Beemster, G.T.; Ljung, K.; De Smet, I.; Van Isterdael, G.; Naudts, M.; Iida, R.; Gruissem, W.; Tasaka, M. Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell 2005, 17, 3035–3050. [Google Scholar] [CrossRef]
- Zhang, Y.; He, P.; Ma, X.; Yang, Z.; Pang, C.; Yu, J.; Wang, G.; Friml, J.; Xiao, G. Auxin-mediated statolith production for root gravitropism. New Phytol. 2019, 224, 761–774. [Google Scholar] [CrossRef]
- Dubrovsky, J.G.; Sauer, M.; Napsucialy-Mendivil, S.; Ivanchenko, M.G.; Friml, J.; Shishkova, S.; Celenza, J.; Benková, E. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc. Nat. Acad. Sci. USA 2008, 105, 8790–8794. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, M.; Salguero, J.; Lloret, P.G. Auxin modulated initiation of lateral roots is linked to pericycle cell length in maize. Front. Plant Sci. 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, M.J.; Williams, M.E.; Nusbaum, H.C.; Sussex, I.M. Formation of lateral root meristems is a two-stage process. Development 1995, 121, 3303–3310. [Google Scholar] [CrossRef] [PubMed]
- Ottenschläger, I.; Wolff, P.; Wolverton, C.; Bhalerao, R.P.; Sandberg, G.; Ishikawa, H.; Evans, M.; Palme, K. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc. Natl. Acad. Sci. USA 2003, 100, 2987–2991. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Romero, J.; Carme Espunya, M.; Platara, M.; Ariño, J.; Carmen Martínez, M. A role for protein kinase CK2 in plant development: Evidence obtained using a dominant-negative mutant. Plant J. 2008, 55, 118–130. [Google Scholar] [CrossRef] [PubMed]
- González-García, M.-P.; Vilarrasa-Blasi, J.; Zhiponova, M.; Divol, F.; Mora-García, S.; Russinova, E.; Caño-Delgado, A.I. Brassinosteroids control meristem size by promoting cell cycle progression in Arabidopsis roots. Development 2011, 138, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, A.; Kawamura, E.; Wasteneys, G.O. Strategies for imaging microtubules in plant cells. In Cytoskeleton Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2009; pp. 243–262. [Google Scholar]
- Cools, T.; Iantcheva, A.; Maes, S.; Van den Daele, H.; De Veylder, L. A replication stress-induced synchronization method for Arabidopsis thaliana root meristems. Plant J. 2010, 64, 705–714. [Google Scholar] [CrossRef]
- Rawlinson, C.; Kamphuis, L.G.; Gummer, J.P.; Singh, K.B.; Trengove, R.D. A rapid method for profiling of volatile and semi-volatile phytohormones using methyl chloroformate derivatisation and GC–MS. Metabolomics 2015, 11, 1922–1933. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, L.; Talarico, E.; Cabeiras-Freijanes, L.; Madeo, M.L.; Muto, A.; Minervino, M.; Lucini, L.; Miras-Moreno, B.; Sofo, A.; Araniti, F. Coumarin Interferes with Polar Auxin Transport Altering Microtubule Cortical Array Organization in Arabidopsis thaliana (L.) Heynh. Root Apical Meristem. Int. J. Mol. Sci. 2021, 22, 7305. https://doi.org/10.3390/ijms22147305
Bruno L, Talarico E, Cabeiras-Freijanes L, Madeo ML, Muto A, Minervino M, Lucini L, Miras-Moreno B, Sofo A, Araniti F. Coumarin Interferes with Polar Auxin Transport Altering Microtubule Cortical Array Organization in Arabidopsis thaliana (L.) Heynh. Root Apical Meristem. International Journal of Molecular Sciences. 2021; 22(14):7305. https://doi.org/10.3390/ijms22147305
Chicago/Turabian StyleBruno, Leonardo, Emanuela Talarico, Luz Cabeiras-Freijanes, Maria Letizia Madeo, Antonella Muto, Marco Minervino, Luigi Lucini, Begoña Miras-Moreno, Adriano Sofo, and Fabrizio Araniti. 2021. "Coumarin Interferes with Polar Auxin Transport Altering Microtubule Cortical Array Organization in Arabidopsis thaliana (L.) Heynh. Root Apical Meristem" International Journal of Molecular Sciences 22, no. 14: 7305. https://doi.org/10.3390/ijms22147305
APA StyleBruno, L., Talarico, E., Cabeiras-Freijanes, L., Madeo, M. L., Muto, A., Minervino, M., Lucini, L., Miras-Moreno, B., Sofo, A., & Araniti, F. (2021). Coumarin Interferes with Polar Auxin Transport Altering Microtubule Cortical Array Organization in Arabidopsis thaliana (L.) Heynh. Root Apical Meristem. International Journal of Molecular Sciences, 22(14), 7305. https://doi.org/10.3390/ijms22147305











