Effects of Chronic Low-Dose Internal Radiation on Immune-Stimulatory Responses in Mice
Abstract
:1. Introduction
2. Results
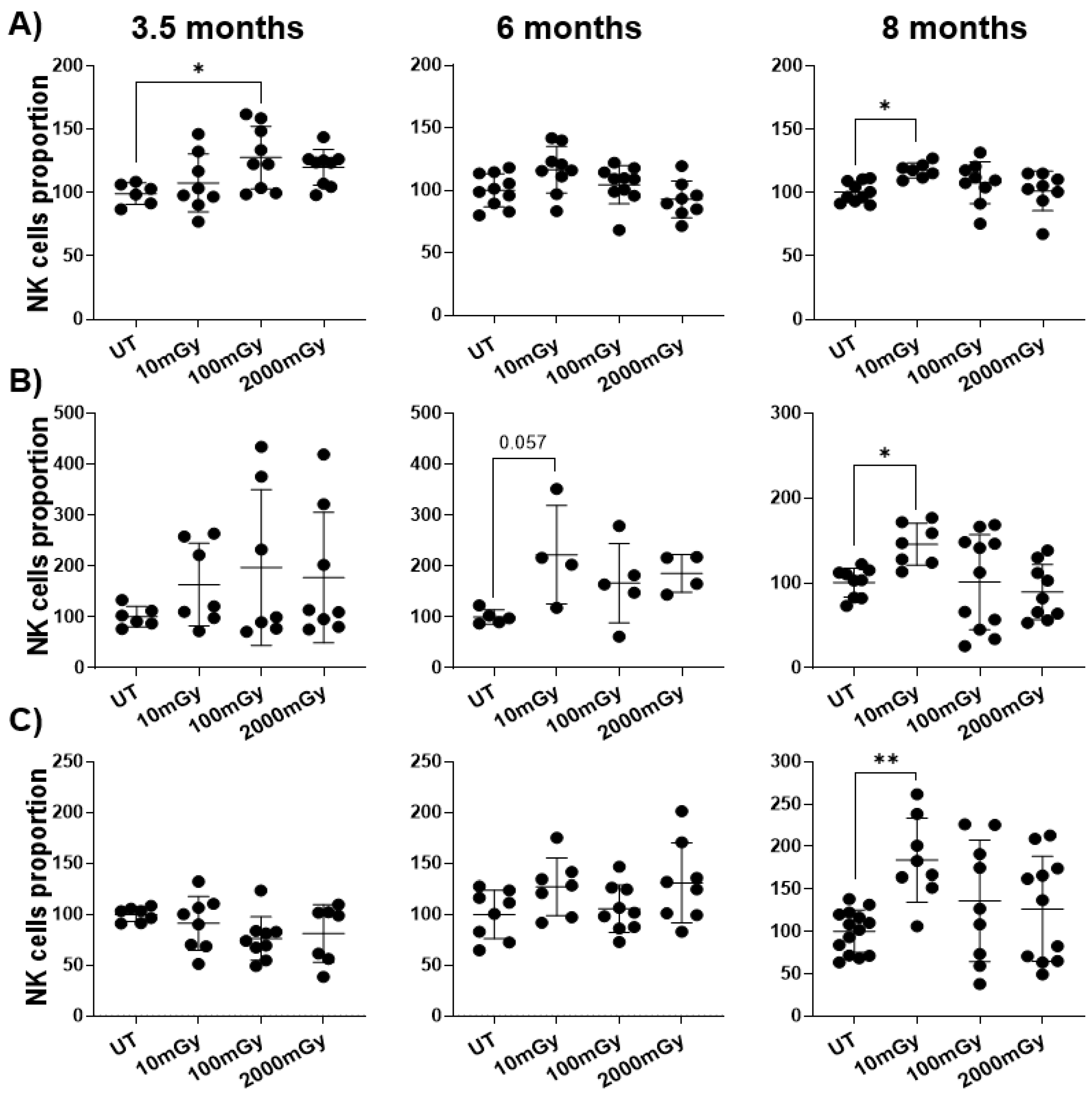
2.1. LDR Affects Immune Cell Frequency
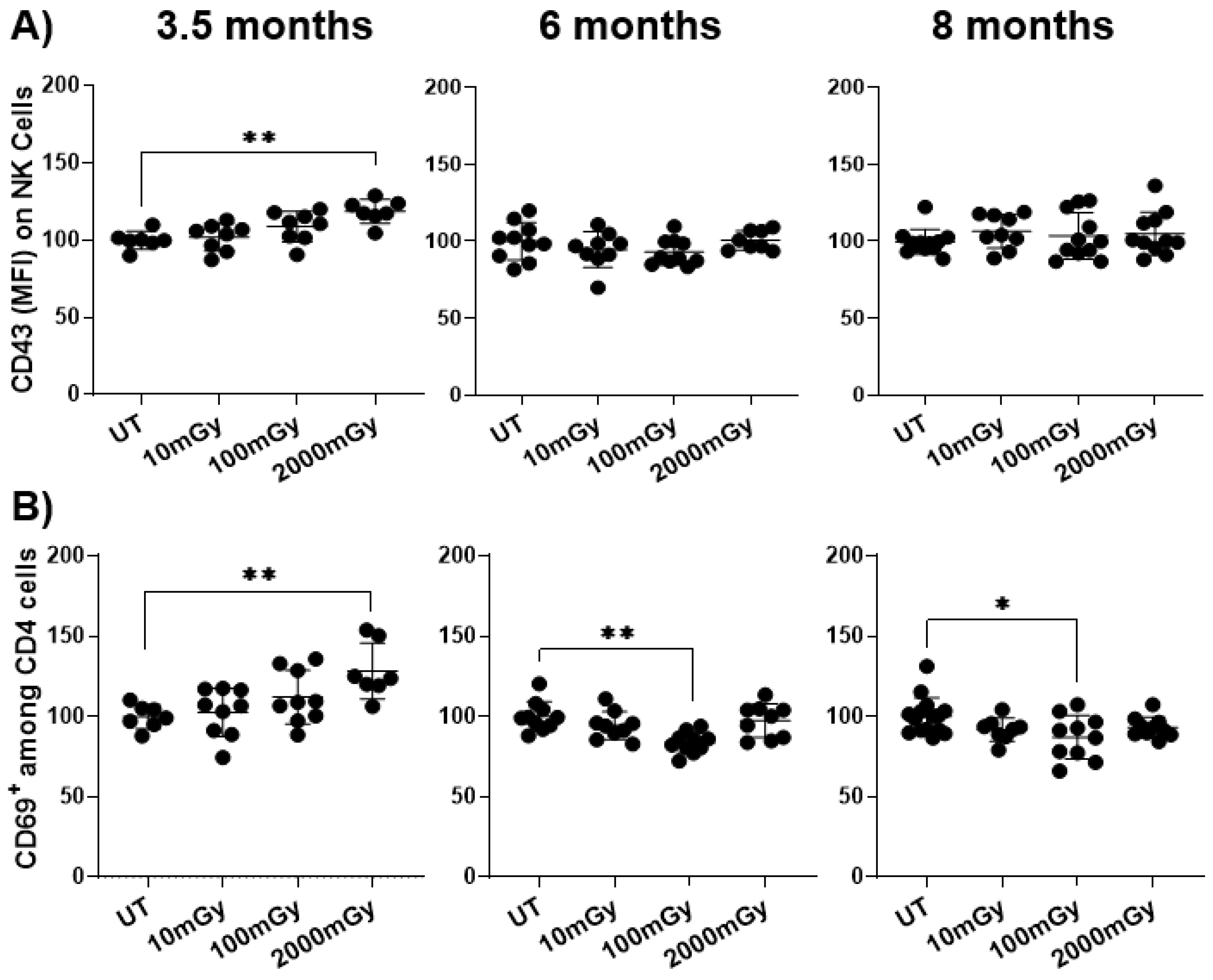
2.2. LDR Influences Immune Cell Activation Status
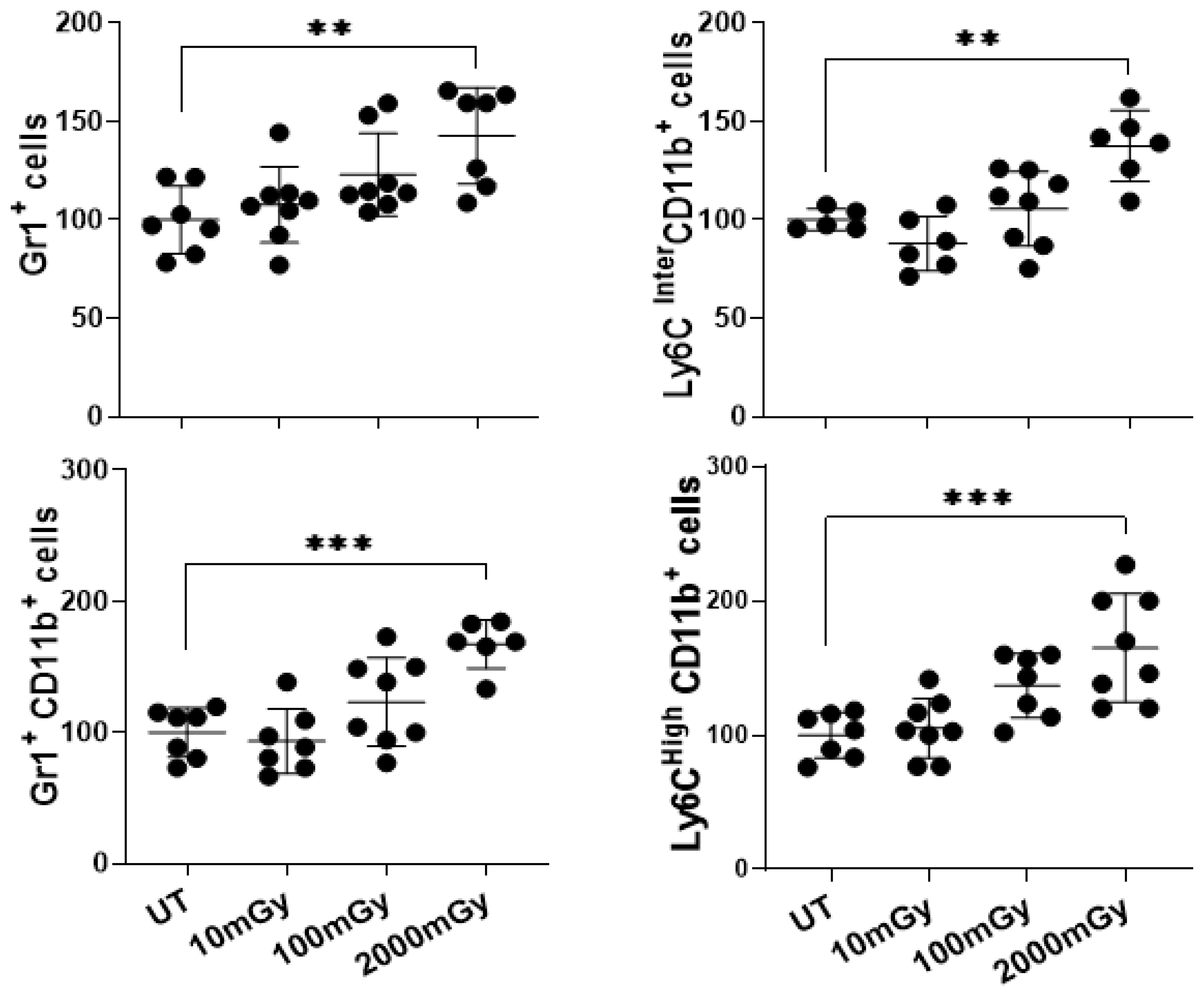
2.3. LDR Induces Inflammatory Responses
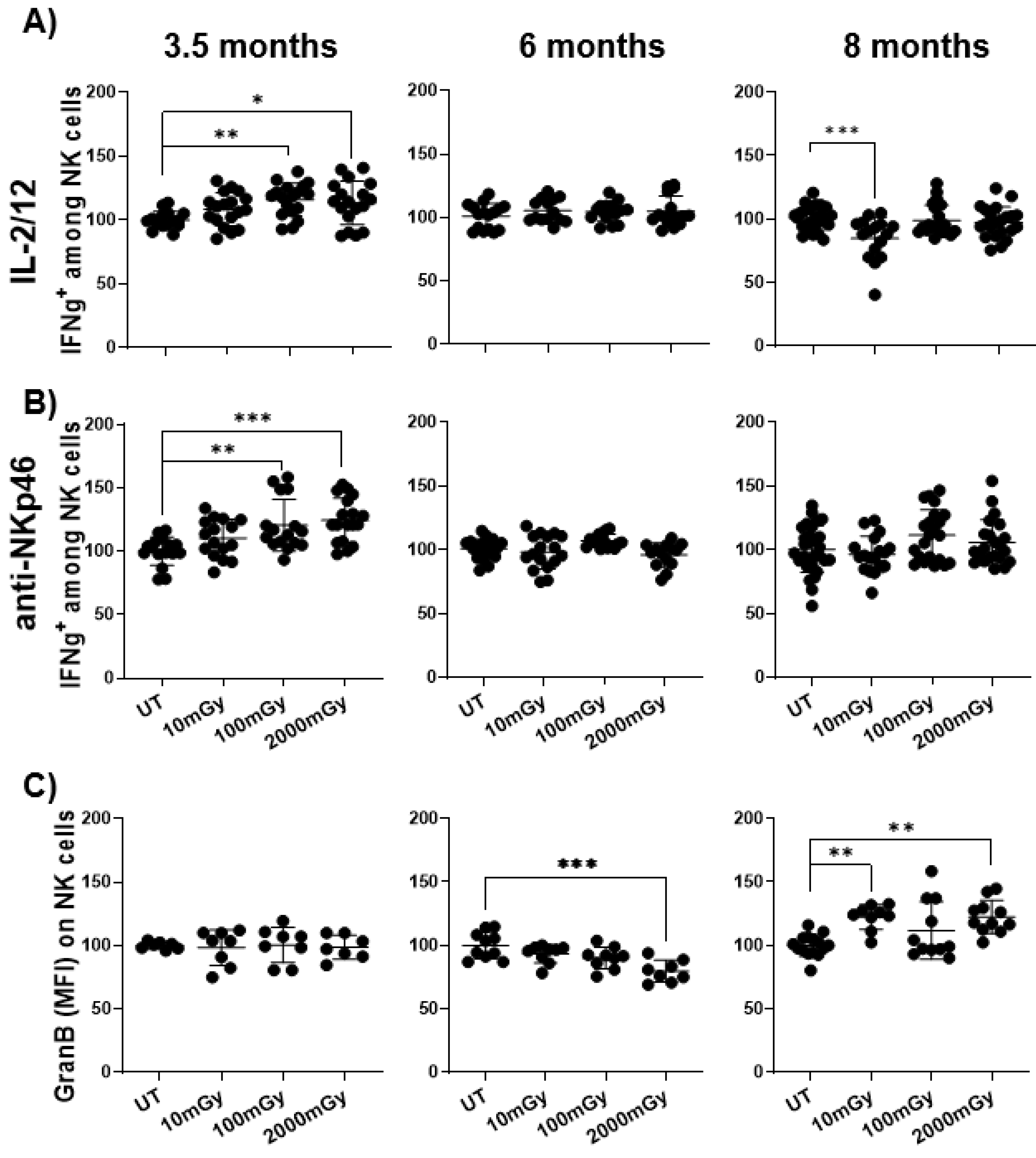
2.4. LDR Affects the Function of Immune Cells
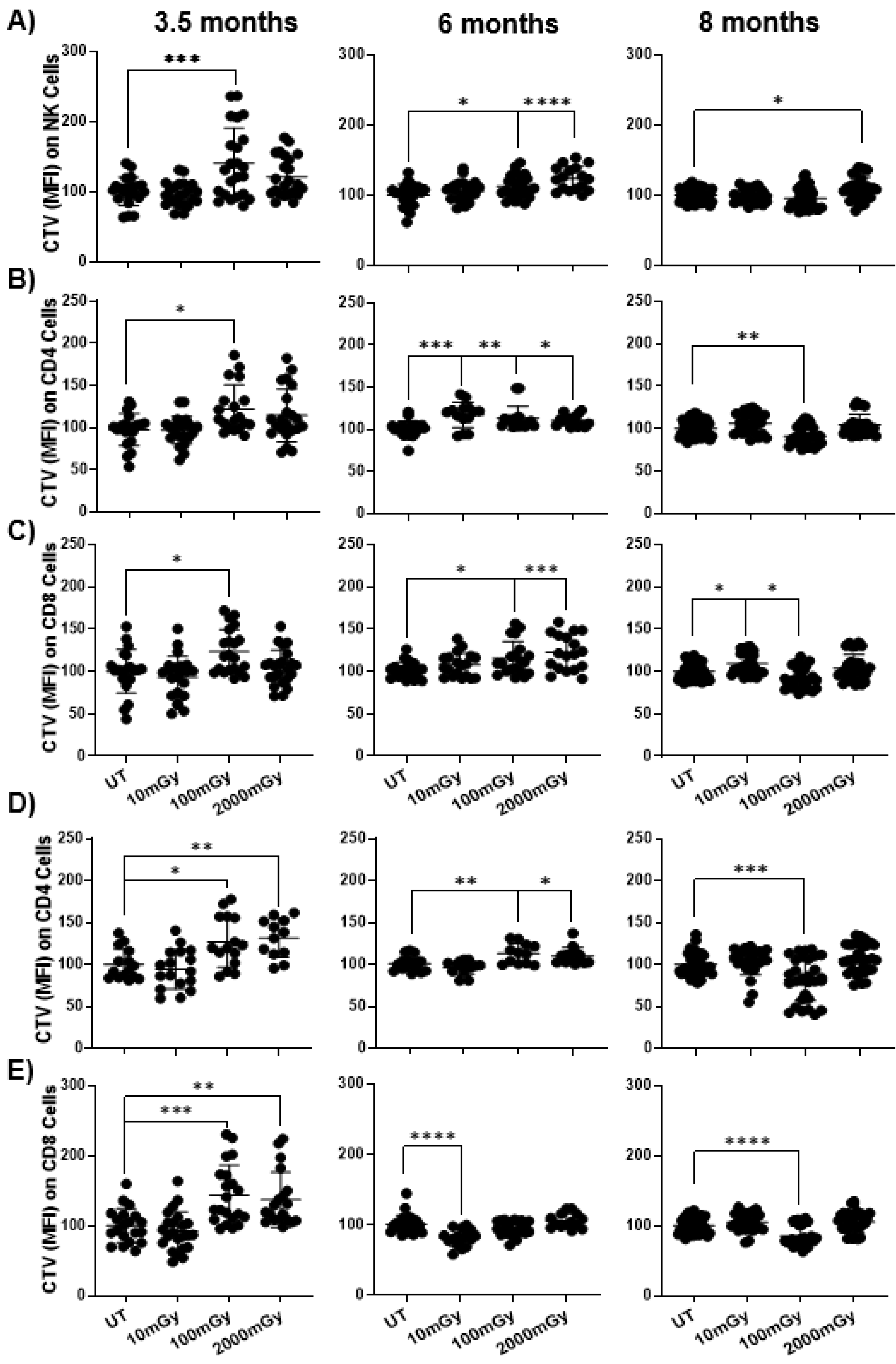
2.5. LDR Suppresses Immune Cell Proliferation
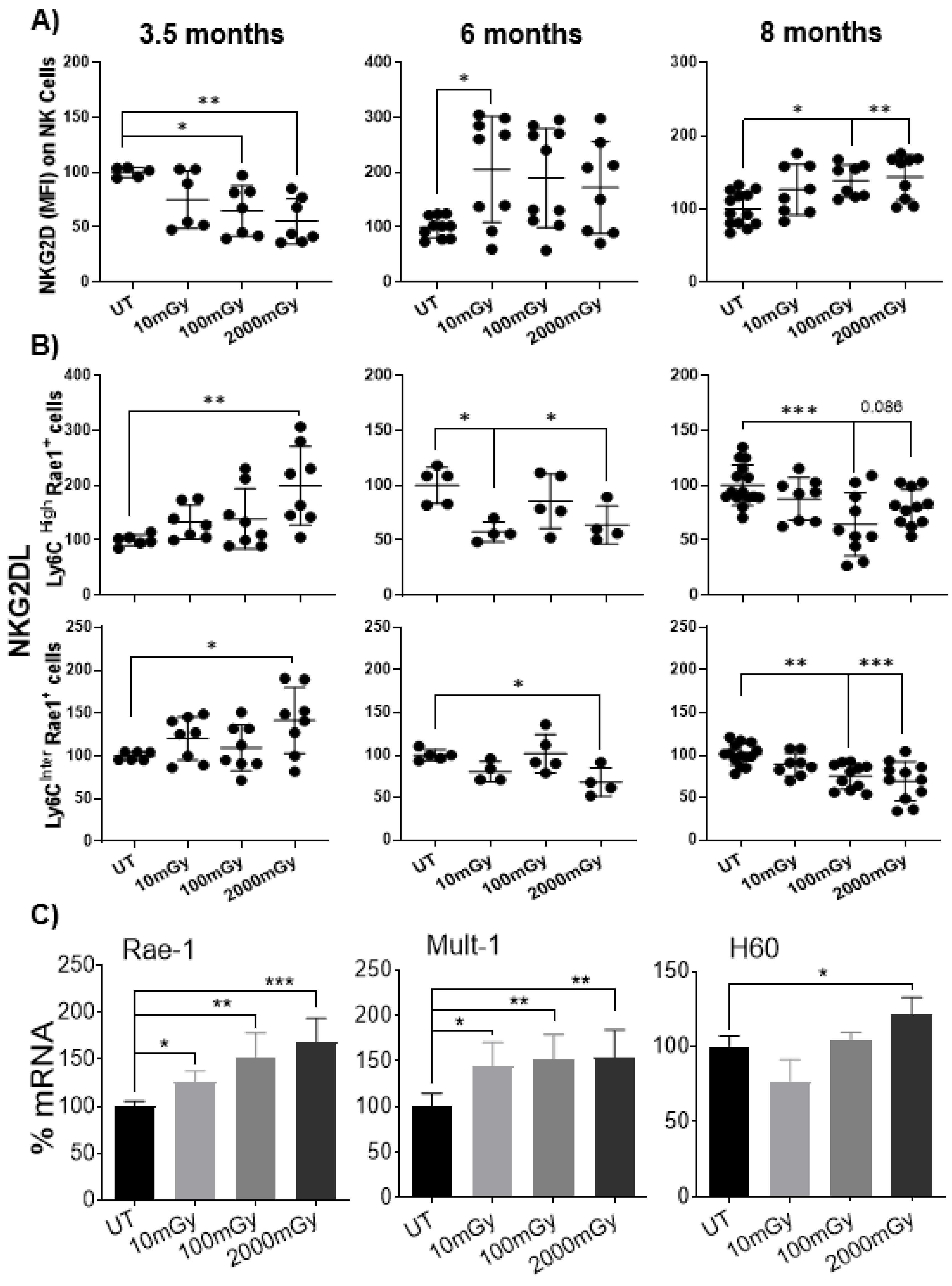
2.6. LDR Regulates Cross-Talk between NKG2D Receptor and Its Ligand
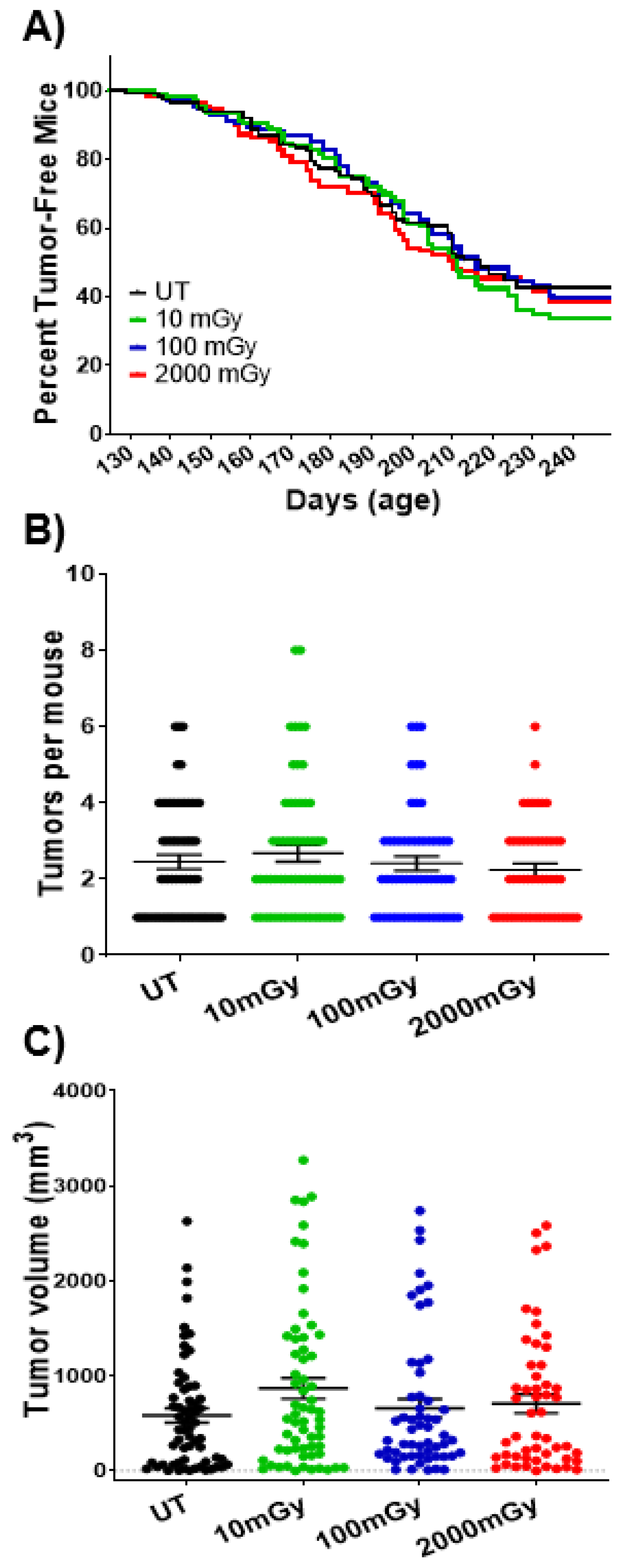
2.7. LDR Has No Impact on Tumorigenesis at Organismal Level
3. Discussion
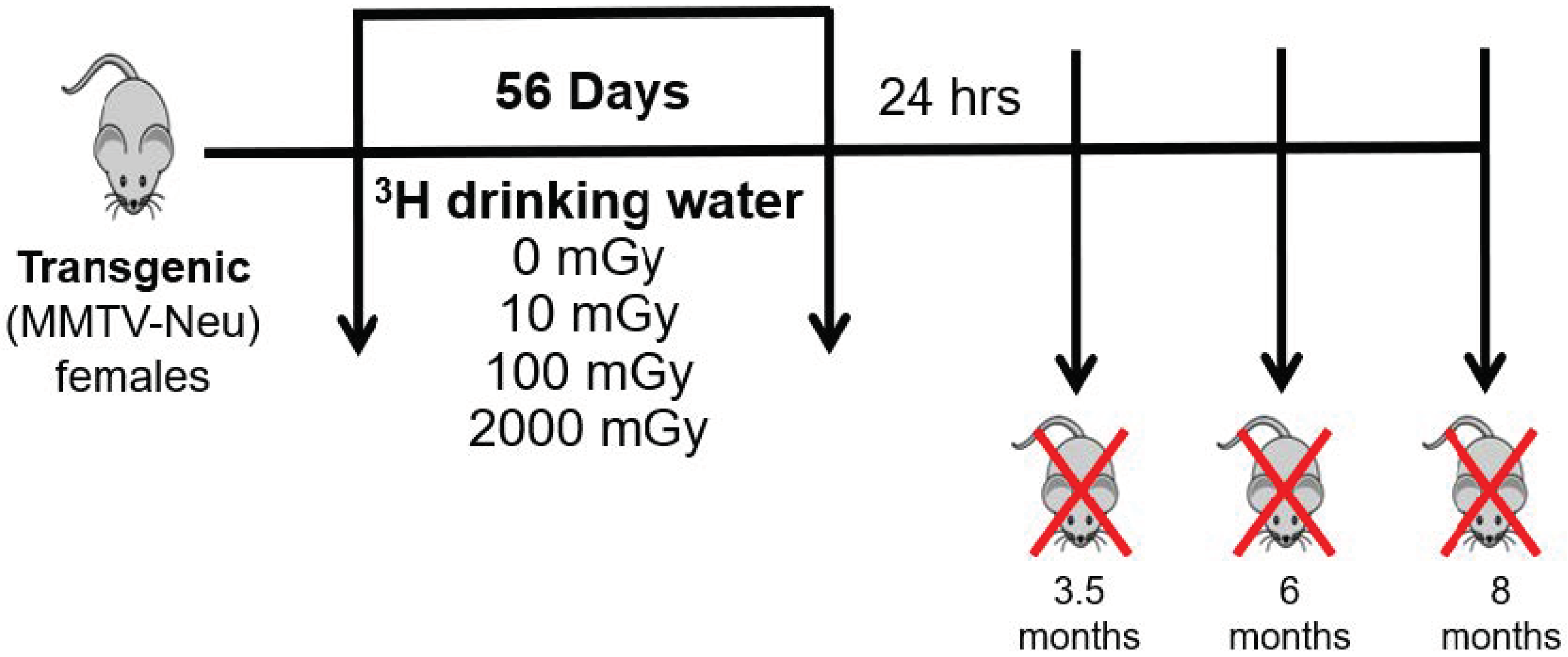
4. Methodology
4.1. Mice
4.2. Tritium Exposure
4.3. Cell Isolation
4.4. In Vitro Proliferation and Functional Assay
4.5. Antibodies and Flow Cytometry
4.6. RNA Extraction and Quantitative Real-Time PCR
4.7. Statistical Analysis
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kathren, R.L. Historical development of the linear nonthreshold dose-response model as applied to radiation. Pierce Law Rev. 2002, 1, 5. [Google Scholar]
- Valentin, J. Low-Dose Extrapolation of Radiation-Related Cancer Risk; Elsevier: London, UK, 2006. [Google Scholar]
- Brenner, D.J.; Hall, E.J. Computed tomography—An increasing source of radiation exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [Green Version]
- Kojima, S.; Tsukimoto, M.; Shimura, N.; Koga, H.; Murata, A.; Takara, T. Treatment of Cancer and Inflammation With Low-Dose Ionizing Radiation: Three Case Reports. Dose Response 2017, 15. [Google Scholar] [CrossRef]
- Scott, B.R. Radiation-hormesis phenotypes, the related mechanisms and implications for disease prevention and therapy. J. Cell Commun. Signal. 2014, 8, 341–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaiserman, A.; Koliada, A.; Zabuga, O.; Socol, Y. Health impacts of low-dose ionizing radiation: Current scientific debates and regulatory issues. Dose Response 2018, 16. [Google Scholar] [CrossRef] [Green Version]
- Fliedner, T.M.; Graessle, D.H.; Meineke, V.; Feinendegen, L.E. Hemopoietic response to low dose-rates of ionizing radiation shows stem cell tolerance and adaptation. Dose Response 2012, 10. [Google Scholar] [CrossRef]
- Kiscsatári, L.; Sárközy, M.; Kővári, B.; Varga, Z.; Gömöri, K.; Morvay, N.; Leprán, I.; Hegyesi, H.; Fábián, G.; Cserni, B. High-dose radiation induced heart damage in a rat model. In Vivo 2016, 30, 623–631. [Google Scholar] [PubMed]
- Donnelly, E.H.; Nemhauser, J.B.; Smith, J.M.; Kazzi, Z.N.; Farfán, E.B.; Chang, A.S.; Naeem, S.F. Acute radiation syndrome: Assessment and management. South. Med. J. 2010, 103, 541–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, D.J.; Doll, R.; Goodhead, D.T.; Hall, E.J.; Land, C.E.; Little, J.B.; Lubin, J.H.; Preston, D.L.; Preston, R.J.; Puskin, J.S. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proc. Natl. Acad. Sci. USA 2003, 100, 13761–13766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seong, K.M.; Seo, S.; Lee, D.; Kim, M.-J.; Lee, S.-S.; Park, S.; Jin, Y.W. Is the linear no-threshold dose-response paradigm still necessary for the assessment of health effects of low dose radiation? J. Korean Med. Sci. 2016, 31, S10–S23. [Google Scholar] [CrossRef] [PubMed]
- Pennington, C.W.; Siegel, J.A. The linear no-threshold model of low-dose radiogenic cancer: A failed fiction. Dose Response 2019, 17. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, E.J. The linear no-threshold (LNT) dose response model: A comprehensive assessment of its historical and scientific foundations. Chem. Biol. Interact. 2019, 301, 6–25. [Google Scholar] [CrossRef] [PubMed]
- Costantini, D.; Borremans, B. The linear no-threshold model is less realistic than threshold or hormesis-based models: An evolutionary perspective. Chem. Biol. Interact. 2019, 301, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Wang, Y.; Du, L.; Xu, C.; Liu, Y.; He, N.; Wang, J.; Liu, Q. Research progress on the biological effects of low-dose radiation in China. Dose Response 2019, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burk, R.J., Jr. Radiation Risk in Perspective: Position Statement of the Health Physics Society; Health Physics Society: McLean, VA, USA, 2004. [Google Scholar]
- Calabrese, E.J.; Bachmann, K.A.; Bailer, A.J.; Bolger, P.M.; Borak, J.; Cai, L.; Cedergreen, N.; Cherian, M.G.; Chiueh, C.C.; Clarkson, T.W. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose–response framework. Toxicol. Appl. Pharmacol. 2007, 222, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Miura, Y. Oxidative stress, radiation-adaptive responses, and aging. J. Radiat. Res. 2004, 45, 357–372. [Google Scholar] [CrossRef] [Green Version]
- Wolff, S. Is radiation all bad? The search for adaptation. Radiat. Res. 1992, 131, 117–123. [Google Scholar] [CrossRef]
- Vaiserman, A.M. Hormesis and epigenetics: Is there a link? Ageing Res. Rev. 2011, 10, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Sakai, K.; Ogata, H.; Magae, J. Prolongation of life span in the accelerated aging klotho mouse model, by low-dose-rate continuous gamma irradiation. Radiat. Res. 2013, 179, 717–724. [Google Scholar] [CrossRef]
- Cameron, B.D.; Sekhar, K.R.; Ofori, M.; Freeman, M.L. The role of Nrf2 in the response to normal tissue radiation injury. Radiat. Res. 2018, 190, 99–106. [Google Scholar] [CrossRef]
- Galván, I.; Bonisoli-Alquati, A.; Jenkinson, S.; Ghanem, G.; Wakamatsu, K.; Mousseau, T.A.; Møller, A.P. Chronic exposure to low-dose radiation at Chernobyl favours adaptation to oxidative stress in birds. Funct. Ecol. 2014, 28, 1387–1403. [Google Scholar] [CrossRef] [Green Version]
- Bernal, A.J.; Dolinoy, D.C.; Huang, D.; Skaar, D.A.; Weinhouse, C.; Jirtle, R.L. Adaptive radiation-induced epigenetic alterations mitigated by antioxidants. FASEB J. 2013, 27, 665–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seong, K.M.; Kim, C.S.; Lee, B.-S.; Nam, S.Y.; Yang, K.H.; Kim, J.-Y.; Park, J.-J.; Min, K.-J.; Jin, Y.-W. Low-dose radiation induces Drosophila innate immunity through Toll pathway activation. J. Radiat. Res. 2012, 1202230254-1202230254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, H.M.; Hong, E.H.; Cho, S.-J.; Nam, S.Y.; Kim, J.Y. Preventative and Therapeutic Effects of Low-dose Ionizing Radiation on the Allergic Response of Rat Basophilic Leukemia Cells. Sci. Rep. 2019, 9, 16079. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.S. Ionising radiation and cancer risks: What have we learned from epidemiology? Int. J. Radiat. Biol. 2009, 85, 467–482. [Google Scholar] [CrossRef] [Green Version]
- Dobrzyński, L.; Fornalski, K.W.; Feinendegen, L.E. Cancer mortality among people living in areas with various levels of natural background radiation. Dose Response 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Portess, D.I.; Bauer, G.; Hill, M.A.; O’Neill, P. Low-dose irradiation of nontransformed cells stimulates the selective removal of precancerous cells via intercellular induction of apoptosis. Cancer Res. 2007, 67, 1246–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornalski, K.W.; Dobrzyński, L. The cancer mortality in high natural radiation areas in Poland. Dose Response 2012, 10. [Google Scholar] [CrossRef]
- Cuttler, J.M.; Moore, E.R.; Hosfeld, V.D.; Nadolski, D.L. Treatment of Alzheimer Disease With CT Scans: A Case Report. Dose Response 2016, 14. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Luan, Y.; Shieh, M.; Chen, S.; Kung, H.; Soong, K.; Yeh, Y.; Chou, T.; Mong, S.; Wu, J. Effects of cobalt-60 exposure on health of Taiwan residents suggest new approach needed in radiation protection. Dose Response 2007, 5, 63–75. [Google Scholar] [CrossRef]
- Scott, B.R. Residential radon appears to prevent lung cancer. Dose Response 2011, 9, 444–464. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.E. Epidemiological Evidence for Possible Radiation Hormesis from Radon Exposure: A Case-Control Study Conducted in Worcester, MA. Dose Response 2011, 9, 59–75. [Google Scholar] [CrossRef] [Green Version]
- Luckey, T. The health effects of low-dose ionizing radiation. J. Am. Physicians Surg. 2008, 13, 39. [Google Scholar]
- Cuttler, J.M.; Pollycove, M. Nuclear energy and health: And the benefits of low-dose radiation hormesis. Dose Response 2009, 7, 52–89. [Google Scholar] [CrossRef]
- Bogdándi, E.N.; Balogh, A.; Felgyinszki, N.; Szatmári, T.; Persa, E.; Hildebrandt, G.; Sáfrány, G.; Lumniczky, K. Effects of low-dose radiation on the immune system of mice after total-body irradiation. Radiat. Res. 2010, 174, 480–489. [Google Scholar] [CrossRef] [PubMed]
- El-Saghire, H.; Michaux, A.; Thierens, H.; Baatout, S. Low doses of ionizing radiation induce immune-stimulatory responses in isolated human primary monocytes. Int. J. Mol. Med. 2013, 32, 1407–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiassi-Nejad, M.; Zakeri, F.; Assaei, R.G.; Kariminia, A. Long-term immune and cytogenetic effects of high level natural radiation on Ramsar inhabitants in Iran. J. Environ. Radioact. 2004, 74, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Manda, K.; Glasow, A.; Paape, D.; Hildebrandt, G. Effects of ionizing radiation on the immune system with special emphasis on the interaction of dendritic and T cells. Front. Oncol. 2012, 2, 102. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Liu, Z.; Wang, D.; Han, Y.; Hu, S.; Xie, Y.; Liu, Y.; Zhu, M.; Guan, H.; Gu, Y. Effects of low dose radiation on immune cells subsets and cytokines in mice. Toxicol. Res. 2020, 9, 249–262. [Google Scholar] [CrossRef]
- Lei, R.; Zhao, T.; Li, Q.; Wang, X.; Ma, H.; Deng, Y. Carbon ion irradiated neural injury induced the peripheral immune effects in vitro or in vivo. Int. J. Mol. Sci. 2015, 16, 28334–28346. [Google Scholar] [CrossRef]
- Wu, Q.; Allouch, A.; Martins, I.; Modjtahedi, N.; Deutsch, E.; Perfettini, J.L. Macrophage biology plays a central role during ionizing radiation-elicited tumor response. Biomed. J. 2017, 40, 200–211. [Google Scholar] [CrossRef]
- Wunderlich, R.; Ernst, A.; Rödel, F.; Fietkau, R.; Ott, O.; Lauber, K.; Frey, B.; Gaipl, U. Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clin. Exp. Immunol. 2015, 179, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Teresa Pinto, A.; Laranjeiro Pinto, M.; Patrícia Cardoso, A.; Monteiro, C.; Teixeira Pinto, M.; Filipe Maia, A.; Castro, P.; Figueira, R.; Monteiro, A.; Marques, M.; et al. Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci. Rep. 2016, 6, 18765. [Google Scholar] [CrossRef] [Green Version]
- Kojima, S.; Nakayama, K.; Ishida, H. Low dose gamma-rays activate immune functions via induction of glutathione and delay tumor growth. J. Radiat. Res. 2004, 45, 33–39. [Google Scholar] [CrossRef]
- Hayase, H.; Ohshima, Y.; Takahashi, M.; Kojima, S. The enhancement of Th1 immunity and the suppression of tumour growth by low-dose γ-radiation. Int. J. Low Radiat. 2008, 5, 275–289. [Google Scholar] [CrossRef]
- Ogata, H. A review of some epidemiological studies on cancer risk from low-dose radiation or other carcinogenic agents. Radiat. Prot. Dosim. 2011, 146, 268–271. [Google Scholar] [CrossRef]
- Scott, B.R. Low-dose radiation risk extrapolation fallacy associated with the linear-no-threshold model. Hum. Exp. Toxicol. 2008, 27, 163–168. [Google Scholar] [CrossRef]
- Fantozzi, A.; Christofori, G. Mouse models of breast cancer metastasis. Breast Cancer Res. 2006, 8, 212. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.J.; Sinn, E.; Pattengale, P.K.; Wallace, R.; Leder, P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 1988, 54, 105–115. [Google Scholar] [CrossRef]
- Quaglino, E.; Mastini, C.; Forni, G.; Cavallo, F. ErbB2 transgenic mice: A tool for investigation of the immune prevention and treatment of mammary carcinomas. Curr. Protoc. Immunol. 2008. [Google Scholar] [CrossRef]
- Little, M.; Wakeford, R. Systematic review of epidemiological studies of exposure to tritium. J. Radiol. Prot. 2008, 28, 9. [Google Scholar] [CrossRef]
- Roch-Lefèvre, S.; Grégoire, E.; Martin-Bodiot, C.; Flegal, M.; Fréneau, A.; Blimkie, M.; Bannister, L.; Wyatt, H.; Barquinero, J.-F.; Roy, L. Cytogenetic damage analysis in mice chronically exposed to low-dose internal tritium beta-particle radiation. Oncotarget 2018, 9, 27397. [Google Scholar] [CrossRef]
- Guéguen, Y.; Priest, N.D.; Dublineau, I.; Bannister, L.; Benderitter, M.; Durand, C.; Ebrahimian, T.G.; Grégoire, E.; Grison, S.; Ibanez, C. In vivo animal studies help achieve international consensus on standards and guidelines for health risk estimates for chronic exposure to low levels of tritium in drinking water. Environ. Mol. Mutagenesis 2018, 59, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Priest, N.D.; Blimkie, M.S.; Wyatt, H.; Bugden, M.; Bannister, L.A.; Gueguen, Y.; Jourdain, J.-R.; Klokov, D. Tritium (3 H) retention in mice: Administered as HTO, DTO or as 3 H-labeled amino-acids. Health Phys. 2017, 112, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Krijgsman, D.; Hokland, M.; Kuppen, P.J. The role of natural killer T cells in cancer—A phenotypical and functional approach. Front. Immunol. 2018, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.; Rodríguez-Fernández, J.L.; Navarro, F.; Sancho, D.; Frade, J.M.; Mellado, M.; Martínez, A.C.; Cabañas, C.; Sánchez-Madrid, F. Signaling through CD43 induces natural killer cell activation, chemokine release, and PYK-2 activation. Blood J. Am. Soc. Hematol. 1999, 94, 2767–2777. [Google Scholar] [CrossRef]
- Cibrián, D.; Sánchez-Madrid, F. CD69: From activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, K.J.; Hudson, A.L.; Back, M.; Eade, T.; Diakos, C.I. Radiation, inflammation and the immune response in cancer. Mamm. Genome 2018, 29, 843–865. [Google Scholar] [CrossRef] [Green Version]
- Rose, S.; Misharin, A.; Perlman, H. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytom. Part A 2012, 81, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Edwards, C.M.; Mundy, G.R. Gr-1+ CD11b+ myeloid-derived suppressor cells: Formidable partners in tumor metastasis. J. Bone Miner. Res. 2010, 25, 1701–1706. [Google Scholar] [CrossRef] [Green Version]
- Frey, B.; Hehlgans, S.; Rödel, F.; Gaipl, U.S. Modulation of inflammation by low and high doses of ionizing radiation: Implications for benign and malign diseases. Cancer Lett. 2015, 368, 230–237. [Google Scholar] [CrossRef]
- Yang, G.; Kong, Q.; Wang, G.; Jin, H.; Zhou, L.; Yu, D.; Niu, C.; Han, W.; Li, W.; Cui, J. Low-dose ionizing radiation induces direct activation of natural killer cells and provides a novel approach for adoptive cellular immunotherapy. Cancer Biother. Radiopharm. 2014, 29, 428–434. [Google Scholar] [CrossRef] [Green Version]
- Wensveen, F.M.; Jelenčić, V.; Polić, B. NKG2D: A master regulator of immune cell responsiveness. Front. Immunol. 2018, 9, 441. [Google Scholar] [CrossRef]
- Guerra, N.; Lanier, L.L. Emerging Concepts on the NKG2D Receptor-Ligand Axis in Health and Diseases. Front. Immunol. 2020, 11, 562. [Google Scholar] [CrossRef]
- Marcus, A.; Gowen, B.G.; Thompson, T.W.; Iannello, A.; Ardolino, M.; Deng, W.; Wang, L.; Shifrin, N.; Raulet, D.H. Recognition of tumors by the innate immune system and natural killer cells. Adv. Immunol. 2014, 122, 91–128. [Google Scholar] [PubMed] [Green Version]
- Raulet, D.H. Roles of the NKG2D immunoreceptor and its ligands. Nat. Rev. Immunol. 2003, 3, 781–790. [Google Scholar] [CrossRef]
- Zingoni, A.; Molfetta, R.; Fionda, C.; Soriani, A.; Paolini, R.; Cippitelli, M.; Cerboni, C.; Santoni, A. NKG2D and its ligands:“one for all, all for one”. Front. Immunol. 2018, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Heo, W.; Nam, J.; Jeung, Y.H.; Bae, J. The combination of ionizing radiation and proteasomal inhibition by bortezomib enhances the expression of NKG2D ligands in multiple myeloma cells. J. Radiat. Res. 2018, 59, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Champsaur, M.; Lanier, L.L. Effect of NKG2D ligand expression on host immune responses. Immunol. Rev. 2010, 235, 267–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raulet, D.H.; Gasser, S.; Gowen, B.G.; Deng, W.; Jung, H. Regulation of ligands for the NKG2D activating receptor. Annu. Rev. Immunol. 2013, 31, 413–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oppenheim, D.E.; Roberts, S.J.; Clarke, S.L.; Filler, R.; Lewis, J.M.; Tigelaar, R.E.; Girardi, M.; Hayday, A.C. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat. Immunol. 2005, 6, 928–937. [Google Scholar] [CrossRef]
- Calabrese, E.J. How the US National Academy of Sciences misled the world community on cancer risk assessment: New findings challenge historical foundations of the linear dose response. Arch. Toxicol. 2013, 87, 2063–2081. [Google Scholar] [CrossRef]
- Socol, Y.; Dobrzyński, L. Atomic bomb survivors life-span study: Insufficient statistical power to select radiation carcinogenesis model. Dose Response 2015, 13. [Google Scholar] [CrossRef]
- Doss, M. The Conclusion of the BEIR VII Report Endorsing the Linear No-Threshold Model Is No Longer Valid Due to Advancement of Knowledge. J. Nucl. Med. 2018, 59, 1777. [Google Scholar] [CrossRef] [Green Version]
- Siegel, J.A.; Sacks, B.; Greenspan, B. Radiation dose does indeed matter: Proof that invalidates the linear no-threshold model. J. Nucl. Med. 2018, 59, 1779–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MotherSill, C.; Seymour, C. Changing paradigms in radiobiology. Mutat. Res. Rev. Mutat. Res. 2012, 750, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.L. Paradigm shifts in radiation biology: Their impact on intervention for radiation-induced disease. Radiat. Res. 2005, 164, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Canter, R.J.; Grossenbacher, S.K.; Foltz, J.A.; Sturgill, I.R.; Park, J.S.; Luna, J.I.; Kent, M.S.; Culp, W.T.; Chen, M.; Modiano, J.F. Radiotherapy enhances natural killer cell cytotoxicity and localization in pre-clinical canine sarcomas and first-in-dog clinical trial. J. Immunother. Cancer 2017, 5, 98. [Google Scholar] [CrossRef] [Green Version]
- Sonn, C.H.; Choi, J.R.; Kim, T.-J.; Yu, Y.-B.; Kim, K.; Shin, S.C.; Park, G.-H.; Shirakawa, T.; Kim, H.S.; Lee, K.-M. Augmentation of natural cytotoxicity by chronic low-dose ionizing radiation in murine natural killer cells primed by IL-2. J. Radiat. Res. 2012, 53, 823–829. [Google Scholar] [CrossRef]
- Rodel, F.; Frey, B.; Gaipl, U.; Keilholz, L.; Fournier, C.; Manda, K.; Schollnberger, H.; Hildebrandt, G.; Rodel, C. Modulation of inflammatory immune reactions by low-dose ionizing radiation: Molecular mechanisms and clinical application. Curr. Med. Chem. 2012, 19, 1741–1750. [Google Scholar] [CrossRef]
- Stoecklein, V.M.; Osuka, A.; Ishikawa, S.; Lederer, M.R.; Wanke-Jellinek, L.; Lederer, J.A. Radiation exposure induces inflammasome pathway activation in immune cells. J. Immunol. 2015, 194, 1178–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooque, A.; Afrin, F.; Adhikari, J.S.; Dwarakanath, B.S.R. Polarization of macrophages towards M1 phenotype by a combination of 2-deoxy-d-glucose and radiation: Implications for tumor therapy. Immunobiology 2016, 221, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.-S.; Chen, F.-H.; Wang, C.-C.; Huang, H.-L.; Jung, S.-M.; Wu, C.-J.; Lee, C.-C.; McBride, W.H.; Chiang, C.-S.; Hong, J.-H. Macrophages from irradiated tumors express higher levels of iNOS, arginase-I and COX-2, and promote tumor growth. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Gu, J.; Yu, D.; Wang, G.; Zhou, L.; Zhang, X.; Zhao, Y.; Chen, X.; Zheng, S.; Liu, Q. Low-dose radiation induces cell proliferation in human embryonic lung fibroblasts but not in lung cancer cells: Importance of ERK1/2 and AKT signaling pathways. Dose Response 2016, 14. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.E.; Nagar, S.; Kim, G.J.; Morgan, W.F. Radiation-induced genomic instability: Radiation quality and dose response. Health Phys. 2003, 85, 23–29. [Google Scholar] [CrossRef]
- McMillan, T.J. Residual DNA damage: What is left over and how does this determine cell fate? Eur. J. Cancer 1992, 28, 267–269. [Google Scholar] [CrossRef]
- Joshi, G.; Nelson, W.; Revell, S.; Shaw, C. X-ray-induced chromosome damage in live mammalian cells, and improved measurements of its effects on their colony-forming ability. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1982, 41, 161–181. [Google Scholar] [CrossRef]
- David, E.; Wolfson, M.; Fraifeld, V.E. Background radiation impacts human longevity and cancer mortality: Reconsidering the linear no-threshold paradigm. Biogerontology 2021, 22, 189–195. [Google Scholar] [PubMed]
- Koana, T.; Okada, M.O.; Ogura, K.; Tsujimura, H.; Sakai, K. Reduction of background mutations by low-dose X irradiation of Drosophila spermatocytes at a low dose rate. Radiat. Res. 2007, 167, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Gasser, S.; Orsulic, S.; Brown, E.J.; Raulet, D.H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005, 436, 1186–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coudert, J.D.; Zimmer, J.; Tomasello, E.; Cebecauer, M.; Colonna, M.; Vivier, E.; Held, W. Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand–expressing tumor cells. Blood 2005, 106, 1711–1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, M.; Simonetta, F.; Baker, J.; Pierini, A.; Wenokur, A.S.; Morrison, A.R.; Murphy, W.J.; Negrin, R.S. Regulation of murine NK cell exhaustion through the activation of the DNA damage repair pathway. JCI Insight 2019, 4, e127729. [Google Scholar] [CrossRef]
- Gill, S.; Vasey, A.E.; De Souza, A.; Baker, J.; Smith, A.T.; Kohrt, H.E.; Florek, M.; Gibbs, K.D., Jr.; Tate, K.; Ritchie, D.S. Rapid development of exhaustion and down-regulation of eomesodermin limit the antitumor activity of adoptively transferred murine natural killer cells. Blood J. Am. Soc. Hematol. 2012, 119, 5758–5768. [Google Scholar] [CrossRef]
- Elpek, K.G.; Rubinstein, M.P.; Bellemare-Pelletier, A.; Goldrath, A.W.; Turley, S.J. Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Rα complexes. Proc. Natl. Acad. Sci. USA 2010, 107, 21647–21652. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, N.; Kim, M.-J.; Kim, R.-K.; Kaushik, N.K.; Seong, K.M.; Nam, S.-Y.; Lee, S.-J. Low-dose radiation decreases tumor progression via the inhibition of the JAK1/STAT3 signaling axis in breast cancer cell lines. Sci. Rep. 2017, 7, 43361. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.-K.; Kim, M.-J.; Seong, K.M.; Kaushik, N.; Suh, Y.; Yoo, K.-C.; Cui, Y.-H.; Jin, Y.W.; Nam, S.Y.; Lee, S.-J. Beneficial effects of low dose radiation in response to the oncogenic KRAS induced cellular transformation. Sci. Rep. 2015, 5, 15809. [Google Scholar] [CrossRef] [Green Version]
| Tissue | Dose/Time-Point | 3.5 mo | 6 mo | 8 mo |
|---|---|---|---|---|
| Spleens | 10 mGy | |||
| 100 mGy | ||||
| 2000 mGy | ||||
| Lungs | 10 mGy | |||
| 100 mGy | ||||
| 2000 mGy | ||||
| Mammary Glands | 10 mGy | |||
| 100 mGy | ||||
| 2000 mGy |
 Non-statistical increase, p > 0.05,
Non-statistical increase, p > 0.05,  Statistical increase, p < 0.05.
Statistical increase, p < 0.05.| Cell Type | Dose/Time-Point | 3.5 mo | 6 mo | 8 mo |
|---|---|---|---|---|
| NK cells (IL-2 stimulation) | 10 mGy | |||
| 100 mGy | ||||
| 2000 mGy | ||||
| CD4 cells (IL-2 stimulation) | 10 mGy | |||
| 100 mGy | ||||
| 2000 mGy | ||||
| CD8 cells (IL-2 stimulation) | 10 mGy | |||
| 100 mGy | ||||
| 2000 mGy | ||||
| CD4 cells (CD3/CD28 stimulation) | 10 mGy | |||
| 100 mGy | ||||
| 2000 mGy | ||||
| CD8 cells (CD3/CD28 stimulation) | 10 mGy | |||
| 100 mGy | ||||
| 2000 mGy |
 Non-statistical decrease, p > 0.05,
Non-statistical decrease, p > 0.05,  Statistical decrease, p < 0.05,
Statistical decrease, p < 0.05,  Non-statistical increase, p > 0.05,
Non-statistical increase, p > 0.05,  Statistical increase, p < 0.05.
Statistical increase, p < 0.05.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.U.H.; Blimkie, M.; Yang, D.S.; Serran, M.; Pack, T.; Wu, J.; Kang, J.-Y.; Laakso, H.; Lee, S.-H.; Le, Y. Effects of Chronic Low-Dose Internal Radiation on Immune-Stimulatory Responses in Mice. Int. J. Mol. Sci. 2021, 22, 7303. https://doi.org/10.3390/ijms22147303
Khan AUH, Blimkie M, Yang DS, Serran M, Pack T, Wu J, Kang J-Y, Laakso H, Lee S-H, Le Y. Effects of Chronic Low-Dose Internal Radiation on Immune-Stimulatory Responses in Mice. International Journal of Molecular Sciences. 2021; 22(14):7303. https://doi.org/10.3390/ijms22147303
Chicago/Turabian StyleKhan, Abrar Ul Haq, Melinda Blimkie, Doo Seok Yang, Mandy Serran, Tyler Pack, Jin Wu, Ji-Young Kang, Holly Laakso, Seung-Hwan Lee, and Yevgeniya Le. 2021. "Effects of Chronic Low-Dose Internal Radiation on Immune-Stimulatory Responses in Mice" International Journal of Molecular Sciences 22, no. 14: 7303. https://doi.org/10.3390/ijms22147303
APA StyleKhan, A. U. H., Blimkie, M., Yang, D. S., Serran, M., Pack, T., Wu, J., Kang, J.-Y., Laakso, H., Lee, S.-H., & Le, Y. (2021). Effects of Chronic Low-Dose Internal Radiation on Immune-Stimulatory Responses in Mice. International Journal of Molecular Sciences, 22(14), 7303. https://doi.org/10.3390/ijms22147303







