Does the Interaction between Local and Systemic Inflammation Provide a Link from Psychology and Lifestyle to Tissue Health in Musculoskeletal Conditions?
Abstract
1. Introduction
2. The Role of Local Inflammation in Musculoskeletal Connective Tissue Health
2.1. Overview of Musculoskeletal Connective Tissue
2.2. Injury-Induced Acute Inflammation and Normal Tissue Repair
2.3. Inflammation Induced by Injury to Adjacent Structures
2.4. Exercise-Induced Inflammation in Muscles
2.5. Chronic Inflammation and Fibrotic Muscle Repair
3. The Role of Systemic Inflammation in Musculoskeletal Connective Tissue Health
3.1. The Interplay between Systemic and Local Inflammation in Musculoskeletal Tissues
3.2. The Role of Systemic Inflammation in Regulating Fibrogenesis
| Condition | Systemic Inflammation/Fibrotic Proteins | Muscle Inflammation | Muscle Fibrosis |
|---|---|---|---|
| Musculoskeletal/neuromuscular conditions | |||
| Complex regional pain syndrome | <6 months after symptoms onset:
| ≤4 weeks after initiation in animal model; local and/or nearby muscles (lower limb):
| Chronic phase; local and nearby muscles (upper and lower limbs):
|
| Low back pain | Acute phase (<2 weeks):
| Late/chronic phase (>6 months); nearby muscle (multifidus):
| Late/chronic phase (>6 months); nearby muscle (multifidus):
|
| Rheumatoid arthritis | Early/late disease stage:
| Late/chronic disease stage; nearby muscles (lower limb):
| Late/chronic disease stage; nearby muscles (lower limb):
|
| Work-related overuse injury/repetitive strain injury | Early/acute phase (≤3 weeks):
| Early/acute phase; local and nearby muscles:
| Early/acute phase; local and nearby muscles:
|
| Duchenne muscular dystrophy | Early/late disease stage:
| Early/late disease stage; various muscles:
| Early/late disease stage; various muscles:
|
| Chronic metabolic/lifestyle-related conditions | |||
| Alcoholism/alcoholic myopathy |
|
|
|
| Cancer related Cachexia |
|
|
|
| Chronic Obstructive Pulmonary Disease |
|
|
|
| Diabetes |
|
|
|
| Obesity |
|
|
|
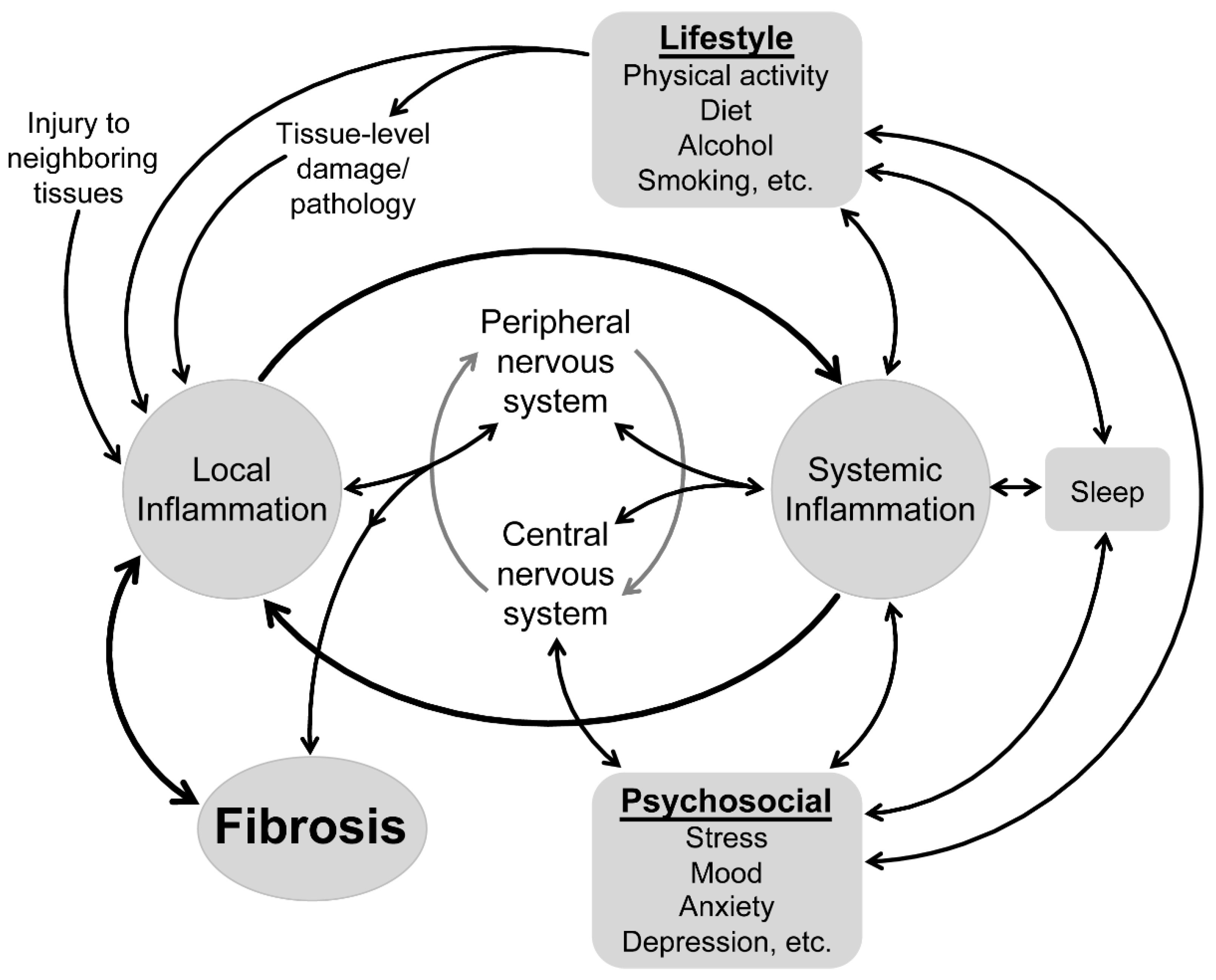
4. Does the Impact of Systemic Inflammation on Local Inflammation Provide a Link between Psychological and Lifestyle Factors in Health of Musculoskeletal Connective Tissues?
4.1. Links between Psychological Factors and Systemic Inflammation
4.2. Links between Lifestyle Factors and Systemic Inflammation
4.3. Reconsidering Health of Musculoskeletal Connective Tissues from a Bio-Psycho-Social and Lifestyle Perspective
5. New Opportunities to Understand and Enhance the Effects of Treatments
6. Conclusions
Funding
Conflicts of Interest
References
- Zügel, M.; Maganaris, C.N.; Wilke, J.; Jurkat-Rott, K.; Klingler, W.; Wearing, S.C.; Findley, T.; Barbe, M.F.; Steinacker, J.M.; Vleeming, A.; et al. Fascial tissue research in sports medicine: From molecules to tissue adaptation, injury and diagnostics: Consensus statement. Br. J. Sports Med. 2018, 52, 1497. [Google Scholar] [CrossRef]
- Toumi, H.; F’Guyer, S.; Best, T.M. The role of neutrophils in injury and repair following muscle stretch. J. Anat. 2006, 208, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018, 371, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Barbe, M.F.; Hilliard, B.A.; Fisher, P.W.; White, A.R.; Delany, S.P.; Iannarone, V.J.; Harris, M.Y.; Amin, M.; Cruz, G.E.; Popoff, S.N. Blocking substance P signaling reduces musculotendinous and dermal fibrosis and sensorimotor declines in a rat model of overuse injury. Connect. Tissue Res. 2020, 61, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Steensberg, A.; Schjerling, P. Muscle-derived interleukin-6: Possible biological effects. J. Physiol. 2001, 536, 329–337. [Google Scholar] [CrossRef]
- Hodges, P.W.; James, G.; Blomster, L.; Hall, L.; Schmid, A.B.; Shu, C.; Little, C.; Melrose, J. Can proinflammatory cytokine gene expression explain multifidus muscle fiber changes after an intervertebral disc lesion? Spine 2014, 39, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Caro, R. 2019 Ejtm special on muscle fascia. Eur. J. Transl. Myol. 2019, 29, 8060. [Google Scholar] [CrossRef]
- Gillies, A.R.; Lieber, R.L. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011, 44, 318–331. [Google Scholar] [CrossRef]
- Dick, M.K.; Miao, J.H.; Limaiem, F. Histology, Fibroblast. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- McCracken, J.M.; Allen, L.A. Regulation of human neutrophil apoptosis and lifespan in health and disease. J. Cell Death 2014, 7, 15–23. [Google Scholar] [CrossRef]
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef]
- Tidball, J.G.; Wehling-Henricks, M. Shifts in macrophage cytokine production drive muscle fibrosis. Nat. Med. 2015, 21, 665–666. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wehling-Henricks, M.; Samengo, G.; Tidball, J.G. Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging Cell 2015, 14, 678–688. [Google Scholar] [CrossRef]
- Welc, S.S.; Wehling-Henricks, M.; Antoun, J.; Ha, T.T.; Tous, I.; Tidball, J.G. Differential effects of myeloid cell PPARdelta and IL-10 in regulating macrophage recruitment, phenotype, and regeneration following acute muscle injury. J. Immunol. 2020, 205, 1664–1677. [Google Scholar] [CrossRef]
- Hu, W.; Jiang, Z.; Zhang, Y.; Liu, Q.; Fan, J.; Luo, N.; Dong, X.; Yu, X. Characterization of infiltrating macrophages in high glucose-induced peritoneal fibrosis in rats. Mol. Med. Rep. 2012, 6, 93–99. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Roszer, T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat. Inflamm 2015, 2015, 816460. [Google Scholar] [CrossRef]
- Lech, M.; Anders, H.J. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim. Biophys. Acta 2013, 1832, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hu, P. Skeletal muscle regeneration is modulated by inflammation. J. Orthop. Translat. 2018, 13, 25–32. [Google Scholar] [CrossRef]
- Mann, C.J.; Perdiguero, E.; Kharraz, Y.; Aguilar, S.; Pessina, P.; Serrano, A.L.; Munoz-Canoves, P. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle 2011, 1, 21. [Google Scholar] [CrossRef]
- Dagdeviren, S.; Jung, D.Y.; Lee, E.; Friedline, R.H.; Noh, H.L.; Kim, J.H.; Patel, P.R.; Tsitsilianos, N.; Tsitsilianos, A.V.; Tran, D.A.; et al. Altered interleukin-10 signaling in skeletal muscle regulates obesity-mediated inflammation and insulin resistance. Mol. Cell Biol. 2016, 36, 2956–2966. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Ahearn, J.M. Acute-phase proteins and inflammation: Immunological and clinical implications. In Measuring Immunity; Lotze, M.T., Thomson, A.W., Eds.; Academic Press: London, UK, 2005; pp. 131–143. [Google Scholar]
- Jain, S.; Gautam, V.; Naseem, S. Acute-phase proteins: As diagnostic tool. J. Pharm. Bioallied Sci. 2011, 3, 118–127. [Google Scholar] [CrossRef]
- Hides, J.A.; Stokes, M.J.; Saide, M.; Jull, G.A.; Cooper, D.H. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine 1994, 19, 165–172. [Google Scholar] [CrossRef]
- Shahidi, B.; Fisch, K.M.; Gibbons, M.C.; Ward, S.R. Increased fibrogenic gene expression in multifidus muscles of patients with chronic versus acute lumbar spine pathology. Spine 2020, 45, E189–E195. [Google Scholar] [CrossRef]
- Hodges, P.W.; James, G.; Blomster, L.; Hall, L.; Schmid, A.; Shu, C.; Little, C.; Melrose, J. Multifidus muscle changes after back injury are characterized by structural remodeling of muscle, adipose and connective tissue, but not muscle atrophy: Molecular and morphological evidence. Spine 2015, 40, 1057–1071. [Google Scholar] [CrossRef]
- James, G.; Klyne, D.M.; Millecamps, M.; Stone, L.S.; Hodges, P.W. ISSLS prize in basic science 2019: Physical activity attenuates fibrotic alterations to the multifidus muscle associated with intervertebral disc degeneration. Eur. Spine J. 2019. [Google Scholar] [CrossRef]
- James, G.; Millecamps, M.; Stone, L.S.; Hodges, P.W. Dysregulation of the inflammatory mediators in the multifidus muscle after spontaneous intervertebral disc degeneration SPARC-null mice is ameliorated by physical activity. Spine 2018, 43, E1184–E1194. [Google Scholar] [CrossRef]
- Shahidi, B.; Hubbard, J.C.; Gibbons, M.C.; Ruoss, S.; Zlomislic, V.; Allen, R.T.; Garfin, S.R.; Ward, S.R. Lumbar multifidus muscle degenerates in individuals with chronic degenerative lumbar spine pathology. J. Orthop. Res. 2017, 35, 2700–2706. [Google Scholar] [CrossRef] [PubMed]
- James, G.; Sluka, K.A.; Blomster, L.; Hall, L.; Schmid, A.B.; Shu, C.C.; Little, C.B.; Melrose, J.; Hodges, P.W. Macrophage polarization contributes to local inflammation and structural change in the multifidus muscle after intervertebral disc injury. Eur. Spine J. 2018, 27, 1744–1756. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.P. Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc. Immunol. Rev. 2006, 12, 6–33. [Google Scholar]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J. Physiol. 1999, 515, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, A.I.; Shephard, R.J.; Shek, P.N. The cytokine response to physical activity and training. Sports Med. 2001, 31, 115–144. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.R.; Pedersen, B.K. The biological roles of exercise-induced cytokines: IL-6, IL-8, and IL-15. Appl. Physiol. Nutr. Metab. 2007, 32, 833–839. [Google Scholar] [CrossRef]
- Munoz-Canoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef] [PubMed]
- Hennigar, S.R.; McClung, J.P.; Pasiakos, S.M. Nutritional interventions and the IL-6 response to exercise. FASEB J. 2017, 31, 3719–3728. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the immune system: Regulation, integration, and adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef]
- Barbe, M.F.; Barr, A.E.; Gorzelany, I.; Amin, M.; Gaughan, J.P.; Safadi, F.F. Chronic repetitive reaching and grasping results in decreased motor performance and widespread tissue responses in a rat model of MSD. J. Orthop. Res. 2003, 21, 167–176. [Google Scholar] [CrossRef]
- Teixeira, C.F.; Zamuner, S.R.; Zuliani, J.P.; Fernandes, C.M.; Cruz-Hofling, M.A.; Fernandes, I.; Chaves, F.; Gutierrez, J.M. Neutrophils do not contribute to local tissue damage, but play a key role in skeletal muscle regeneration, in mice injected with Bothrops asper snake venom. Muscle Nerve 2003, 28, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Duffield, J.S.; Forbes, S.J.; Constandinou, C.M.; Clay, S.; Partolina, M.; Vuthoori, S.; Wu, S.; Lang, R.; Iredale, J.P. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Investig. 2005, 115, 56–65. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Yao, B.; Yang, S.; Jiang, L.; Wang, S.; Fan, X.; Yin, H.; Wong, K.; Miyazawa, T.; Chen, J.; et al. CSF-1 signaling mediates recovery from acute kidney injury. J. Clin. Investig. 2012, 122, 4519–4532. [Google Scholar] [CrossRef]
- Kjaer, M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef]
- Barr, A.E.; Barbe, M.F. Pathophysiological tissue changes associated with repetitive movement: A review of the evidence. Phys. Ther. 2002, 82, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Contreras, O.; Rebolledo, D.L.; Oyarzun, J.E.; Olguin, H.C.; Brandan, E. Connective tissue cells expressing fibro/adipogenic progenitor markers increase under chronic damage: Relevance in fibroblast-myofibroblast differentiation and skeletal muscle fibrosis. Cell Tissue Res. 2016, 364, 647–660. [Google Scholar] [CrossRef]
- Klingberg, F.; Hinz, B.; White, E.S. The myofibroblast matrix: Implications for tissue repair and fibrosis. J. Pathol. 2013, 229, 298–309. [Google Scholar] [CrossRef]
- Ito, Y.; Goldschmeding, R.; Kasuga, H.; Claessen, N.; Nakayama, M.; Yuzawa, Y.; Sawai, A.; Matsuo, S.; Weening, J.J.; Aten, J. Expression patterns of connective tissue growth factor and of TGF-beta isoforms during glomerular injury recapitulate glomerulogenesis. Am. J. Physiol. Renal. Physiol. 2010, 299, F545–F558. [Google Scholar] [CrossRef]
- Song, J.J.; Aswad, R.; Kanaan, R.A.; Rico, M.C.; Owen, T.A.; Barbe, M.F.; Safadi, F.F.; Popoff, S.N. Connective tissue growth factor (CTGF) acts as a downstream mediator of TGF-beta1 to induce mesenchymal cell condensation. J. Cell Physiol. 2007, 210, 398–410. [Google Scholar] [CrossRef]
- Kaasboll, O.J.; Gadicherla, A.K.; Wang, J.H.; Monsen, V.T.; Hagelin, E.M.V.; Dong, M.Q.; Attramadal, H. Connective tissue growth factor (CCN2) is a matricellular preproprotein controlled by proteolytic activation. J. Biol. Chem. 2018, 293, 17953–17970. [Google Scholar] [CrossRef]
- Lipson, K.E.; Wong, C.; Teng, Y.; Spong, S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenes. Tissue Repair 2012, 5, S24. [Google Scholar] [CrossRef] [PubMed]
- Barbe, M.F.; Hilliard, B.A.; Delany, S.P.; Iannarone, V.J.; Harris, M.Y.; Amin, M.; Cruz, G.E.; Barreto-Cruz, Y.; Tran, N.; Day, E.P.; et al. Blocking CCN2 reduces progression of sensorimotor declines and fibrosis in a rat model of chronic repetitive overuse. J. Orthop. Res. 2019, 37, 2004–2018. [Google Scholar] [CrossRef] [PubMed]
- Bickelhaupt, S.; Erbel, C.; Timke, C.; Wirkner, U.; Dadrich, M.; Flechsig, P.; Tietz, A.; Pfohler, J.; Gross, W.; Peschke, P.; et al. Effects of CTGF blockade on attenuation and reversal of radiation-induced pulmonary fibrosis. J. Natl. Cancer. Inst. 2017, 109. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Wirkner, U.; Bickelhaupt, S.; Lopez Perez, R.; Tietz, A.; Lipson, K.E.; Seeley, T.W.; Huber, P.E. Radiation-induced pulmonary gene expression changes are attenuated by the CTGF antibody Pamrevlumab. Respir. Res. 2018, 19, 14. [Google Scholar] [CrossRef]
- Morales, M.G.; Cabello-Verrugio, C.; Santander, C.; Cabrera, D.; Goldschmeding, R.; Brandan, E. CTGF/CCN-2 over-expression can directly induce features of skeletal muscle dystrophy. J. Pathol. 2011, 225, 490–501. [Google Scholar] [CrossRef]
- Ohara, Y.; Chew, S.H.; Misawa, N.; Wang, S.; Somiya, D.; Nakamura, K.; Kajiyama, H.; Kikkawa, F.; Tsuyuki, Y.; Jiang, L.; et al. Connective tissue growth factor-specific monoclonal antibody inhibits growth of malignant mesothelioma in an orthotopic mouse model. Oncotarget 2018, 9, 18494–18509. [Google Scholar] [CrossRef]
- Barbe, M.F.; Hilliard, B.A.; Amin, M.; Harris, M.Y.; Hobson, L.J.; Cruz, G.E.; Popoff, S.N. Blocking CTGF/CCN2 reduces established skeletal muscle fibrosis in a rat model of overuse injury. FASEB J. 2020, 34, 6554–6569. [Google Scholar] [CrossRef]
- Benatti, F.B.; Pedersen, B.K. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat. Rev. Rheumatol. 2015, 11, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jun, H.S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Barbe, M.F.; Elliott, M.B.; Abdelmagid, S.M.; Amin, M.; Popoff, S.N.; Safadi, F.F.; Barr, A.E. Serum and tissue cytokines and chemokines increase with repetitive upper extremity tasks. J. Orthop. Res. 2008, 26, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Barbe, M.F.; Gallagher, S.; Popoff, S.N. Serum biomarkers as predictors of stage of work-related musculoskeletal disorders. J. Am. Acad. Orthop. Surg. 2013, 21, 644–646. [Google Scholar] [CrossRef]
- Tuttle, C.S.L.; Thang, L.A.N.; Maier, A.B. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res. Rev. 2020, 64, 101185. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Choi, I.; Lee, Y.H. Implications of skeletal muscle extracellular matrix remodeling in metabolic disorders: Diabetes perspective. Int. J. Mol. Sci. 2020, 21, 3845. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Walton, K.L.; Winbanks, C.E.; Murphy, K.T.; Thomson, R.E.; Makanji, Y.; Qian, H.; Lynch, G.S.; Harrison, C.A.; Gregorevic, P. Elevated expression of activins promotes muscle wasting and cachexia. FASEB J. 2014, 28, 1711–1723. [Google Scholar] [CrossRef]
- Martinez-Huenchullan, S.; McLennan, S.V.; Verhoeven, A.; Twigg, S.M.; Tam, C.S. The emerging role of skeletal muscle extracellular matrix remodelling in obesity and exercise. Obes. Rev. 2017, 18, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.Z.; Folco, E.J. Inflammatory concepts of obesity. Int. J. Inflam 2011, 2011, 529061. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Klyne, D.M.; Barbe, M.F.; Hodges, P.W. Systemic inflammatory profiles and their relationships with demographic, behavioural and clinical features in acute low back pain. Brain Behav. Immun. 2017, 60, 84–92. [Google Scholar] [CrossRef]
- Klyne, D.M.; Barbe, M.F.; van den Hoorn, W.; Hodges, P.W. ISSLS prize in clinical science 2018: Longitudinal analysis of inflammatory, psychological, and sleep-related factors following an acute low back pain episode-the good, the bad, and the ugly. Eur. Spine J. 2018, 27, 763–777. [Google Scholar] [CrossRef]
- Klyne, D.M.; Hodges, P.W. Circulating adipokines in predicting the transition from acute to persistent low back pain. Pain Med. 2020, 21, 2975–2985. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Schiltenwolf, M.; Buchner, M. The role of TNF-alpha in patients with chronic low back pain-a prospective comparative longitudinal study. Clin. J. Pain 2008, 24, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.H.; Silvis, C.; Deshpande, N.; Nystrom, G.; Frost, R.A. Endotoxin stimulates In Vivo expression of inflammatory cytokines tumor necrosis factor alpha, interleukin-1beta, -6, and high-mobility-group protein-1 in skeletal muscle. Shock 2003, 19, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.A.; Nystrom, G.J.; Lang, C.H. Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R698–R709. [Google Scholar] [CrossRef]
- Plomgaard, P.; Bouzakri, K.; Krogh-Madsen, R.; Mittendorfer, B.; Zierath, J.R.; Pedersen, B.K. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 2005, 54, 2939–2945. [Google Scholar] [CrossRef]
- Langen, R.C.; Schols, A.M.; Kelders, M.C.; van der Velden, J.L.; Wouters, E.F.; Janssen-Heininger, Y.M. Muscle wasting and impaired muscle regeneration in a murine model of chronic pulmonary inflammation. Am. J. Respir. Cell Mol. Biol. 2006, 35, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Mojumdar, K.; Liang, F.; Lemaire, C.; Li, T.; Richardson, J.; Divangahi, M.; Qureshi, S.; Petrof, B.J. Toll-like receptor 4 ablation in mdx mice reveals innate immunity as a therapeutic target in Duchenne muscular dystrophy. Hum. Mol. Genet. 2015, 24, 2147–2162. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Maejima, Y.; Saito, M.; Sakamoto, K.; Horita, S.; Shimomura, K.; Inoue, S.; Kotani, J. TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice. Sci. Rep. 2020, 10, 694. [Google Scholar] [CrossRef] [PubMed]
- Verzola, D.; Bonanni, A.; Sofia, A.; Montecucco, F.; D’Amato, E.; Cademartori, V.; Parodi, E.L.; Viazzi, F.; Venturelli, C.; Brunori, G.; et al. Toll-like receptor 4 signalling mediates inflammation in skeletal muscle of patients with chronic kidney disease. J. Cachexia Sarcopenia Muscle 2017, 8, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Ollewagen, T.; Myburgh, K.H.; van de Vyver, M.; Smith, C. Rheumatoid cachexia: The underappreciated role of myoblast, macrophage and fibroblast interplay in the skeletal muscle niche. J. Biomed. Sci. 2021, 28, 15. [Google Scholar] [CrossRef]
- Rudrapatna, S.; Bhatt, M.; Wang, K.W.; Bierbrier, R.; Wang, P.W.; Banfield, L.; Elsheikh, W.; Sims, E.D.; Peterson, D.; Thabane, L.; et al. Obesity and muscle-macrophage crosstalk in humans and mice: A systematic review. Obes. Rev. 2019, 20, 1572–1596. [Google Scholar] [CrossRef] [PubMed]
- Abdelmagid, S.M.; Barr, A.E.; Rico, M.; Amin, M.; Litvin, J.; Popoff, S.N.; Safadi, F.F.; Barbe, M.F. Performance of repetitive tasks induces decreased grip strength and increased fibrogenic proteins in skeletal muscle: Role of force and inflammation. PLoS ONE 2012, 7, e38359. [Google Scholar] [CrossRef]
- Rani, S.; Barbe, M.F.; Barr, A.E.; Litivn, J. Role of TNF alpha and PLF in bone remodeling in a rat model of repetitive reaching and grasping. J. Cell Physiol. 2010, 225, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Barbe, M.F.; Gallagher, S.; Massicotte, V.S.; Tytell, M.; Popoff, S.N.; Barr-Gillespie, A.E. The interaction of force and repetition on musculoskeletal and neural tissue responses and sensorimotor behavior in a rat model of work-related musculoskeletal disorders. BMC Musculoskelet. Disord. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Fisher, P.W.; Zhao, Y.; Rico, M.C.; Massicotte, V.S.; Wade, C.K.; Litvin, J.; Bove, G.M.; Popoff, S.N.; Barbe, M.F. Increased CCN2, substance P and tissue fibrosis are associated with sensorimotor declines in a rat model of repetitive overuse injury. J. Cell Commun. Signal 2015, 9, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.G.; Fisher, P.W.; Lambi, A.G.; Wade, C.K.; Barr-Gillespie, A.E.; Popoff, S.N.; Barbe, M.F. Increased serum and musculotendinous fibrogenic proteins following persistent low-grade inflammation in a rat model of long-term upper extremity overuse. PLoS ONE 2013, 8, e71875. [Google Scholar] [CrossRef] [PubMed]
- Barbe, M.F.; Harris, M.Y.; Cruz, G.E.; Amin, M.; Billett, N.M.; Dorotan, J.T.; Day, E.P.; Kim, S.Y.; Bove, G.M. Key indicators of repetitive overuse-induced neuromuscular inflammation and fibrosis are prevented by manual therapy in a rat model. BMC Musculoskelet. Disord. 2021, 22, 417. [Google Scholar] [CrossRef]
- Fedorczyk, J.M.; Barr, A.E.; Rani, S.; Gao, H.G.; Amin, M.; Amin, S.; Litvin, J.; Barbe, M.F. Exposure-dependent increases in IL-1beta, substance P, CTGF, and tendinosis in flexor digitorum tendons with upper extremity repetitive strain injury. J. Orthop. Res. 2010, 28, 298–307. [Google Scholar] [CrossRef]
- Hilliard, B.A.; Amin, M.; Popoff, S.N.; Barbe, M.F. Force dependent effects of chronic overuse on fibrosis-related genes and proteins in skeletal muscles. Connect. Tissue Res. 2021, 62, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Beddy, D.; Mulsow, J.; Watson, R.W.; Fitzpatrick, J.M.; O’Connell, P.R. Expression and regulation of connective tissue growth factor by transforming growth factor beta and tumour necrosis factor alpha in fibroblasts isolated from strictures in patients with Crohn’s disease. Br. J. Surg. 2006, 93, 1290–1296. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, Y.; Lu, J.; Wu, N. Connective tissue growth factor promotes interleukin-1beta-mediated synovial inflammation in knee osteoarthritis. Mol. Med. Rep. 2013, 8, 877–882. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, S.C.; Hsu, C.J.; Chen, H.T.; Tsou, H.K.; Chuang, S.M.; Tang, C.H. CTGF increases IL-6 expression in human synovial fibroblasts through integrin-dependent signaling pathway. PLoS ONE 2012, 7, e51097. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, K.; Paramel, G.V.; Kienesberger, P.C. Lysophosphatidic acid signaling in obesity and insulin resistance. Nutrients 2018, 10, 399. [Google Scholar] [CrossRef]
- Davies, M.R.; Lee, L.; Feeley, B.T.; Kim, H.T.; Liu, X. Lysophosphatidic acid-induced RhoA signaling and prolonged macrophage infiltration worsens fibrosis and fatty infiltration following rotator cuff tears. J. Orthop. Res. 2017, 35, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, F.S.; Córdova-Casanova, A.; Brandan, E. The linkage between inflammation and fibrosis in muscular dystrophies: The axis autotaxin-lysophosphatidic acid as a new therapeutic target? J. Cell Commun. Signal 2021. [Google Scholar] [CrossRef] [PubMed]
- Vial, C.; Zúñiga, L.M.; Cabello-Verrugio, C.; Cañón, P.; Fadic, R.; Brandan, E. Skeletal muscle cells express the profibrotic cytokine connective tissue growth factor (CTGF/CCN2), which induces their dedifferentiation. J. Cell Physiol. 2008, 215, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Parkitny, L.; McAuley, J.H.; Di Pietro, F.; Stanton, T.R.; O’Connell, N.E.; Marinus, J.; van Hilten, J.J.; Moseley, G.L. Inflammation in complex regional pain syndrome: A systematic review and meta-analysis. Neurology 2013, 80, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Laferriere, A.; Millecamps, M.; Xanthos, D.N.; Xiao, W.H.; Siau, C.; de Mos, M.; Sachot, C.; Ragavendran, J.V.; Huygen, F.J.; Bennett, G.J.; et al. Cutaneous tactile allodynia associated with microvascular dysfunction in muscle. Mol. Pain 2008, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Arai, T.; Iwatsuki, K.; Urano, H.; Kurahashi, T.; Kato, S.; Yamamoto, M.; Hirata, H. Pathological mechanism of musculoskeletal manifestations associated with CRPS type II: An animal study. Pain 2014, 155, 1976–1985. [Google Scholar] [CrossRef]
- Guo, T.Z.; Wei, T.; Huang, T.T.; Kingery, W.S.; Clark, J.D. Oxidative stress contributes to fracture/cast-induced inflammation and pain in a rat model of complex regional pain syndrome. J. Pain 2018, 19, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Hulsman, N.M.; Geertzen, J.H.; Dijkstra, P.U.; van den Dungen, J.J.; den Dunnen, W.F. Myopathy in CRPS-I: Disuse or neurogenic? Eur. J. Pain 2009, 13, 731–736. [Google Scholar] [CrossRef]
- Huffman, K.M.; Jessee, R.; Andonian, B.; Davis, B.N.; Narowski, R.; Huebner, J.L.; Kraus, V.B.; McCracken, J.; Gilmore, B.F.; Tune, K.N.; et al. Molecular alterations in skeletal muscle in rheumatoid arthritis are related to disease activity, physical inactivity, and disability. Arthritis Res. Ther. 2017, 19, 12. [Google Scholar] [CrossRef]
- Altomonte, L.; Zoli, A.; Mirone, L.; Scolieri, P.; Magaro, M. Serum levels of interleukin-1b, tumour necrosis factor-a and interleukin-2 in rheumatoid arthritis. Correlation with disease activity. Clin. Rheumatol. 1992, 11, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.E.; Choy, E.H. C-reactive protein and implications in rheumatoid arthritis and associated comorbidities. Semin. Arthritis Rheum. 2021, 51, 219–229. [Google Scholar] [CrossRef]
- Wei, S.T.; Sun, Y.H.; Zong, S.H.; Xiang, Y.B. Serum levels of IL-6 and TNF-α may correlate with activity and severity of rheumatoid arthritis. Med. Sci. Monit. 2015, 21, 4030–4038. [Google Scholar] [CrossRef] [PubMed]
- Oyenihi, A.B.; Ollewagen, T.; Myburgh, K.H.; Powrie, Y.S.L.; Smith, C. Redox status and muscle pathology in rheumatoid arthritis: Insights from various rat hindlimb muscles. Oxid. Med. Cell Longev. 2019, 2019, 2484678. [Google Scholar] [CrossRef] [PubMed]
- Bove, G.M.; Harris, M.Y.; Zhao, H.Q.; Barbe, M.F. Manual therapy as an effective treatment for fibrosis in a rat model of upper extremity overuse injury. J. Neurol. Sci. 2016, 361, 168–180. [Google Scholar] [CrossRef]
- Carp, S.J.; Barbe, M.F.; Winter, K.A.; Amin, M.; Barr, A.E. Inflammatory biomarkers increase with severity of upper-extremity overuse disorders. Clin. Sci. 2007, 112, 305–314. [Google Scholar] [CrossRef]
- Gold, J.E.; Mohamed, F.B.; Ali, S.; Barbe, M.F. Serum and MRI biomarkers in mobile device texting: A pilot study. Hum. Factors 2014, 56, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, J.I.; Verbeek, J.H.; Everts, V.; Straub, J.P.; Frings-Dresen, M.H. Serum markers of collagen metabolism: Construction workers compared to sedentary workers. Occup. Environ. Med. 2005, 62, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, J.I.; Verbeek, J.H.; Straub, J.P.; Everts, V.; Frings-Dresen, M.H. Physical workload of student nurses and serum markers of collagen metabolism. Scand. J. Work Environ. Health 2002, 28, 168–175. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rechardt, M.; Shiri, R.; Karppinen, J.; Jula, A.; Heliovaara, M.; Viikari-Juntura, E. Lifestyle and metabolic factors in relation to shoulder pain and rotator cuff tendinitis: A population-based study. BMC Musculoskelet. Disord. 2010, 11, 165. [Google Scholar] [CrossRef]
- Rechardt, M.; Shiri, R.; Lindholm, H.; Karppinen, J.; Viikari-Juntura, E. Associations of metabolic factors and adipokines with pain in incipient upper extremity soft tissue disorders: A cross-sectional study. BMJ Open 2013, 3, e003036. [Google Scholar] [CrossRef] [PubMed]
- Abdelmagid, S.M.; Barbe, M.F.; Rico, M.C.; Salihoglu, S.; Arango-Hisijara, I.; Selim, A.H.; Anderson, M.G.; Owen, T.A.; Popoff, S.N.; Safadi, F.F. Osteoactivin, an anabolic factor that regulates osteoblast differentiation and function. Exp. Cell Res. 2008, 314, 2334–2351. [Google Scholar] [CrossRef]
- Larsson, B.; Rosendal, L.; Kristiansen, J.; Sjogaard, G.; Sogaard, K.; Ghafouri, B.; Abdiu, A.; Kjaer, M.; Gerdle, B. Responses of algesic and metabolic substances to 8 h of repetitive manual work in myalgic human trapezius muscle. Pain 2008, 140, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Rosendal, L.; Larsson, B.; Kristiansen, J.; Peolsson, M.; Sogaard, K.; Kjaer, M.; Sorensen, J.; Gerdle, B. Increase in muscle nociceptive substances and anaerobic metabolism in patients with trapezius myalgia: Microdialysis in rest and during exercise. Pain 2004, 112, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Rufo, A.; Del Fattore, A.; Capulli, M.; Carvello, F.; De Pasquale, L.; Ferrari, S.; Pierroz, D.; Morandi, L.; De Simone, M.; Rucci, N.; et al. Mechanisms inducing low bone density in Duchenne muscular dystrophy in mice and humans. J. Bone Miner. Res. 2011, 26, 1891–1903. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, B.; De Bleecker, J.L. Cytokines and chemokines as regulators of skeletal muscle inflammation: Presenting the case of Duchenne muscular dystrophy. Mediat. Inflamm. 2013, 2013, 540370. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.P.; Misyak, S.A.; Robertson, J.L.; Bassaganya-Riera, J.; Grange, R.W. Dysregulated intracellular signaling and inflammatory gene expression during initial disease onset in Duchenne muscular dystrophy. Am. J. Phys. Med. Rehabil. 2009, 88, 502–522. [Google Scholar] [CrossRef]
- Villalta, S.A.; Deng, B.; Rinaldi, C.; Wehling-Henricks, M.; Tidball, J.G. IFN-gamma promotes muscle damage in the mdx mouse model of Duchenne muscular dystrophy by suppressing M2 macrophage activation and inhibiting muscle cell proliferation. J. Immunol. 2011, 187, 5419–5428. [Google Scholar] [CrossRef]
- Villalta, S.A.; Nguyen, H.X.; Deng, B.; Gotoh, T.; Tidball, J.G. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum. Mol. Genet. 2009, 18, 482–496. [Google Scholar] [CrossRef]
- Song, Y.; Yao, S.; Liu, Y.; Long, L.; Yang, H.; Li, Q.; Liang, J.; Li, X.; Lu, Y.; Zhu, H.; et al. Expression levels of TGF-beta1 and CTGF are associated with the severity of Duchenne muscular dystrophy. Exp. Ther. Med. 2017, 13, 1209–1214. [Google Scholar] [CrossRef]
- Gonzalez-Reimers, E.; Fernandez-Rodriguez, C.M.; Santolaria-Fernandez, F.; de la Vega-Prieto, M.J.; Martin-Gonzalez, C.; Gomez-Rodriguez, M.A.; Aleman-Valls, M.R.; Rodriguez-Gaspar, M. Interleukin-15 and other myokines in chronic alcoholics. Alcohol Alcohol. 2011, 46, 529–533. [Google Scholar] [CrossRef][Green Version]
- Imhof, A.; Froehlich, M.; Brenner, H.; Boeing, H.; Pepys, M.B.; Koenig, W. Effect of alcohol consumption on systemic markers of inflammation. Lancet 2001, 357, 763–767. [Google Scholar] [CrossRef]
- Achur, R.N.; Freeman, W.M.; Vrana, K.E. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J. Neuroimmune Pharmacol. 2010, 5, 83–91. [Google Scholar] [CrossRef]
- Simon, L.; Jolley, S.E.; Molina, P.E. Alcoholic Myopathy: Pathophysiologic Mechanisms and Clinical Implications. Alcohol Res. 2017, 38, 207–217. [Google Scholar]
- Molina, P.E.; Lang, C.H.; McNurlan, M.; Bagby, G.J.; Nelson, S. Chronic alcohol accentuates simian acquired immunodeficiency syndrome-associated wasting. Alcohol. Clin. Exp. Res. 2008, 32, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.E.; McNurlan, M.; Rathmacher, J.; Lang, C.H.; Zambell, K.L.; Purcell, J.; Bohm, R.P.; Zhang, P.; Bagby, G.J.; Nelson, S. Chronic alcohol accentuates nutritional, metabolic, and immune alterations during asymptomatic simian immunodeficiency virus infection. Alcohol. Clin. Exp. Res. 2006, 30, 2065–2078. [Google Scholar] [CrossRef] [PubMed]
- Dodd, T.; Simon, L.; LeCapitaine, N.J.; Zabaleta, J.; Mussell, J.; Berner, P.; Ford, S.; Dufour, J.; Bagby, G.J.; Nelson, S.; et al. Chronic binge alcohol administration accentuates expression of pro-fibrotic and inflammatory genes in the skeletal muscle of simian immunodeficiency virus-infected macaques. Alcohol. Clin. Exp. Res. 2014, 38, 2697–2706. [Google Scholar] [CrossRef]
- Dekeyser, G.J.; Clary, C.R.; Otis, J.S. Chronic alcohol ingestion delays skeletal muscle regeneration following injury. Regen. Med. Res. 2013, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, V.S.; Fitzgerald, L.W.; Bathe, O.F. Cancer-associated muscle wasting-candidate mechanisms and molecular pathways. Int. J. Mol. Sci. 2020, 21, 9268. [Google Scholar] [CrossRef]
- Shukla, S.K.; Markov, S.D.; Attri, K.S.; Vernucci, E.; King, R.J.; Dasgupta, A.; Grandgenett, P.M.; Hollingsworth, M.A.; Singh, P.K.; Yu, F.; et al. Macrophages potentiate STAT3 signaling in skeletal muscles and regulate pancreatic cancer cachexia. Cancer Lett. 2020, 484, 29–39. [Google Scholar] [CrossRef]
- Dalle, S.; Koppo, K. Is inflammatory signaling involved in disease-related muscle wasting? Evidence from osteoarthritis, chronic obstructive pulmonary disease and type II diabetes. Exp. Gerontol. 2020, 137, 110964. [Google Scholar] [CrossRef]
- Hansen, M.J.; Chen, H.; Jones, J.E.; Langenbach, S.Y.; Vlahos, R.; Gualano, R.C.; Morris, M.J.; Anderson, G.P. The lung inflammation and skeletal muscle wasting induced by subchronic cigarette smoke exposure are not altered by a high-fat diet in mice. PLoS ONE 2013, 8, e80471. [Google Scholar] [CrossRef]
- Scott, A.; Wang, X.; Road, J.D.; Reid, W.D. Increased injury and intramuscular collagen of the diaphragm in COPD: Autopsy observations. Eur. Respir. J. 2006, 27, 51–59. [Google Scholar] [CrossRef]
- Patsouris, D.; Cao, J.J.; Vial, G.; Bravard, A.; Lefai, E.; Durand, A.; Durand, C.; Chauvin, M.A.; Laugerette, F.; Debard, C.; et al. Insulin resistance is associated with MCP1-mediated macrophage accumulation in skeletal muscle in mice and humans. PLoS ONE 2014, 9, e110653. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Yannakoulia, M.; Chrysohoou, C.; Stefanadis, C. The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis 2005, 183, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Zorena, K.; Jachimowicz-Duda, O.; Slezak, D.; Robakowska, M.; Mrugacz, M. Adipokines and obesity. Potential link to metabolic disorders and chronic complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef]
- Edwards, R.R.; Kronfli, T.; Haythornthwaite, J.A.; Smith, M.T.; McGuire, L.; Page, G.G. Association of catastrophizing with interleukin-6 responses to acute pain. Pain 2008, 140, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Lotrich, F.E. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience 2013, 246, 199–229. [Google Scholar] [CrossRef]
- Kubera, M.; Obuchowicz, E.; Goehler, L.; Brzeszcz, J.; Maes, M. In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 744–759. [Google Scholar] [CrossRef]
- Slavich, G.M.; Irwin, M.R. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol. Bull. 2014, 140, 774–815. [Google Scholar] [CrossRef]
- Marsland, A.L.; Walsh, C.; Lockwood, K.; John-Henderson, N.A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav. Immun. 2017, 64, 208–219. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctot, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Segerstrom, S.C.; Miller, G.E. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol. Bull. 2004, 130, 601–630. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Miller, A.H. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am. J. Psychiatry 2003, 160, 1554–1565. [Google Scholar] [CrossRef]
- Grebe, K.M.; Takeda, K.; Hickman, H.D.; Bailey, A.L.; Embry, A.C.; Bennink, J.R.; Yewdell, J.W. Cutting edge: Sympathetic nervous system increases proinflammatory cytokines and exacerbates influenza A virus pathogenesis. J. Immunol. 2010, 184, 540–544. [Google Scholar] [CrossRef]
- Pariante, C.M. The glucocorticoid receptor: Part of the solution or part of the problem? J. Psychopharmacol. 2006, 20, 79–84. [Google Scholar] [CrossRef]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef]
- Cirulli, F.; Laviola, G.; Ricceri, L. Risk factors for mental health: Translational models from behavioural neuroscience. Neurosci. Biobehav. Rev. 2009, 33, 493–497. [Google Scholar] [CrossRef][Green Version]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11. [Google Scholar] [CrossRef]
- Fasick, V.; Spengler, R.N.; Samankan, S.; Nader, N.D.; Ignatowski, T.A. The hippocampus and TNF: Common links between chronic pain and depression. Neurosci. Biobehav. Rev. 2015, 53, 139–159. [Google Scholar] [CrossRef]
- Bauer, M.E.; Teixeira, A.L. Inflammation in psychiatric disorders: What comes first? Ann. N. Y. Acad. Sci. 2019, 1437, 57–67. [Google Scholar] [CrossRef]
- Felger, J.C. Imaging the role of inflammation in mood and anxiety-related disorders. Curr. Neuropharmacol. 2018, 16, 533–558. [Google Scholar] [CrossRef]
- Quan, N.; Banks, W.A. Brain-immune communication pathways. Brain Behav. Immun. 2007, 21, 727–735. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef]
- Hoogland, I.C.; Houbolt, C.; van Westerloo, D.J.; van Gool, W.A.; van de Beek, D. Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflamm. 2015, 12, 114. [Google Scholar] [CrossRef]
- Capuron, L.; Miller, A.H. Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacol. Ther. 2011, 130, 226–238. [Google Scholar] [CrossRef]
- Dantzer, R. Cytokine-induced sickness behaviour: A neuroimmune response to activation of innate immunity. Eur. J. Pharmacol. 2004, 500, 399–411. [Google Scholar] [CrossRef] [PubMed]
- de Brouwer, S.J.; Kraaimaat, F.W.; Sweep, F.C.; Creemers, M.C.; Radstake, T.R.; van Laarhoven, A.I.; van Riel, P.L.; Evers, A.W. Experimental stress in inflammatory rheumatic diseases: A review of psychophysiological stress responses. Arthritis Res. Ther. 2010, 12, R89. [Google Scholar] [CrossRef]
- Zautra, A.J.; Hoffman, J.; Potter, P.; Matt, K.S.; Yocum, D.; Castro, L. Examination of changes in interpersonal stress as a factor in disease exacerbations among women with rheumatoid arthritis. Ann. Behav. Med. 1997, 19, 279–286. [Google Scholar] [CrossRef]
- Wallace, D.J. The role of stress and trauma in rheumatoid arthritis and systemic lupus erythematosus. Semin. Arthritis Rheum. 1987, 16, 153–157. [Google Scholar] [CrossRef]
- Blackburn-Munro, G.; Blackburn-Munro, R.E. Chronic pain, chronic stress and depression: Coincidence or consequence? J. Neuroendocrinol. 2001, 13, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, C.G.; Geha, P. Chronic pain and chronic stress: Two sides of the same coin? Chronic Stress 2017, 1. [Google Scholar] [CrossRef]
- Cutolo, M.; Straub, R.H. Stress as a risk factor in the pathogenesis of rheumatoid arthritis. Neuroimmunomodulat 2006, 13, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Boscarino, J.A. Posttraumatic stress disorder and physical illness: Results from clinical and epidemiologic studies. Ann. N. Y. Acad. Sci. 2004, 1032, 141–153. [Google Scholar] [CrossRef]
- Straub, R.H.; Bijlsma, J.W.J.; Cutolo, M. How psychological stress via hormones and nerve fibers may exacerbate rheumatoid arthritis: Comment on the review by Straub et al.—Reply. Arthritis Rheum. 2006, 54, 682–683. [Google Scholar] [CrossRef]
- Harbuz, M. Neuroendocrine function and chronic inflammatory stress. Exp. Physiol. 2002, 87, 519–525. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F. Immune regulation of central nervous system functions: From sickness responses to pathological pain. J. Intern. Med. 2005, 257, 139–155. [Google Scholar] [CrossRef]
- Strouse, T.B. The relationship between cytokines and pain/depression: A review and current status. Curr. Pain Headache Rep. 2007, 11, 98–103. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Xin, D.L.; Hadrevi, J.; Elliott, M.E.; Amin, M.; Harris, M.Y.; Barr-Gillespie, A.E.; Barbe, M.F. Effectiveness of conservative interventions for sickness and pain behaviors induced by a high repetition high force upper extremity task. BMC Neurosci. 2017, 18, 36. [Google Scholar] [CrossRef]
- Mullington, J.M.; Simpson, N.S.; Meier-Ewert, H.K.; Haack, M. Sleep loss and inflammation. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Opp, M.R. Sleep health: Reciprocal regulation of sleep and innate immunity. Neuropsychopharmacol 2017, 42, 129–155. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R. Sleep and inflammation: Partners in sickness and in health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, S.; Lange, T.; Benedict, C.; Nowell, M.A.; Jones, S.A.; Scheller, J.; Rose-John, S.; Born, J. Sleep enhances IL-6 trans-signaling in humans. FASEB J. 2006, 20, 2174–2176. [Google Scholar] [CrossRef]
- Redwine, L.; Dang, J.; Hall, M.; Irwin, M. Disordered sleep, nocturnal cytokines, and immunity in alcoholics. Psychosom. Med. 2003, 65, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Redwine, L.; Hauger, R.L.; Gillin, J.C.; Irwin, M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J. Clin. Endocrinol. Metab. 2000, 85, 3597–3603. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Lotsikas, A.; Zachman, K.; Kales, A.; Prolo, P.; Wong, M.L.; Licinio, J.; Gold, P.W.; et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J. Clin. Endocrinol. Metab. 1999, 84, 2603–2607. [Google Scholar] [CrossRef]
- Minkel, J.D.; Banks, S.; Htaik, O.; Moreta, M.C.; Jones, C.W.; McGlinchey, E.L.; Simpson, N.S.; Dinges, D.F. Sleep deprivation and stressors: Evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion 2012, 12, 1015–1020. [Google Scholar] [CrossRef]
- Irwin, M.R.; Wang, M.; Ribeiro, D.; Cho, H.J.; Olmstead, R.; Breen, E.C.; Martinez-Maza, O.; Cole, S. Sleep loss activates cellular inflammatory signaling. Biol. Psychiatry 2008, 64, 538–540. [Google Scholar] [CrossRef]
- Irwin, M.R.; Wang, M.; Campomayor, C.O.; Collado-Hidalgo, A.; Cole, S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch. Intern. Med. 2006, 166, 1756–1762. [Google Scholar] [CrossRef]
- Irwin, M.R.; Witarama, T.; Caudill, M.; Olmstead, R.; Breen, E.C. Sleep loss activates cellular inflammation and signal transducer and activator of transcription (STAT) family proteins in humans. Brain Behav. Immun. 2015, 47, 86–92. [Google Scholar] [CrossRef]
- Abedelmalek, S.; Chtourou, H.; Aloui, A.; Aouichaoui, C.; Souissi, N.; Tabka, Z. Effect of time of day and partial sleep deprivation on plasma concentrations of IL-6 during a short-term maximal performance. Eur. J. Appl. Physiol. 2013, 113, 241–248. [Google Scholar] [CrossRef]
- Frey, D.J.; Fleshner, M.; Wright, K.P., Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav. Immun. 2007, 21, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Chennaoui, M.; Sauvet, F.; Drogou, C.; Van Beers, P.; Langrume, C.; Guillard, M.; Gourby, B.; Bourrilhon, C.; Florence, G.; Gomez-Merino, D. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-alpha) levels in healthy men. Cytokine 2011, 56, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Haack, M.; Sanchez, E.; Mullington, J.M. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep 2007, 30, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Haack, M. The sleep-immune crosstalk in health and disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Zoumakis, M.; Papanicolaou, D.A.; Bixler, E.O.; Prolo, P.; Lin, H.M.; Vela-Bueno, A.; Kales, A.; Chrousos, G.P. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism 2002, 51, 887–892. [Google Scholar] [CrossRef]
- Cho, H.J.; Seeman, T.E.; Kiefe, C.I.; Lauderdale, D.S.; Irwin, M.R. Sleep disturbance and longitudinal risk of inflammation: Moderating influences of social integration and social isolation in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Brain Behav. Immun. 2015, 46, 319–326. [Google Scholar] [CrossRef]
- Irwin, M.R. Why sleep is important for health: A psychoneuroimmunology perspective. Annu. Rev. Psychol. 2015, 66, 143–172. [Google Scholar] [CrossRef]
- Krueger, J.M. The role of cytokines in sleep regulation. Curr. Pharm. Des. 2008, 14, 3408–3416. [Google Scholar] [CrossRef]
- Mathur, N.; Pedersen, B.K. Exercise as a mean to control low-grade systemic inflammation. Mediat. Inflamm. 2008, 2008, 109502. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Geffken, D.F.; Cushman, M.; Burke, G.L.; Polak, J.F.; Sakkinen, P.A.; Tracy, R.P. Association between physical activity and markers of inflammation in a healthy elderly population. Am. J. Epidemiol. 2001, 153, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Halle, M.; Korsten-Reck, U.; Wolfarth, B.; Berg, A. Low-grade systemic inflammation in overweight children: Impact of physical fitness. Exerc. Immunol. Rev. 2004, 10, 66–74. [Google Scholar]
- Fischer, C.P.; Berntsen, A.; Perstrup, L.B.; Eskildsen, P.; Pedersen, B.K. Plasma levels of interleukin-6 and C-reactive protein are associated with physical inactivity independent of obesity. Scand. J. Med. Sci. Sports 2007, 17, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.T.; Janssen, I.; Ardern, C.I. Physical inactivity, excess adiposity and premature mortality. Obes. Rev. 2003, 4, 257–290. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef]
- Gonzalez-Reimers, E.; Santolaria-Fernandez, F.; Martin-Gonzalez, M.C.; Fernandez-Rodriguez, C.M.; Quintero-Platt, G. Alcoholism: A systemic proinflammatory condition. World J. Gastroenterol. 2014, 20, 14660–14671. [Google Scholar] [CrossRef]
- Crews, F.T.; Bechara, R.; Brown, L.A.; Guidot, D.M.; Mandrekar, P.; Oak, S.; Qin, L.; Szabo, G.; Wheeler, M.; Zou, J. Cytokines and alcohol. Alcohol. Clin. Exp. Res. 2006, 30, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Zakhari, S.; Jung, M.K. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J. Gastroenterol. 2010, 16, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jonsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106, S5–S78. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Albers, R.; Bosco, N.; Bourdet-Sicard, R.; Haller, D.; Holgate, S.T.; Jönsson, L.S.; Latulippe, M.E.; Marcos, A.; et al. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br. J. Nutr. 2013, 109, S1–S34. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Lee, J.; Taneja, V.; Vassallo, R. Cigarette smoking and inflammation: Cellular and molecular mechanisms. J. Dent. Res. 2012, 91, 142–149. [Google Scholar] [CrossRef]
- Shields, G.S.; Spahr, C.M.; Slavich, G.M. Psychosocial interventions and immune system function: A systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 2020, 77, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Tuglu, C.; Kara, S.H.; Caliyurt, O.; Vardar, E.; Abay, E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology 2003, 170, 429–433. [Google Scholar] [CrossRef]
- Livingston, W.S.; Rusch, H.L.; Nersesian, P.V.; Baxter, T.; Mysliwiec, V.; Gill, J.M. Improved sleep in military personnel is associated with changes in the expression of inflammatory genes and improvement in depression symptoms. Front. Psychiatry 2015, 6. [Google Scholar] [CrossRef]
- Heinzelmann, M.; Lee, H.; Rak, H.; Livingston, W.; Barr, T.; Baxter, T.; Scattergood-Keepper, L.; Mysliwiec, V.; Gill, J. Sleep restoration is associated with reduced plasma C-reactive protein and depression symptoms in military personnel with sleep disturbance after deployment. Sleep Med. 2014, 15, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef]
- Woods, J.A.; Wilund, K.R.; Martin, S.A.; Kistler, B.M. Exercise, inflammation and aging. Aging Dis. 2012, 3, 130–140. [Google Scholar] [PubMed]
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1 alpha, myokines and exercise. Bone 2015, 80, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Starkie, R.; Ostrowski, S.R.; Jauffred, S.; Febbraio, M.; Pedersen, B.K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003, 17, 884–886. [Google Scholar] [CrossRef]
- Flynn, M.G.; McFarlin, B.K.; Markofski, M.M. The anti-inflammatory actions of exercise training. Am. J. Lifestyle Med. 2007, 1, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.T.G.; Barr-Gillespie, A.E.; Klyne, D.M.; Harris, M.Y.; Amin, M.; Paul, R.W.; Cruz, G.E.; Zhao, H.; Gallagher, S.; Barbe, M.F. Forced treadmill running reduces systemic inflammation yet worsens upper limb discomfort in a rat model of work-related musculoskeletal disorders. BMC Musculoskelet. Disord. 2020, 21, 57. [Google Scholar] [CrossRef]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of physical exercise on cognitive functioning and wellbeing: Biological and psychological benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Chennaoui, M.; Arnal, P.J.; Sauvet, F.; Leger, D. Sleep and exercise: A reciprocal issue? Sleep Med. Rev. 2015, 20, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.M.; Ramos, D.; Xavier, R.F.; Ito, J.T.; Souza, A.P.; Fernandes, R.A.; Cecchini, R.; Rossi e Silva, R.C.; Macchione, M.; Toledo-Arruda, A.C.; et al. Nasal and systemic inflammatory profile after short term smoking cessation. Respir. Med. 2014, 108, 999–1006. [Google Scholar] [CrossRef]
- Ajime, T.T.; Serre, J.; Wust, R.C.I.; Messa, G.A.M.; Poffe, C.; Swaminathan, A.; Maes, K.; Janssens, W.; Troosters, T.; Degens, H.; et al. Two weeks of smoking cessation reverse cigarette smoke-induced skeletal muscle atrophy and mitochondrial dysfunction in mice. Nicotine Tob. Res. 2021, 23, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Willemse, B.W.; Postma, D.S.; Timens, W.; ten Hacken, N.H. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur. Respir. J. 2004, 23, 464–476. [Google Scholar] [CrossRef]
- Mann, R.E.; Smart, R.G.; Govoni, R. The epidemiology of alcoholic liver disease. Alcohol Res. Health 2003, 27, 209–219. [Google Scholar]
- Li, W.; Lin, E.L.; Liangpunsakul, S.; Lan, J.; Chalasani, S.; Rane, S.; Puri, P.; Kamath, P.S.; Sanyal, A.J.; Shah, V.H.; et al. Alcohol abstinence does not fully reverse abnormalities of mucosal-associated invariant T cells in the blood of patients with alcoholic hepatitis. Clin. Transl. Gastroenterol. 2019, 10, e00052. [Google Scholar] [CrossRef]
- Donohue, T.M., Jr. Alcohol-induced steatosis in liver cells. World J. Gastroenterol. 2007, 13, 4974–4978. [Google Scholar] [CrossRef]
- Meyer, R.E.; Murray, R.F.; Babor, T.F.; Farquhar, J.W.; Greenlick, M.R.; Helzer, J.E.; Holder, H.D.; Kellam, S.G.; Li, T.K.; Woody, G.E.; et al. Prevention and treatment of alcohol-related problems: Research opportunities. Committee to identify research opportunities in the prevention and treatment of alcohol-related problems. J. Stud. Alcohol 1992, 53, 5–16. [Google Scholar] [CrossRef]
- Taylor, G.; McNeill, A.; Girling, A.; Farley, A.; Lindson-Hawley, N.; Aveyard, P. Change in mental health after smoking cessation: Systematic review and meta-analysis. BMJ 2014, 348, g1151. [Google Scholar] [CrossRef]
- Kohler, O.; Benros, M.E.; Nordentoft, M.; Farkouh, M.E.; Iyengar, R.L.; Mors, O.; Krogh, J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: A systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 2014, 71, 1381–1391. [Google Scholar] [CrossRef]
- Kojima, M.; Kojima, T.; Suzuki, S.; Oguchi, T.; Oba, M.; Tsuchiya, H.; Sugiura, F.; Kanayama, Y.; Furukawa, T.A.; Tokudome, S.; et al. Depression, inflammation, and pain in patients with rheumatoid arthritis. Arthritis Rheum. 2009, 61, 1018–1024. [Google Scholar] [CrossRef]
- Cho, M.; Lee, T.Y.; Kwak, Y.B.; Yoon, Y.B.; Kim, M.; Kwon, J.S. Adjunctive use of anti-inflammatory drugs for schizophrenia: A meta-analytic investigation of randomized controlled trials. Aust. N. Z. J. Psychiatry 2019, 53, 742–759. [Google Scholar] [CrossRef] [PubMed]
- Kietrys, D.M.; Barr, A.E.; Barbe, M.F. Exposure to repetitive tasks induces motor changes related to skill acquisition and inflammation in rats. J. Motor. Behav. 2011, 43, 465–476. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bove, G.M.; Delany, S.P.; Hobson, L.; Cruz, G.E.; Harris, M.Y.; Amin, M.; Chapelle, S.L.; Barbe, M.F. Manual therapy prevents onset of nociceptor activity, sensorimotor dysfunction, and neural fibrosis induced by a volitional repetitive task. Pain 2019, 160, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.J.; Wang, J.; Ando, T. Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv. Exp. Med. Biol. 1999, 461, 117–127. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klyne, D.M.; Barbe, M.F.; James, G.; Hodges, P.W. Does the Interaction between Local and Systemic Inflammation Provide a Link from Psychology and Lifestyle to Tissue Health in Musculoskeletal Conditions? Int. J. Mol. Sci. 2021, 22, 7299. https://doi.org/10.3390/ijms22147299
Klyne DM, Barbe MF, James G, Hodges PW. Does the Interaction between Local and Systemic Inflammation Provide a Link from Psychology and Lifestyle to Tissue Health in Musculoskeletal Conditions? International Journal of Molecular Sciences. 2021; 22(14):7299. https://doi.org/10.3390/ijms22147299
Chicago/Turabian StyleKlyne, David M., Mary F. Barbe, Greg James, and Paul W. Hodges. 2021. "Does the Interaction between Local and Systemic Inflammation Provide a Link from Psychology and Lifestyle to Tissue Health in Musculoskeletal Conditions?" International Journal of Molecular Sciences 22, no. 14: 7299. https://doi.org/10.3390/ijms22147299
APA StyleKlyne, D. M., Barbe, M. F., James, G., & Hodges, P. W. (2021). Does the Interaction between Local and Systemic Inflammation Provide a Link from Psychology and Lifestyle to Tissue Health in Musculoskeletal Conditions? International Journal of Molecular Sciences, 22(14), 7299. https://doi.org/10.3390/ijms22147299






