CO2-Sensitive Connexin Hemichannels in Neurons and Glia: Three Different Modes of Signalling?
Abstract
:1. Introduction
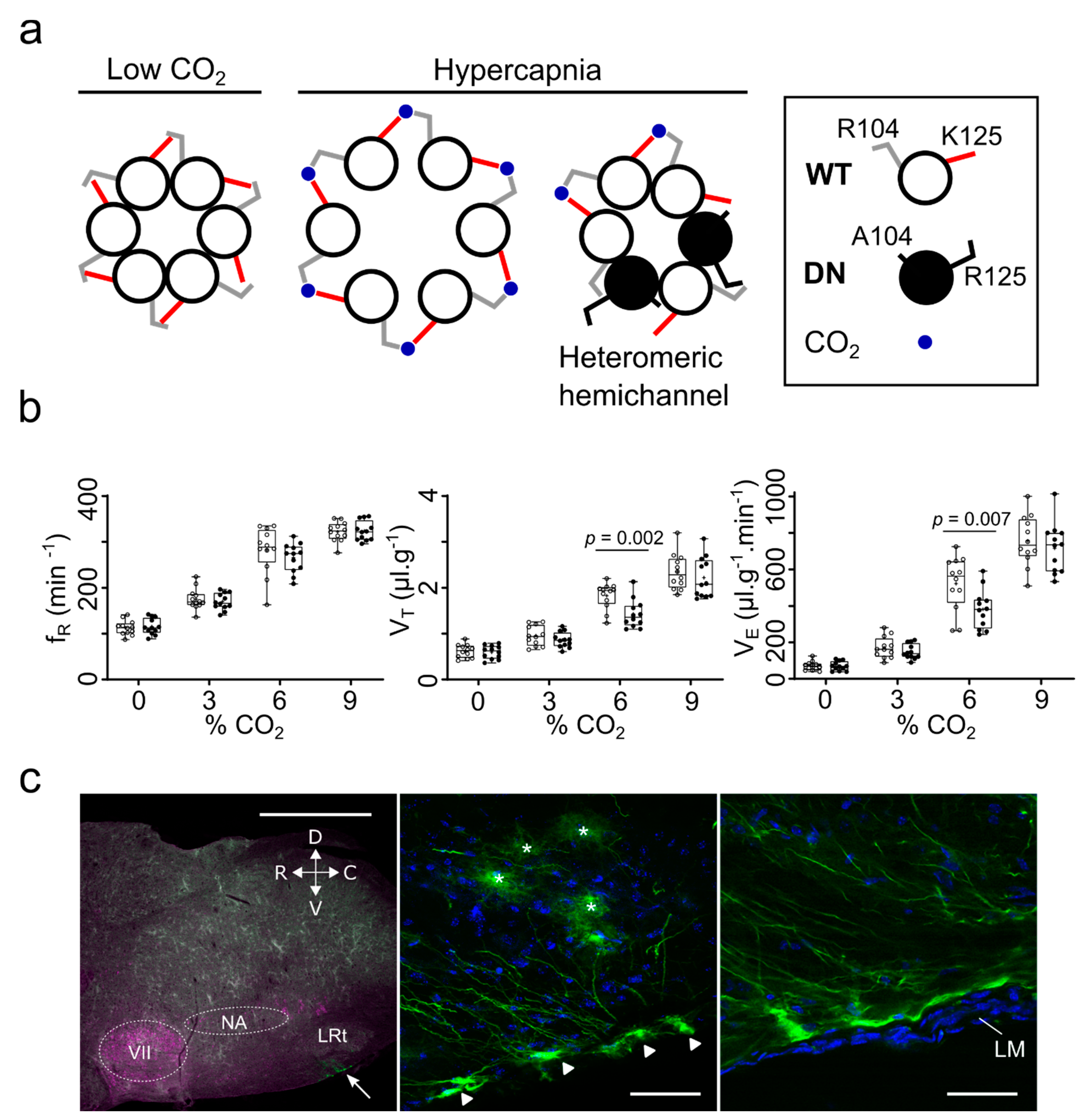
CO2 Sensing by Connexins
2. Physiological Consequences of Cx26-Mediated CO2 Sensitivity
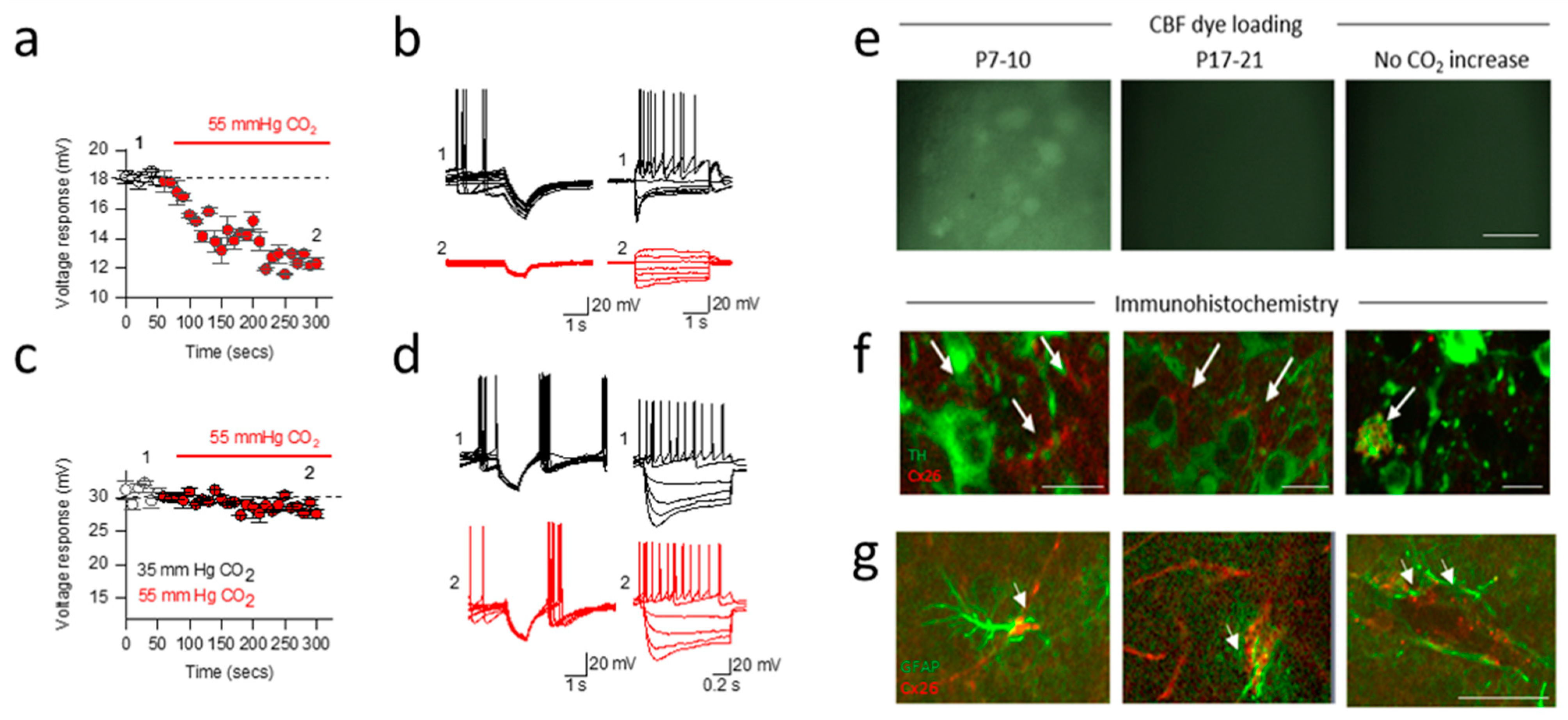
2.1. Cx26 in Glial Cells
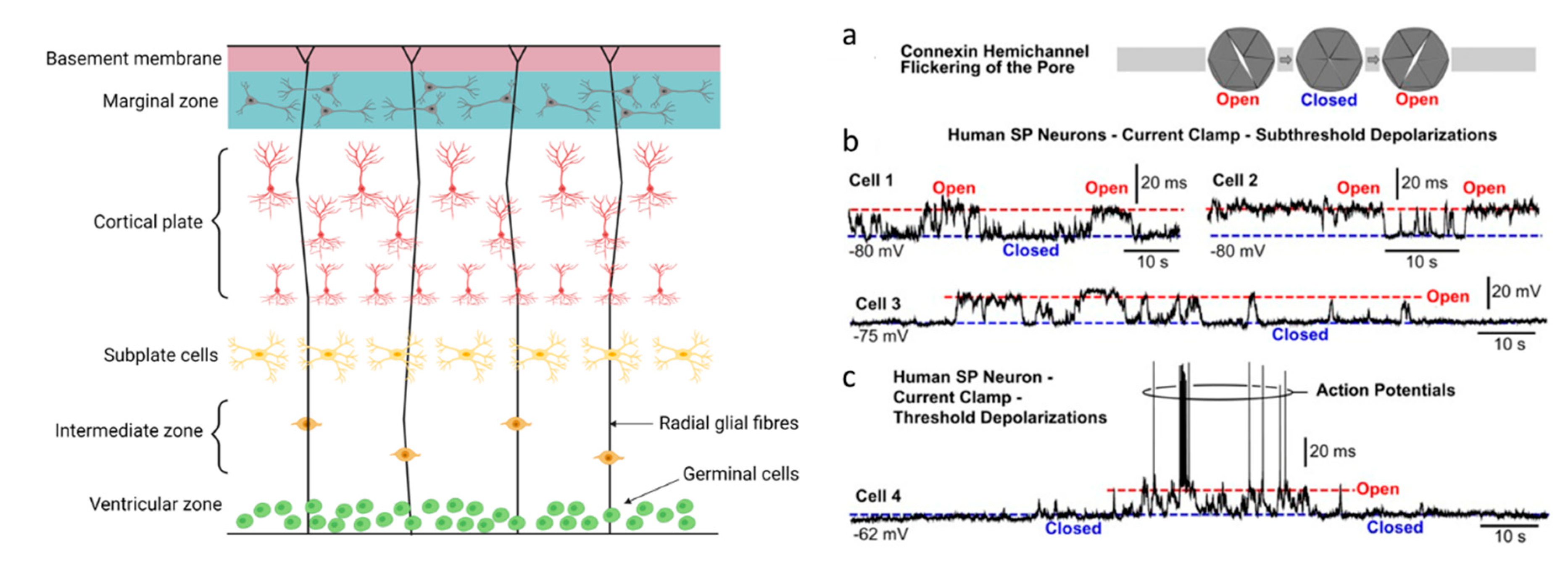
2.2. Cx26 and Cx32 Are Expressed in Developing Cerebral Cortical Neurons
2.3. Cx26 in Adult Neurons
2.4. Developmental Shift in Cx26 Expression Mediates a Change in Signalling in the Substantia Nigra
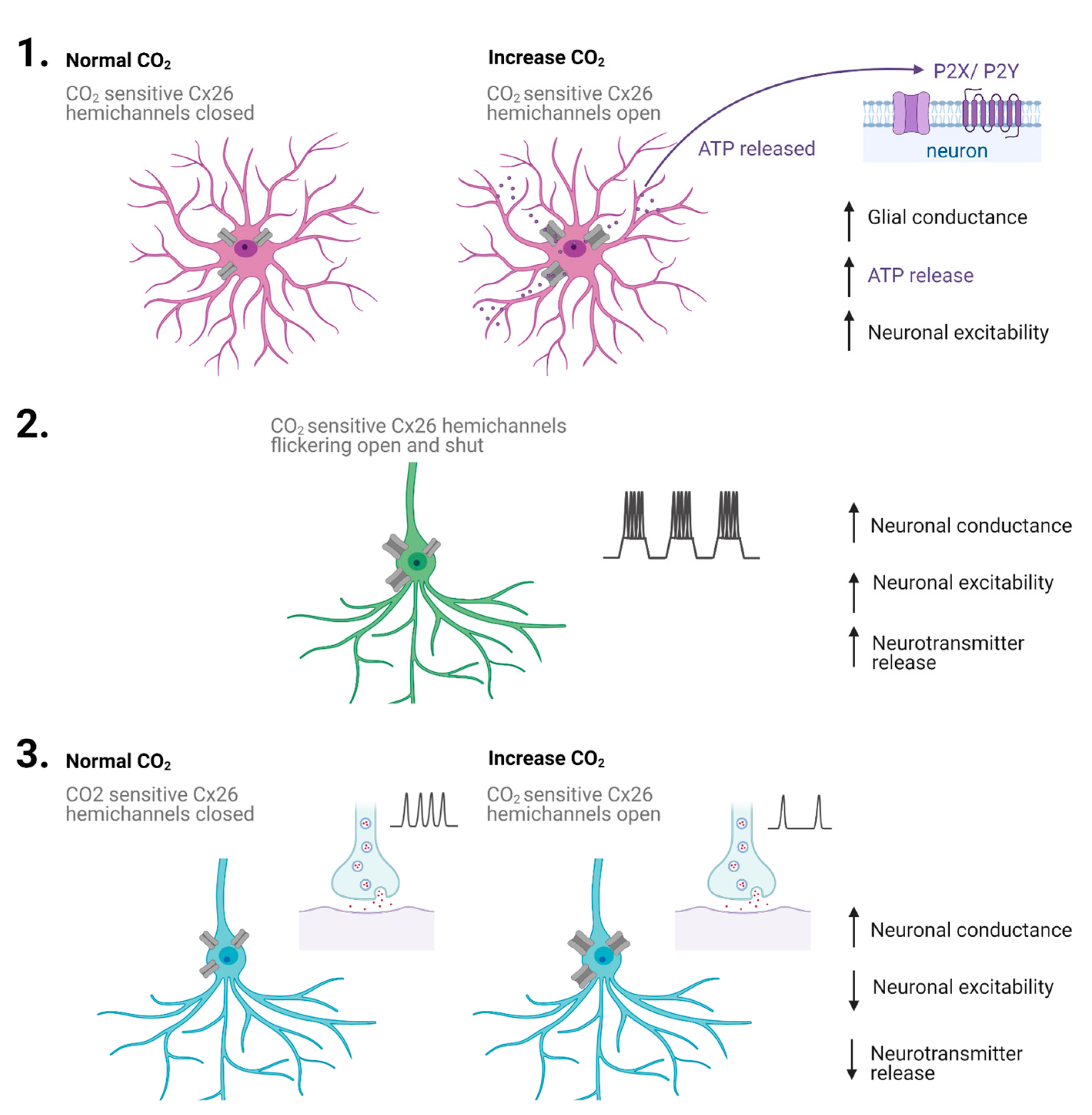
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Owens, R.L.; Malhotra, A. Sleep-disordered breathing and COPD: The overlap syndrome. Respir. Care 2010, 55, 1333–1346. [Google Scholar] [PubMed]
- Van Thienen, R.; Hespel, P. Enhanced muscular oxygen extraction in athletes exaggerates hypoxemia during exercise in hypoxia. J. Appl. Physiol. 2016, 120, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Gourine, A.V.; Kasymov, V.; Marina, N.; Tang, F.; Figueiredo, M.F.; Lane, S.; Teschemacher, A.G.; Spyer, K.M.; Deisseroth, K.; Kasparov, S. Astrocytes Control Breathing Through pH-Dependent Release of ATP. Science 2010, 329, 571–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosford, P.S.; Mosienko, V.; Kishi, K.; Jurisic, G.; Seuwen, K.; Kinzel, B.; Ludwig, M.G.; Wells, J.A.; Christie, I.N.; Koolen, L.; et al. CNS distribution, signalling properties and central effects of G-protein coupled receptor 4. Neuropharmacology 2018, 138, 381–392. [Google Scholar] [CrossRef]
- Kumar, S.; Tepper, K.; Kaniyappan, S.; Biernat, J.; Wegmann, S.; Mandelkow, E.-M.; Müller, D.J.; Mandelkow, E. Stages and Conformations of the Tau Repeat Domain during Aggregation and Its Effect on Neuronal Toxicity. J. Biol. Chem. 2014, 289, 20318–20332. [Google Scholar] [CrossRef] [Green Version]
- Loeschcke, H.H. Central chemosensitivity and the reaction theory. J. Physiol. 1982, 332, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Trapp, S.; Aller, M.I.; Wisden, W.; Gourine, A.V. A Role for TASK-1 (KCNK3) Channels in the Chemosensory Control of Breathing. J. Neurosci. 2008, 28, 8844–8850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Benamer, N.; Zanella, S.; Kumar, N.N.; Shi, Y.; Bévengut, M.; Penton, D.; Guyenet, P.G.; Lesage, F.; Gestreau, C.; et al. TASK-2 Channels Contribute to pH Sensitivity of Retrotrapezoid Nucleus Chemoreceptor Neurons. J. Neurosci. 2013, 33, 16033–16044. [Google Scholar] [CrossRef] [Green Version]
- Eldridge, F.L.; Kiley, J.P.; Millhorn, D.E. Respiratory responses to medullary hydrogen ion changes in cats: Different effects of respiratory and metabolic acidoses. J. Physiol. 1985, 358, 285–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shams, H. Differential effects of CO2 and H+ as central stimuli of respiration in the cat. J. Appl. Physiol. 1985, 58, 357–364. [Google Scholar] [CrossRef]
- Dale, N. Dynamic ATP signalling and neural development. J. Physiol. 2008, 586, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Huckstepp, R.T.R.; Eason, R.; Sachdev, A.; Dale, N. CO2-dependent opening of connexin 26 and related β connexins. J. Physiol. 2010, 588, 3921–3931. [Google Scholar] [CrossRef]
- Pearson, R.A.; Dale, N.; Llaudet, E.; Mobbs, P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron 2005, 46, 731–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stout, C.E.; Costantin, J.L.; Naus, C.C.G.; Charles, A.C. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 2002, 277, 10482–10488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissman, T.A.; Riquelme, P.A.; Ivic, L.; Flint, A.C.; Kriegstein, A.R. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron 2004, 43, 647–661. [Google Scholar] [CrossRef] [Green Version]
- Moore, A.R.; Zhou, W.-L.; Sirois, C.L.; Belinsky, G.S.; Zecevic, N.; Antic, S.D. Connexin hemichannels contribute to spontaneous electrical activity in the human fetal cortex. Proc. Natl. Acad. Sci. USA 2014, 111, E3919–E3928. [Google Scholar] [CrossRef] [Green Version]
- Hill, E.; Dale, N.; Wall, M.J. Moderate Changes in CO2 Modulate the Firing of Neurons in the VTA and Substantia Nigra. iScience 2020, 23, 101343. [Google Scholar] [CrossRef]
- de Wolf, E.; van de Wiel, J.; Cook, J.; Dale, N. Altered CO2 sensitivity of connexin26 mutant hemichannels in vitro. Physiol. Rep. 2016, 4, e13038. [Google Scholar] [CrossRef]
- Huckstepp, R.T.R.; Bihi, R.I.; Eason, R.; Spyer, K.M.; Dicke, N.; Willecke, K.; Marina, N.; Gourine, A.V.; Dale, N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J. Physiol. 2010, 588, 3901–3920. [Google Scholar] [CrossRef]
- Meigh, L.; Greenhalgh, S.A.; Rodgers, T.L.; Cann, M.J.; Roper, D.I.; Dale, N. CO2 directly modulates connexin 26 by formation of carbamate bridges between subunits. eLife 2013, 2, e01213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dospinescu, V.; Nijjar, S.; Spanos, F.; Cook, J.; de Wolf, E.; Biscotti, M.; Gerdol, M.; Dale, N. Structural determinants of CO2-sensitivity in the β connexin family suggested by evolutionary analysis. Commun. Biol. 2019, 2, 331. [Google Scholar] [CrossRef] [Green Version]
- Meigh, L.; Cook, D.; Zhang, J.; Dale, N. Rational design of new NO and redox sensitivity into connexin26 hemichannels. Open Biol. 2015, 5, 140208. [Google Scholar] [CrossRef] [Green Version]
- Brotherton, D.H.; Savva, C.G.; Ragan, T.J.; Linthwaite, V.L.; Cann, M.J.; Dale, N.; Cameron, A.D. Conformational changes and channel gating induced by CO2 binding to Connexin26. bioRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Nijjar, S.; Maddison, D.; Meigh, L.; de Wolf, E.; Rodgers, T.; Cann, M.J.; Dale, N. Opposing modulation of Cx26 gap junctions and hemichannels by CO2. J. Physiol. 2021, 599, 103–118. [Google Scholar] [CrossRef]
- Nagy, J.I.; Lynn, B.D.; Tress, O.; Willecke, K.; Rash, J.E. Connexin26 expression in brain parenchymal cells demonstrated by targeted connexin ablation in transgenic mice. Eur. J. Neurosci. 2011, 34, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Wiel, J.; Meigh, L.; Bhandare, A.; Cook, J.; Nijjar, S.; Huckstepp, R.; Dale, N. Connexin26 mediates CO2-dependent regulation of breathing via glial cells of the medulla oblongata. Commun. Biol. 2020, 3, 521. [Google Scholar] [CrossRef]
- Gourine, A.V.; Llaudet, E.; Dale, N.; Spyer, K.M. ATP is a mediator of chemosensory transduction in the central nervous system. Nature 2005, 436, 108–111. [Google Scholar] [CrossRef]
- Dale, N. CO2 sensing by connexin26 and its role in the control of breathing. Interface Focus 2021, 11, 20200029. [Google Scholar] [CrossRef]
- Nadarajah, B.; Jones, A.; Evans, W.; Parnavelas, J. Differential Expression of Connexins during Neocortical Development and Neuronal Circuit Formation. J. Neurosci. 1997, 17, 3096–3111. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; White, J.; McKimm, E.; Milosevic, M.; Antic, S. Mechanisms of Spontaneous Electrical Activity in the Developing Cerebral Cortex—Mouse Subplate Zone. Cereb. Cortex 2018, 29, 3363–3379. [Google Scholar] [CrossRef] [PubMed]
- Kocovic, D.; Limaye, P.; Colburn, L.; Singh, M.; Milosevic, M.; Tadic, J.; Petronijevic, M.; Vrzic-Petronijevic, S.; Andjus, P.; Antic, S. Cadmium versus Lanthanum Effects on Spontaneous Electrical Activity and Expression of Connexin Isoforms Cx26, Cx36, and Cx45 in the Human Fetal Cortex. Cereb. Cortex 2019, 30, 1244–1259. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Arce, F.T.; Ramachandran, S.; Lal, R. Nanomechanics of hemichannel conformations: Connexin flexibility underlying channel opening and closing. J. Biol. Chem. 2006, 281, 23207–23217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mlinar, B.; Enyeart, J. Block of current through T-type calcium channels by trivalent metal cations and nickel in neural rat and human cells. J. Physiol. 1993, 469, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.E.; Saez, J.C.; Bukauskas, F.F.; Bennett, M.V. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. USA 2003, 100, 11388–11393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, B.; Zonta, F.; Farkas, T.; Litman, T.; Nielsen, M.; MacAulay, N. Structural determinants underlying permeant discrimination of the Cx43 hemichannel. J. Biol. Chem. 2019, 294, 16789–16803. [Google Scholar] [CrossRef]
- Su, X.; Chen, J.; Liu, L.; Huang, Q.; Zhang, L.; Li, X.; He, X.; Lu, W.; Sun, S.; Li, H.; et al. Neonatal Cx26 removal impairs neocortical development and leads to elevated anxiety. Proc. Natl. Acad. Sci. USA 2017, 114, 3228–3233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grace, A.A.; Bunney, B.S. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons-3. Evidence for electrotonic coupling. Neuroscience 1983, 10, 333–348. [Google Scholar] [CrossRef]
- Vandecasteele, M.; Glowinski, J.; Venance, L. Electrical Synapses between Dopaminergic Neurons of the Substantia Nigra Pars Compacta. J. Neurosci. 2005, 25, 291–298. [Google Scholar] [CrossRef]
- Vandecasteele, M.; Deniau, J.-M.; Glowinski, J.; Venance, L. Electrical synapses in basal ganglia. Rev. Neurosci. 2007, 18, 15–35. [Google Scholar] [CrossRef]
- Vandecasteele, M.; Glowinski, J.; Venance, L. Connexin mRNA expression in single dopaminergic neurons of substantia nigra pars compacta. Neurosci. Res. 2006, 56, 419–426. [Google Scholar] [CrossRef]
- Hill, E.; Dale, N.; Wall, M.J. Detecting CO2-Sensitive Hemichannels in Neurons in Acute Brain Slices. STAR Protoc. 2020, 1, 100139. [Google Scholar] [CrossRef]
- Bouarab, C.; Thompson, B.; Polter, A.M. VTA GABA Neurons at the Interface of Stress and Reward. Front. Neural Circuits 2019, 13, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, M.; Root, D.H. Glutamate neurons within the midbrain dopamine regions. Neuroscience 2014, 282, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Root, D.H.; Estrin, D.J.; Morales, M. Aversion or Salience Signaling by Ventral Tegmental Area Glutamate Neurons. iScience 2018, 2, 51–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takata, Y.; Oishi, Y.; Zhou, X.-Z.; Hasegawa, E.; Takahashi, K.; Cherasse, Y.; Sakurai, T.; Lazarus, M. Sleep and Wakefulness Are Controlled by Ventral Medial Midbrain/Pons GABAergic Neurons in Mice. J. Neurosci. 2018, 38, 10080–10092. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.H.; Zell, V.; Gutierrez-Reed, N.; Wu, J.; Ressler, R.; Shenasa, M.A.; Johnson, A.B.; Fife, K.H.; Faget, L.; Hnasko, T.S. Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat. Commun. 2016, 7, 13697. [Google Scholar] [CrossRef] [PubMed]
- Eshel, N.; Bukwich, M.; Rao, V.; Hemmelder, V.; Tian, J.; Uchida, N. Arithmetic and local circuitry underlying dopamine prediction errors. Nature 2015, 525, 243–246. [Google Scholar] [CrossRef]
- Eban-Rothschild, A.; Rothschild, G.; Giardino, W.J.; Jones, J.R.; de Lecea, L. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat. Neurosci. 2016, 19, 1356–1366. [Google Scholar] [CrossRef]
- Fifel, K.; Meijer, J.H.; Deboer, T. Circadian and Homeostatic Modulation of Multi-Unit Activity in Midbrain Dopaminergic Structures. Sci. Rep. 2018, 8, 7765. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.V.; Petko, A.K.; Paladini, C.A. Differential expression of long-term potentiation among identified inhibitory inputs to dopamine neurons. J. Neurophysiol. 2017, 118, 1998–2008. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.R.; Yvon, C.; Turiault, M.; Mirzabekov, J.J.; Doehner, J.; Labouèbe, G.; Deisseroth, K.; Tye, K.M.; Lüscher, C. GABA neurons of the VTA drive conditioned place aversion. Neuron 2012, 73, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, W.; Ma, Y.; Tossell, K.; Harris, J.J.; Harding, E.C.; Ba, W.; Miracca, G.; Wang, D.; Li, L.; et al. GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nat. Neurosci. 2019, 22, 106–119. [Google Scholar] [CrossRef] [PubMed]
- van Zessen, R.; Phillips, J.; Budygin, E.; Stuber, G. Activation of VTA GABA Neurons Disrupts Reward Consumption. Neuron 2012, 73, 1184–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, E.; Dale, N.; Wall, M.J. CO2-Sensitive Connexin Hemichannels in Neurons and Glia: Three Different Modes of Signalling? Int. J. Mol. Sci. 2021, 22, 7254. https://doi.org/10.3390/ijms22147254
Hill E, Dale N, Wall MJ. CO2-Sensitive Connexin Hemichannels in Neurons and Glia: Three Different Modes of Signalling? International Journal of Molecular Sciences. 2021; 22(14):7254. https://doi.org/10.3390/ijms22147254
Chicago/Turabian StyleHill, Emily, Nicholas Dale, and Mark J. Wall. 2021. "CO2-Sensitive Connexin Hemichannels in Neurons and Glia: Three Different Modes of Signalling?" International Journal of Molecular Sciences 22, no. 14: 7254. https://doi.org/10.3390/ijms22147254
APA StyleHill, E., Dale, N., & Wall, M. J. (2021). CO2-Sensitive Connexin Hemichannels in Neurons and Glia: Three Different Modes of Signalling? International Journal of Molecular Sciences, 22(14), 7254. https://doi.org/10.3390/ijms22147254







