Metabolite Profiling and Transcriptome Analysis Provide Insight into Seed Coat Color in Brassica juncea
Abstract
:1. Introduction
2. Results
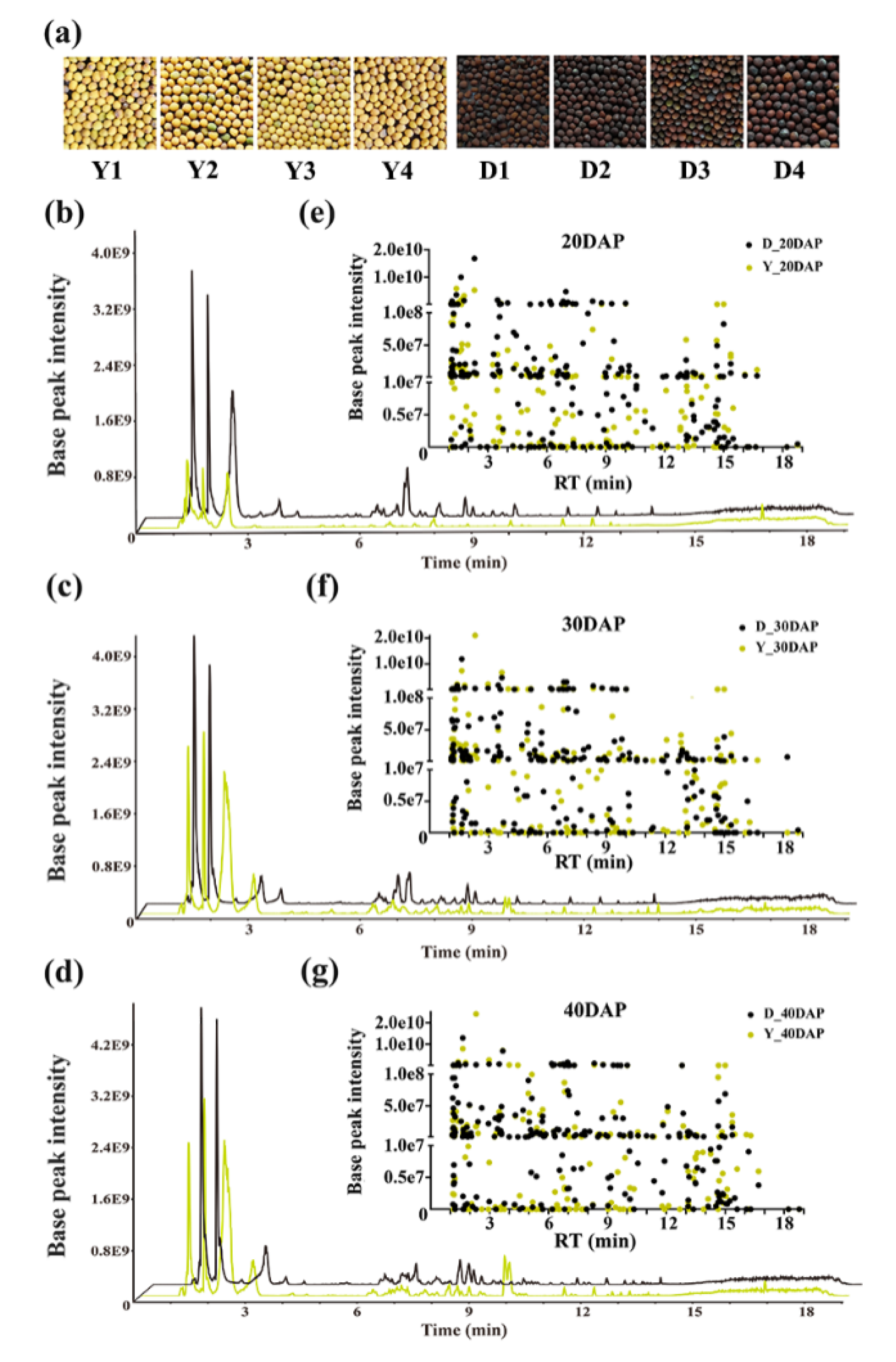
2.1. Metabolite Profiling of Developing B. juncea Seeds
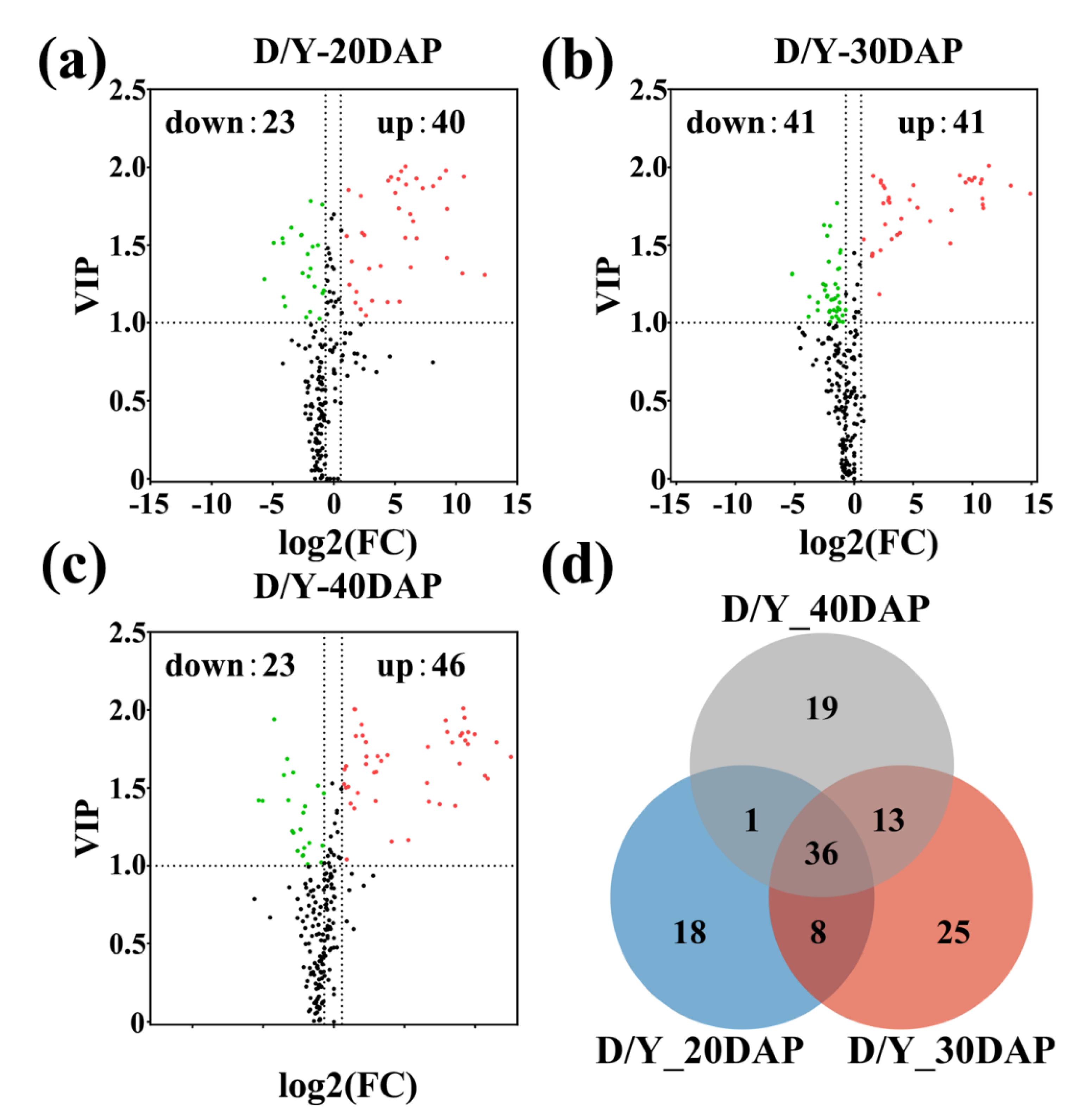
2.2. Differential Metabolic Profiling Analysis Based on OPLS-DA
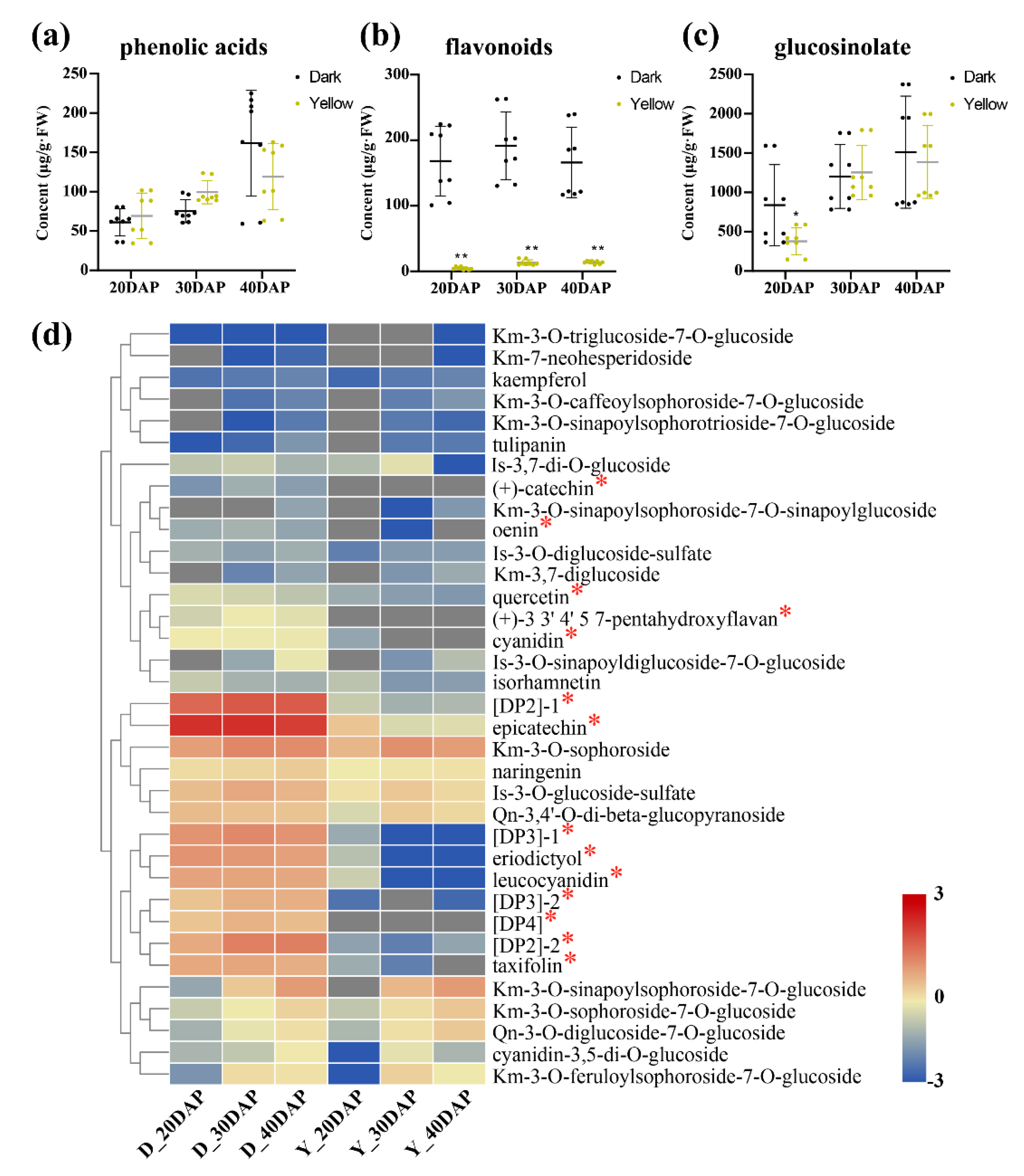
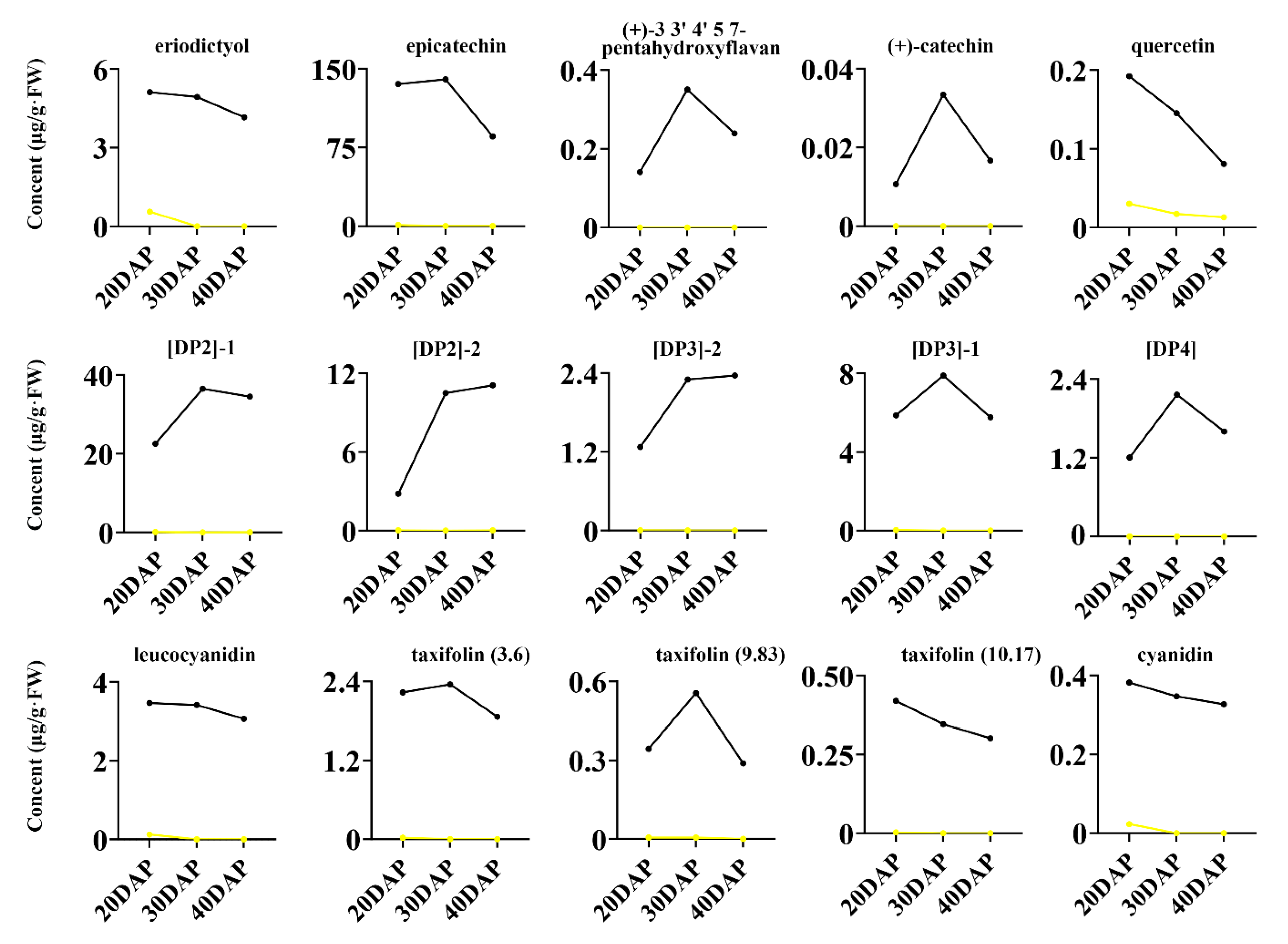
2.3. Metabolite Accumulation Patterns
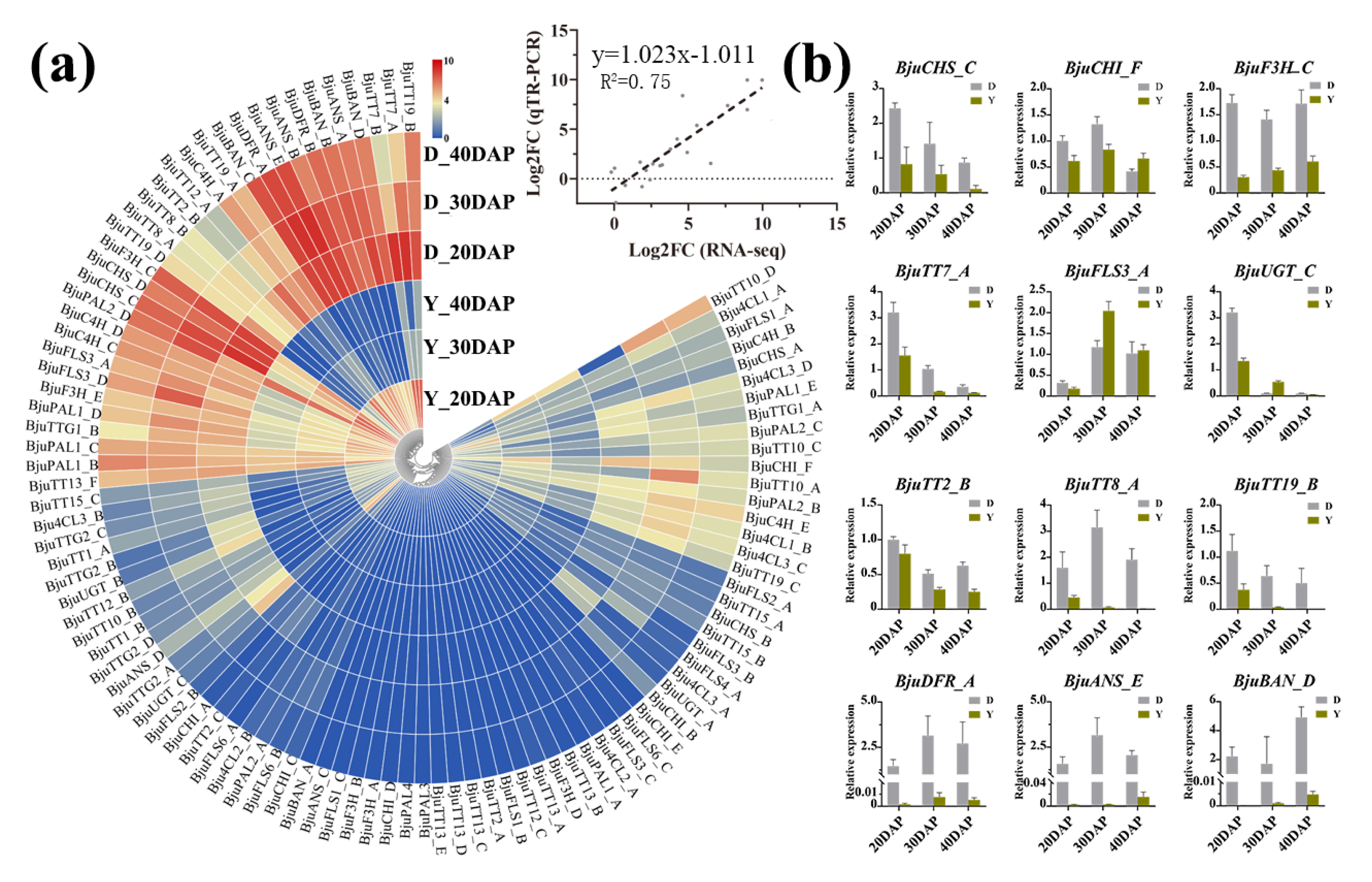
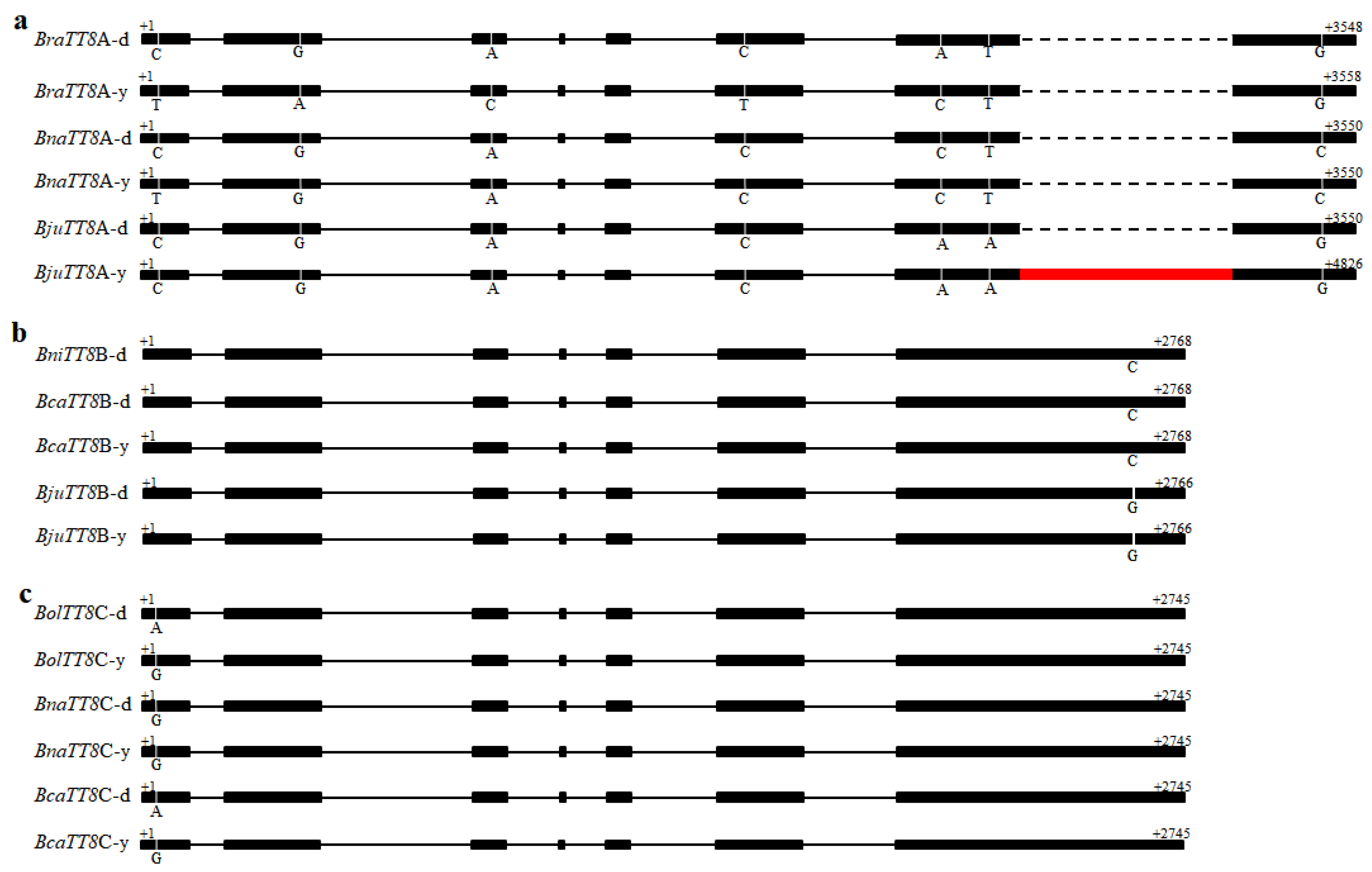
2.4. Transcriptome Analysis of Flavonoid Biosynthesis Genes in B. juncea
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Culture Conditions
4.2. Chemicals and Calibration Curve
4.3. Metabolite Extraction and Analysis
4.4. Statistical Analysis
4.5. Identification of Flavonoid Biosynthesis Genes in B. juncea
4.6. Transcriptome Sequencing Analysis of Differentially Expressed Genes in the Flavonoid Pathway
4.7. Reverse-Transcription Quantitative PCR (RT-qPCR) Analysis
4.8. Gene Cloning and Multi-Sequence Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.H.; Liu, D.Y.; Wang, X.W.; Ji, C.M.; Cheng, F.; Liu, B.N.; Hu, Z.Y.; Chen, S.; Pental, D.; Ju, Y.H.; et al. The genome sequence of allopolyploid Brassica juncea and analysis of differential homoeolog gene expression influencing selection. Nat. Genet. 2018, 50, 1616. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Chen, B.; Lu, G.; Wang, H.; Xu, K.; Gao, G.; Song, Y. Genetic diversity in oil and vegetable mustard (Brassica juncea) landraces revealed by SRAP markers. Genet. Resour. Crop Evol. 2009, 56, 1011. [Google Scholar] [CrossRef]
- Gladis, T.; Hammer, K. Die Gaterslebener Brassica-Kollektion—Brassica juncea, B. napus, B. nigra und B. rapa. Feddes Repert. 1992, 103, 469–507. [Google Scholar] [CrossRef]
- Oram, R.; Kirk, J.; Veness, P.; Hurlstone, C.; Edlington, J.; Halsall, D. Breeding Indian mustard [Brassica juncea (L.) Czern.] for cold-pressed, edible oil production—A review. Aust. J. Agric. Res. 2005, 56, 581–596. [Google Scholar] [CrossRef]
- Liu, Z.; Guan, C.; Chen, S.; Liu, S.; Yang, L. Transfer of superior traits from Brassica juncea into Brassica napus. Agric. Sci. Technol. Hunan 2010, 11, 49–52. [Google Scholar]
- Yan, M.; Liu, Z.; Guan, C.; Chen, S.; Yuan, M.; Liu, X. Inheritance and molecular markers for the seed coat color in Brassica juncea. Front. Agric. China 2009, 3, 1–6. [Google Scholar] [CrossRef]
- Hong, M.; Hu, K.; Tian, T.; Li, X.; Chen, L.; Zhang, Y.; Yi, B.; Wen, J.; Ma, C.; Shen, J.; et al. Transcriptomic Analysis of Seed Coats in Yellow-Seeded Brassica napus Reveals Novel Genes That Influence Proanthocyanidin Biosynthesis. Front. Plant Sci. 2017, 8, 1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padmaja, L.K.; Agarwal, P.; Gupta, V.; Mukhopadhyay, A.; Sodhi, Y.S.; Pental, D.; Pradhan, A.K. Natural mutations in two homoeologous TT8 genes control yellow seed coat trait in allotetraploid Brassica juncea (AABB). Theor. Appl. Genet. 2014, 127, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Akhov, L.; Ashe, P.; Tan, Y.; Datla, R.; Selvaraj, G. Proanthocyanidin biosynthesis in the seed coat of yellow-seeded, canola quality Brassica napus YN01-429 is constrained at the committed step catalyzed by dihydroflavonol 4-reductase. Botany 2009, 87, 616–625. [Google Scholar] [CrossRef] [Green Version]
- Auger, B.; Marnet, N.; Gautier, V.; Maia-Grondard, A.; Leprince, F.; Renard, M.; Guyot, S.; Nesi, N.; Routaboul, J.M. A detailed survey of seed coat flavonoids in developing seeds of Brassica napus L. J. Agric. Food Chem. 2010, 58, 6246–6256. [Google Scholar] [CrossRef]
- Jiang, J.; Shao, Y.; Li, A.; Lu, C.; Zhang, Y.; Wang, Y. Phenolic composition analysis and gene expression in developing seeds of yellow-and black-seeded Brassica napus. J. Integr. Plant Biol. 2013, 55, 537–551. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Hong, M.; Zhang, Y.; Zu, F.; Wen, J.; Yi, B.; Ma, C.; Shen, J.; Tu, J. A large insertion in bHLH transcription factor BrTT8 resulting in yellow seed coat in Brassica rapa. PLoS ONE 2012, 7, e44145. [Google Scholar] [CrossRef]
- Qu, C.; Fu, F.; Lu, K.; Zhang, K.; Wang, R.; Xu, X.; Wang, M.; Lu, J.; Wan, H.; Zhanglin, T.; et al. Differential accumulation of phenolic compounds and expression of related genes in black- and yellow-seeded Brassica napus. J. Exp. Bot. 2013, 64, 2885–2898. [Google Scholar] [CrossRef] [Green Version]
- Qu, C.; Yin, N.; Chen, S.; Wang, S.; Chen, X.; Zhao, H.; Shen, S.; Fu, F.; Zhou, B.; Xu, X.; et al. Comparative Analysis of the Metabolic Profiles of Yellow- versus Black-Seeded Rapeseed Using UPLC-HESI-MS/MS and Transcriptome Analysis. J. Agric. Food Chem. 2020, 68, 3033–3049. [Google Scholar] [CrossRef]
- Wang, J.; Xian, X.; Xu, X.; Qu, C.; Lu, K.; Li, J.; Liu, L. Genome-Wide Association Mapping of Seed Coat Color in Brassica napus. J. Agric. Food Chem. 2017, 65, 5229–5237. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, G.S.; Chen, S.L.; Chen, Y.J.; Jiang, J.J.; Wang, Y.P. Correlation Analysis of Phenolic Contents and Antioxidation in Yellow- and Black-Seeded Brassica napus. Molecules 2018, 23, 1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.; Huang, T.; Tian, B.; Zhan, J. Advances in Biosynthesis and Biological Functions of Proanthocyanidins in Horticultural Plants. Foods 2020, 9, 1774. [Google Scholar] [CrossRef] [PubMed]
- Lepiniec, L.; Debeaujon, I.; Routaboul, J.M.; Baudry, A.; Pourcel, L.; Nesi, N.; Caboche, M. Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol. 2006, 57, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, B.; Zhang, G.; Chen, L.; Dong, Q.; Wen, J.; Mysore, K.S.; Zhao, J. Regulation of anthocyanin and proanthocyanidin biosynthesis by Medicago truncatula bHLH transcription factor MtTT8. New Phytol. 2016, 210, 905–921. [Google Scholar] [CrossRef]
- Pang, Y.; Peel, G.J.; Wright, E.; Wang, Z.; Dixon, R.A. Early steps in proanthocyanidin biosynthesis in the model legume Medicago truncatula. Plant Physiol. 2007, 145, 601–615. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Li, X.; Jia, J.; Yuan, G.; Chen, S.; Qi, D.; Cheng, L.; Liu, G. bHLH92 from sheepgrass acts as a negative regulator of anthocyanin/proanthocyandin accumulation and influences seed dormancy. J. Exp. Bot. 2019, 70, 269–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Reichelt, M.; Hidalgo, W.; Agnolet, S.; Schneider, B. Tissue-specific distribution of secondary metabolites in rapeseed (Brassica napus L.). PLoS ONE 2012, 7, e48006. [Google Scholar] [CrossRef]
- Llorach, R.; Gil-Izquierdo, A.; Ferreres, F.; Tomás-Barberán, F.A. HPLC-DAD-MS/MS ESI Characterization of Unusual Highly Glycosylated Acylated Flavonoids from Cauliflower (Brassica oleracea L. var. botrytis) Agroindustrial Byproducts. J. Agric. Food Chem. 2003, 51, 3895–3899. [Google Scholar] [CrossRef] [PubMed]
- Harbaum, B.; Hubbermann, E.M.; Wolff, C.; Herges, R.; Zhu, Z.; Schwarz, K. Identification of flavonoids and hydroxycinnamic acids in pak choi varieties (Brassica campestris L. ssp. chinensis var. communis) by HPLC-ESI-MSn and NMR and their quantification by HPLC-DAD. J. Agric. Food Chem. 2007, 55, 8251–8260. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.W.; Wang, S.X.; Jia, L.D.; Zhu, M.C.; Yang, J.; Zhou, B.J.; Yin, J.M.; Lu, K.; Wang, R.; Li, J.N.; et al. Identification and Characterization of Major Constituents in Different-Colored Rapeseed Petals by UPLC-HESI-MS/MS. J. Agric. Food Chem. 2019, 67, 11053–11065. [Google Scholar] [CrossRef]
- Bajpai, P.K.; Reichelt, M.; Augustine, R.; Gershenzon, J.; Bisht, N.C. Heterotic patterns of primary and secondary metabolites in the oilseed crop Brassica juncea. Heredity 2019, 123, 318–336. [Google Scholar] [CrossRef]
- Lin, L.Z.; Sun, J.H.; Chen, P.; Harnly, J. UHPLC-PDA-ESI/HRMS/MSn Analysis of Anthocyanins, Flavonol Glycosides, and Hydroxycinnamic Acid Derivatives in Red Mustard Greens (Brassica juncea Coss Variety). J. Agric. Food Chem. 2011, 59, 12059–12072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tohge, T.; Souza, L.P.; Fernie, A.R. Current understanding of the pathways of flavonoid biosynthesis in model and crop plants. J. Exp. Bot. 2017, 68, 4013–4028. [Google Scholar] [CrossRef]
- Appelhagen, I.; Thiedig, K.; Nordholt, N.; Schmidt, N.; Huep, G.; Sagasser, M.; Weisshaar, B. Update on transparent testa mutants from Arabidopsis thaliana: Characterisation of new alleles from an isogenic collection. Planta 2014, 240, 955–970. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, M.B.; Noel, J.P. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 2003, 20, 79. [Google Scholar] [CrossRef]
- Jiang, W.; Yin, Q.; Wu, R.; Zheng, G.; Liu, J.; Dixon, R.A.; Pang, Y. Role of a chalcone isomerase-like protein in flavonoid biosynthesis in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 7165–7179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenbohm, C.; Martens, S.; Eder, C.; Forkmann, G.; Weisshaar, B. Identification of the Arabidopsis thaliana flavonoid 3′-hydroxylase gene and functional expression of the encoded P450 enzyme. Biol. Chem. 2000, 381, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Nesi, N.; Debeaujon, I.; Jond, C.; Pelletier, G.; Caboche, M.; Lepiniec, L. The TT8 gene encodes a basic helix-loop-helix domain protein required for expression of DFR and BAN genes in Arabidopsis siliques. Plant Cell 2000, 12, 1863–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Lu, Y.; Yuan, Y.; Liu, S.; Guan, C.; Chen, S.; Liu, Z. De novo transcriptome of Brassica juncea seed coat and identification of genes for the biosynthesis of flavonoids. PLoS ONE 2013, 8, e71110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saigo, T.; Wang, T.; Watanabe, M.; Tohge, T. Diversity of anthocyanin and proanthocyanin biosynthesis in land plants. Curr. Opin. Plant Biol. 2020, 55, 93–99. [Google Scholar] [CrossRef]
- Zou, S.; Wu, J.; Shahid, M.Q.; He, Y.; Lin, S.; Liu, Z.; Yang, X. Identification of key taste components in loquat using widely targeted metabolomics. Food Chem. 2020, 323, 126822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Liu, L.L.; Zhou, D.G.; Liu, X.J.; Liu, Z.S.; Yan, M.L. Genome-wide identification and expression analysis of anthocyanin biosynthetic genes in Brassica juncea. J. Integr. Agric. 2020, 19, 1250–1260. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colanero, S.; Perata, P.; Gonzali, S. The Gene Encodes an R3-MYB Protein Repressing Anthocyanin Synthesis in Tomato Plants. Front. Plant Sci. 2018, 9, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Gao, Y.; Xie, W.; Gong, L.; Lu, K.; Wang, W.; Li, Y.; Liu, X.; Zhang, H.; Dong, H.; et al. Genome-wide association analyses provide genetic and biochemical insights into natural variation in rice metabolism. Nat. Genet. 2014, 46, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, G.; Li, L.; Li, Y.; Zhou, B.; Yan, H. Identification and expression analysis of BrTT8 during anthocyanin biosynthesis and exposure to abiotic stress in turnip (Brassica rapa subsp. rapa ‘Tsuda’). Sci. Hort. 2020, 268, 109332. [Google Scholar] [CrossRef]
- Onkokesung, N.; Reichelt, M.; Doorn, A.; Schuurink, R.C.; Loon, J.J.A.; Dicke, M. Modulation of flavonoid metabolites in Arabidopsis thaliana through overexpression of the MYB75 transcription factor: Role of kaempferol-3,7-dirhamnoside in resistance to the specialist insect herbivore Pieris brassicae. J. Exp. Bot. 2014, 65, 2203–2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008, 53, 814–827. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Huang, J. Arabidopsis TT19 functions as a carrier to transport anthocyanin from the cytosol to tonoplasts. Mol. Plant 2012, 5, 387–400. [Google Scholar] [CrossRef]
- Xu, W.; Bobet, S.; Gourrierec, J.; Grain, D.; Vos, D.; Berger, A.; Salsac, F.; Kelemen, Z.; Boucherez, J.; Rolland, A.; et al. TRANSPARENT TESTA 16 and 15 act through different mechanisms to control proanthocyanidin accumulation in Arabidopsis testa. J. Exp. Bot. 2017, 68, 2859–2870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debeaujon, I.; Peeters, A.J.M.; Léon-Kloosterziel, K.M.; Koornneef, M. The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 2001, 13, 853–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Su, J.; Zhu, Y.; Yao, G.; Allan, A.C.; Ampomah-Dwamena, C.; Shu, Q.; Kui, L.; Zhang, S.; Wu, J. The involvement of PybZIPa in light-induced anthocyanin accumulation via the activation of PyUFGT through binding to tandem G-boxes in its promoter. Hortic. Res. 2019, 6, 134. [Google Scholar] [CrossRef] [Green Version]
- Ying, S.; Su, M.; Wu, Y.; Zhou, L.; Fu, R.; Li, Y.; Guo, H.; Luo, J.; Wang, S.; Zhang, Y. Trichome regulator SlMIXTA-like directly manipulates primary metabolism in tomato fruit. Plant Biotechnol. J. 2020, 18, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Nishiyama, Y.; Hirai, M.Y.; Yano, M.; Nakajima, J.; Awazuhara, M.; Inoue, E.; Takahashi, H.; Goodenowe, D.B.; Kitayama, M.; et al. Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant J. 2005, 42, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Kim, J.K.; Kim, H.; Kim, Y.J.; Park, Y.J.; Kim, S.J.; Kim, C.; Park, S.U. Transcriptome analysis and metabolic profiling of green and red kale (Brassica oleracea var. acephala) seedlings. Food Chem. 2018, 241, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.; Yang, X.; Tong, C.; Edwards, D.; Parkin, I.A.P.; Zhao, M.; Ma, J.; Yu, J.; Huang, S.; et al. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 2014, 5, 3930. [Google Scholar] [CrossRef] [PubMed]
- Paritosh, K.; Yadava, S.K.; Singh, P.; Bhayana, L.; Mukhopadhyay, A.; Gupta, V.; Bisht, N.C.; Zhang, J.; Kudrna, D.A.; Copetti, D.; et al. A chromosome-scale assembly of allotetraploid Brassica juncea (AABB) elucidates comparative architecture of the A and B genomes. Plant Biotechnol. J. 2020, 19, 602–614. [Google Scholar] [CrossRef]
- Qu, C.; Zhao, H.; Fu, F.; Wang, Z.; Zhang, K.; Zhou, Y.; Wang, X.; Wang, R.; Xu, X.; Tang, Z.; et al. Genome-Wide Survey of Flavonoid Biosynthesis Genes and Gene Expression Analysis between Black- and Yellow-Seeded Brassica napus. Front. Plant Sci. 2016, 7, 1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, M.L.; Liu, X.J.; Liu, Z.S.; Guan, C.Y.; Xiong, X.H. Cloning and Expression Analysis of Dihydroflavonol 4-Reductase Gene in Brassica juncea. Acta Agron. Sin. 2008, 34, 1–7. [Google Scholar]
- Kitamura, S.; Matsuda, F.; Tohge, T.; Yonekura-Sakakibara, K.; Yamazaki, M.; Saito, K.; Narumi, I. Metabolic profiling and cytological analysis of proanthocyanidins in immature seeds of Arabidopsis thaliana flavonoid accumulation mutants. Plant J. 2010, 62, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, P.; Cui, D.; Wu, L.; Parkin, I.; Saberianfar, R.; Menassa, R.; Pan, H.; Westcott, N.; Gruber, M.Y. The Arabidopsis tt19-4 mutant differentially accumulates proanthocyanidin and anthocyanin through a 3’ amino acid substitution in glutathione S-transferase. Plant Cell Environ. 2011, 34, 374–388. [Google Scholar] [CrossRef] [PubMed]
- Gratacós-Cubarsí, M.; Ribas-Agustí, A.; García-Regueiro, J.A.; Castellari, M. Simultaneous evaluation of intact glucosinolates and phenolic compounds by UPLC-DAD-MS/MS in Brassica oleracea L. var. botrytis. Food Chem. 2010, 121, 257–263. [Google Scholar] [CrossRef]
- Martínezsánchez, A.; Gilizquierdo, A.; Gil, M.I.; Ferreres, F.A. Comparative Study of Flavonoid Compounds, Vitamin C, and Antioxidant Properties of Baby Leaf Brassicaceae Species. J. Agric. Food Chem. 2008, 56, 2330. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Badr, A.; Desjardins, Y.; Gosselin, A.; Angers, P. Characterization of industrial broccoli discards (Brassica oleracea var. italica) for their glucosinolate, polyphenol and flavonoid contents using UPLC MS/MS and spectrophotometric methods. Food Chem. 2018, 245, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Nagaharu, U. Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilisation. J. Japan Bot. 1935, 7, 389–452. [Google Scholar]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Han, Y.; Vimolmangkang, S.; Soria-Guerra, R.E.; Rosales-Mendoza, S.; Zheng, D.; Lygin, A.V.; Korban, S.S. Ectopic expression of apple F3’H genes contributes to anthocyanin accumulation in the Arabidopsis tt7 mutant grown under nitrogen stress. Plant Physiol. 2010, 153, 806–820. [Google Scholar] [CrossRef] [Green Version]
- Fraser, C.M.; Chapple, C. The phenylpropanoid pathway in Arabidopsis. Arab. Book 2011, 9, e0152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, H.; Wang, J.; Sun, R.; Wu, J.; Liu, S.; Bai, Y.; Mun, J.H.; Bancroft, I.; Cheng, F.; et al. The genome of the mesopolyploid crop species Brassica rapa. Nat. Genet. 2011, 43, 1035–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Cai, X.; Wu, J.; Liu, M.; Grob, S.; Cheng, F.; Liang, J.; Cai, C.; Liu, Z.; Liu, B.; et al. Improved Brassica rapa reference genome by single-molecule sequencing and chromosome conformation capture technologies. Hortic. Res. 2018, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Perumal, S.; Koh, C.S.; Jin, L.; Buchwaldt, M.; Higgins, E.E.; Zheng, C.; Sankoff, D.; Robinson, S.J.; Kagale, S.; Navabi, Z.K.; et al. A high-contiguity Brassica nigra genome localizes active centromeres and defines the ancestral Brassica genome. Nat. Plants 2020, 6, 929–941. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, N.; Li, R.; Ma, X.; Zhang, L. Comparative transcriptome and flavonoids components analysis reveal the structural genes responsible for the yellow seed coat color of Brassica rapa L. PeerJ 2021, 9, e10770. [Google Scholar] [CrossRef]
- Zhai, Y.; Yu, K.; Cai, S.; Hu, L.; Amoo, O.; Xu, L.; Yang, Y.; Ma, B.; Jiao, Y.; Zhang, C.; et al. Targeted mutagenesis of BnTT8 homologs controls yellow seed coat development for effective oil production in Brassica napus L. Plant Biotechnol. J. 2020, 18, 1153–1168. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.U.; Park, J.I.; Jung, H.J.; Hur, Y.; Nou, I.S. Anthocyanin biosynthesis for cold and freezing stress tolerance and desirable color in Brassica rapa. Funct. Integr. Genomics 2015, 15, 383–394. [Google Scholar] [CrossRef]
- Mushtaq, M.A.; Pan, Q.; Chen, D.; Zhang, Q.; Ge, X.; Li, Z. Comparative Leaves Transcriptome Analysis Emphasizing on Accumulation of Anthocyanins in Brassica: Molecular Regulation and Potential Interaction with Photosynthesis. Front. Plant Sci. 2016, 7, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Hu, Z.; Zhu, M.; Zhu, Z.; Wang, Z.; Tian, S.; Chen, G. Anthocyanin Accumulation and Molecular Analysis of Correlated Genes in Purple Kohlrabi (Brassica oleracea var. gongylodes L.). J. Agric. Food Chem. 2015, 63, 4160–4169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Lu, Y.; Yuan, Y.X.; Zhang, X.W.; Geng, J.F.; Chen, Y.; Cloutier, S.; McVetty, P.B.E.; Li, G.Y. Map-based cloning and characterization of a gene controlling hairiness and seed coat color traits in Brassica rapa. Plant Mol. Biol. 2009, 69, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Seitz, C.; Ameres, S.; Schlangen, K.; Forkmann, G.; Halbwirth, H. Multiple evolution of flavonoid 3′,5′-hydroxylase. Planta 2015, 242, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Werck-Reichhart, D. A P450-centric view of plant evolution. Plant J. 2011, 66, 194–211. [Google Scholar] [CrossRef]
- Tsugawa, H.; Nakabayashi, R.; Mori, T.; Yamada, Y.; Takahashi, M.; Rai, A.; Sugiyama, R.; Yamamoto, H.; Nakaya, T.; Yamazaki, M.; et al. A cheminformatics approach to characterize metabolomes in stable-isotope-labeled organisms. Nat. Methods 2019, 16, 295–298. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.; Liu, Y.; Jiang, W.; Meng, X.; Zhang, W.; Chen, W.; Peng, D.; Xing, S. UPLC/MS-based untargeted metabolomics reveals the changes of metabolites profile of Salvia miltiorrhiza bunge during Sweating processing. Sci. Rep. 2020, 10, 19524. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Ye, L.; Kang, Z.; Jia, D.; Yang, L.; Zhang, B. LC-MS-Based Metabolomic Approach Revealed the Significantly Different Metabolic Profiles of Five Commercial Truffle Species. Front. Microbiol. 2019, 10, 2227. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Pucker, B.; Reiher, F.; Schilbert, H.M. Automatic Identification of Players in the Flavonoid Biosynthesis with Application on the Biomedicinal Plant Croton tiglium. Plants 2020, 9, 1103. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Alerding, A.B.; Crosby, K.C.; Bandara, A.B.; Westwood, J.H.; Winkel, B.S.J. Functional analysis of a predicted flavonol synthase gene family in Arabidopsis. Plant Physiol. 2008, 147, 1046–1061. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Chandna, R.; Augustine, R.; Bisht, N.C. Evaluation of Candidate Reference Genes for Gene Expression Normalization in Brassica juncea Using Real Time Quantitative RT-PCR. PLoS ONE 2012, 7, e36918. [Google Scholar] [CrossRef]
- Lu, K.; Li, T.; He, J.; Chang, W.; Zhang, R.; Liu, M.; Yu, M.N.; Fan, Y.H.; Ma, J.Q.; Sun, W.; et al. qPrimerDB: A thermodynamics-based gene-specific qPCR primer database for 147 organisms. Nucleic Acids Res. 2018, 46, D1229–D1236. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| ID | Type 1 | Proposed Compound 2 | RT 3 | Average Mass | D/Y-20DAP | D/Y-30DAP | D/Y-40DAP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FC 4 | VIP-Value 5 | p-Value | FC | VIP-Value | p-Value | FC | VIP-Value | p-Value | |||||
| 30 | 1 | 2,5-dihydroxybenzoic acid | 3.49 | 153.0182 | 608.61 | 1.417 | 0.007 | 281.69 | 1.417 | 0.023 | 106.51 | 1.411 | 0.069 |
| 105 | 2 | eriodictyol | 6.98 | 287.0565 | 45.634 | 1.974 | 0 | 2732.3 | 1.974 | 0 | 615.06 | 1.951 | 0 |
| 106 | 2 | epicatechin | 6.97 | 289.0723 | 60.921 | 1.889 | 0 | 494.35 | 1.889 | 0 | 243.94 | 1.934 | 0 |
| 108 | 2 | (+)-3,3′,4′,5,7-pentahydroxyflavan | 5.58 | 289.0728 | 4.677 | 1.817 | 0 | 4.753 | 1.817 | 0 | 2.729 | 2.005 | 0 |
| 110 | 2 | (+)-catechin | 13.16 | 289.0728 | 3.430 | 1.130 | 0.170 | 9.129 | 1.130 | 0 | 4.856 | 1.795 | 0 |
| 118 | 2 | quercetin | 13.12 | 301.0365 | 2.310 | 1.854 | 0.002 | 4.801 | 1.854 | 0 | 2.937 | 1.833 | 0 |
| 182 | 2 | oenin | 12.69 | 491.1215 | 79.572 | 1.358 | 0 | 15.839 | 1.358 | 0 | 7.814 | 1.415 | 0 |
| 197 | 2 | [DP2]-2 | 9.52 | 577.1362 | 41.518 | 1.135 | 0.265 | 1168.5 | 1.135 | 0 | 263.83 | 1.858 | 0 |
| 198 | 2 | [DP2]-1 | 6.30 | 577.1363 | 114.18 | 1.536 | 0 | 527.47 | 1.869 | 0 | 549.83 | 1.851 | 0 |
| 233 | 2 | [DP3]-2 | 9.41 | 865.2006 | 110.57 | 1.544 | 0 | 1783.3 | 1.544 | 0 | 643.99 | 1.805 | 0 |
| 235 | 2 | [DP3]-1 | 7.47 | 865.2016 | 57.917 | 1.547 | 0 | 1854.9 | 1.547 | 0 | 1926.2 | 1.559 | 0 |
| 249 | 2 | [DP4] | 7.84 | 1153.2640 | 5.695 | 1.564 | 0 | 8.064 | 1.564 | 0 | 7.948 | 1.603 | 0 |
| 261 | 2 | Is-3-O-glucoside-7-O-glucoside | 5.16 | 639.1576 | 8.740 | 1.142 | 0.215 | 25.923 | 1.142 | 0 | 8.558 | 1.703 | 0 |
| 309 | 2 | leucocyanidin | 3.6 | 305.0656 | 38.740 | 1.923 | 0 | 1650 | 1.900 | 0 | 9.504 | 1.780 | 0 |
| 310 | 2 | taxifolin | 3.6 | 303.0533 | 39.700 | 1.736 | 0 | 9818 | 1.880 | 0 | 11.54 | 1.790 | 0 |
| 311 | 2 | taxifolin | 9.83 | 303.0533 | 22.080 | 1.914 | 0 | 41.79 | 1.740 | 0 | 6.659 | 1.760 | 0 |
| 312 | 2 | taxifolin | 10.17 | 303.0533 | 32.850 | 1.837 | 0 | 3.086 | 1.940 | 0 | 2.323 | 1.70 | 0 |
| 313 | 2 | cyanidin | 3.6 | 285.0397 | 280.200 | 1.878 | 0 | 7.383 | 1.790 | 0 | 8.928 | 1.660 | 0 |
| 33 | 4 | pimelic acid | 6.3 | 159.0653 | 0.239 | 1.298 | 0.001 | 0.173 | 1.298 | 0 | 0.276 | 1.011 | 0.003 |
| 171 | 4 | LysoPE 16:0 | 15.36 | 452.2786 | 0.261 | 1.072 | 0.010 | 0.027 | 1.072 | 0 | 0.108 | 1.420 | 0 |
| 7 | 5 | 4-Chlorophenol | 3.25 | 127.0024 | 0.340 | 1.234 | 0 | 0.425 | 1.234 | 0 | 0.573 | 1.129 | 0.033 |
| 129 | 5 | thymidine-5′-monophosphate | 13.13 | 321.0446 | 2.090 | 1.558 | 0.119 | 4.714 | 1.558 | 0 | 10.239 | 1.673 | 0 |
| 287 | 6 | un13 | 12.11 | 461.1082 | 14.225 | 1.366 | 0 | 32.625 | 1.366 | 0 | 4.923 | 1.653 | 0 |
| 288 | 6 | un14 | 11.91 | 461.1083 | 90.449 | 1.653 | 0 | 85.958 | 1.653 | 0 | 96.966 | 1.531 | 0 |
| 294 | 6 | un20 | 3.47 | 368.0614 | 154.720 | 1.866 | 0 | 5.568 | 1.866 | 0 | 1693.3 | 1.578 | 0 |
| 295 | 6 | un21 | 3.6 | 403.0335 | 109.03 | 1.928 | 0 | 5.966 | 1.928 | 0 | 3.931 | 1.907 | 0 |
| 296 | 6 | un22 | 3.54 | 437.1093 | 58.71 | 2.006 | 0 | 985.41 | 2.006 | 0 | 581.94 | 2.01 | 0 |
| 297 | 6 | un23 | 5.09 | 447.1416 | 413.2 | 1.929 | 0 | 14.701 | 1.929 | 0 | 38.925 | 1.165 | 0 |
| 298 | 6 | un24 | 5.68 | 447.1416 | 1613.9 | 1.94 | 0 | 12.554 | 1.94 | 0 | 17.16 | 1.156 | 0 |
| 299 | 6 | un25 | 3.29 | 468.042 | 576.27 | 1.979 | 0 | 1897.9 | 1.979 | 0 | 736.6 | 1.858 | 0 |
| 300 | 6 | un26 | 3.56 | 524.1769 | 25.956 | 1.936 | 0 | 5.574 | 1.936 | 0 | 2.827 | 2.005 | 0 |
| 301 | 6 | un27 | 7.07 | 579.1514 | 613.78 | 1.733 | 0 | 30570 | 1.733 | 0 | 6037.4 | 1.699 | 0 |
| 302 | 6 | un28 | 9.63 | 623.1410 | 5.023 | 1.578 | 0 | 847.06 | 1.578 | 0 | 334.75 | 1.793 | 0 |
| 303 | 6 | un29 | 6.43 | 645.1224 | 77.343 | 1.7 | 0 | 697.36 | 1.7 | 0 | 506.53 | 1.836 | 0 |
| 304 | 6 | un30 | 8.09 | 720.1609 | 5292.1 | 1.308 | 0 | 299.54 | 1.308 | 0 | 183.1 | 1.395 | 0 |
| 305 | 6 | un31 | 10.18 | 720.1609 | 1484.9 | 1.317 | 0 | 1971.1 | 1.317 | 0 | 392.68 | 1.385 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, S.; Tang, Y.; Zhang, C.; Yin, N.; Mao, Y.; Sun, F.; Chen, S.; Hu, R.; Liu, X.; Shang, G.; et al. Metabolite Profiling and Transcriptome Analysis Provide Insight into Seed Coat Color in Brassica juncea. Int. J. Mol. Sci. 2021, 22, 7215. https://doi.org/10.3390/ijms22137215
Shen S, Tang Y, Zhang C, Yin N, Mao Y, Sun F, Chen S, Hu R, Liu X, Shang G, et al. Metabolite Profiling and Transcriptome Analysis Provide Insight into Seed Coat Color in Brassica juncea. International Journal of Molecular Sciences. 2021; 22(13):7215. https://doi.org/10.3390/ijms22137215
Chicago/Turabian StyleShen, Shulin, Yunshan Tang, Chao Zhang, Nengwen Yin, Yuanyi Mao, Fujun Sun, Si Chen, Ran Hu, Xueqin Liu, Guoxia Shang, and et al. 2021. "Metabolite Profiling and Transcriptome Analysis Provide Insight into Seed Coat Color in Brassica juncea" International Journal of Molecular Sciences 22, no. 13: 7215. https://doi.org/10.3390/ijms22137215
APA StyleShen, S., Tang, Y., Zhang, C., Yin, N., Mao, Y., Sun, F., Chen, S., Hu, R., Liu, X., Shang, G., Liu, L., Lu, K., Li, J., & Qu, C. (2021). Metabolite Profiling and Transcriptome Analysis Provide Insight into Seed Coat Color in Brassica juncea. International Journal of Molecular Sciences, 22(13), 7215. https://doi.org/10.3390/ijms22137215









