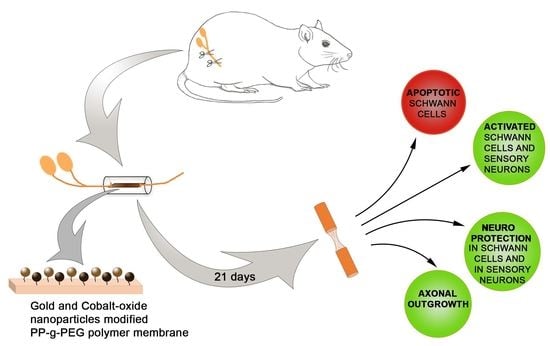
Gold and Cobalt Oxide Nanoparticles Modified Poly-Propylene Poly-Ethylene Glycol Membranes in Poly (ε-Caprolactone) Conduits Enhance Nerve Regeneration in the Sciatic Nerve of Healthy Rats
Abstract
1. Introduction
2. Results
2.1. SEM and EDS Analysis of the Membranes
2.2. Macroscopic Analyses of the Regenerated Matrix
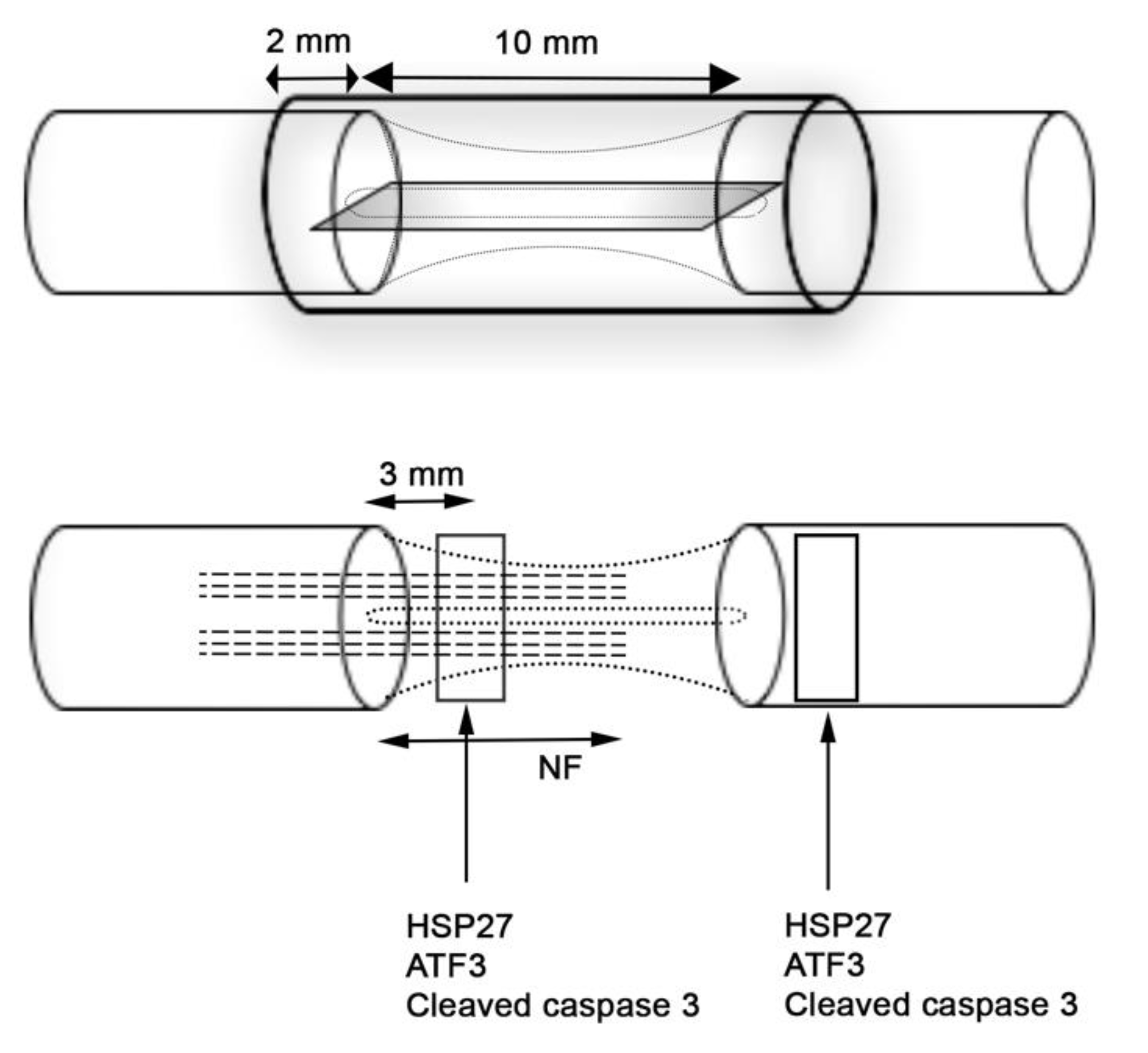
2.3. Immunohistochemical Analysis of the Thickest Cable of Regenerative Matrix
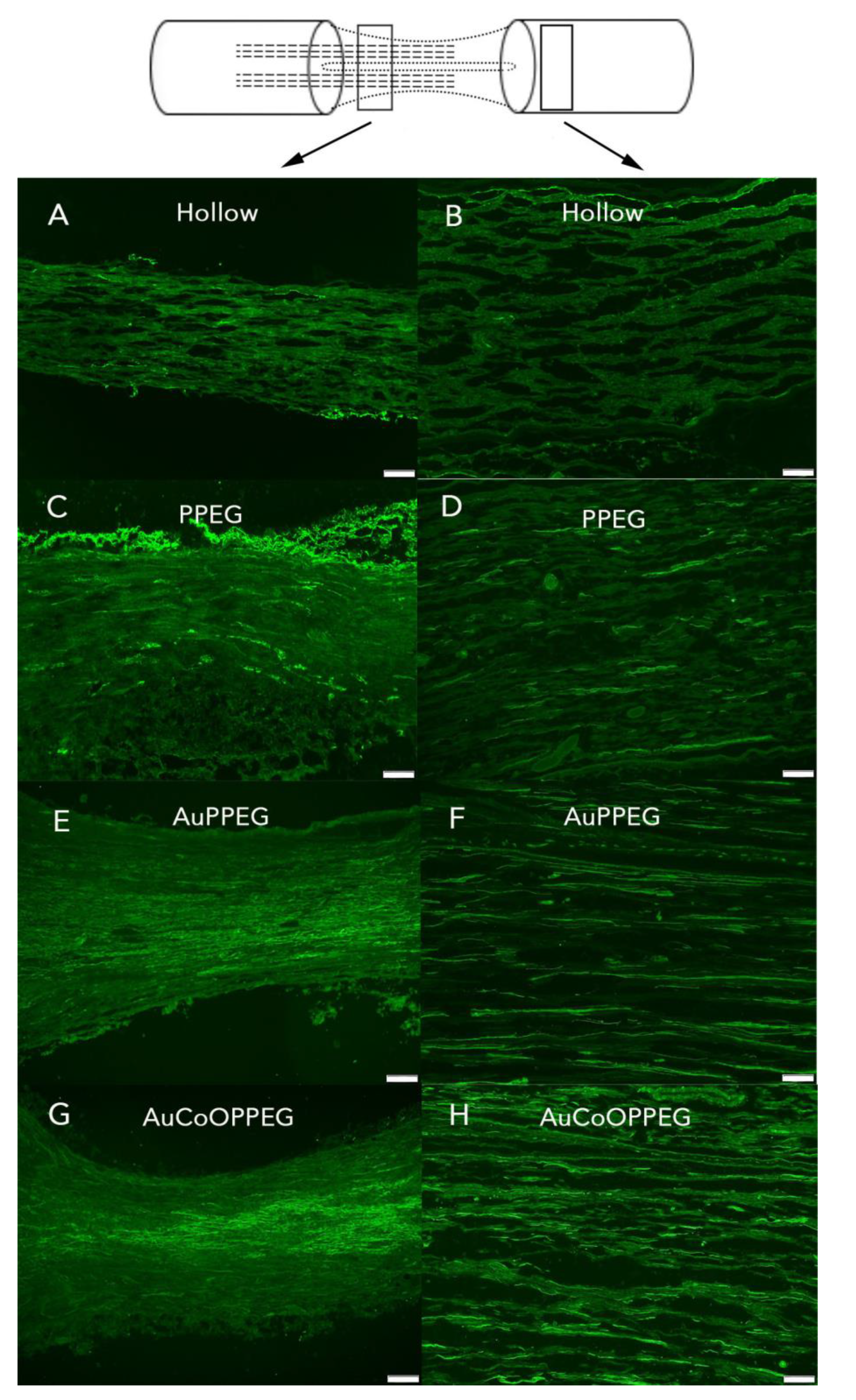
2.3.1. Axonal Outgrowth
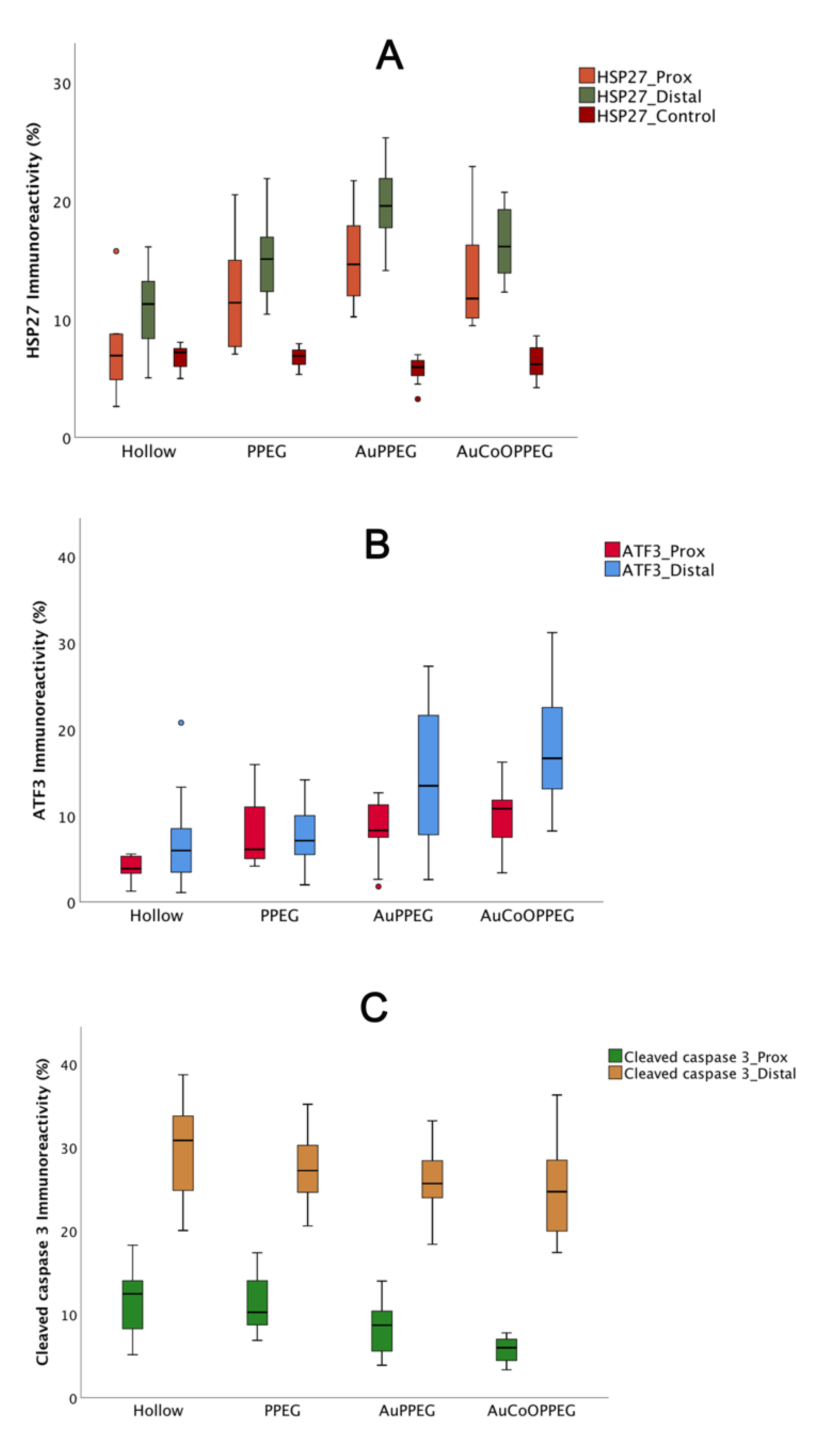
2.3.2. HSP27 Immunoreactivity
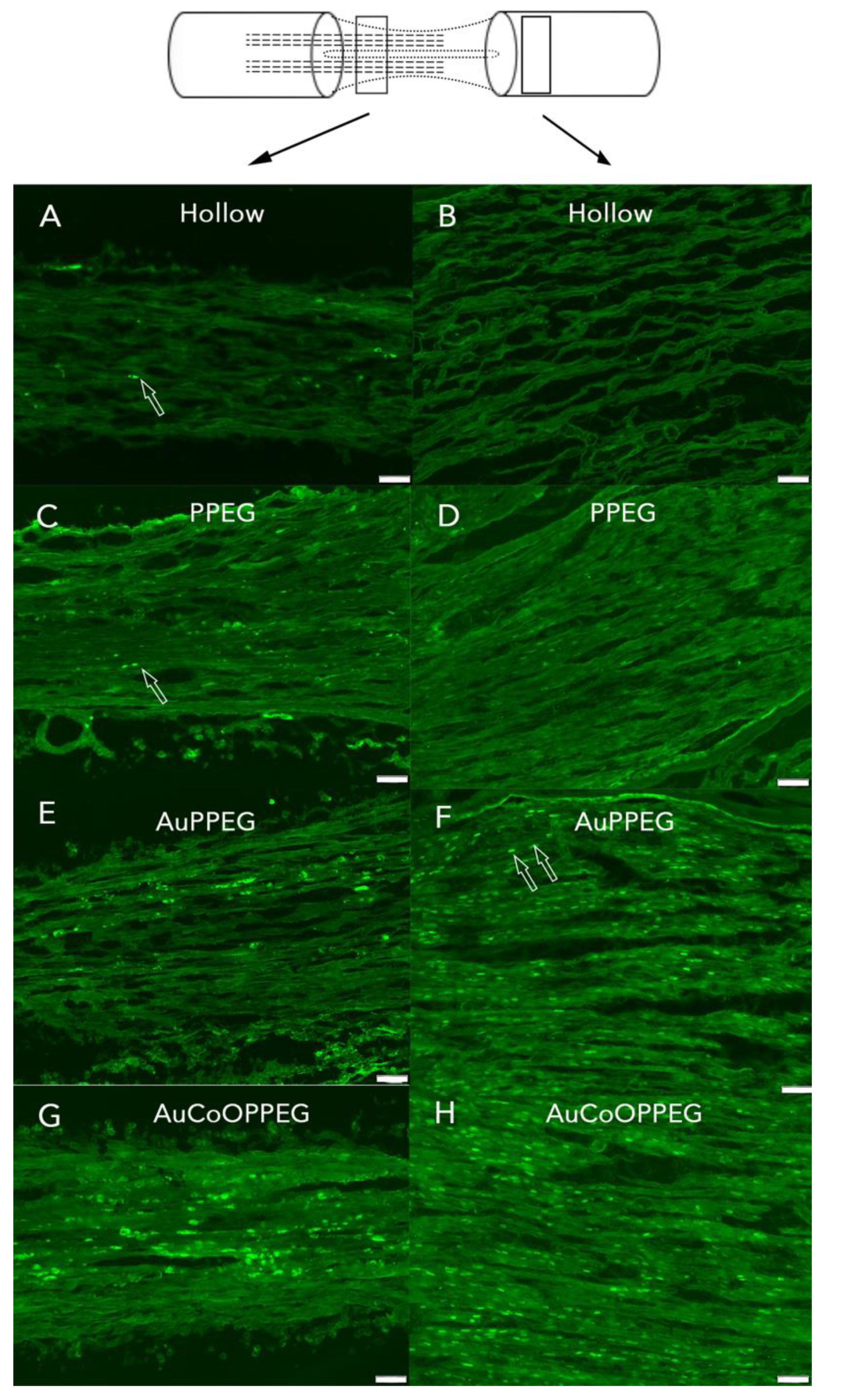
2.3.3. ATF3 Immunoreactivity
2.3.4. Cleaved Caspase 3 Immunoreactivity
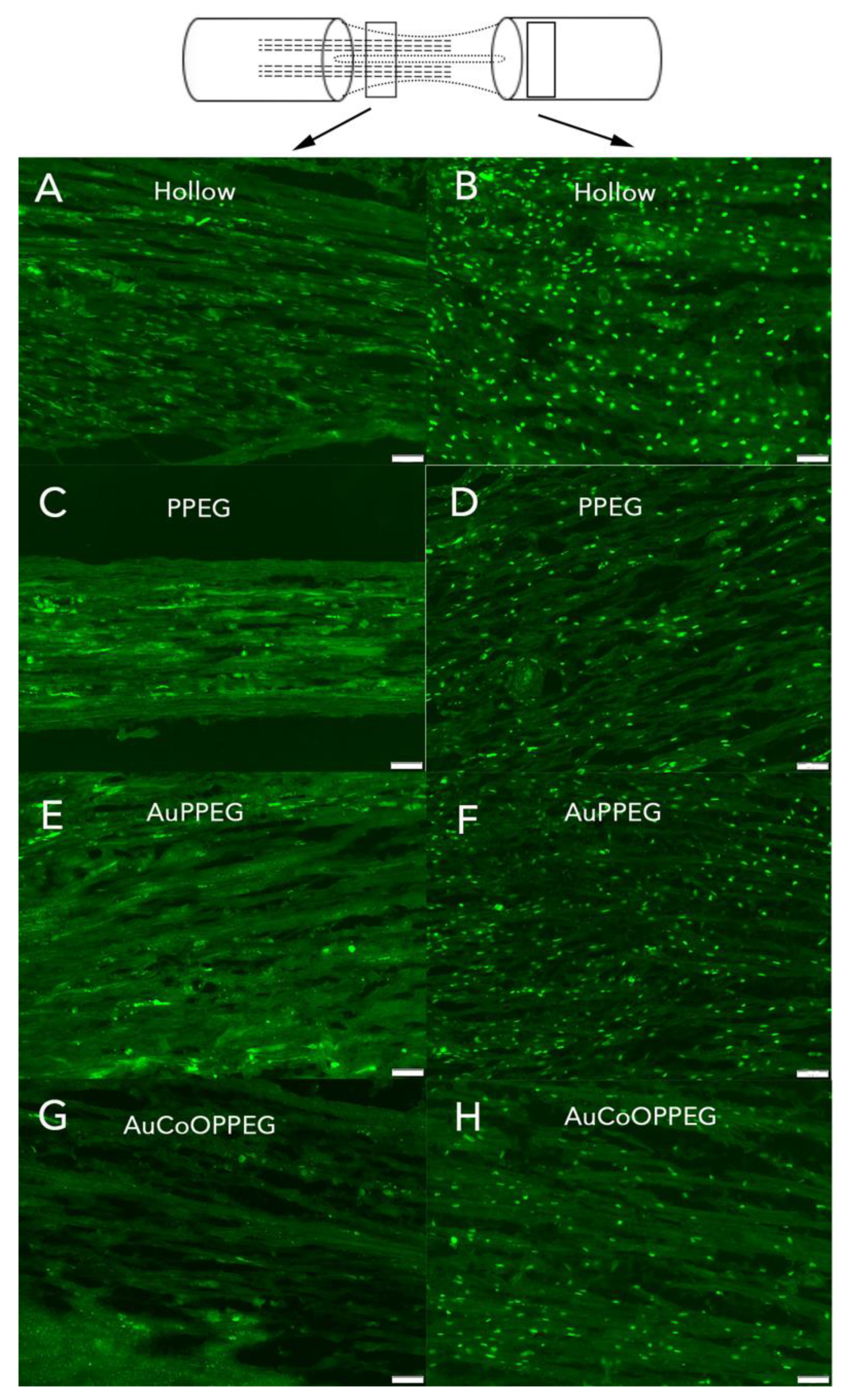
2.3.5. DAPI Positive Cells
2.4. Immunohistochemical Analysis of the Thinner Cable of Regenerative Matrix
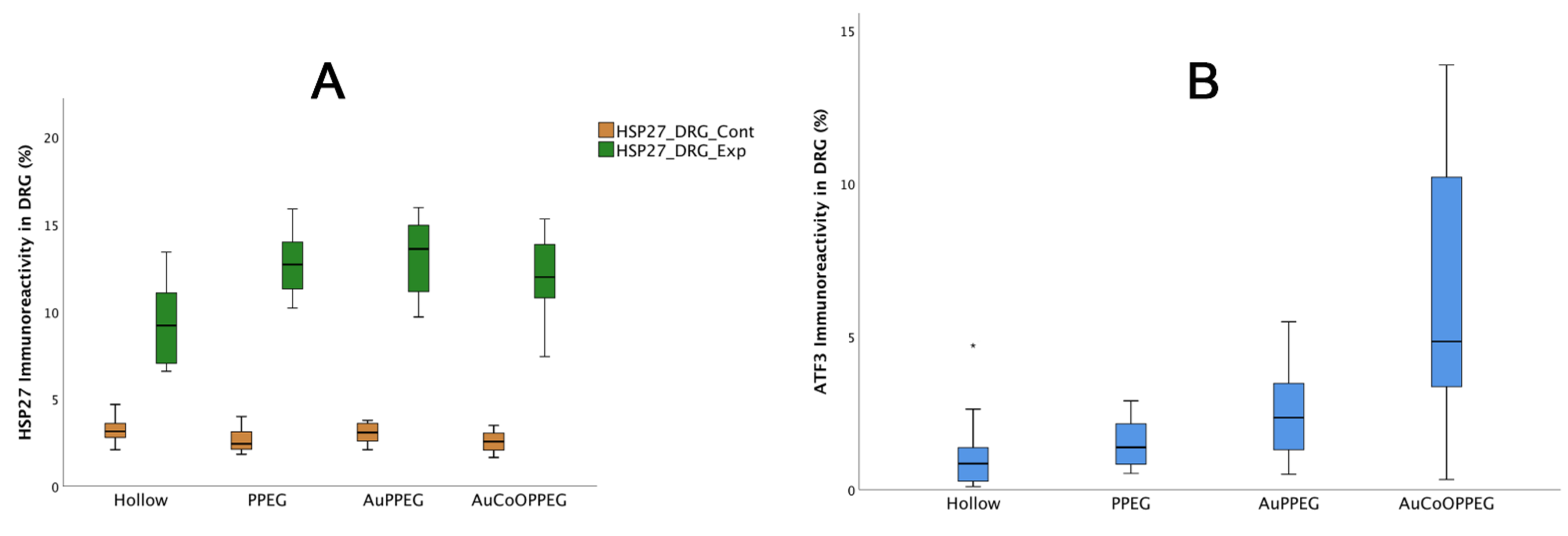
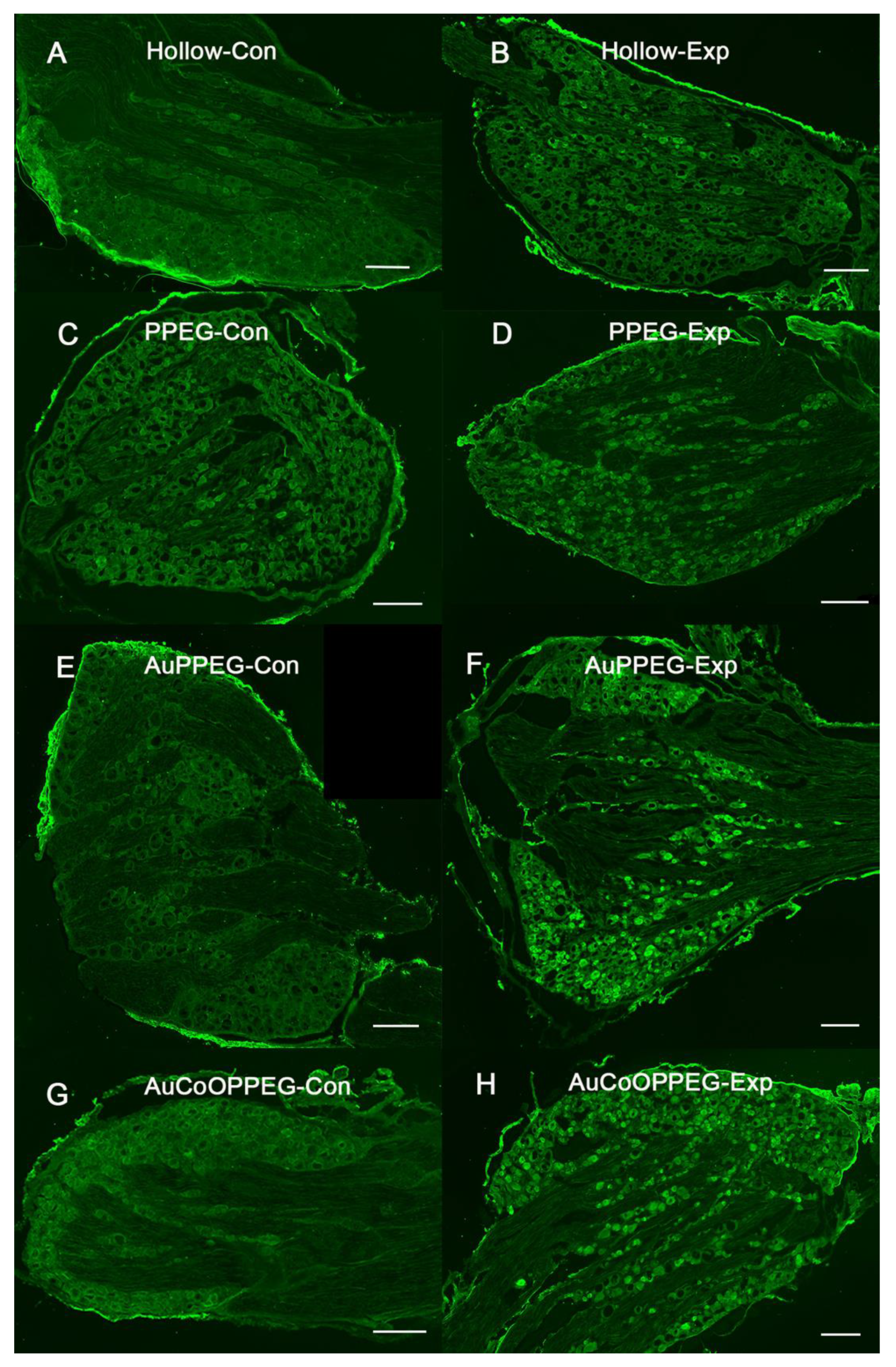
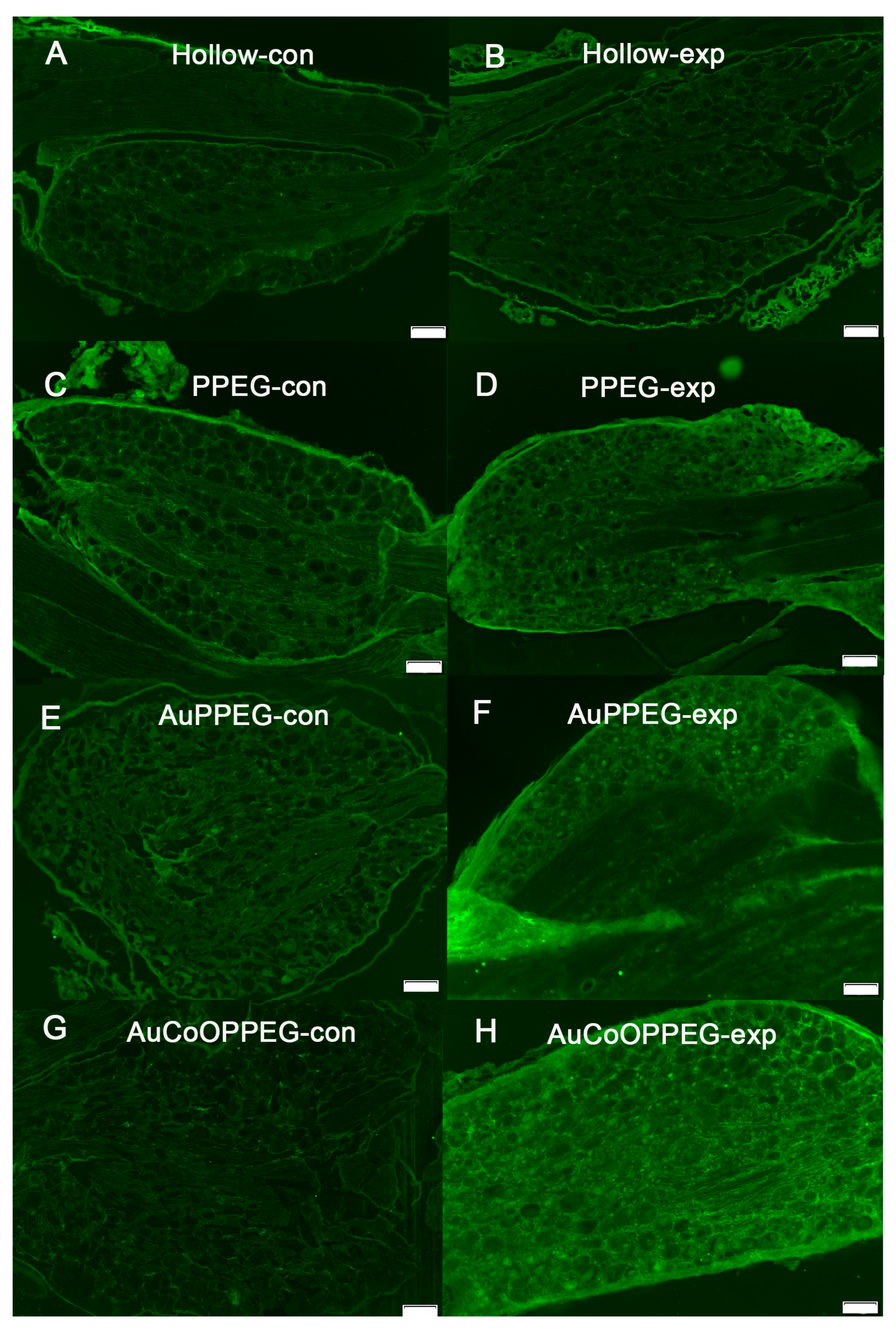
2.5. Immunohistochemical Analysis of DRG
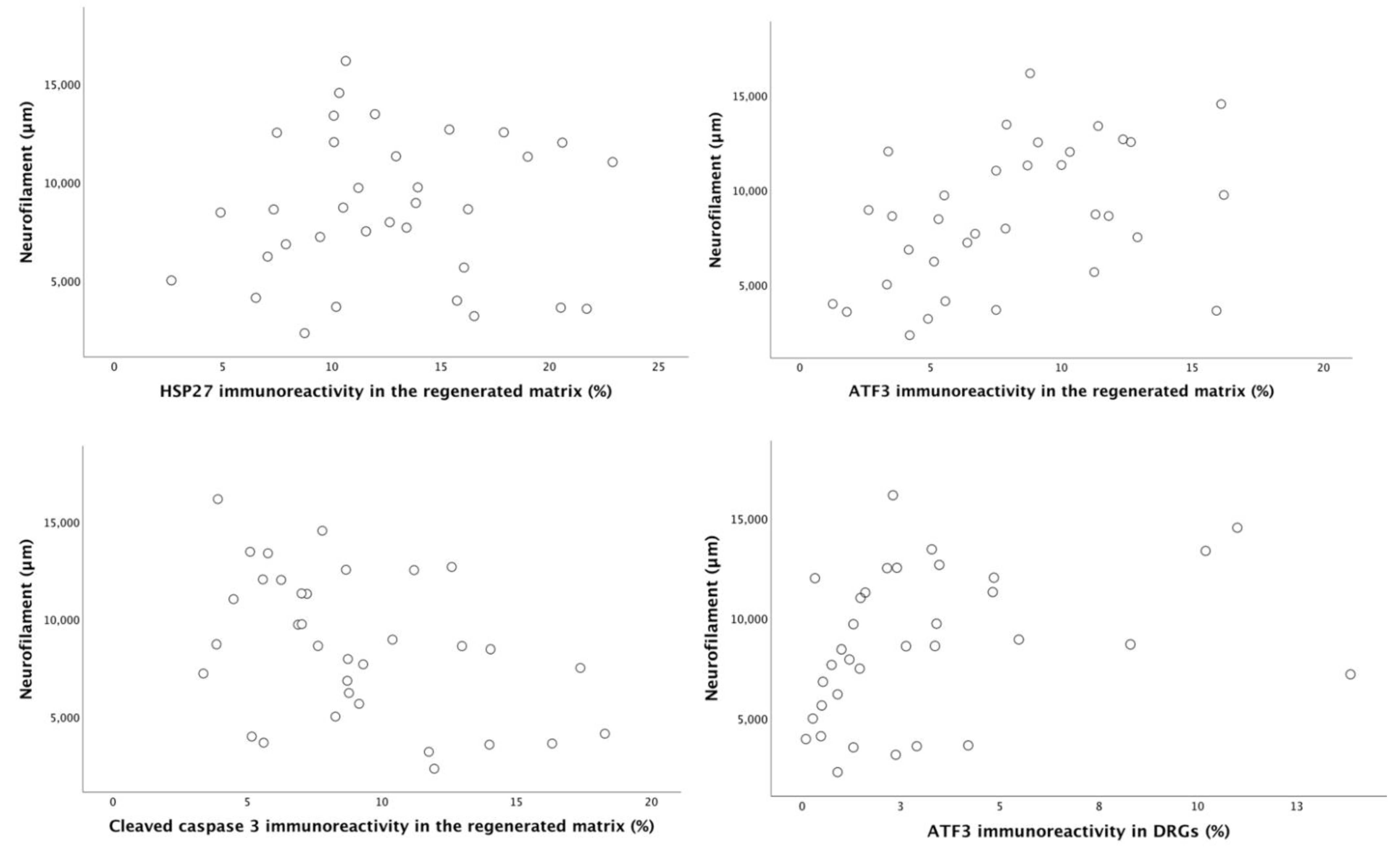
2.6. Correlation and Regression Analyses
3. Discussion
4. Materials and Method
4.1. Pilot Study with Different Types of Nanoparticles and Membranes
4.2. Production of Poly (ε-caprolactone) (PCL) Conduits with Modified Membranes
4.2.1. Preparation of Poly (ε-caprolactone) (PCL) Conduits
4.2.2. Synthesis of Polypropylene-Polyethylene Glycol (PP-g-PEG) Amphiphilic Copolymer
4.2.3. Synthesis of Gold Nanoparticle-Embedded PP-g-PEG Amphiphilic Graft Copolymers
4.2.4. Synthesis of Gold and Cobalt-Oxide Nanoparticle-Embedded PP-g-PEG Amphiphilic Graft Copolymers
4.2.5. Modified Nano-Composite PCL Conduit Formation
4.3. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectrometry (EDS) Analysis of the Membranes
4.4. Animals
4.5. Surgery
4.6. Harvest of Specimens
4.7. Regenerative Matrix
4.8. Immunohistochemistry
4.9. Imaging and Analyses
4.9.1. HSP27 Immunoreactivity Analysis
4.9.2. Analysis of ATF3 and Cleaved Caspase 3 Immunoreactivity:
4.10. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dahlin, L.B.; Wiberg, M. Nerve injuries of the upper extremity and hand. EFORT Open Rev. 2017, 2, 158–170. [Google Scholar] [CrossRef]
- Leversedge, F.J.; Zoldos, J.; Nydick, J.; Kao, D.S.; Thayer, W.; MacKay, B.; McKee, D.; Hoyen, H.; Safa, B.; Buncke, G.M. A Multicenter Matched Cohort Study of Processed Nerve Allograft and Conduit in Digital Nerve Reconstruction. J. Hand Surg. Am. 2020, 45, 1148–1156. [Google Scholar] [CrossRef]
- Raza, C.; Riaz, H.A.; Anjum, R.; Shakeel, N.U.A. Repair strategies for injured peripheral nerve: Review. Life Sci. 2020, 243, 117308. [Google Scholar] [CrossRef]
- Saeki, M.; Tanaka, K.; Imatani, J.; Okamoto, H.; Watanabe, K.; Nakamura, T.; Gotani, H.; Ohi, H.; Nakamura, R.; Hirata, H. Efficacy and safety of novel collagen conduits filled with collagen filaments to treat patients with peripheral nerve injury: A multicenter, controlled, open-label clinical trial. Injury 2018, 49, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Reitan, I.; Dahlin, L.B.; Rosberg, H.E. Patient-reported quality of life and hand disability in elderly patients after a traumatic hand injury—A retrospective study. Health Qual. Life Outcomes 2019, 17, 148. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Srivastava, A.K. Nerve conduits as replacements of autografts in peripheral nerve surgery: Still a work in progress. Neurol. India 2019, 67, 115–117. [Google Scholar] [CrossRef]
- Pabari, A.; Lloyd-Hughes, H.; Seifalian, A.M.; Mosahebi, A. Nerve conduits for peripheral nerve surgery. Plast. Reconstr. Surg. 2014, 133, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, L.B.; Danielsen, N.; Ochi, M.; Lundborg, G. Axonal growth in mesothelial chambers: Effects of a proximal preconditioning lesion and/or predegeneration of the distal nerve stump. Exp. Neurol. 1988, 99, 655–663. [Google Scholar] [CrossRef]
- Hazer, D.B.; Kilicay, E.; Hazer, B. Poly(3-hydroxyalkanoate)s: Diversification and biomedical applications: A state of the art review. Mater. Sci. Eng. C 2012, 32, 637–647. [Google Scholar] [CrossRef]
- Hazer, D.B.; Bal, E.; Nurlu, G.; Benli, K.; Balci, S.; Ozturk, F.; Hazer, B. In vivo application of poly-3-hydroxyoctanoate as peripheral nerve graft. J. Zhejiang Univ Sci. B 2013, 14, 993–1003. [Google Scholar] [CrossRef]
- Sakar, M.; Korkusuz, P.; Demirbilek, M.; Cetinkaya, D.U.; Arslan, S.; Denkbas, E.B.; Temucin, C.M.; Bilgic, E.; Hazer, D.B.; Bozkurt, G. The effect of poly(3-hydroxybutyrate-co-3- hydroxyhexanoate) (PHBHHx) and human mesenchymal stem cell (hMSC) on axonal regeneration in experimental sciatic nerve damage. Int. J. Neurosci. 2014, 124, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Chen, K.C.; He, T.; Yu, W.; Huang, S.; Xu, K. Scaffolds from block polyurethanes based on poly(varepsilon-caprolactone) (PCL) and poly(ethylene glycol) (PEG) for peripheral nerve regeneration. Biomaterials 2014, 35, 4266–4277. [Google Scholar] [CrossRef] [PubMed]
- Haastert-Talini, K.; Geuna, S.; Dahlin, L.B.; Meyer, C.; Stenberg, L.; Freier, T.; Heimann, C.; Barwig, C.; Pinto, L.F.; Raimondo, S.; et al. Chitosan tubes of varying degrees of acetylation for bridging peripheral nerve defects. Biomaterials 2013, 34, 9886–9904. [Google Scholar] [CrossRef] [PubMed]
- Shapira, Y.; Tolmasov, M.; Nissan, M.; Reider, E.; Koren, A.; Biron, T.; Bitan, Y.; Livnat, M.; Ronchi, G.; Geuna, S.; et al. Comparison of results between chitosan hollow tube and autologous nerve graft in reconstruction of peripheral nerve defect: An experimental study. Microsurgery 2016, 36, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Bellamkonda, R.V. Tissue-engineered scaffolds are effective alternatives to autografts for bridging peripheral nerve gaps. Tissue Eng. 2003, 9, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Assaf, K.; Leal, C.V.; Derami, M.S.; de Rezende Duek, E.A.; Ceragioli, H.J.; de Oliveira, A.L.R. Sciatic nerve repair using poly(epsilon-caprolactone) tubular prosthesis associated with nanoparticles of carbon and graphene. Brain Behav. 2017, 7, e00755. [Google Scholar] [CrossRef] [PubMed]
- Bockelmann, J.; Klinkhammer, K.; von Holst, A.; Seiler, N.; Faissner, A.; Brook, G.A.; Klee, D.; Mey, J. Functionalization of electrospun poly(epsilon-caprolactone) fibers with the extracellular matrix-derived peptide GRGDS improves guidance of schwann cell migration and axonal growth. Tissue Eng. Part A 2011, 17, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Pierucci, A.; Duek, E.A.; de Oliveira, A.L. Expression of basal lamina components by Schwann cells cultured on poly(lactic acid) (PLLA) and poly(caprolactone) (PCL) membranes. J. Mater. Sci. Mater. Med. 2009, 20, 489–495. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, W.; Zhu, C.; Zhang, X.; Ye, D.; Zhang, W.; Zhou, Y.; Jiang, X.; Zhang, Z. Sciatic nerve regeneration in rats by a promising electrospun collagen/poly(epsilon-caprolactone) nerve conduit with tailored degradation rate. BMC Neurosci. 2011, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, L.; Stossel, M.; Ronchi, G.; Geuna, S.; Yin, Y.; Mommert, S.; Martensson, L.; Metzen, J.; Grothe, C.; Dahlin, L.B.; et al. Regeneration of long-distance peripheral nerve defects after delayed reconstruction in healthy and diabetic rats is supported by immunomodulatory chitosan nerve guides. BMC Neurosci. 2017, 18, 53. [Google Scholar] [CrossRef]
- Stenberg, L.; Kodama, A.; Lindwall-Blom, C.; Dahlin, L.B. Nerve regeneration in chitosan conduits and in autologous nerve grafts in healthy and in type 2 diabetic Goto-Kakizaki rats. Eur. J. Neurosci. 2016, 43, 463–473. [Google Scholar] [CrossRef]
- Frost, H.K.; Andersson, T.; Johansson, S.; Englund-Johansson, U.; Ekstrom, P.; Dahlin, L.B.; Johansson, F. Electrospun nerve guide conduits have the potential to bridge peripheral nerve injuries in vivo. Sci. Rep. 2018, 8, 16716. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.K.; Kodama, A.; Ekstrom, P.; Dahlin, L.B. G-CSF prevents caspase 3 activation in Schwann cells after sciatic nerve transection, but does not improve nerve regeneration. Neuroscience 2016, 334, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.; Razavi, S.; Seyedebrahimi, R.; Reisi, P.; Kazemi, M. Regeneration of Rat Sciatic Nerve Using PLGA Conduit Containing Rat ADSCs with Controlled Release of BDNF and Gold Nanoparticles. J. Mol. Neurosci. 2020, 71, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Baranes, K.; Shevach, M.; Shefi, O.; Dvir, T. Gold Nanoparticle-Decorated Scaffolds Promote Neuronal Differentiation and Maturation. Nano Lett. 2016, 16, 2916–2920. [Google Scholar] [CrossRef]
- Lin, Y.L.; Jen, J.C.; Hsu, S.H.; Chiu, I.M. Sciatic nerve repair by microgrooved nerve conduits made of chitosan-gold nanocomposites. Surg. Neurol. 2008, 70 (Suppl. S1), S9–S18. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sharma, M.; Saharia, D.; Sarma, K.K.; Sarma, M.G.; Borthakur, B.B.; Bora, U. In vivo studies of silk based gold nano-composite conduits for functional peripheral nerve regeneration. Biomaterials 2015, 62, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Dogra, V.; Kaur, G.; Jindal, S.; Kumar, R.; Kumar, S.; Singhal, N.K. Bactericidal effects of metallosurfactants based cobalt oxide/hydroxide nanoparticles against Staphylococcus aureus. Sci. Total Environ. 2019, 681, 350–364. [Google Scholar] [CrossRef]
- Long, N.V.; Yang, Y.; Teranishi, T.; Thi, C.M.; Cao, Y.; Nogami, M. Biomedical Applications of Advanced Multifunctional Magnetic Nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 10091–10107. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Dash, S.K.; Kar Mahapatra, S.; Tripathy, S.; Ghosh, T.; Das, B.; Das, D.; Pramanik, P.; Roy, S. Chitosan-modified cobalt oxide nanoparticles stimulate TNF-alpha-mediated apoptosis in human leukemic cells. J. Biol. Inorg. Chem. 2014, 19, 399–414. [Google Scholar] [CrossRef]
- Huang, X.; Cai, H.; Zhou, H.; Li, T.; Jin, H.; Evans, C.E.; Cai, J.; Pi, J. Cobalt oxide nanoparticle-synergized protein degradation and phototherapy for enhanced anticancer therapeutics. Acta Biomater. 2020, 121, 605–620. [Google Scholar] [CrossRef]
- Kilic, M.S.; Korkut, S.; Hazer, B.; Erhan, E. Development and operation of gold and cobalt oxide nanoparticles containing polypropylene based enzymatic fuel cell for renewable fuels. Biosens. Bioelectron. 2014, 61, 500–505. [Google Scholar] [CrossRef]
- Kalaycı, Ö.A.; Cömert, F.B.; Hazer, B. Synthesis, characterization, and antibacterial activity of metal nanoparticles embedded into amphiphilic comb-type graft copolymers. Polym. Bull. 2010, 65, 215–226. [Google Scholar] [CrossRef]
- Kocer, H.; Borcakli, M.; Demirel, S.; Hazer, B. Production of bacterial polyesters from some various new substrates by Alcaligenes eutrophus and Pseudomonas oleovorans. Turk. J. Chem. 2003, 27, 365–374. [Google Scholar]
- Toraman, T.; Hazer, B. Synthesis and Characterization of the Novel Thermoresponsive Conjugates Based on Poly(3-hydroxy alkanoates). J. Polym. Environ. 2014, 22, 159–166. [Google Scholar] [CrossRef]
- Erduranlı, H.; Hazer, B.; Borcaklı, M. Post Polymerization of Saturated and Unsaturated Poly(3-hydroxy alkanoate)s. Macromol. Symp. 2008, 269, 161–169. [Google Scholar] [CrossRef]
- Öztürk, T.; Atalar, M.N.; Göktaş, M.; Hazer, B. One-step synthesis of block-graft copolymers via simultaneous reversible-addition fragmentation chain transfer and ring-opening polymerization using a novel macroinitiator. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2651–2659. [Google Scholar] [CrossRef]
- Saito, H.; Dahlin, L.B. Expression of ATF3 and axonal outgrowth are impaired after delayed nerve repair. BMC Neurosci. 2008, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [PubMed]
- Cattin, A.L.; Lloyd, A.C. The multicellular complexity of peripheral nerve regeneration. Curr. Opin. Neurobiol. 2016, 39, 38–46. [Google Scholar] [CrossRef]
- Stetler, R.A.; Gao, Y.; Signore, A.P.; Cao, G.; Chen, J. HSP27: Mechanisms of cellular protection against neuronal injury. Curr. Mol. Med. 2009, 9, 863–872. [Google Scholar] [CrossRef]
- Costigan, M.; Mannion, R.J.; Kendall, G.; Lewis, S.E.; Campagna, J.A.; Coggeshall, R.E.; Meridith-Middleton, J.; Tate, S.; Woolf, C.J. Heat shock protein 27: Developmental regulation and expression after peripheral nerve injury. J. Neurosci. 1998, 18, 5891–5900. [Google Scholar] [CrossRef]
- Meyer, C.; Stenberg, L.; Gonzalez-Perez, F.; Wrobel, S.; Ronchi, G.; Udina, E.; Suganuma, S.; Geuna, S.; Navarro, X.; Dahlin, L.B.; et al. Chitosan-film enhanced chitosan nerve guides for long-distance regeneration of peripheral nerves. Biomaterials 2016, 76, 33–51. [Google Scholar] [CrossRef]
- Pourhamidi, K.; Skarstrand, H.; Dahlin, L.B.; Rolandsson, O. HSP27 concentrations are lower in patients with type 1 diabetes and correlate with large nerve fiber dysfunction. Diabetes Care 2014, 37, e49–e50. [Google Scholar] [CrossRef][Green Version]
- Zimmerman, M.; Rolandsson Enes, S.; Skarstrand, H.; Pourhamidi, K.; Gottsater, A.; Wollmer, P.; Rolandsson, O.; Westergren-Thorsson, G.; Dahlin, L.B. Temporal trend of autonomic nerve function and HSP27, MIF and PAI-1 in type 1 diabetes. J. Clin. Transl. Endocrinol. 2017, 8, 15–21. [Google Scholar] [CrossRef]
- Stenberg, L.; Hazer Rosberg, D.B.; Kohyama, S.; Suganuma, S.; Dahlin, L.B. HSP27 expression and axonal outgrowth after immediate or delayed nerve repair in healthy and diabetic Goto-Kakizaki rats. Int. J. Mol. Sci. 2021, unpublished, manuscript submitted. [Google Scholar]
- Read, D.E.; Gorman, A.M. Heat shock protein 27 in neuronal survival and neurite outgrowth. Biochem. Biophys. Res. Commun. 2009, 382, 6–8. [Google Scholar] [CrossRef]
- Wiberg, R.; Novikova, L.N.; Kingham, P.J. Evaluation of apoptotic pathways in dorsal root ganglion neurons following peripheral nerve injury. Neuroreport 2018, 29, 779–785. [Google Scholar] [CrossRef]
- Lindwall, C.; Kanje, M. Retrograde axonal transport of JNK signaling molecules influence injury induced nuclear changes in p-c-Jun and ATF3 in adult rat sensory neurons. Mol. Cell Neurosci. 2005, 29, 269–282. [Google Scholar] [CrossRef]
- Zigmond, R.E.; Echevarria, F.D. Macrophage biology in the peripheral nervous system after injury. Prog. Neurobiol. 2019, 173, 102–121. [Google Scholar] [CrossRef]
- Lundborg, G.; Rosen, B.; Dahlin, L.; Danielsen, N.; Holmberg, J. Tubular versus conventional repair of median and ulnar nerves in the human forearm: Early results from a prospective, randomized, clinical study. J. Hand Surg. Am. 1997, 22, 99–106. [Google Scholar] [CrossRef]
- Dietzmeyer, N.; Forthmann, M.; Grothe, C.; Haastert-Talini, K. Modification of tubular chitosan-based peripheral nerve implants: Applications for simple or more complex approaches. Neural. Regen. Res. 2020, 15, 1421–1431. [Google Scholar]
- Rebowe, R.; Rogers, A.; Yang, X.; Kundu, S.C.; Smith, T.L.; Li, Z. Nerve Repair with Nerve Conduits: Problems, Solutions, and Future Directions. J. Hand Microsurg. 2018, 10, 61–65. [Google Scholar] [CrossRef]
- Pan, D.; Mackinnon, S.E.; Wood, M.D. Advances in the repair of segmental nerve injuries and trends in reconstruction. Muscle Nerve 2020, 61, 726–739. [Google Scholar] [CrossRef]
- Wlaszczuk, A.; Marcol, W.; Kucharska, M.; Wawro, D.; Palen, P.; Lewin-Kowalik, J. Poly(D,L-Lactide-Co-Glycolide) Tubes With Multifilament Chitosan Yarn or Chitosan Sponge Core in Nerve Regeneration. J. Oral Maxillofac. Surg. 2016, 74, 2327.e1–2327.e12. [Google Scholar] [CrossRef]
- Daly, W.T.; Yao, L.; Abu-rub, M.T.; O’Connell, C.; Zeugolis, D.I.; Windebank, A.J.; Pandit, A.S. The effect of intraluminal contact mediated guidance signals on axonal mismatch during peripheral nerve repair. Biomaterials 2012, 33, 6660–6671. [Google Scholar] [CrossRef]
- Papastefanaki, F.; Jakovcevski, I.; Poulia, N.; Djogo, N.; Schulz, F.; Martinovic, T.; Ciric, D.; Loers, G.; Vossmeyer, T.; Weller, H.; et al. Intraspinal Delivery of Polyethylene Glycol-coated Gold Nanoparticles Promotes Functional Recovery After Spinal Cord Injury. Mol. Ther 2015, 23, 993–1002. [Google Scholar] [CrossRef]
- Hazer, D.B.; Hazer, B.; Dincer, N. Soft tissue response to the presence of polypropylene-G-poly(ethylene glycol) comb-type graft copolymers containing gold nanoparticles. J. Biomed. Biotechnol. 2011, 2011, 956169. [Google Scholar] [CrossRef]
- Hazer, D.B.; Sakar, M.; Dere, Y.; Altinkanat, G.; Ziyal, M.I.; Hazer, B. Antimicrobial Effect of Polymer-Based Silver Nanoparticle Coated Pedicle Screws: Experimental Research on Biofilm Inhibition in Rabbits. Spine 2016, 41, E323–E329. [Google Scholar] [CrossRef]
- Evangelista, M.S.; Perez, M.; Salibian, A.A.; Hassan, J.M.; Darcy, S.; Paydar, K.Z.; Wicker, R.B.; Arcaute, K.; Mann, B.K.; Evans, G.R. Single-lumen and multi-lumen poly(ethylene glycol) nerve conduits fabricated by stereolithography for peripheral nerve regeneration in vivo. J. Reconstr. Microsurg. 2015, 31, 327–335. [Google Scholar]
- Mokarizadeh, A.; Mehrshad, A.; Mohammadi, R. Local Polyethylene Glycol in Combination with Chitosan Based Hybrid Nanofiber Conduit Accelerates Transected Peripheral Nerve Regeneration. J. Investig. Surg. 2016, 29, 167–174. [Google Scholar] [CrossRef]
- Paviolo, C.; Stoddart, P.R. Gold Nanoparticles for Modulating Neuronal Behavior. Nanomaterials 2017, 7, 92. [Google Scholar] [CrossRef]
- Saderi, N.; Rajabi, M.; Akbari, B.; Firouzi, M.; Hassannejad, Z. Fabrication and characterization of gold nanoparticle-doped electrospun PCL/chitosan nanofibrous scaffolds for nerve tissue engineering. J. Mater. Sci. Mater. Med. 2018, 29, 134. [Google Scholar] [CrossRef]
- Soderstjerna, E.; Bauer, P.; Cedervall, T.; Abdshill, H.; Johansson, F.; Johansson, U.E. Silver and gold nanoparticles exposure to in vitro cultured retina--studies on nanoparticle internalization, apoptosis, oxidative stress, glial- and microglial activity. PLoS ONE 2014, 9, e105359. [Google Scholar] [CrossRef]
- Balcı, M.A.; Hazer, B.; Guven, O.; Cavicchi, K.; Cakmak, M. Synthesis and characterization of novel comb-type amphiphilic graft copolymers containing polypropylene and polyethylene glycol. Polym. Bull. 2010, 64, 691–705. [Google Scholar] [CrossRef]
- Hazer, B.; Ashby, R.D. Synthesis of a novel tannic acid-functionalized polypropylene as antioxidant active-packaging materials. Food Chem. 2021, 344, 128644. [Google Scholar] [CrossRef]
- Stenberg, L.; Kanje, M.; Dolezal, K.; Dahlin, L.B. Expression of activating transcription factor 3 (ATF 3) and caspase 3 in Schwann cells and axonal outgrowth after sciatic nerve repair in diabetic BB rats. Neurosci. Lett. 2012, 515, 34–38. [Google Scholar] [CrossRef]
- Woeffler-Maucler, C.; Beghin, A.; Ressnikoff, D.; Bezin, L.; Marinesco, S. Automated immunohistochemical method to quantify neuronal density in brain sections: Application to neuronal loss after status epilepticus. J. Neurosci. Methods 2014, 225, 32–41. [Google Scholar] [CrossRef] [PubMed]
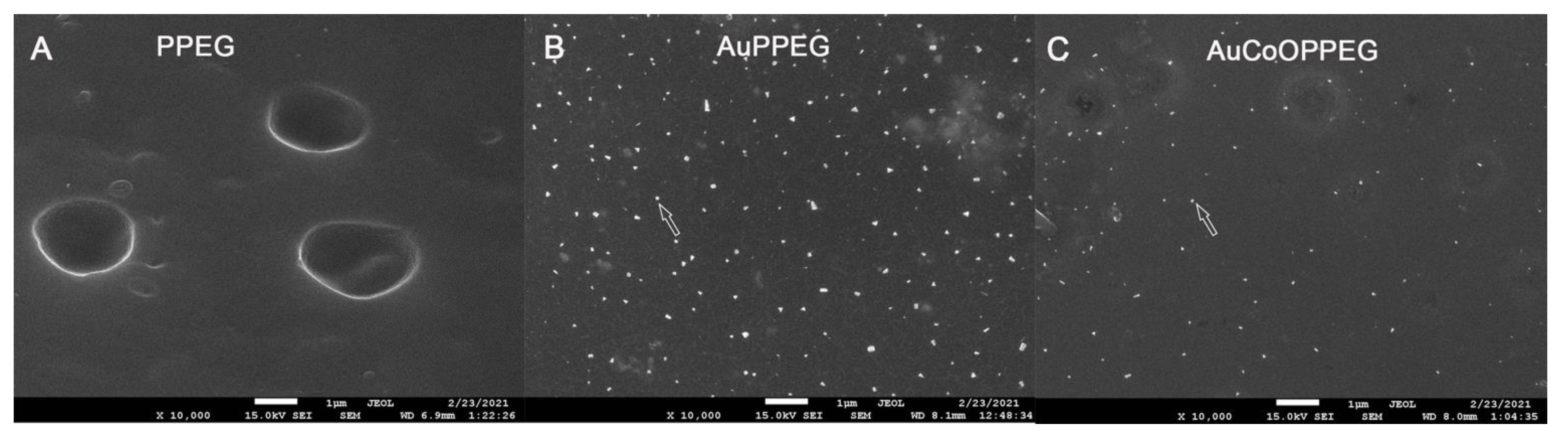
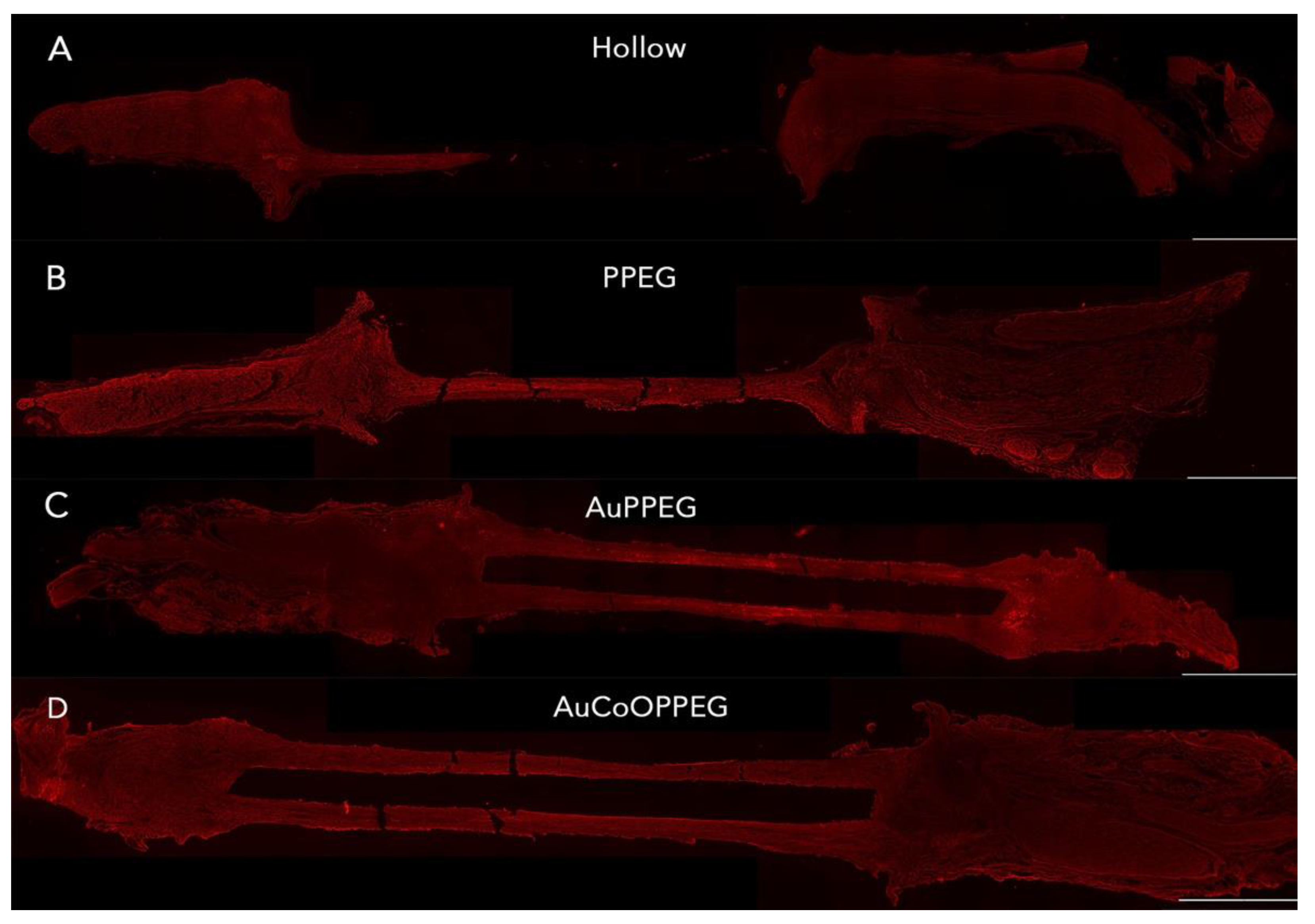
| PCL Conduits without/with Modifications, i.e., Inserted Membranes | p-Values | |||||
|---|---|---|---|---|---|---|
| Hollow(n = 10) | PPEG (n = 10) | AuPPEG (n = 10) | AuCoOPPEG (n = 10) | |||
| Thickness of regenerative matrix (see foot note) | No matrix | 4 | 2 | 0 | 0 | 0.015 |
| Thickness < 1 mm (loose) | 6 | 4 | 4 | 4 | ||
| Thickness ≥ 1 mm (dense) | 0 | 4 | 6 a | 6 a | ||
| Number of cables in regenerative matrix | No cable | 4 | 2 | 0 | 0 | 0.022 |
| One full length cable | 6 | 4 | 6 | 4 | ||
| Two definable full-length cables | 0 | 4 | 4 a | 6 a | ||
| PCL Conduits without/with Modifications, i.e., Inserted Membranes | p-Values | |||||
|---|---|---|---|---|---|---|
| Hollow (n = 10) | PPEG (n = 10) | AuPPEG (n = 10) | AuCoOPPEG (n = 10) | |||
| Length of axonal outgrowth (NF; µm) | 4559 (3562–8493) | 7169 (4266–9200) | 10,113 (5156–12,866) | 11,164 (8682–12,366) a,b | 0.016 | |
| HSP27 immunoreactivity (% of total area) in sciatic nerve at control side | 7.2 (5.8–7.6) | 6.9 (6.2–7.5) | 5.9 (5.0–6.6) | 6.2 (5.3–7.7) | 0.15 | |
| HSP27 immunoreactivity (% of total area) in sciatic nerve at experimental side | At 3 mm | 6.9 (4.3–10.5) | 11.4 (7.6–15.8) | 14.6 (11.6–18.2) a | 11.7 (10.1–17.3) a | 0.023 |
| At distal site | 11.3 (8.3–13.5) | 15.1 (12.2–17.1) a | 19.6 (17.5–22.0) a,b | 16.1 (13.5–19.6) a,b | 0.001 | |
| ATF3 immunoreactivity (% of total number of DAPI positive cells) | At 3 mm | 3.9 (2.8–5.4) | 6.1 (4.9–11.9) a | 8.3 (6.2–11.5) a | 10.8 (7.2–12.9) a | 0.022 |
| At distal site | 5.6 (3.0–9.7) | 7.1 (5.3–11) | 13.4 (7.5–21.8) a | 16.6 (13–23.4) a,b | 0.002 | |
| Cleaved caspase 3 immunoreactivity (% of total number of DAPI positive cells) | At 3 mm | 12.4 (7.5–15.0) | 10.2 (8.7–15.1) | 8.7 (5.5–10.9) | 6 (4.3–7.1) a,b,c | 0.004 |
| At distal site | 30.8 (24.4–34.2) | 27.2 (23.9–30.7) | 25.7 (23.8–29) | 24.7 (19.7–28.5) | 0.23 | |
| DAPI positive cells (no/mm2) | At 3 mm | 4094 (3513–5753) | 3486 (3005–4252) | 3472 (2784–4396) | 3842 (3020–4413) | 0.38 |
| At distal site | 3505 (2849–3776) | 3515 (3022–3875) | 3692 (2775–4286) | 4073 (3767–4381) a,b | 0.03 | |
| PCL Conduits without/with Modifications, i.e., Inserted Membranes | Length of Axonal Outgrowth (NF; µm) | HSP27 Immunoreactivity (% of Total Area) at 3 mm Proximal Site | ATF3 Immunoreactivity (% of Total Number of DAPI Positive Cells) at 3 mm Proximal Site | Cleaved Caspase 3 Immunoreactivity (% of Total Number of DAPI Positive Cells) at 3 mm Proximal Site |
|---|---|---|---|---|
| Hollow (n = 0) | - | - | - | - |
| PPEG (n = 4) | 2556 (1351–4161) | 13.1 (8.1–19.0) | 3.4 (2.1–9.1) | 12.6 (5.1–15.2) |
| AuPPEG (n = 4) | 2897 (836–4267) | 19.3 (17.0–21.9) | 4.5 (2.5–7.4) | 6.9 (3.2–15.3) |
| AuCoOPPEG (n = 6) | 5279 (368–8930) | 14.3 (12.3–20.7) | 5.8 (2.5–9.6) | 5.7 (2.4–8.0) |
| PCL Conduits without/with Modifications, i.e., Inserted Membranes | p Values | ||||
|---|---|---|---|---|---|
| Hollow (n = 10) | PPEG (n = 10) | AuPPEG (n = 10) | AuCoOPPEG (n = 10) | ||
| HSP27 immunoreactivity (control L4 and L5 DRG; %) | 3.1 (2.8–3.6) | 2.4 (2.1–3.3) | 3.0 (2.5–3.6) | 2.6 (2.0–3.0) | 0.18 |
| HSP27 immunoreactivity (experimental L4 and L5 DRG; %) | 9.2 (7.0–11.1) | 12.7 (11.2–14.1) a | 13.6 (10.9–15.1) a | 12.0 (10.7–13.8) a | 0.005 |
| HSP27 ratio (Exp/Control) | 3.0 (2.0–3.8) | 4.35 (3.7–6.7) a | 4.3 (4.2–4.9) a | 4.5 (3.6–6.7) a | 0.015 |
| ATF3-labelled sensory neurons (experimental L4 and L5 DRG; %) | 0.8 (0.23–1.6) | 1.4 (0.8–2.2) | 2.3 (1.3–3.6) a | 4.8 (2.9–10.4) a,b | 0.002 |
| Primary Antibody | Secondary Antibody | ||
|---|---|---|---|
| Sciatic nerve | Neurofilament | Monoclonal mouse anti-human neurofilament (1:80; Dako, Glostrup, Denmark) | Alexa Fluor 594-goat anti-mouse IgG (1:500, Invitrogen, Molecular Probes, Eugene, OR, USA) |
| Heat Shock Protein 27 (HSP27) | Polyclonal goat anti-HSP-27(1:200, Santa Cruz Biotechnology, Dallas, TX, USA) | Alexa Flour 488-donkey anti-goat IgG (1:500, Invitrogen, Molecular Probes, Eugene, OR, USA) | |
| Activating transcription factor 3 (ATF3) | Monoclonal mouse anti-ATF3 (1:200, Santa Cruz Biotechnology, Dallas, TX, USA) | Alexa Fluor 488-goat anti-mouse IgG (1:500, Invitrogen, Molecular Probes, Eugene, OR, USA) | |
| Cleaved caspase 3 | Monoclonal rabbit anti-cleaved caspase 3 (1:200, Cell signalling Technology, Denvers, MA, USA). | Alexa Fluor 488-goat anti-rabbit IgG (1:500, Invitrogen, Molecular Probes, Eugene, OR, USA) | |
| Dorsal root ganglia (DRG) | Heat Shock Protein 27 (HSP27) | Polyclonal rabbit anti-HSP-27 (1:200, Enzo, Farmingdale, NY, USA) | Alexa Fluor 488-goat anti-rabbit IgG (1:250, Invitrogen, Molecular Probes, Eugene, OR, USA) |
| Activating transcription factor 3 (ATF3) | Monoclonal mouse anti-ATF3 (1:200, Santa Cruz Biotechnology, Dallas, TX, USA) | Alexa Fluor 488-goat anti-mouse IgG (1:500, Invitrogen, Molecular Probes, Eugene, OR, USA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazer Rosberg, D.B.; Hazer, B.; Stenberg, L.; Dahlin, L.B. Gold and Cobalt Oxide Nanoparticles Modified Poly-Propylene Poly-Ethylene Glycol Membranes in Poly (ε-Caprolactone) Conduits Enhance Nerve Regeneration in the Sciatic Nerve of Healthy Rats. Int. J. Mol. Sci. 2021, 22, 7146. https://doi.org/10.3390/ijms22137146
Hazer Rosberg DB, Hazer B, Stenberg L, Dahlin LB. Gold and Cobalt Oxide Nanoparticles Modified Poly-Propylene Poly-Ethylene Glycol Membranes in Poly (ε-Caprolactone) Conduits Enhance Nerve Regeneration in the Sciatic Nerve of Healthy Rats. International Journal of Molecular Sciences. 2021; 22(13):7146. https://doi.org/10.3390/ijms22137146
Chicago/Turabian StyleHazer Rosberg, Derya Burcu, Baki Hazer, Lena Stenberg, and Lars B. Dahlin. 2021. "Gold and Cobalt Oxide Nanoparticles Modified Poly-Propylene Poly-Ethylene Glycol Membranes in Poly (ε-Caprolactone) Conduits Enhance Nerve Regeneration in the Sciatic Nerve of Healthy Rats" International Journal of Molecular Sciences 22, no. 13: 7146. https://doi.org/10.3390/ijms22137146
APA StyleHazer Rosberg, D. B., Hazer, B., Stenberg, L., & Dahlin, L. B. (2021). Gold and Cobalt Oxide Nanoparticles Modified Poly-Propylene Poly-Ethylene Glycol Membranes in Poly (ε-Caprolactone) Conduits Enhance Nerve Regeneration in the Sciatic Nerve of Healthy Rats. International Journal of Molecular Sciences, 22(13), 7146. https://doi.org/10.3390/ijms22137146