Behind Brain Metastases Formation: Cellular and Molecular Alterations and Blood–Brain Barrier Disruption
Abstract
1. Introduction
2. Results
2.1. SS Promotes In Vivo-Like BBB Properties
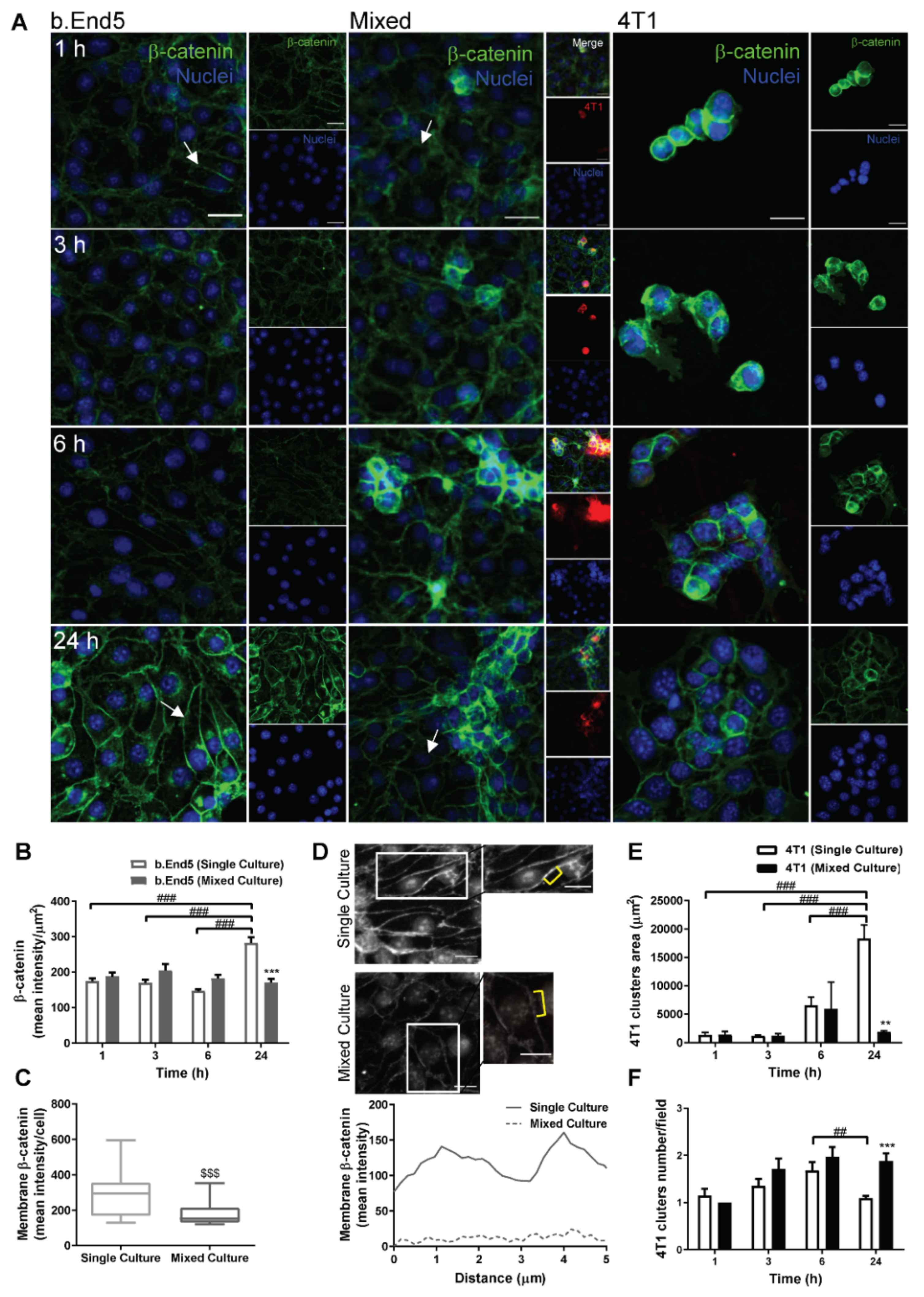
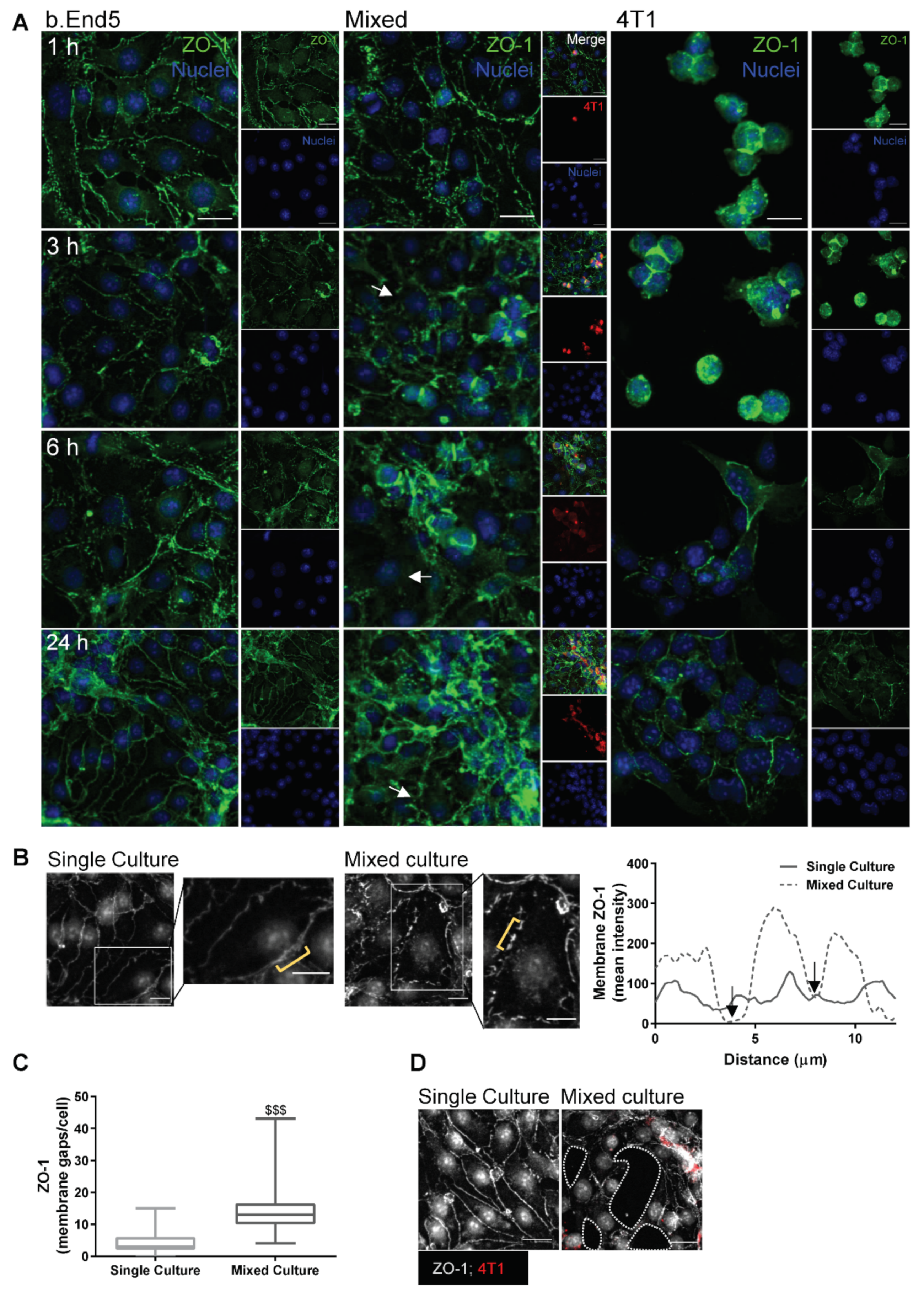
2.2. Adherens and Tight Junctional Complexes Are Compromised during BMEC–BCC Interaction
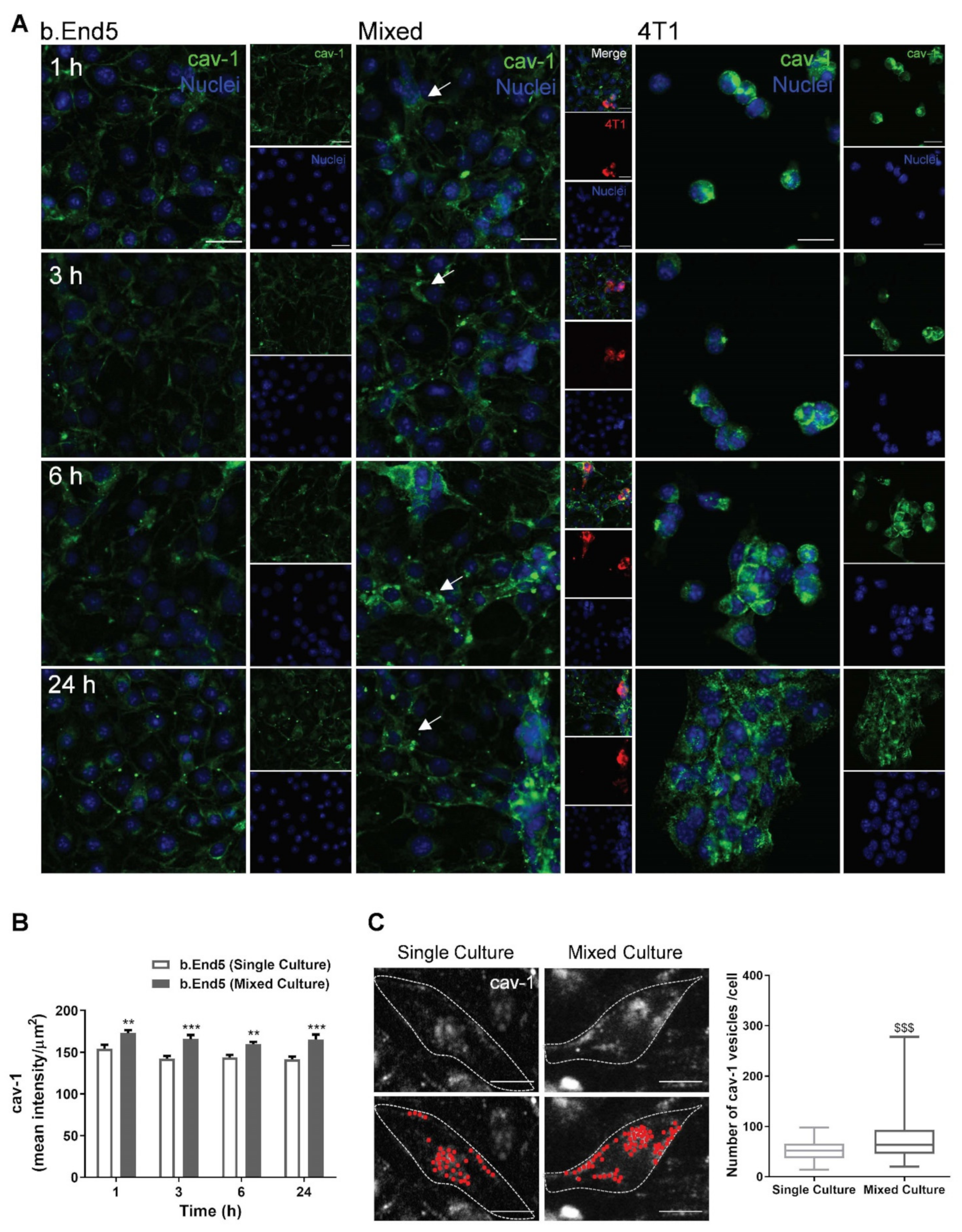
2.3. BBB Transcellular Permeability Increases with BMEC–BCC Interaction
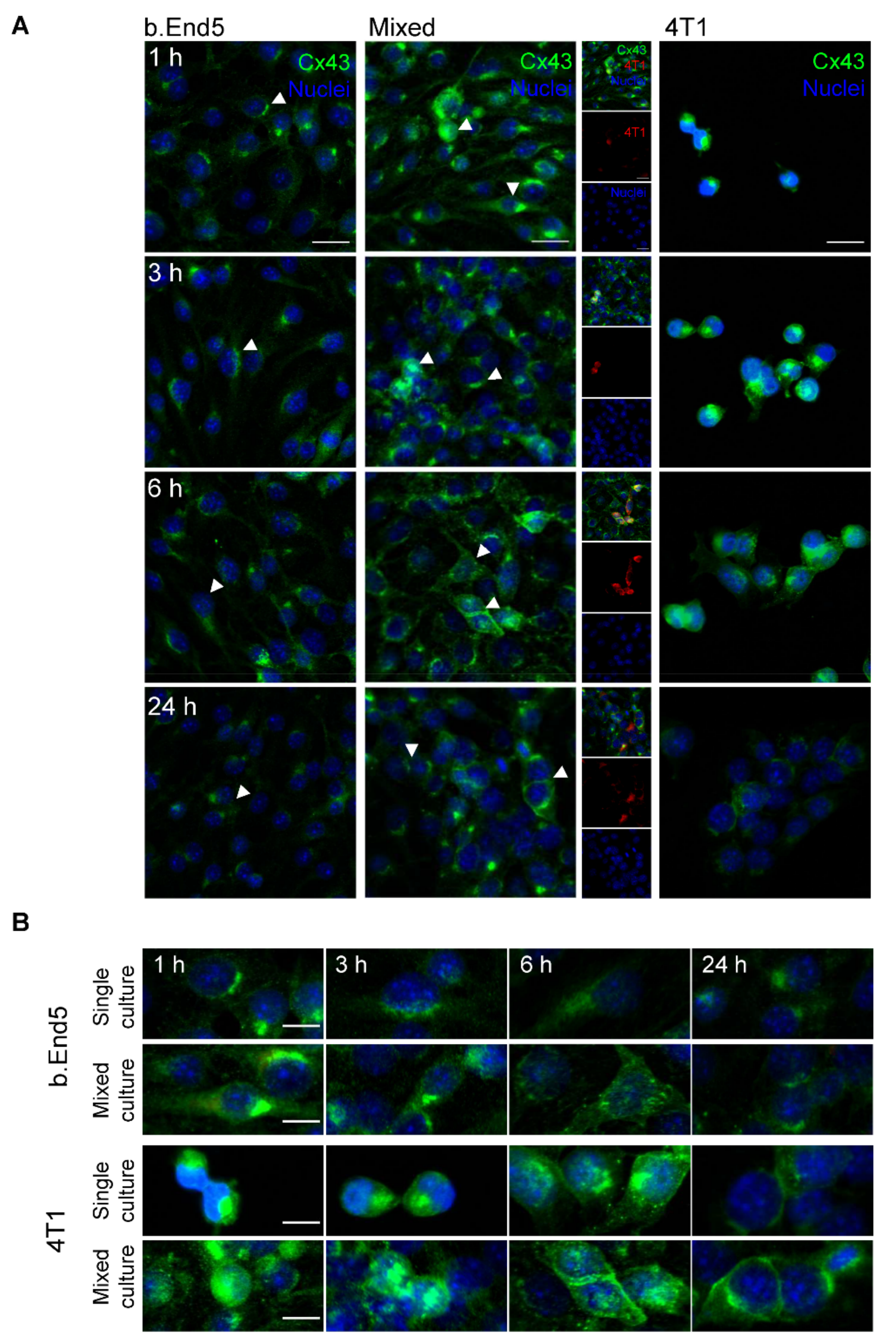
2.4. The GJs Protein Cx43 Is Involved in BMECs-BCCs Interaction
2.5. BMEC–BCC Interaction Leads to Cytoskeleton Alterations
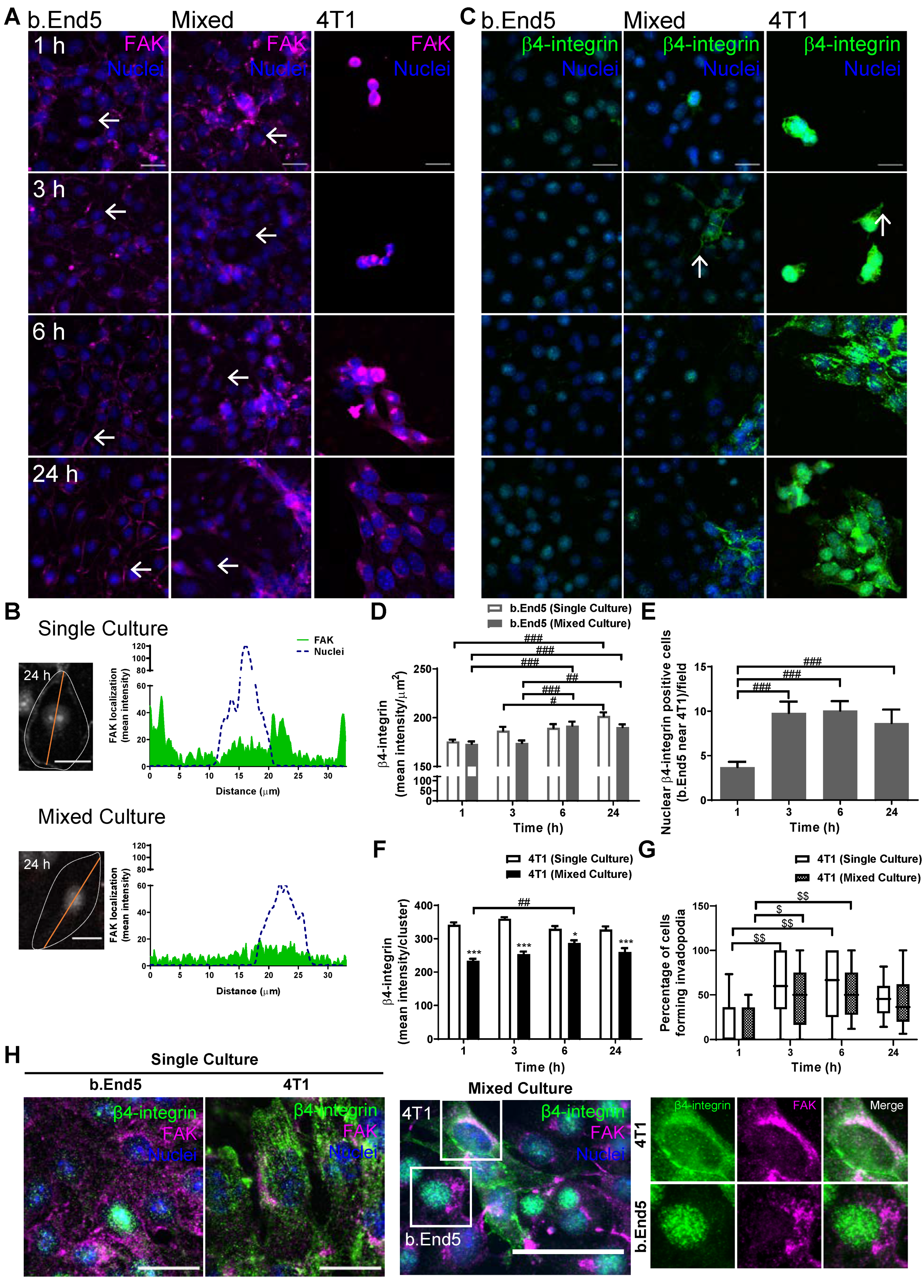
2.6. Adhesion-Related Signaling Pathway Activation Occurs during BMEC–BCC Interaction
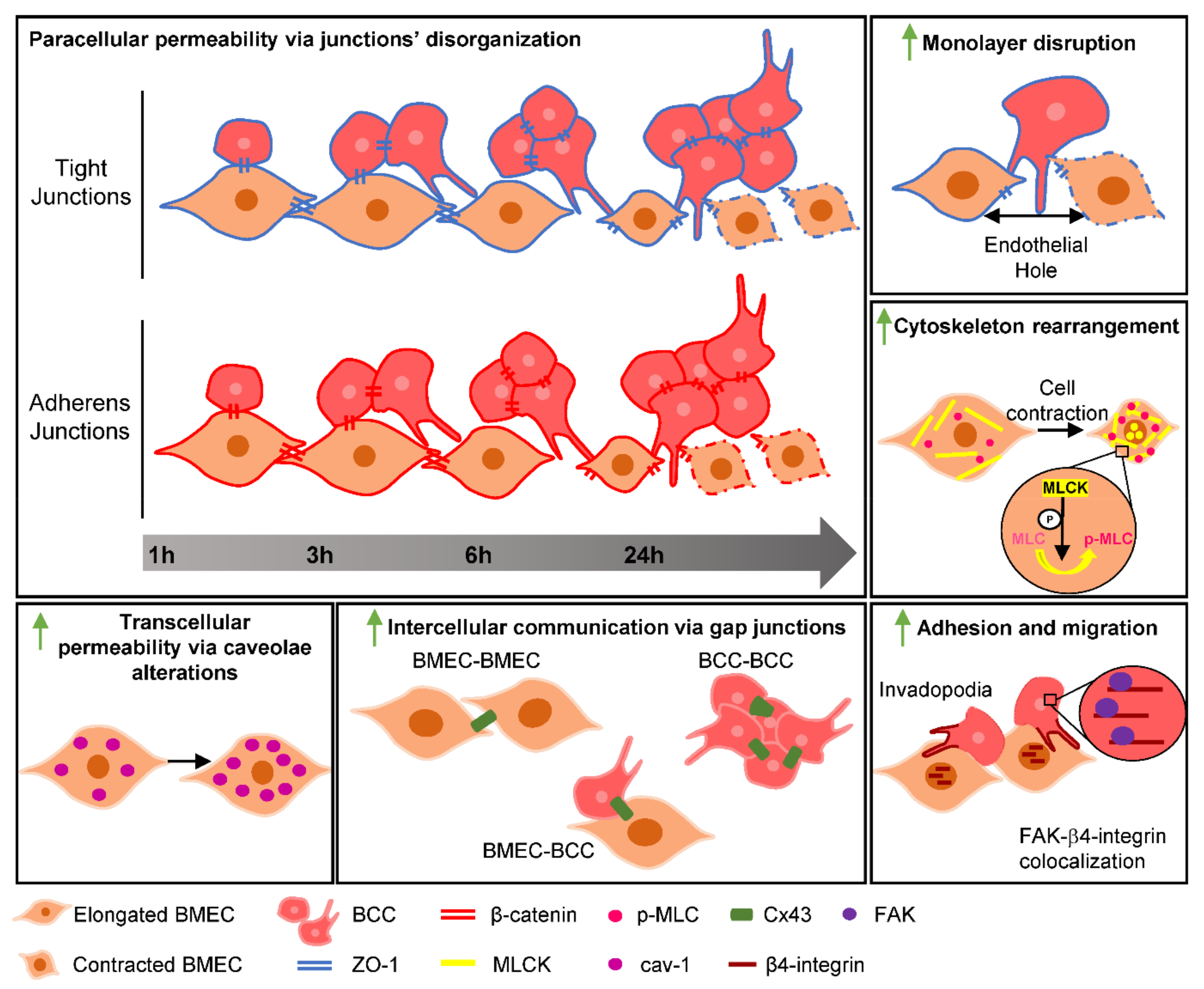
3. Discussion
4. Materials and Methods
4.1. Cell Culture Conditions
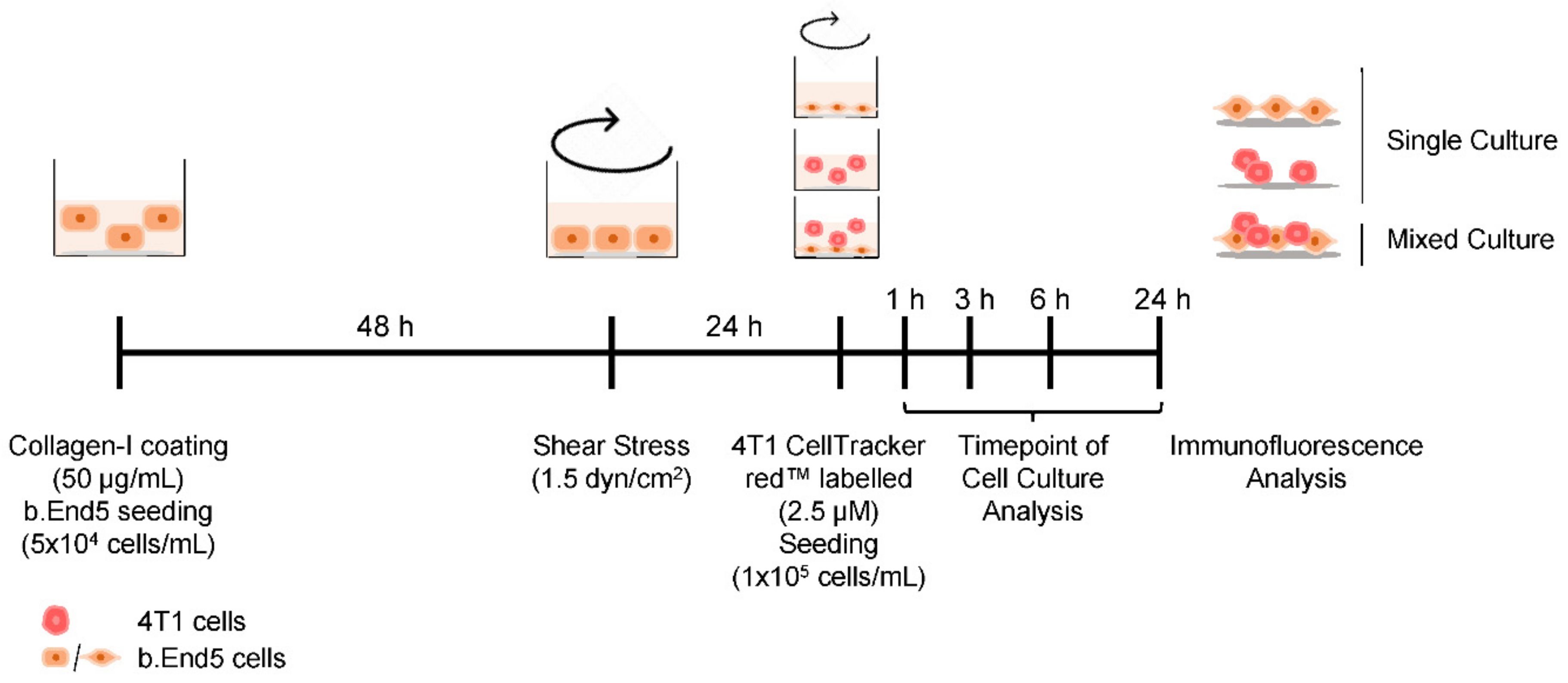
4.2. SS Application
4.3. BC Brain Metastasis Formation In Vitro Model Establishment
4.4. Immunofluorescence
4.5. Image Acquisition and Data Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AJ | Adherens Junction |
| BBB | Blood–Brain Barrier |
| BC | Breast Cancer |
| BCCs | Breast Cancer Cells |
| BMECs | Brain Microvascular Endothelial Cells |
| BSA | Bovine Serum Albumin |
| Cav-1 | Caveolin-1 |
| Cx43 | Connexin 43 |
| ECs | Endothelial Cells |
| FAK | Focal Adhesion Kinase |
| FBS | Foetal Bovine Serum |
| GJ | Gap Junction |
| MLC | Myosin Light Chain |
| MLCK | Myosin Light Chain Kinase |
| PBS | Phosphate Buffer Solution |
| p-MLC | Phosphorylated Myosin Light Chain |
| SS | Shear Stress |
| TJ | Tight Junction |
| TEM | Transendothelial Migration |
| ZO-1 | Zonula Occludens-1 |
References
- Sung, H.; Ferlay, F.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kimberly, D.; Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar]
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The lingering mysteries of metastatic recurrence in breast cancer. Br. J. Cancer 2021, 124, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Branco, V.; Pimentel, J.; Brito, M.A.; Carvalho, C. Thioredoxin, glutathione and related molecules in tumors of the nervous system. Curr. Med. Chem. 2020, 27, 1878–1900. [Google Scholar] [CrossRef]
- Krishnan, M.; Krishnamurthy, J.; Shonka, N. Targeting the sanctuary site: Options when breast cancer metastasizes to the brain. Oncology 2019, 33, 683730. [Google Scholar]
- Martin, A.M.; Cagney, D.N.; Catalano, P.J.; Warren, L.E.; Bellon, J.R.; Punglia, R.S.; Claus, E.B.; Lee, E.Q.; Wen, P.Y.; Haas-Kogan, D.A.; et al. Brain metastases in newly diagnosed breast cancer: A population-based study. JAMA Oncol. 2017, 3, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Custodio-Santos, T.; Videira, M.; Brito, M.A. Brain metastasization of breast cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 132–147. [Google Scholar] [CrossRef]
- Sökeland, G.; Schumacher, U. The functional role of integrins during intra- and extravasation within the metastatic cascade. Mol. Cancer 2019, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.A.; Blache, C.A.; Bajana, S.; Hasan, N.; Kamal, M.; Morita, Y.; Gupta, V.; Tsolmon, B.; Suh, K.S.; Gorenstein, D.G.; et al. The effect of soluble E-selectin on tumor progression and metastasis. BMC Cancer 2016, 16, 331. [Google Scholar]
- Blecharz, K.G.; Colla, R.; Rohde, V.; Vajkoczy, P. Control of the blood-brain barrier function in cancer cell metastasis. Biol. Cell 2015, 107, 342–371. [Google Scholar] [CrossRef]
- Schaller, M.D. Cellular functions of FAK kinases: Insight into molecular mechanisms and novel functions. J. Cell Sci. 2010, 123, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Kouam, P.N.; Bühler, H.; Hero, T.; Irenäus, A.; Adamietz, I.A. The increased adhesion of tumor cells to endothelial cells after irradiation can be reduced by FAK-inhibition. Radiat. Oncol. 2019, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.L.; Chu, P.Y.; Lai, I.R.; Wang, M.Y.; Tseng, H.Y.; Guan, J.L.; Liou, J.Y.; Shen, T.L. An EGFR/Src-dependent β4 integrin/FAK complex contributes to malignancy of breast cancer. Sci. Rep. 2015, 5, 16408. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Nam, J.-O.; Jean, C.; Lawson, C.; Walsh, C.T.; Goka, E.; Lim, S.-T.; Tomar, A.; Tancioni, I.; Uryu, S.; et al. VEGF-induced vascular permeability is mediated by FAK. Dev. Cell 2012, 22, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Jean, C.; Chen, X.L.; Nam, J.-O.; Tancioni, I.; Uryu, S.; Lawson, C.; Ward, K.K.; Walsh, C.T.; Miller, N.L.G.; Ghassemian, M.; et al. Inhibition of endothelial FAK activity prevents tumor metastasis by enhancing barrier function. J. Cell Biol. 2014, 204, 247–263. [Google Scholar] [CrossRef]
- Cardoso, F.L.; Brites, D.; Brito, M.A. Looking at the blood-brain barrier: Molecular anatomy and possible investigation approaches. Brain Res. Rev. 2010, 64, 328–363. [Google Scholar] [CrossRef]
- Garcia, A.R.; Godinho-Pereira, J.; Figueira, I.; Malhó, R.; Brito, M.A. Replicating the blood-brain barrier properties in an in vitro model: Effects of hydrocortisone and/or shear stress. Arch. Anat. 2019, 8, 4–20. [Google Scholar]
- Cucullo, L.; Hossain, M.; Puvenna, V.; Marchi, N.; Janigro, D. The role of shear stress in Blood-Brain Barrier endothelial physiology. BMC Neurosci. 2011, 12, 40. [Google Scholar] [CrossRef]
- Figueira, I.; Galego, S.; Custódio-Santos, T.; Vicente, R.; Molnár, K.; Haskó, J.; Malhó, R.; Videira, M.; Wilhelm, I.; Krizbai, I.; et al. Picturing breast cancer brain metastasis development to unravel molecular players and cellular crosstalk. Cancers 2021, 13, 910. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.S.; Robertson, A.E.; Stoletov, K.; Leith, S.J.; Chin, C.A.; Chien, A.E.; Hague, M.N.; Ablack, A.; Carmine-Simmen, K.; McPherson, V.A.; et al. Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep. 2014, 8, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, D.; Strilic, B.; Sivaraj, K.K.; Wettschureck, N.; Offermanns, S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 2013, 24, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Strilic, B.; Offermanns, S. Intravascular survival and extravasation of tumor cells. Cancer Cell 2017, 32, 282–293. [Google Scholar] [CrossRef]
- Herman, H.; Fazakas, C.; Hasko, J.; Molnar, K.; Meszaros, A.; Nyul-Toth, A.; Szabo, G.; Erdelyi, F.; Ardelean, A.; Hermenean, A.; et al. Paracellular and transcellular migration of metastatic cells through the cerebral endothelium. J. Cell. Mol. Med. 2019, 23, 2619–2631. [Google Scholar] [CrossRef] [PubMed]
- Haskó, J.; Fazakas, C.; Molnár, K.; Mészáros, Á.; Patai, R.; Szabó, G.; Erdélyi, F.; Nyúl-Tóth, A.; Győri, F.; Kozma, M.; et al. Response of the neurovascular unit to brain metastatic breast cancer cells. Acta Neuropathol. Commun. 2019, 7, 133. [Google Scholar] [CrossRef] [PubMed]
- Reymond, N.; d’Agua, B.B.; Ridley, A.J. Crossing the endothelial barrier during metastasis. Nat. Rev. Cancer 2013, 13, 858–870. [Google Scholar] [CrossRef]
- Shen, Q.; Wu, M.H.; Yuan, S.Y. Endothelial contractile cytoskeleton and microvascular permeability. Cell Health Cytoskelet. 2009, 2009, 43–50. [Google Scholar]
- Zhou, X.; Liu, Y.; You, J.; Zhang, H.; Zhang, X.; Ye, L. Myosin light-chain kinase contributes to the proliferation and migration of breast cancer cells through cross-talk with activated ERK1/2. Cancer Lett. 2008, 270, 312–327. [Google Scholar] [CrossRef] [PubMed]
- Khuon, S.; Liang, L.; Dettman, R.W.; Sporn, P.H.; Wysolmerski, R.B.; Chew, T.L. Myosin light chain kinase mediates transcellular intravasation of breast cancer cells through the underlying endothelial cells: A three-dimensional FRET study. J. Cell Sci. 2010, 123, 431–440. [Google Scholar] [CrossRef]
- Andreone, B.J.; Chow, B.W.; Tata, A.; Lacoste, B.; Ben-Zvi, A.; Bullock, K.; Deik, A.A.; Ginty, D.D.; Clish, C.B.; Gu, C. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron 2017, 94, 581–594.e5. [Google Scholar] [CrossRef]
- Qian, X.L.; Pan, Y.H.; Huang, Q.Y.; Shi, Y.B.; Huang, Q.Y.; Hu, Z.Z.; Xiong, L.X. Caveolin-1: A multifaceted driver of breast cancer progression and its application in clinical treatment. OncoTargets Ther. 2019, 12, 1539–1552. [Google Scholar] [CrossRef]
- Li, S.; Chen, Y.; Zhang, Y.; Jiang, X.; Jiang, Y.; Qin, X.; Yang, H.; Wu, C.; Liu, Y. Shear stress promotes anoikis resistance of cancer cells via caveolin-1-dependent extrinsic and intrinsic apoptotic pathways. J. Cell. Physiol. 2019, 234, 3730–3743. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhu, X.; Chen, Y.; Yin, Y.; Ma, T. Tubeimoside V sensitizes human triple negative breast cancer MDA-MB-231 cells to anoikis via regulating caveolin-1-related signaling pathways. Arch. Biochem. Biophys. 2018, 646, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.-T.; Lee, H.-T.; Huang, F.-J.; Aldape, K.D.; Yao, J.; Steeg, P.S.; Chou, C.-Y.; Lu, Z.; Xie, K.; Huang, S. Caveolin-1 upregulation mediates suppression of primary breast tumor growth and brain metastases by stat3 inhibition. Cancer Res. 2011, 71, 4932–4943. [Google Scholar] [CrossRef]
- Wu, J.I.; Wang, L.H. Emerging roles of gap junction proteins connexins in cancer metastasis, chemoresistance and clinical application. J. Biomed. Sci. 2019, 26, 8. [Google Scholar] [CrossRef]
- Johnson, A.M.; Roach, J.P.; Hu, A.; Stamatovic, S.M.; Michal, R.; Zochowski, M.R.; Keep, R.F.; Andjelkovic, A.V. Connexin 43 gap junctions contribute to brain endothelial barrier hyperpermeability in familial cerebral cavernous malformations type III by modulating tight junction structure. FASEB J. 2018, 32, 2615–2629. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.L.; Williams, C.B.; Zambrano, J.N.; Williams, C.J.; Yeh, E.S. Connexin 43 in the development and progression of breast cancer: What’s the connection? Int. J. Oncol. 2017, 51, 1005–1013. [Google Scholar] [CrossRef]
- Naser Al Deen, N.; AbouHaidar, M.; Talhouk, R. Connexin43 as a tumor suppressor: Proposed Connexin43 mRNA-circularRNAs-microRNAs axis towards prevention and early detection in breast cancer. Front. Med. 2019, 6, 192. [Google Scholar] [CrossRef]
- Pollmann, M.-A.; Shao, Q.; Laird, D.W.; Sandig, M. Connexin 43 mediated gap junctional communication enhances breast tumor cell diapedesis in culture. Breast Cancer Res. 2005, 7, R522–R534. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Rigor, R.R.; Shen, Q.; Pivetti, C.D.; Wu, M.H.; Yuan, S.Y. Myosin light chain kinase signaling in endothelial barrier dysfunction. Med. Res. Rev. 2013, 33, 911–933. [Google Scholar] [CrossRef]
- Roy-Luzarraga, M.; Hodivala-Dilke, K. Molecular pathways: Endothelial cell FAK—A target for cancer treatment. Clin. Cancer Res. 2016, 22, 3718. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.F.; Ribeiro, A.S.; Dionisio, M.R.; Sousa, B.; Nobre, A.R.; Albergaria, A.; Santiago-Gomez, A.; Mendes, N.; Gerhard, R.; Schmitt, F.; et al. P-cadherin signals through the laminin receptor alpha6beta4 integrin to induce stem cell and invasive properties in basal-like breast cancer cells. Oncotarget 2014, 5, 679–692. [Google Scholar] [CrossRef]
- Chiang, S.P.; Cabrera, R.M.; Segall, J.E. Tumor cell intravasation. Am. J. Physiol. Cell Physiol. 2016, 311, C1–C14. [Google Scholar] [CrossRef]
- Wang, W.-C.; Zhang, X.-F.; Peng, J.; Li, X.-F.; Wang, A.-L.; Bie, Y.-Q.; Shi, L.-H.; Lin, M.-B.; Zhang, X.-F. Survival Mechanisms and influence factors of circulating tumor cells. Biomed. Res. Int. 2018, 2018, 6304701. [Google Scholar] [CrossRef] [PubMed]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Lintz, M.; Muñoz, A.; Reinhart-King, C.A. The mechanics of single cell and collective migration of tumor cells. J. Biomech. Eng. 2017, 139, 0210051–0210059. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, H.; Zhan, Y.; Fan, S. An emerging tumor invasion mechanism about the collective cell migration. Am. J. Transl. Res. 2019, 11, 5301–5312. [Google Scholar] [PubMed]
- Plutoni, C.; Keil, S.; Zeledon, S.; Delsin, L.E.A.; Decelle, B.; Roux, P.P.; Carréno, S.; Emery, G. Misshapen coordinates protrusion restriction and actomyosin contractility during collective cell migration. Nat. Commun. 2019, 10, 3940. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.A.; Ilina, O.; Vasaturo, A.; Venhuizen, J.-H.; Vullings, M.; Venhuizen, V.; Bilos, A.; Figdor, C.; Span, P.N.; Friedl, P. Leader cell activity and collective invasion by an autocrine nucleotide loop through connexin-43 hemichannels and ADORA1. bioRxiv 2019. Available online: https://www.biorxiv.org/content/10.1101/2019.12.30.888958v1.full (accessed on 12 January 2021).
- Chen, M.B.; Whisler, J.A.; Jeon, J.S.; Kamm, R.D. Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Integr. Biol. 2013, 5, 1262–1271. [Google Scholar] [CrossRef]
- Wrenn, E.D.; Yamamoto, A.; Moore, B.M.; Huang, Y.; McBirney, M.; Thomas, A.J.; Greenwood, E.; Rabena, Y.F.; Rahbar, H.; Partridge, S.C.; et al. Regulation of collective metastasis by nanolumenal signaling. Cell 2020, 183, 395–410.e19. [Google Scholar] [CrossRef] [PubMed]
- Avraham, H.K.; Jiang, S.; Fu, Y.; Nakshatri, H.; Ovadia, H.; Avraham, S. Angiopoietin-2 mediates blood-brain barrier impairment and colonization of triple-negative breast cancer cells in brain. J. Pathol. 2014, 232, 369–381. [Google Scholar] [CrossRef]
- Li, B.; Zhao, W.D.; Tan, Z.M.; Fang, W.G.; Zhu, L.; Chen, Y.H. Involvement of Rho/ROCK signalling in small cell lung cancer migration through human brain microvascular endothelial cells. FEBS Lett. 2006, 580, 4252–4260. [Google Scholar] [CrossRef]
- Fan, J.; Fu, B.M. Quantification of malignant breast cancer cell MDA-MB-231 transmigration across brain and lung microvascular endothelium. Ann. Biomed. Eng. 2016, 44, 2189–2201. [Google Scholar] [CrossRef] [PubMed]
- Weidert, E.; Pohler, S.E.; Gomez, E.W.; Dong, C. Actinomyosin contraction, phosphorylation of VE-cadherin, and actin remodeling enable melanomainduced endothelial cell-cell junction disassembly. PLoS ONE 2014, 9, e108092. [Google Scholar]
- Carman, C.V. Mechanisms for transcellular diapedesis: Probing and pathfinding by ‘invadosome-like protrusions’. J. Cell Sci. 2009, 122, 3025–3035. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Huang, Q.; Wang, F.; Liu, Y.; Wang, Z.; Wang, Z.; Zhang, Q.; Lei, B.; Cheng, Y. The role of caveolin-1 in blood-brain barrier disruption induced by focused ultrasound combined with microbubbles. J. Mol. Neurosci. 2012, 46, 677–687. [Google Scholar] [CrossRef]
- Sotgia, F.; Williams, T.M.; Schubert, W.; Medina, F.; Minetti, C.; Pestell, R.G.; Lisanti, M.P. Caveolin-1 deficiency (−/−) conveys premalignant alterations in mammary epithelia, with abnormal lumen formation, growth factor independence, and cell invasiveness. Am. J. Pathol. 2006, 168, 292–309. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.W.; Hnasko, R.; Schubert, W.; Lisanti, M.P. Role of caveolae and caveolins in health and disease. Physiol. Rev. 2004, 84, 1341–1379. [Google Scholar] [CrossRef]
- Nag, S.; Venugopalan, R.; Stewart, D.J. Increased caveolin-1 expression precedes decreased expression of occludin and claudin-5 during blood–brain barrier breakdown. Acta Neuropathol. 2007, 114, 459–469. [Google Scholar] [CrossRef]
- Arvanitis, C.; Khuon, S.; Spann, R.; Ridge, K.M.; Chew, T.L. Structure and biomechanics of the endothelial transcellular circumferential invasion array in tumor invasion. PLoS ONE 2014, 9, e89758. [Google Scholar] [CrossRef][Green Version]
- Li, Q.; Sarna, S.K. Nuclear myosin II regulates the assembly of preinitiation complec for ICAM-1 gene transcription. Gastroenterology 2009, 137, 1051–1060.e3. [Google Scholar] [CrossRef]
- Thomas, D.; Thiagarajan, P.S.; Rai, V.; Reizes, O.; Lathia, J.; Egelhoff, T. Increased cancer stem cell invasion is mediated by myosin IIB and nuclear translocation. Oncotarget 2016, 7, 47586–47592. [Google Scholar] [CrossRef] [PubMed]
- Stoletov, K.; Strnadel, J.; Zardouzian, E.; Momiyama, M.; Park, F.D.; Kelber, J.A.; Pizzo, D.P.; Hoffman, R.; VandenBerg, S.R.; Klemke, R.L. Role of connexins in metastatic breast cancer and melanoma brain colonization. J. Cell Sci. 2013, 126, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Elzarrad, M.K.; Haroon, A.; Willecke, K.; Dobrowolski, R.; Gillespie, M.N.; Al-Mehdi, A.B. Connexin-43 upregulation in micrometastases and tumor vasculature and its role in tumor cell attachment to pulmonary endothelium. BMC Med. 2008, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.F.; Cheng, Y.K.; Lu, D.Y.; Wang, S.L.; Chang, C.N.; Chang, P.C.; Yeh, W.L. Inhibition of estrogen receptor reduces connexin 43 expression in breast cancers. Toxicol. Appl. Pharmacol. 2018, 338, 182–190. [Google Scholar] [CrossRef]
- Miranti, C.K.; Brugge, J.S. Sensing the environment: A historical perspective on integrin signal transduction. Nat. Cell Biol. 2002, 4, E83–E90. [Google Scholar] [CrossRef]
- Zhao, J.; Guan, J.L. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009, 28, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Infusino, G.A.; Jacobson, J.R. Endothelial FAK as a therapeutic target in disease. Microvasc. Res. 2012, 83, 89–96. [Google Scholar] [CrossRef]
- Guo, X.; Eitnier, R.A.; Beard, R.S., Jr.; Meegan, J.E.; Yang, X.; Aponte, A.M.; Wang, F.; Nelson, P.R.; Wu, M.H. Focal adhesion kinase and Src mediate microvascular hyperpermeability caused by fibrinogen-γC-terminal fragments. PLoS ONE 2020, 15, e0231739. [Google Scholar] [CrossRef]
- Mehta, D.; Tiruppathi, C.; Sandoval, R.; Minshall, R.D.; Holinstat, M.; Malik, A.B. Modulatory role of focal adhesion kinase in regulating human pulmonary arterial endothelial barrier function. J. Physiol. 2002, 539, 779–789. [Google Scholar] [CrossRef]
- Nikolopoulos, S.N.; Blaikie, P.; Yoshioka, T.; Guo, W.; Puri, C.; Tacchetti, C.; Giancotti, F.G. Targeted deletion of the integrin β4 signaling domain suppresses laminin-5-dependent nuclear entry of mitogen-activated protein kinases and NF-kB, causing defects in epidermal growth and migration. Mol. Cell. Biol. 2005, 25, 6090–6102. [Google Scholar] [CrossRef] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Stewart, R.L.; O’Connor, K.L. Clinical significance of the integrin alpha6beta4 in human malignancies. Lab. Investig. 2015, 95, 976–986. [Google Scholar] [CrossRef]
- Ge, D.; Kong, X.; Liu, W.; Zhao, J.; Su, L.; Zhang, S.; Zhang, Y.; Zhao, B.; Miao, J. Phosphorylation and nuclear translocation of integrin β4 induced by a chemical small molecule contribute to apoptosis in vascular endothelial cells. Apoptosis 2013, 18, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Peláez, R.; Pariente, A.; Pérez-Sala, A.; Larrayoz, I.M. Integrins: Moonlighting proteins in invadosome formation. Cancers 2019, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Genna, A.; Lapetina, S.; Lukic, N.; Twafra, S.; Meirson, T.; Sharma, V.P.; Condeelis, J.S.; Hava Gil-Henn, H. Pyk2 and FAK differentially regulate invadopodia formation and function in breast cancer cells. J. Cell Biol. 2018, 217, 375–395. [Google Scholar] [CrossRef]
- Tai, Y.L.; Lai, I.R.; Peng, Y.J.; Ding, S.T.; Shen, T.L. Activation of focal adhesion kinase through an interaction with β4 integrin contributes to tumorigenicity of colon cancer. FEBS Lett. 2016, 590, 1826–1837. [Google Scholar] [CrossRef]
- Colgan, O.C.; Ferguson, G.; Collins, N.T.; Murphy, R.P.; Meade, G.; Cahill, P.A.; Cummins, P.M. Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H3190–H3197. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, R.J.; Cahill, P.A.; Sitzmann, J.V.; Redmond, E.M. Ethanol enhances basal and flow-stimulated nitric oxide synthase activity in vitro by activating an inhibitory guanine nucleotide binding protein. J. Pharmacol. Exp. Ther. 1999, 289, 1293–1300. [Google Scholar]
- Wimmer, I.; Tietz, S.; Nishihara, H.; Deutsch, U.; Sallusto, F.; Gosselet, F.; Lyck, R.; Muller, W.A.; Lassmann, H.; Engelhardt, B. PECAM-1 stabilizes blood-brain barrier integrity and favors paracellular T-cell diapedesis across the blood-brain barrier during neuroinflammation. Front. Immunol. 2019, 10, 711. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, H.; Klopstein, A.; Gruber, I.; Blatti, C.; Lyck, R.; Engelhardt, B. Postarrest stalling rather than crawling favors CD8+ over CD4+ T-cell migration across the blood-brain barrier under flow in vitro. Eur. J. Immunol. 2016, 46, 2187–2203. [Google Scholar] [CrossRef] [PubMed]


| Target Protein | Permeabilisation | Blocking | Primary Antibody | Secondary Antibody |
|---|---|---|---|---|
| β-catenin | 0.3% Triton X-100 | 3% BSA | β-catenin (1:100) Thermo Fisher Scientific, #71-2700, Rabbit | Alexa Fluor® 488 (1:500) Thermo Fisher Scientific, #A21206, Goat Anti-Rabbit |
| β4-Integrin | 0.3% Triton X-100 | 3% BSA | β4-integrin (1:50) Santa Cruz Biotechnology, #sc-514426, Mouse | Alexa Fluor® 488 (1:500) Thermo Fisher Scientific, #A11001 Goat Anti-Mouse |
| Cav-1 | 0.1% Saponin in 3% BSA | 0.1% Saponin in 3% BSA | Caveolin-1 (1:100) Cell Signaling, #3238S, Rabbit | Alexa Fluor® 488 (1:500) Thermo Fisher Scientific, #A21206, Goat Anti-Rabbit |
| Cx43 | 0.3% Triton X-100 | 3% BSA | Cx43 (1:50) Thermo Fisher Scientific, #35-5000, Mouse | Alexa Fluor® 488 (1:500) Thermo Fisher Scientific, #A11001 Goat Anti-Mouse |
| FAK | 0.3% Triton X-100 | 3% BSA | FAK (1:200) Abcam, #ab131435, Rabbit | Alexa Fluor® 488 (1:500) Thermo Fisher Scientific, #A21206, Goat Anti-Rabbit |
| MLCK | 0.3% Triton X-100 | 3% BSA | MLCK (1:100) Thermo Fisher Scientific, #PA515177, Rabbit | Alexa Fluor® 488 (1:500) Thermo Fisher Scientific, #A21206, Goat Anti-Rabbit |
| p-MLC | 0.3% Triton X-100 | 3% BSA | p-MLC (1:400) Thermo Fisher Scientific, #MA5-15163, Mouse | Alexa Fluor® 488 (1:500) Thermo Fisher Scientific, #A11001 Goat Anti-Mouse |
| ZO-1 | 0.3% Triton X-100 | 3% BSA | ZO-1 (1:200) Thermo Fisher Scientific, #40-2200, Rabbit | Alexa Fluor® 488 (1:500) Thermo Fisher Scientific, #A21206, Goat Anti-Rabbit |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godinho-Pereira, J.; Garcia, A.R.; Figueira, I.; Malhó, R.; Brito, M.A. Behind Brain Metastases Formation: Cellular and Molecular Alterations and Blood–Brain Barrier Disruption. Int. J. Mol. Sci. 2021, 22, 7057. https://doi.org/10.3390/ijms22137057
Godinho-Pereira J, Garcia AR, Figueira I, Malhó R, Brito MA. Behind Brain Metastases Formation: Cellular and Molecular Alterations and Blood–Brain Barrier Disruption. International Journal of Molecular Sciences. 2021; 22(13):7057. https://doi.org/10.3390/ijms22137057
Chicago/Turabian StyleGodinho-Pereira, Joana, Ana Rita Garcia, Inês Figueira, Rui Malhó, and Maria Alexandra Brito. 2021. "Behind Brain Metastases Formation: Cellular and Molecular Alterations and Blood–Brain Barrier Disruption" International Journal of Molecular Sciences 22, no. 13: 7057. https://doi.org/10.3390/ijms22137057
APA StyleGodinho-Pereira, J., Garcia, A. R., Figueira, I., Malhó, R., & Brito, M. A. (2021). Behind Brain Metastases Formation: Cellular and Molecular Alterations and Blood–Brain Barrier Disruption. International Journal of Molecular Sciences, 22(13), 7057. https://doi.org/10.3390/ijms22137057







