Protective Effects of a Hyaluronan-Binding Peptide (P15-1) on Mesenchymal Stem Cells in an Inflammatory Environment
Abstract
1. Introduction
2. Results
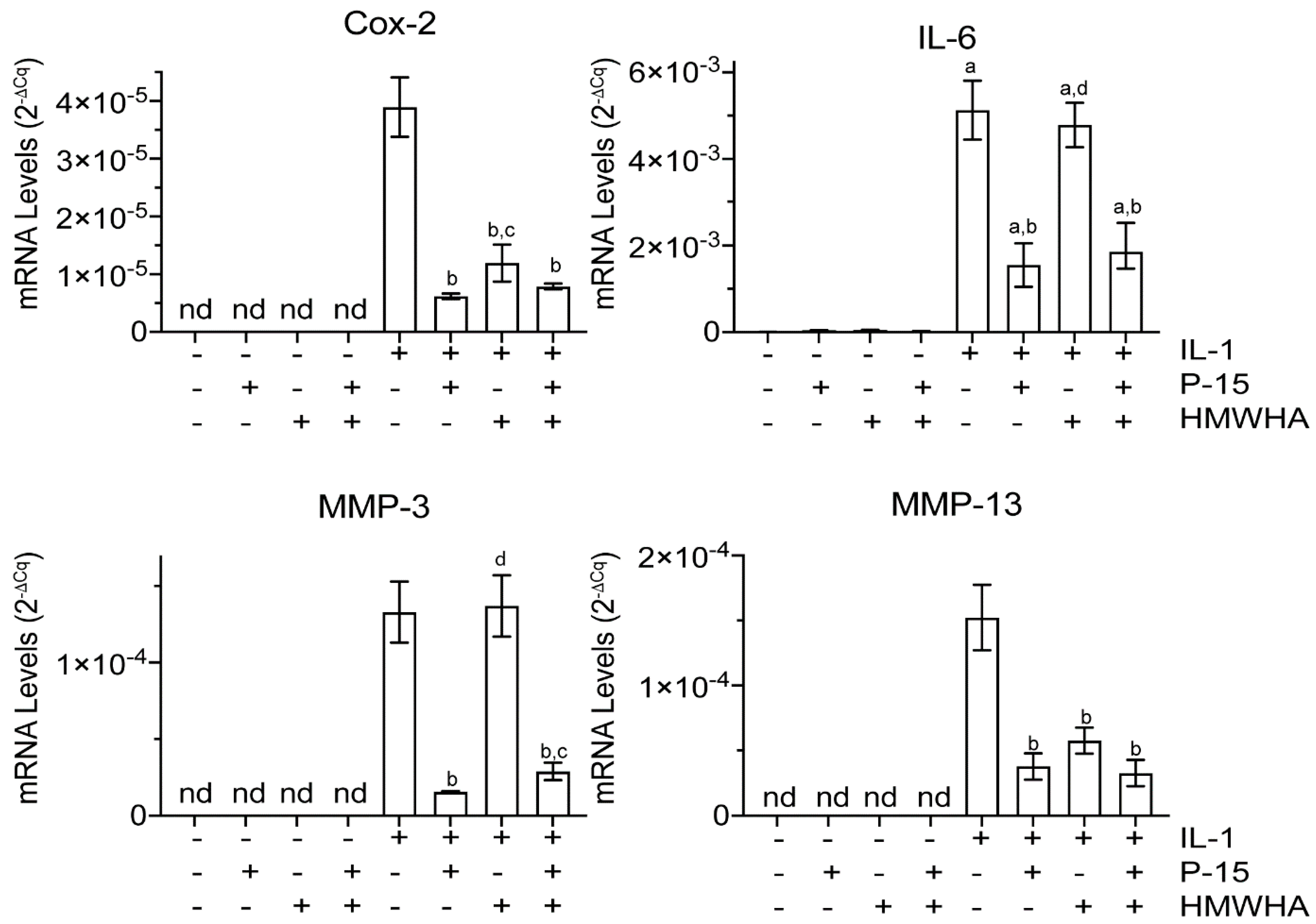
2.1. P15-1 Decreases Catabolic and Inflammatory Events in Human BMSCs
2.2. P15-1 Decreases the mRNA Levels of Inflammatory Receptors TLR2 and TLR4 in IL-1β-Treated Human BMSCs
2.3. P15-1 Affects the mRNA Levels of HA Receptor CD44 and RHAMM Receptor and TSG-6 in Human BMSCs
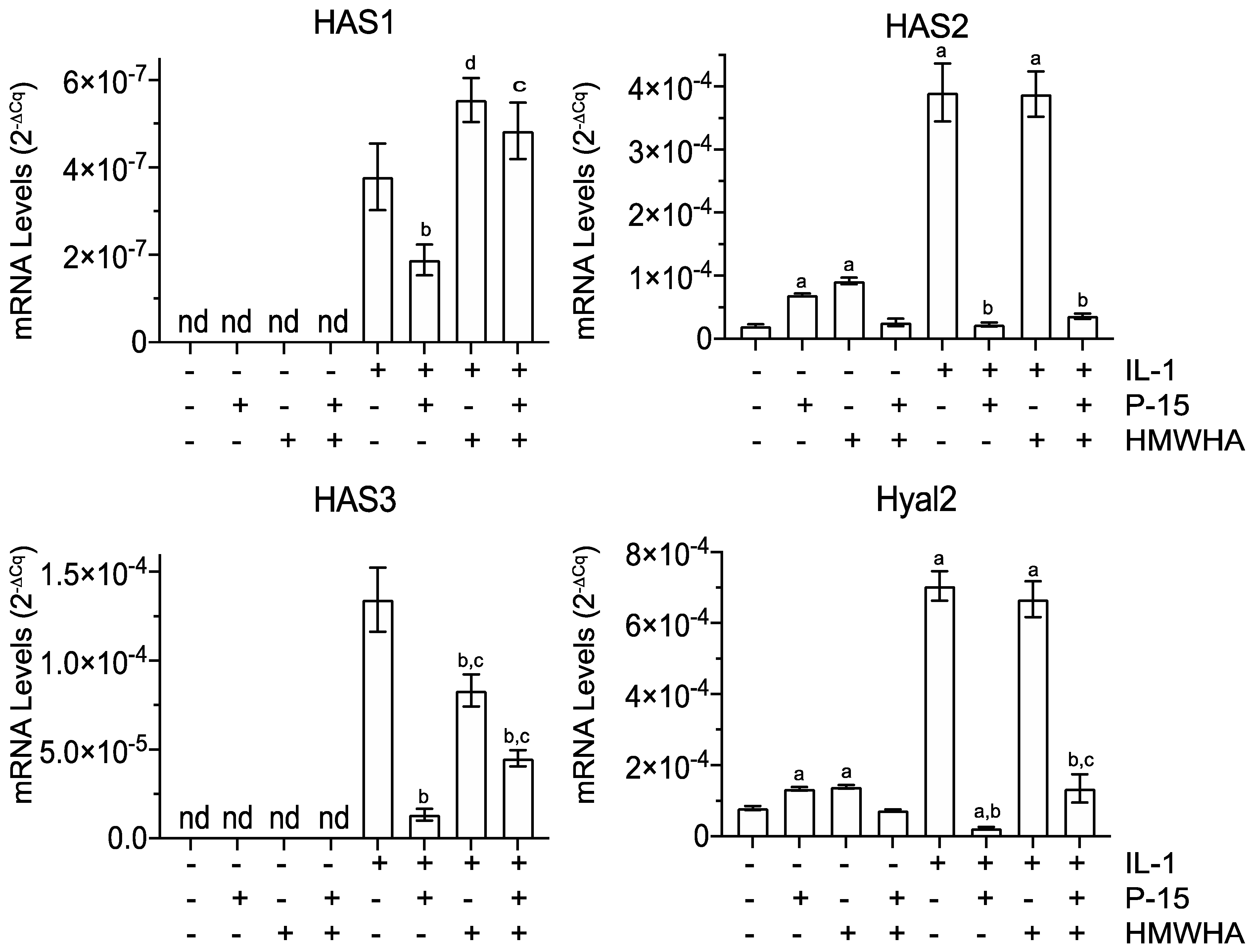
2.4. P15-1 Alters the mRNA Levels of HA-Producing and -Degrading Enzymes in BMSCs
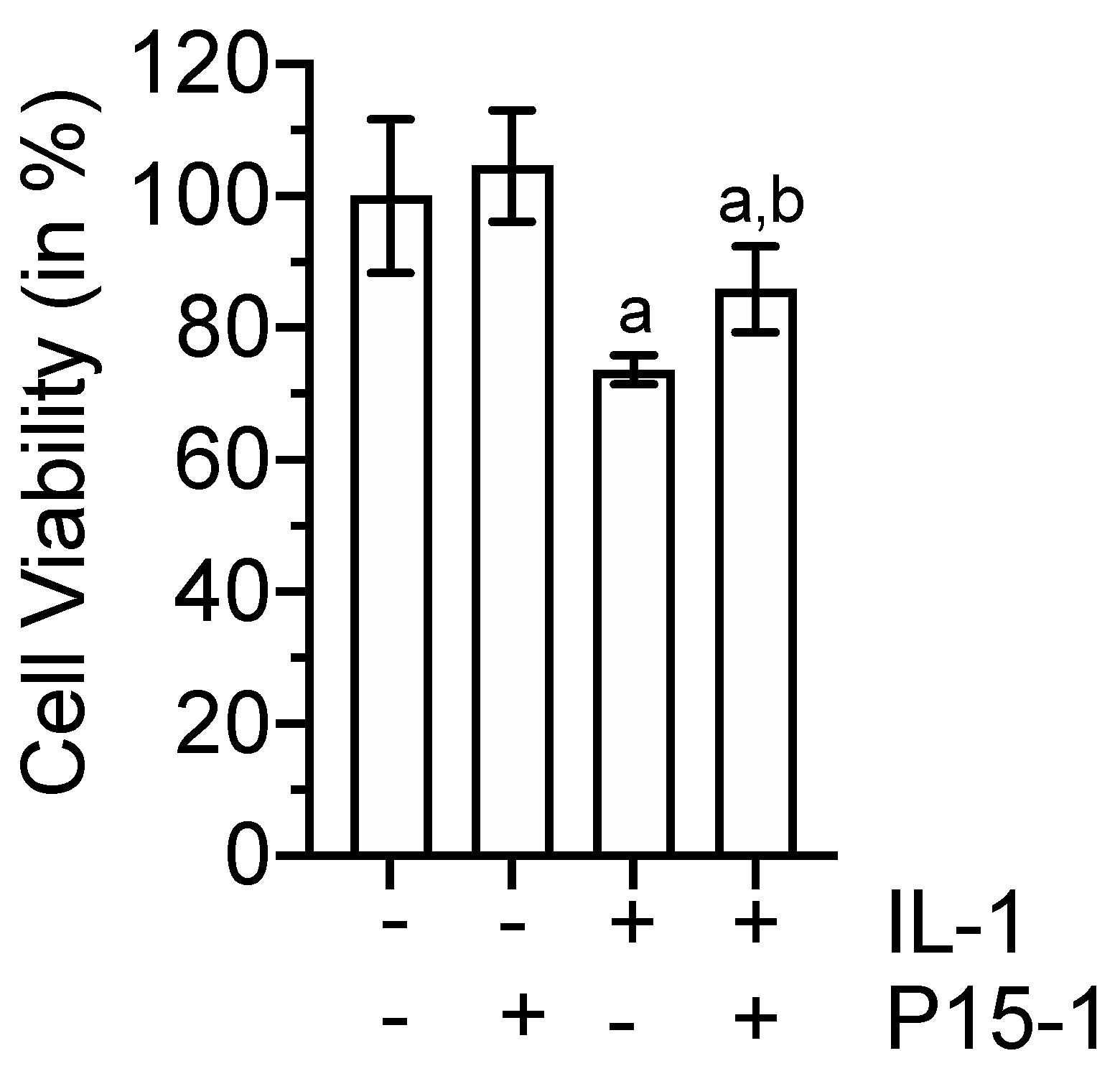
2.5. P15-1 Enhances Viability of IL-1β-Treated BMSCs
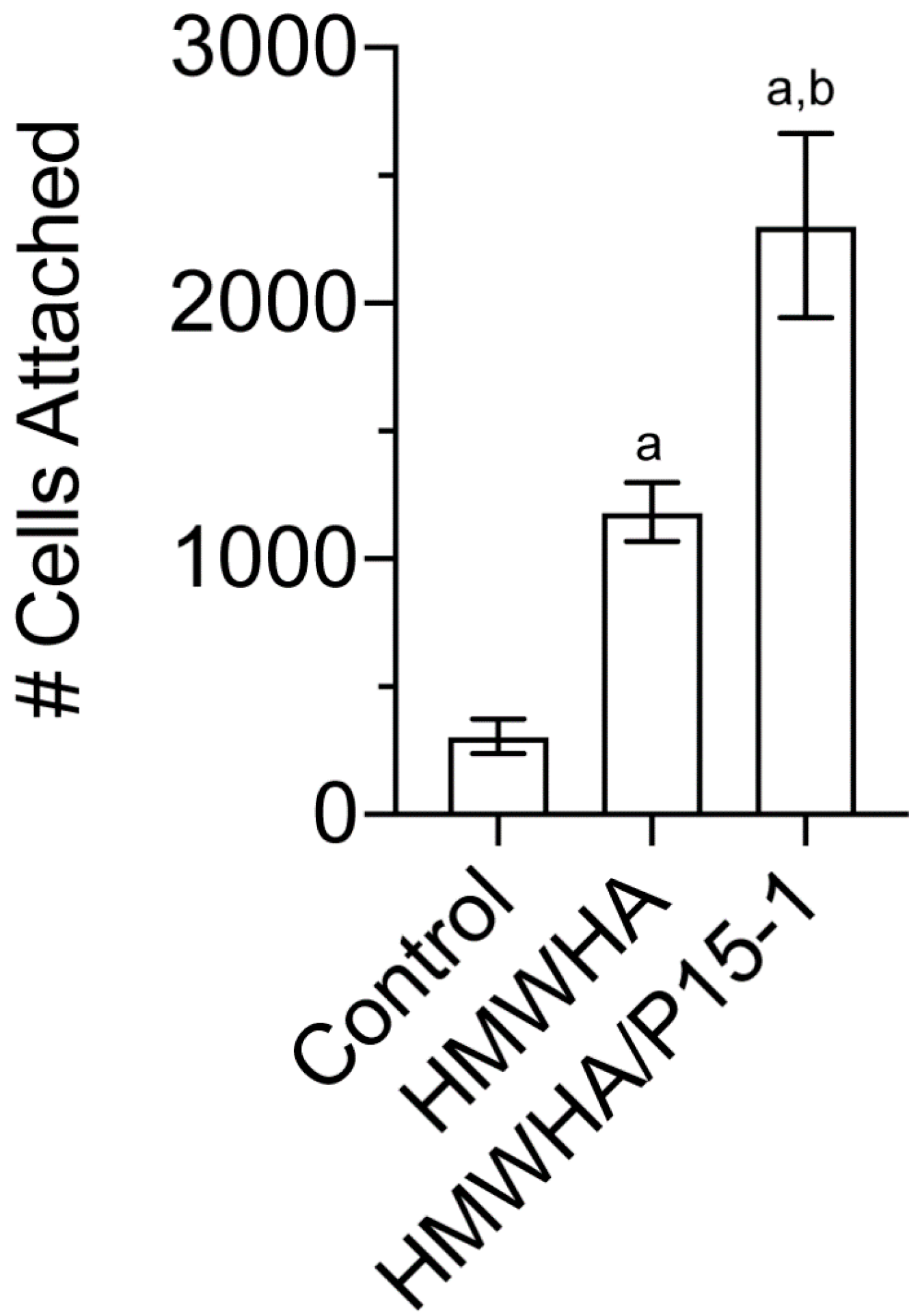
2.6. P15-1 Enhances the Attachment of BMSCs to HA-Coated Tissue Culture Dishes
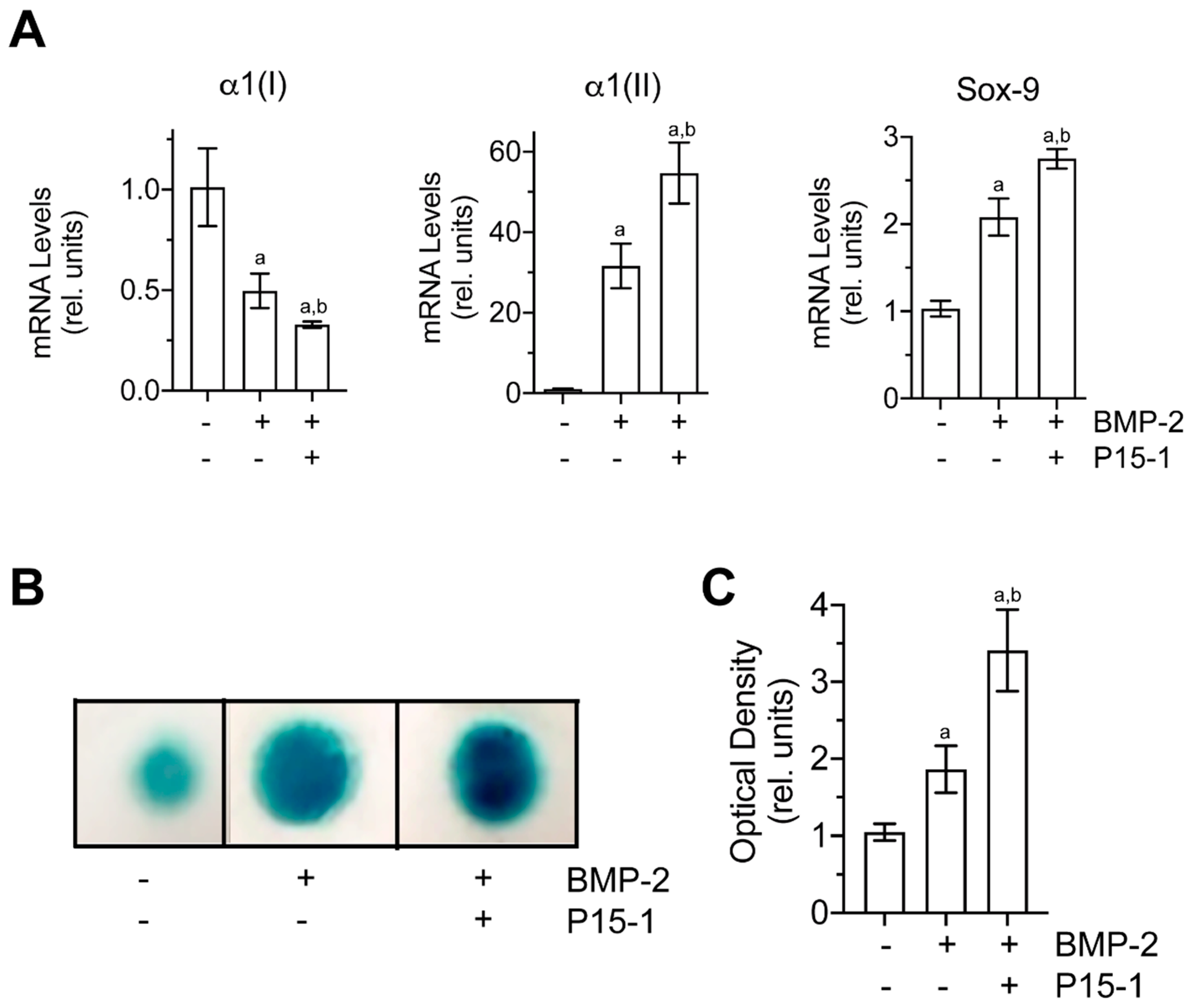
2.7. P15-1 Enhances the Chondrogenic Differentiation of C3H10T1/2 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Human BMSC Culture
4.3. Chondrogenic Differentiation of Murine Multipotential Stem Cell Line C3H10T1/2
4.4. Cell Attachment Assay
4.5. Reverse Transcription–PCR and Real-Time PCR Analysis
4.6. Statistical Analysis
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMP-2 | bone morphogenetic protein-2 |
| BMSCs | bone marrow-derived mesenchymal stem cells |
| BSA | bovine serum albumin |
| Cox-2 | cyclooxygenase-2 |
| ECM | extracellular matrix |
| EU | Endotoxin unit |
| EVs | extracellular vesicles |
| FCS | fetal calf serum |
| FGF | fibroblast growth factor |
| GAG | glycosaminoglycan |
| IL-1β | interleukin-1beta |
| IL-6 | interleukin-6 |
| HA | hyaluronan |
| HAS | hyaluronan synthase |
| HMWHA | high-molecular-weight hyaluronan |
| Hyal2 | hyaluronidase 2 |
| MMP | matrix metalloproteinase |
| MSCs | mesenchymal stem cells |
| OA | osteoarthritis |
| PCR | polymerase chain reaction |
| RHAMM | receptor for hyaluronan-mediated motility |
| SD | standard deviation |
| TLR | Toll-like receptor |
| TSG-6 | tumor necrosis factor-stimulated gene-6 |
References
- Barry, F.; Murphy, M. Mesenchymal stem cells in joint disease and repair. Nat. Rev. Rheumatol. 2013, 9, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells: Time to change the name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef]
- Kurtz, A. Mesenchymal stem cell delivery routes and fate. Int. J. Stem Cells 2008, 1, 1–7. [Google Scholar] [CrossRef]
- Garcia-Olmo, D.; Garcia-Arranz, M.; Herreros, D.; Pascual, I.; Peiro, C.; Rodriguez-Montes, J.A. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis. Colon Rectum 2005, 48, 1416–1423. [Google Scholar] [CrossRef]
- Badiavas, E.V.; Abedi, M.; Butmarc, J.; Falanga, V.; Quesenberry, P. Participation of bone marrow derived cells in cutaneous wound healing. J. Cell. Physiol. 2003, 196, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Geesala, R.; Dhoke, N.R.; Das, A. Cox-2 inhibition potentiates mouse bone marrow stem cell engraftment and differentiation-mediated wound repair. Cytotherapy 2017, 19, 756–770. [Google Scholar] [CrossRef]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef]
- Heldens, G.T.; Blaney Davidson, E.N.; Vitters, E.L.; Schreurs, B.W.; Piek, E.; van den Berg, W.B.; van der Kraan, P.M. Catabolic factors and osteoarthritis-conditioned medium inhibit chondrogenesis of human mesenchymal stem cells. Tissue Eng. Part A 2012, 18, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, N.; Krieg, J.; Weissenberger, M.; Scheller, C.; Steinert, A.F. Rescued chondrogenesis of mesenchymal stem cells under interleukin 1 challenge by foamyviral interleukin 1 receptor antagonist gene transfer. Front. Pharmacol. 2017, 8, 255. [Google Scholar] [CrossRef]
- Kota, D.J.; Prabhakara, K.S.; Cox, C.S.; Olson, S.D. MSCs and hyaluronan: Sticking together for new therapeutic potential? Int. J. Biochem. Cell Biol. 2014, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.M.; Hong, X.; Wong, M.M.; Karamariti, E.; Bhaloo, S.I.; Warren, D.; Kong, W.; Hu, Y.; Xu, Q. Hyaluronan is crucial for stem cell differentiation into smooth muscle lineage. Stem Cells 2016, 34, 1225–1238. [Google Scholar] [CrossRef]
- Coulson-Thomas, V.J.; Gesteira, T.F.; Hascall, V.; Kao, W. Umbilical cord mesenchymal stem cells suppress host rejection: The role of the glycocalyx. J. Biol. Chem. 2014, 289, 23465–23481. [Google Scholar] [CrossRef] [PubMed]
- Cowman, M.K.; Lee, H.-G.; Schwertfeger, K.L.; McCarthy, J.B.; Turley, E.A. The content and size of hyaluronan in biological fluids and tissues. Front. Immunol. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Cao, M.; Liu, H.; He, Y.; Xu, J.; Du, Y.; Liu, Y.; Wang, W.; Cui, L.; Hu, J.; et al. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J. Biol. Chem. 2012, 287, 43094–43107. [Google Scholar] [CrossRef]
- Cowman, M.K. Hyaluronan and hyaluronan fragments. Adv. Carbohydr. Chem. Biochem. 2017, 74, 1–59. [Google Scholar]
- Scheibner, K.A.; Lutz, M.A.; Boodoo, S.; Fenton, M.J.; Powell, J.D.; Horton, M.R. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J. Immunol. 2006, 177, 1272–1281. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a crucial regulator of inflammation. Front. Immunol. 2014, 5, 101. [Google Scholar] [CrossRef]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size matters: Molecular weight specificity of hyaluronan effects in cell biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef]
- Wu, S.C.; Chang, J.K.; Wang, C.K.; Wang, G.J.; Ho, M.L. Enhancement of chondrogenesis of human adipose derived stem cells in a hyaluronan-enriched microenvironment. Biomaterials 2010, 31, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Chen, C.H.; Chang, J.K.; Fu, Y.C.; Wang, C.K.; Eswaramoorthy, R.; Lin, Y.S.; Wang, Y.H.; Lin, S.Y.; Wang, G.J.; et al. Hyaluronan initiates chondrogenesis mainly via CD44 in human adipose-derived stem cells. J. Appl. Physiol. 2013, 114, 1610–1618. [Google Scholar] [CrossRef]
- Wu, S.C.; Chen, C.H.; Wang, J.Y.; Lin, Y.S.; Chang, J.K.; Ho, M.L. Hyaluronan size alters chondrogenesis of adipose-derived stem cells via the CD44/ERK/SOX-9 pathway. Acta Biomater. 2018, 66, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Mitsuhashi, N.; Klein, A.; Barsky, L.W.; Weinberg, K.; Barr, M.L.; Demetriou, A.; Wu, G.D. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells 2006, 24, 928–935. [Google Scholar] [CrossRef]
- Reiprich, S.; Hofbauer, E.; Kiderlen, S.; Clausen-Schaumann, H.; Bocker, W.; Aszodi, A.; Schonitzer, V. Adhesive Properties of the Hyaluronan Pericellular coat in hyaluronan synthases overexpressing mesenchymal stem cells. Int. J. Mol. Sci. 2020, 21, 3827. [Google Scholar] [CrossRef] [PubMed]
- Ducale, A.E.; Ward, S.I.; Dechert, T.; Yager, D.R. Regulation of hyaluronan synthase-2 expression in human intestinal mesenchymal cells: Mechanisms of interleukin-1beta-mediated induction. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G462–G470. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kota, D.J.; DiCarlo, B.; Hetz, R.A.; Smith, P.; Cox, C.S., Jr.; Olson, S.D. Differential MSC activation leads to distinct mononuclear leukocyte binding mechanisms. Sci. Rep. 2014, 4, 4565. [Google Scholar] [CrossRef]
- Li, Y.; Toole, B.P.; Dealy, C.N.; Kosher, R.A. Hyaluronan in limb morphogenesis. Dev. Biol. 2007, 305, 411–420. [Google Scholar] [CrossRef]
- Knudson, C.B. Hyaluronan and CD44: Strategic players for cell-matrix interactions during chondrogenesis and matrix assembly. Birth Defects Res. Part C Embryo Today Rev. 2003, 69, 174–196. [Google Scholar] [CrossRef]
- Knudson, W.; Ishizuka, S.; Terabe, K.; Askew, E.B.; Knudson, C.B. The pericellular hyaluronan of articular chondrocytes. Matrix Biol. 2019, 78–79, 32–46. [Google Scholar] [CrossRef]
- Astachov, L.; Vago, R.; Aviv, M.; Nevo, Z. Hyaluronan and mesenchymal stem cells: From germ layer to cartilage and bone. Front. Biosci. 2011, 16, 261–276. [Google Scholar] [CrossRef]
- Hardwick, C.; Hoare, K.; Owens, R.; Hohn, H.P.; Hook, M.; Moore, D.; Cripps, V.; Austen, L.; Nance, D.M.; Turley, E.A. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J. Cell. Biol. 1992, 117, 1343–1350. [Google Scholar] [CrossRef]
- Tolg, C.; Hamilton, S.R.; Nakrieko, K.A.; Kooshesh, F.; Walton, P.; McCarthy, J.B.; Bissell, M.J.; Turley, E.A. Rhamm−/− fibroblasts are defective in CD44-mediated ERK1,2 motogenic signaling, leading to defective skin wound repair. J. Cell. Biol. 2006, 175, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Liao, J.; Cheong, N.; Longoria, C.; Cao, G.; DeLisser, H.M.; Savani, R.C. The Receptor for Hyaluronan-Mediated Motility (CD168) promotes inflammation and fibrosis after acute lung injury. Matrix Biol. 2019, 78–79, 255–271. [Google Scholar] [CrossRef]
- Wu, K.; Kim, S.; Liu, V.M.; Sabino, A.; Minkhorst, K.; Yazdani, A.; Turley, E.A. Function-blocking RHAMM peptides attenuate fibrosis and promote anti-fibrotic adipokines in a bleomycin-induced murine model of systemic sclerosis. J. Investig. Dermatol. 2021, 141, 1482–1492.e4. [Google Scholar] [CrossRef]
- Leng, Y.; Abdullah, A.; Wendt, M.K.; Calve, S. Hyaluronic acid, CD44 and RHAMM regulate myoblast behavior during embryogenesis. Matrix Biol. 2019, 78–79, 236–254. [Google Scholar] [CrossRef]
- Tolg, C.; Hamilton, S.R.; Zalinska, E.; McCulloch, L.; Amin, R.; Akentieva, N.; Winnik, F.; Savani, R.; Bagli, D.J.; Luyt, L.G.; et al. A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds. Am. J. Pathol. 2012, 181, 1250–1270. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.B.; Tolg, C.; Peart, T.; Symonette, C.; Veiseh, M.; Umoh, J.U.; Holdsworth, D.W.; McCarthy, J.B.; Luyt, L.G.; Bissell, M.J.; et al. Receptor for hyaluronan mediated motility (RHAMM/HMMR) is a novel target for promoting subcutaneous adipogenesis. Integr. Biol. 2017, 9, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Hauser-Kawaguchi, A.; Tolg, C.; Peart, T.; Milne, M.; Turley, E.A.; Luyt, L.G. A truncated RHAMM protein for discovering novel therapeutic peptides. Bioorg. Med. Chem. 2018, 26, 5194–5203. [Google Scholar] [CrossRef]
- Hauser-Kawaguchi, A.; Luyt, L.G.; Turley, E. Design of peptide mimetics to block pro-inflammatory functions of HA fragments. Matrix Biol. 2019, 78–79, 346–356. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, L.; Turley, E.A. Identification of two hyaluronan-binding domains in the hyaluronan receptor RHAMM. J. Biol. Chem. 1993, 268, 8617–8623. [Google Scholar] [CrossRef]
- Yang, B.; Yang, B.L.; Savani, R.C.; Turley, E.A. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 1994, 13, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Shortt, C.; Luyt, L.G.; Turley, E.A.; Cowman, M.K.; Kirsch, T. A Hyaluronan-binding Peptide (P15-1) reduces inflammatory and catabolic events in IL-1beta-treated human articular chondrocytes. Sci. Rep. 2020, 10, 1441. [Google Scholar] [CrossRef] [PubMed]
- Cowman, M.K.; Kirsch, T.; Strauss, E.J.; Turley, E.A.; Toelg, C.; Luyt, L.G. Compositions and Methods for Cartilage Defect Repair Using a RHAMM-Mimetic Peptide. U.S. Patent No. US20170239318A1, 24 August 2017. [Google Scholar]
- Baranova, N.S.; Foulcer, S.J.; Briggs, D.C.; Tilakaratna, V.; Enghild, J.J.; Milner, C.M.; Day, A.J.; Richter, R.P. Inter-alpha-inhibitor impairs TSG-6-induced hyaluronan cross-linking. J. Biol. Chem. 2013, 288, 29642–29653. [Google Scholar] [CrossRef]
- Bayliss, M.T.; Howat, S.L.; Dudhia, J.; Murphy, J.M.; Barry, F.P.; Edwards, J.C.; Day, A.J. Up-regulation and differential expression of the hyaluronan-binding protein TSG-6 in cartilage and synovium in rheumatoid arthritis and osteoarthritis. Osteoarthr. Cartil. 2001, 9, 42–48. [Google Scholar] [CrossRef]
- Chou, C.H.; Attarian, D.E.; Wisniewski, H.G.; Band, P.A.; Kraus, V.B. TSG-6—A double-edged sword for osteoarthritis (OA). Osteoarthr. Cartil. 2018, 26, 245–254. [Google Scholar] [CrossRef]
- Wisniewski, H.G.; Vilcek, J. TSG-6: An IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Rev. 1997, 8, 143–156. [Google Scholar] [CrossRef]
- Zhao, L.; Li, G.; Chan, K.M.; Wang, Y.; Tang, P.F. Comparison of multipotent differentiation potentials of murine primary bone marrow stromal cells and mesenchymal stem cell line C3H10T1/2. Calcif. Tissue Int. 2009, 84, 56–64. [Google Scholar] [CrossRef]
- Kobayashi, M.; Squires, G.R.; Mousa, A.; Tanzer, M.; Zukor, D.J.; Antoniou, J.; Feige, U.; Poole, A.R. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005, 52, 128–135. [Google Scholar] [CrossRef]
- Cowman, M.K.; Shortt, C.; Arora, S.; Fu, Y.; Villavieja, J.; Rathore, J.; Huang, X.; Rakshit, T.; Jung, G.I.; Kirsch, T. Role of hyaluronan in inflammatory effects on human articular chondrocytes. Inflammation 2019, 42, 1808–1820. [Google Scholar] [CrossRef]
- Day, A.J.; Milner, C.M. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019, 78–79, 60–83. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef]
- Torihashi, S.; Ho, M.; Kawakubo, Y.; Komatsu, K.; Nagai, M.; Hirayama, Y.; Kawabata, Y.; Takenaka-Ninagawa, N.; Wanachewin, O.; Zhuo, L.; et al. Acute and temporal expression of tumor necrosis factor (TNF)-alpha-stimulated gene 6 product, TSG6, in mesenchymal stem cells creates microenvironments required for their successful transplantation into muscle tissue. J. Biol. Chem. 2015, 290, 22771–22781. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Castro, E.; Cunningham, C.; Miller, J.; Martuscelli, L.; Aoulad-Ali, S.; Rothwell, N.J.; Kielty, C.M.; Allan, S.M.; Pinteaux, E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res. Ther. 2017, 8, 79. [Google Scholar] [CrossRef]
- Haas, A.R.; Tuan, R.S. Chondrogenic differentiation of murine C3H10T1/2 multipotential mesenchymal cells: II. Stimulation by bone morphogenetic protein-2 requires modulation of N-cadherin expression and function. Differentiation 1999, 64, 77–89. [Google Scholar] [CrossRef] [PubMed]


| Primer Name | Sequences (5′ to 3′) |
|---|---|
| α1(I) | F: 5′-GAGGGCCAAGACGAAGACATC-3′ |
| R: 5′-CAGATCACGTCATCGCACAAC-3′ | |
| α1(II) | F: 5′-GGCAATAGCAGGTTCACGTACA-3′ |
| R: 5′-CGATAACAGTCTTGCCCCACTT-3′ | |
| CD44 | F: 5′-AGCATCGGATTTGAGACCTG-3′ |
| R: 5′-GTTGTTTGCTGCACAGATGG-3′ | |
| Cox-2 | F: 5′-CGGTGAAACTCTGGCTAGACAG-3′ |
| F: 5′-RGCAAACCGTAGATGCTCAGGGA-3′ | |
| HAS1 | F: 5′-ATCCTGCATCAGCGGTCCTC-3′ |
| R: 5′-CTGGTTGTACCAGGCCTCAAGAA-3′ | |
| HAS2 | F: 5′-GAGGACGACTTTATGACCAAGAG-3′ |
| R: 5′-AAAGAGTGTGGTTCCAATTATTCTC-3′ | |
| HAS3 | F: 5′-AGCACCTTCTCGTGCATCATGC-3′ |
| R: 5′-TCCTCCAGGACTCGAAGCATCT-3′ | |
| Hyal2 | F: 5′-CCCAGTCTACGTCTTCACACG-3′ |
| R: 5′-TCCATCTCACTAAGCCCCG-3′ | |
| IL-6 | F: 5′-AGACAGCCACTCACCTCTTCAG-3′ |
| R: 5′-TTCTGCCAGTGCCTCTTTGCTG-3′ | |
| MMP-3 | F: 5′-CACTCACAGACCTGACTCGGTT-3′ |
| R: 5′-AAGCAGGATCACAGTTGGCTGG-3′ | |
| MMP-13 | F: 5′-CCAGTCTCCGAGGAGAAACA-3′ |
| R: 5′-AAAAACAGCTCCGCATCAAC-3′ | |
| Sox-9 | F: 5′-GTACCCGCACTTGCACAAC-3′ |
| R: 5′-TCTCGCTCTCGTTCAGAAGTC-3′ | |
| RHAMM | F: 5′-GGCTGGGAAAAATGCAGAGGATG-3′ |
| R: 5′-CCTTTAGTGCTGACTTGGTCTGC-3′ | |
| TLR-2 | F: 5′-ATCCTCCAATCAGGCTTCTCT-3′ |
| R: 5′-GGACAGGTCAAGGCTTTTTACA-3′ | |
| TLR-4 | F: 5′-CCCTGAGGCATTTAGGCAGCTA-3′ |
| R: 5′-AGGTAGAGAGGTGGCTTAGGCT-3′ | |
| TSG-6 | F: 5′-AATACAAGCTCACCTACGCAG-3′ |
| R: 5′-GGTATCCAACTCTGCCCTTAG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirsch, T.; Zhang, F.; Braender-Carr, O.; Cowman, M.K. Protective Effects of a Hyaluronan-Binding Peptide (P15-1) on Mesenchymal Stem Cells in an Inflammatory Environment. Int. J. Mol. Sci. 2021, 22, 7058. https://doi.org/10.3390/ijms22137058
Kirsch T, Zhang F, Braender-Carr O, Cowman MK. Protective Effects of a Hyaluronan-Binding Peptide (P15-1) on Mesenchymal Stem Cells in an Inflammatory Environment. International Journal of Molecular Sciences. 2021; 22(13):7058. https://doi.org/10.3390/ijms22137058
Chicago/Turabian StyleKirsch, Thorsten, Fenglin Zhang, Olivia Braender-Carr, and Mary K. Cowman. 2021. "Protective Effects of a Hyaluronan-Binding Peptide (P15-1) on Mesenchymal Stem Cells in an Inflammatory Environment" International Journal of Molecular Sciences 22, no. 13: 7058. https://doi.org/10.3390/ijms22137058
APA StyleKirsch, T., Zhang, F., Braender-Carr, O., & Cowman, M. K. (2021). Protective Effects of a Hyaluronan-Binding Peptide (P15-1) on Mesenchymal Stem Cells in an Inflammatory Environment. International Journal of Molecular Sciences, 22(13), 7058. https://doi.org/10.3390/ijms22137058







