Peculiarities of Neurostimulation by Intense Nanosecond Pulsed Electric Fields: How to Avoid Firing in Peripheral Nerve Fibers
Abstract
:1. Introduction
2. Results
2.1. Nerve Excitation by Nano- to Millisecond Pulses
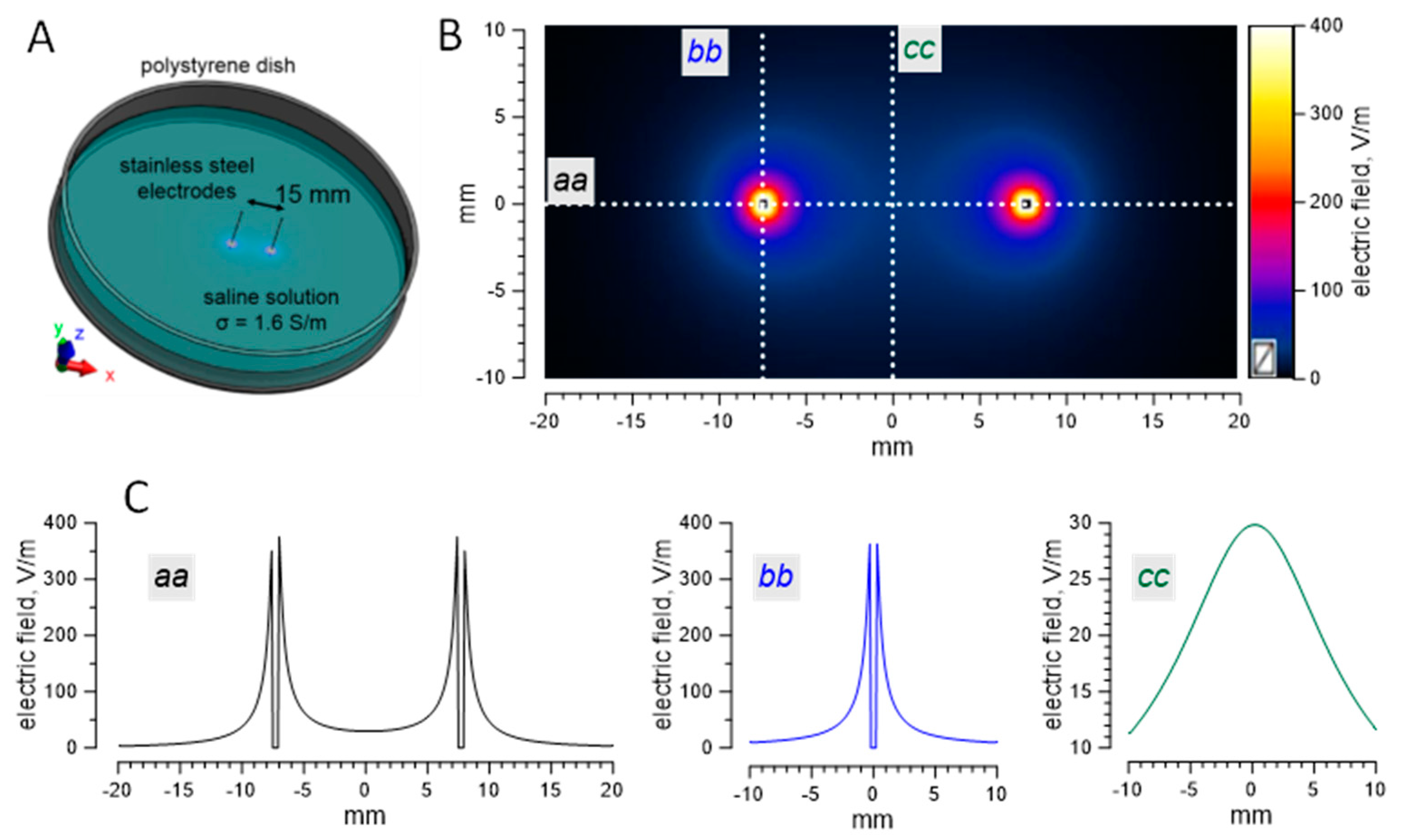
2.2. Placement of the Stimulating Electrodes and PEF Thresholds for Nerve Excitation
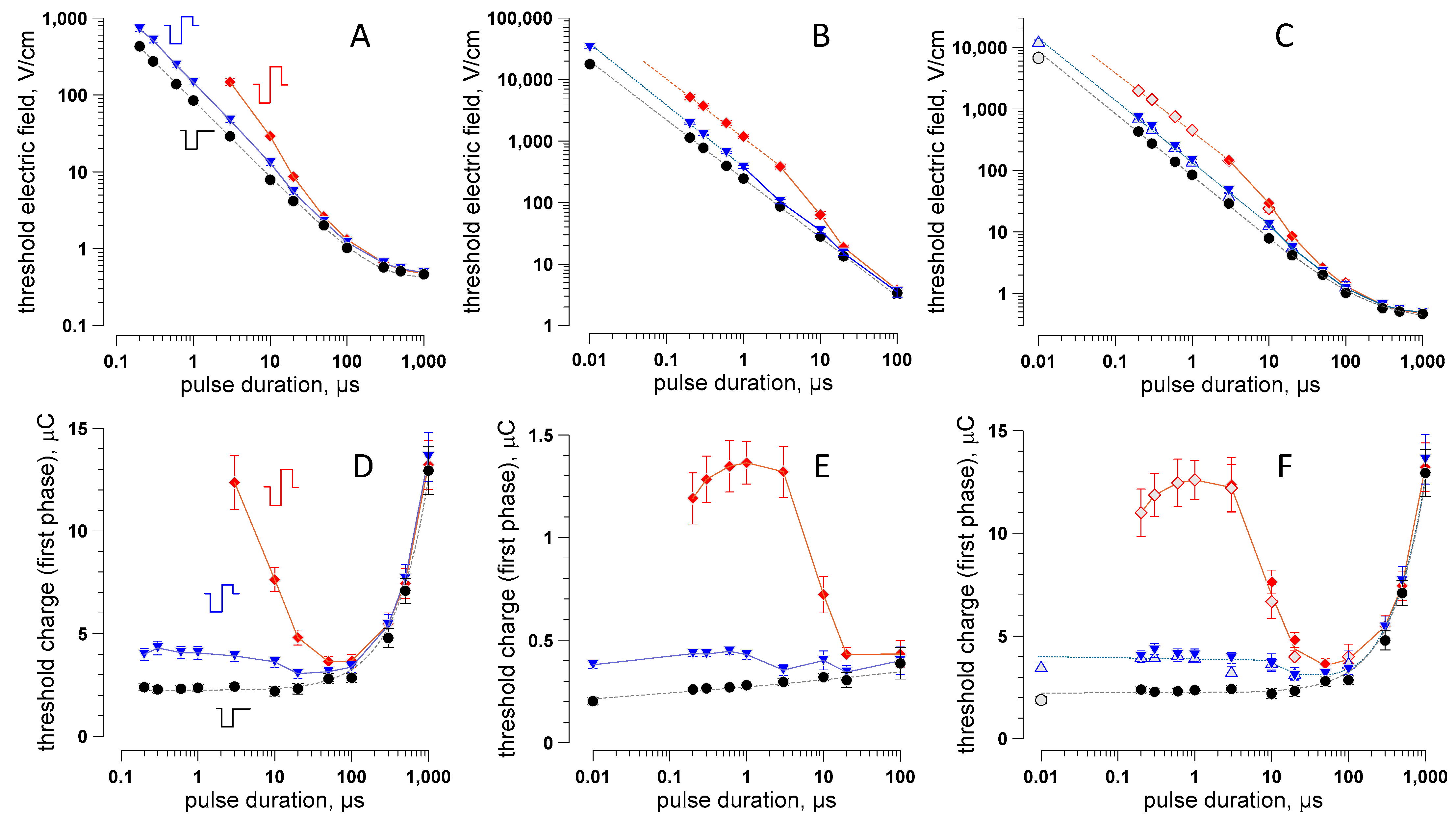
2.3. The Strength–Duration (S–D) Curve from 10 ns to 1 ms for Uni- and Bipolar PEF Stimuli
2.4. The Role of the Electric Charge in Nerve Stimulation by nsPEF
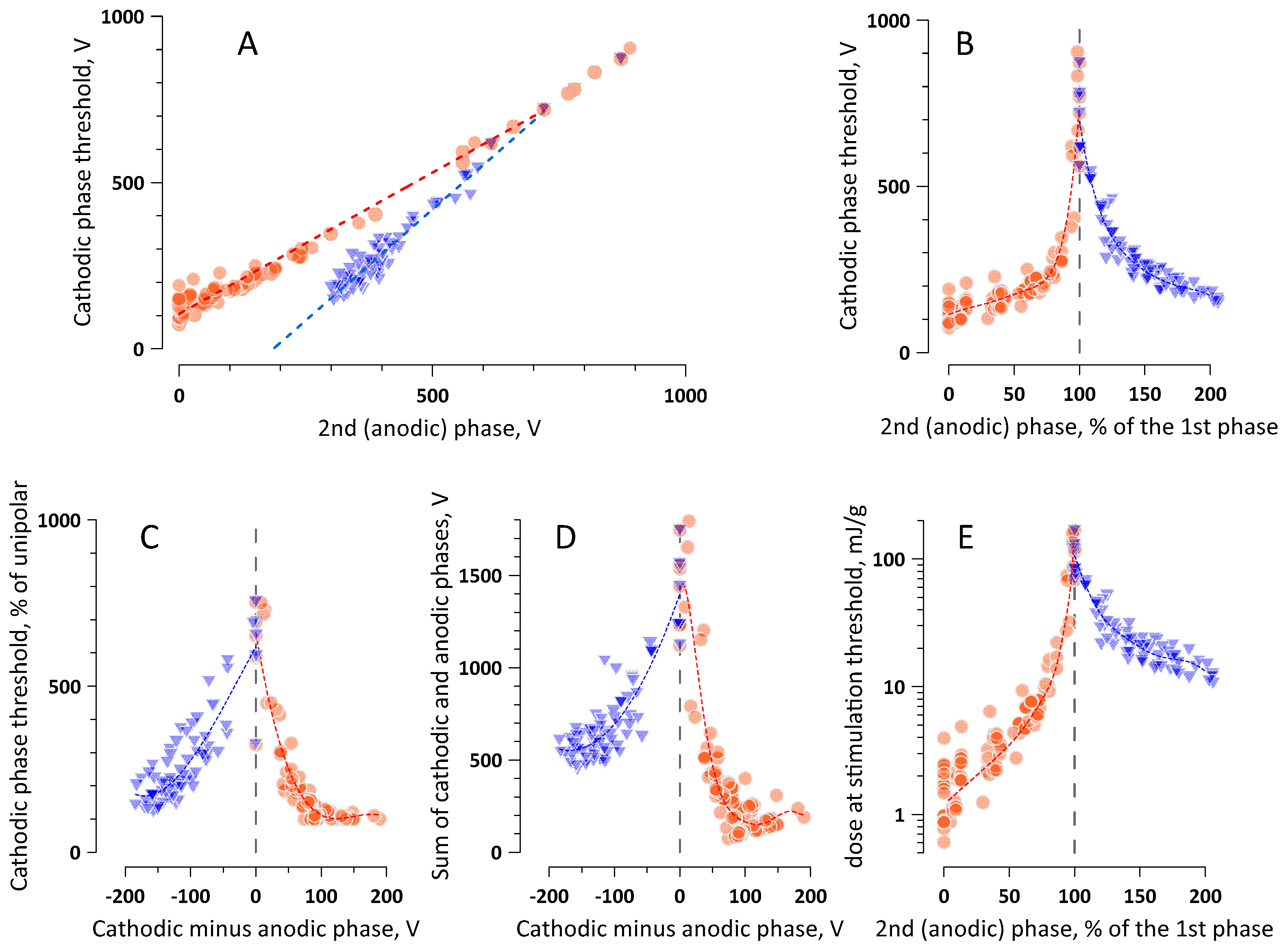
2.5. The Interplay of Phase Amplitudes and Their Ratio in Nerve Excitation by Bipolar nsPEF
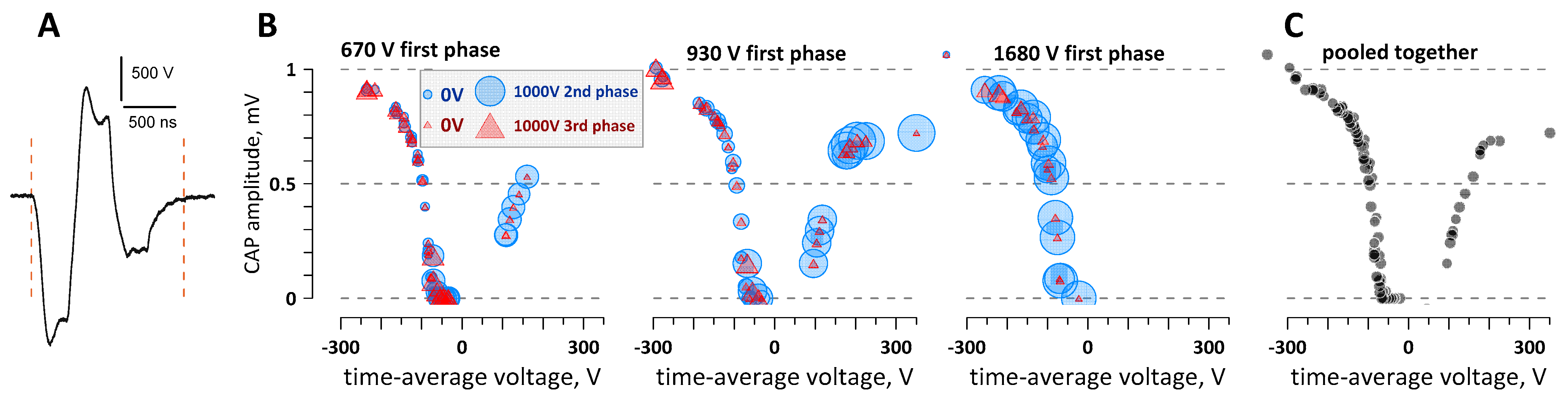
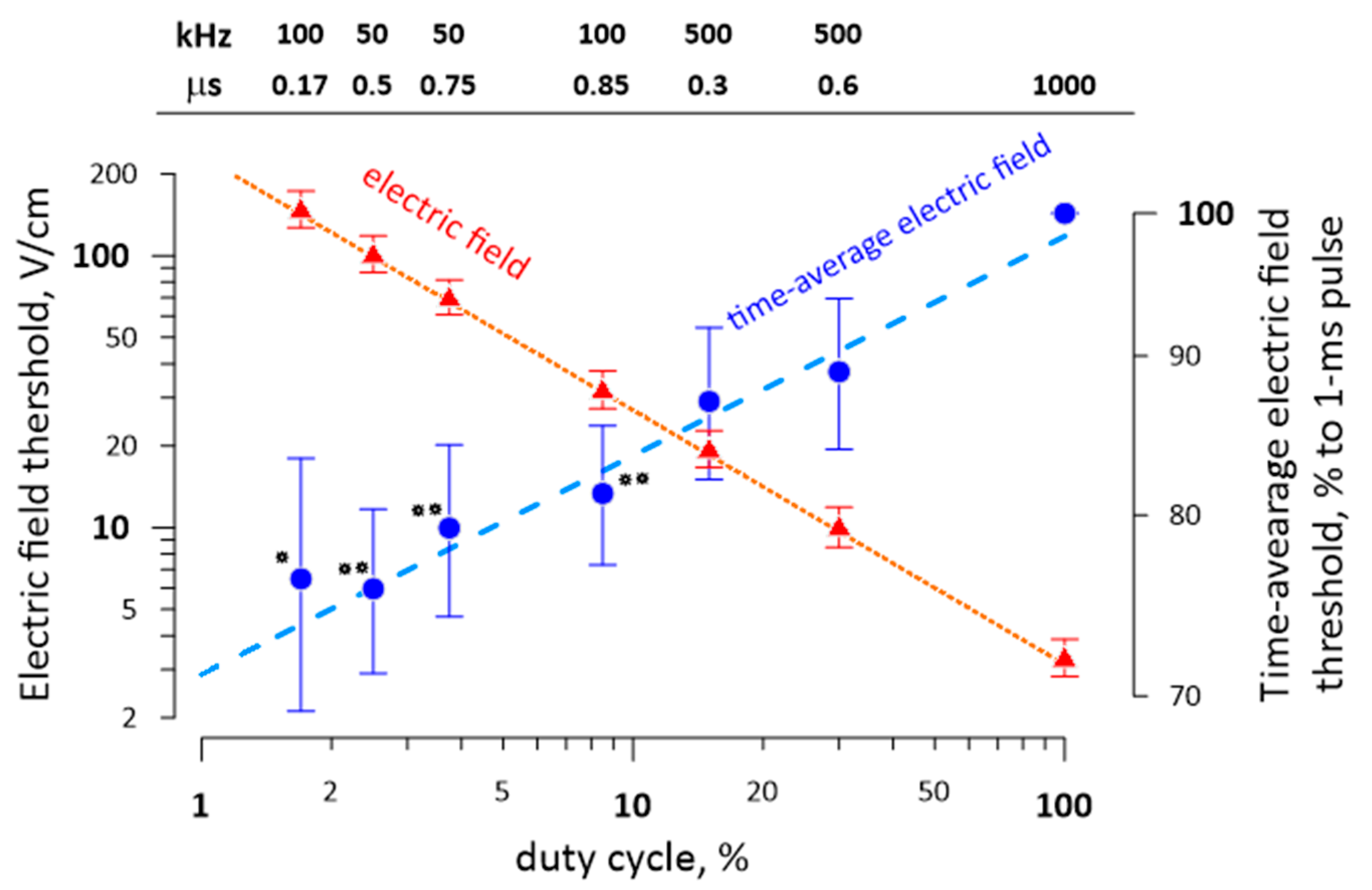
2.6. The Time-Average Voltage across Multiple Pulse Phases (=Net Charge) Predicts the Efficacy of Cathodic and Anodic Stimulation by Complex nsPEF Shapes
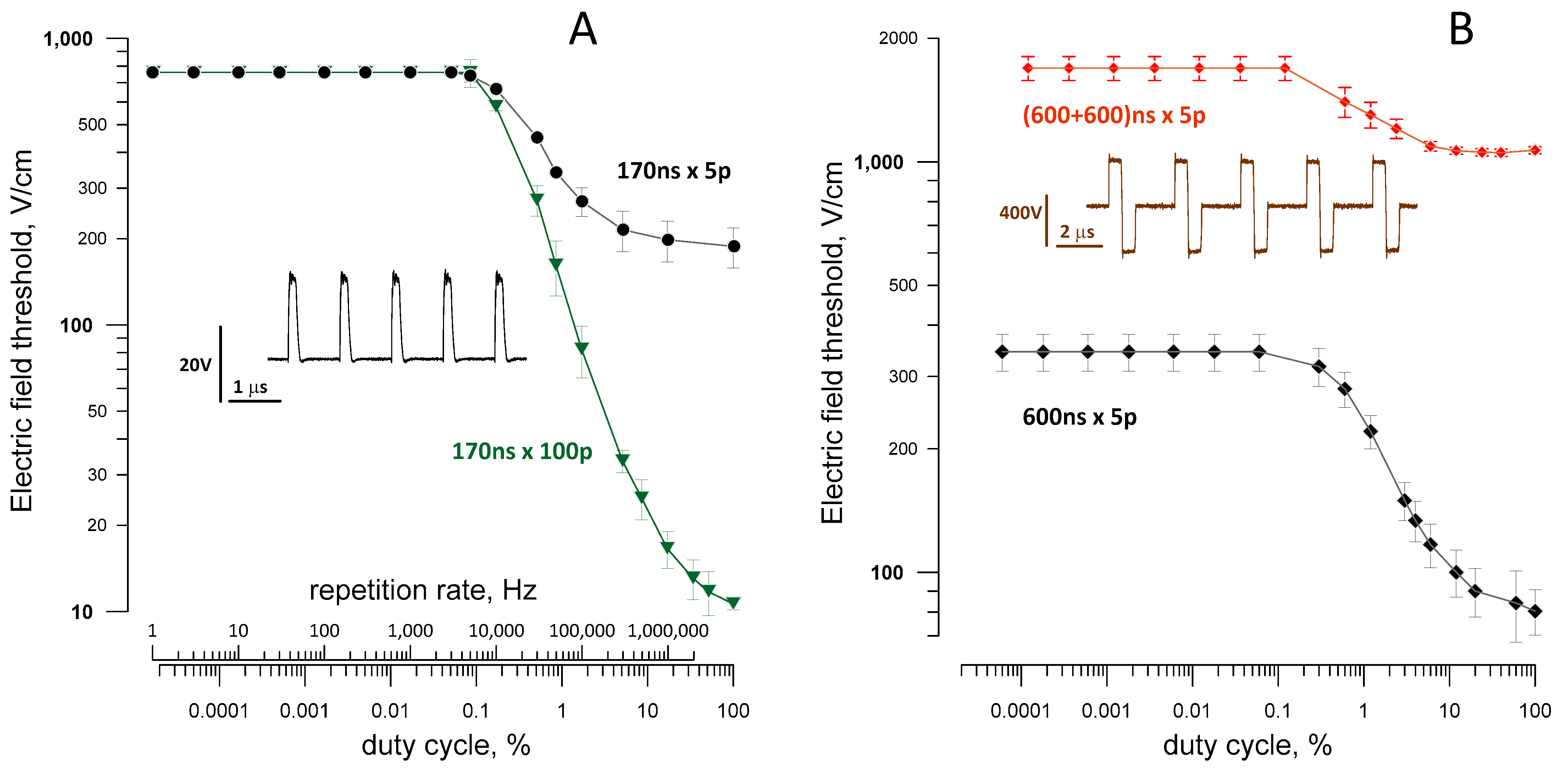
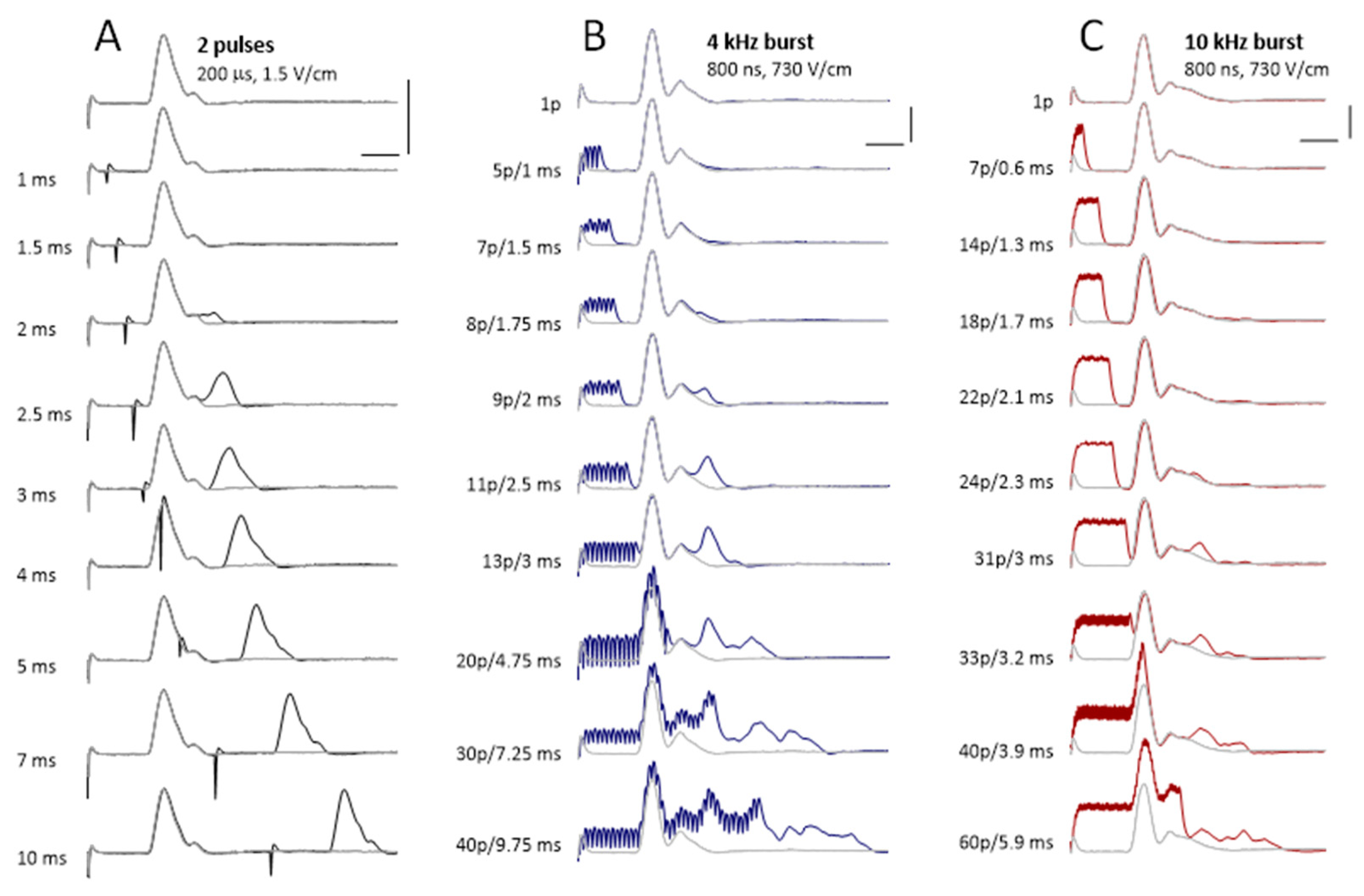
2.7. The Reduction of the Excitation Threshold by High-Rate Bursts of Uni- and Bipolar nsPEF
2.8. Excitation by nsPEF Bursts during the Refractory Period
3. Discussion
4. Materials and Methods
4.1. Nerve Preparation
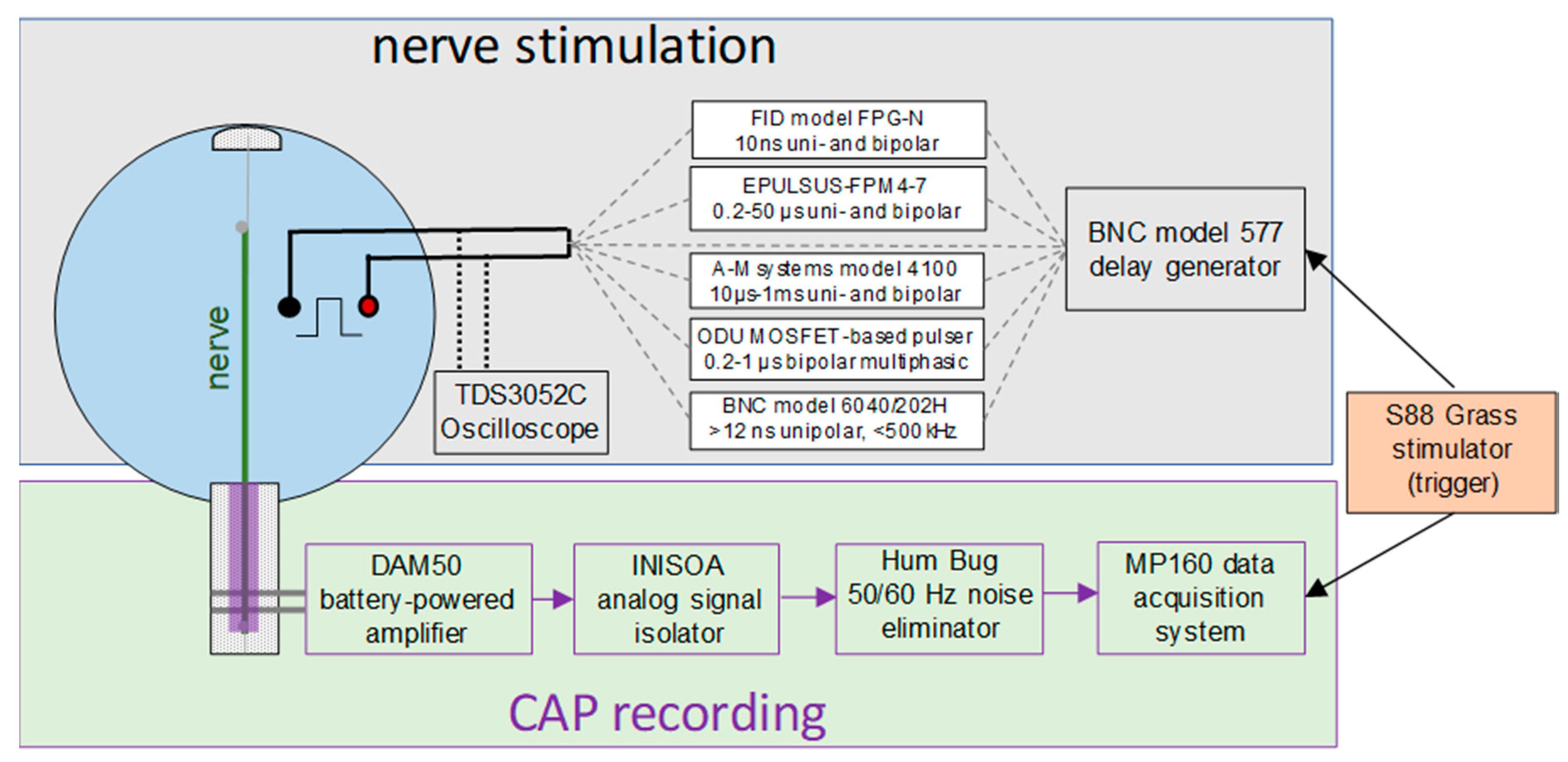
4.2. Nerve Stimulation and CAP Recording
- The FPG20 generator (FID, Burbach, Germany) produced high-voltage (4–20 kV), 10-ns unipolar or (10 + 10)-ns bipolar pulses with a variable phase amplitude ratio.
- The EPULSUS-FPM4-7 generator was custom-built by Energy Pulse Systems (Lisbon, Portugal) to deliver positive polarity rectangular pulses from 200 ns to 50 µs at up to 6.5-kV amplitude and 4-MHz rate. It has 4 independently controlled and programmable output channels, and each of the channels can also serve for the current return. Energizing two electrodes in an alternating fashion generated a bipolar electric field between them (see the waveform in Figure 5B). The voltage output of each channel could be tuned either independently or linked to the output of the other channel.
- The Model 4100 isolated high-power stimulator (A-M systems, Carlsborg, WA, USA) was custom modified for battery-powered operation. It produced nearly rectangular uni- and bipolar stimuli with a phase duration above 1–2 µs and up to 100-V amplitude.
- The Model 6040 mainframe with a 202H high-voltage (300 V) plug-in module (Berkley Nucleonics Corporation, San Rafael, CA, USA) produced 15-ns to 5-ms unipolar pulses at up to a 500-kHz repetition rate (see the waveform in Figure 5A).
- The BNC model 577 digital delay generator could be connected directly to stimulating electrodes to deliver low-voltage (up to 16 V), high-repetition rate (up to 20 MHz) pulses down to 15-ns duration.
4.3. Numerical Simulations of the Electric Field Strength
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAP | Compound action potential |
| H-FIRE | High-frequency irreversible electroporation |
| nsPEF | Nanosecond pulsed electric field |
| PEF | Pulsed electric field |
| TMP | Transmembrane potential |
References
- Sánchez-Velázquez, P.; Castellví, Q.; Villanueva, A.; Quesada, R.; Pañella, C.; Cáceres, M.; Dorcaratto, D.; Andaluz, A.; Moll, X.; Trujillo, M.; et al. Irreversible electroporation of the liver: Is there a safe limit to the ablation volume? Sci. Rep. 2016, 6, 23781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubinsky, J.; Onik, G.; Mikus, P.; Rubinsky, B. Optimal Parameters for the Destruction of Prostate Cancer Using Irre-versible Electroporation. J. Urol. 2008, 180, 2668–2674. [Google Scholar] [CrossRef] [PubMed]
- Nuccitelli, R.; Tran, K.; Sheikh, S.; Athos, B.; Kreis, M.; Nuccitelli, P. Optimized nanosecond pulsed electric field therapy can cause murine malignant melanomas to self-destruct with a single treatment. Int. J. Cancer 2010, 127, 1727–1736. [Google Scholar] [CrossRef]
- Al-Sakere, B.; Andre, F.; Bernat, C.; Connault, E.; Opolon, P.; Davalos, R.V.; Rubinsky, B.; Mir, L.M. Tumor ablation with irreversible electroporation. PLoS ONE 2007, 2, e1135. [Google Scholar] [CrossRef] [Green Version]
- Tracy, C.R.; Kabbani, W.; Cadeddu, J.A. Irreversible electroporation (IRE): A novel method for renal tissue ablation. BJU Int. 2011, 107, 1982–1987. [Google Scholar] [CrossRef]
- Bower, M.; Sherwood, L.; Li, Y.; Martin, R. Irreversible electroporation of the pancreas: Definitive local therapy without systemic effects. J. Surg. Oncol. 2011, 104, 22–28. [Google Scholar] [CrossRef]
- Thomson, K.R.; Cheung, W.; Ellis, S.J.; Federman, D.; Kavnoudias, H.; Loader-Oliver, D.; Roberts, S.; Evans, P.; Ball, C.M.; Haydon, A. Investigation of the Safety of Irreversible Electroporation in Humans. J. Vasc. Interv. Radiol. 2011, 22, 611–621. [Google Scholar] [CrossRef]
- Scheffer, H.J.; Nielsen, K.; de Jong, M.C.; van Tilborg, A.A.; Vieveen, J.M.; Bouwman, A.R.A.; Meijer, S.; van Kuijk, C.; van den Tol, P.M.; Meijerink, M.R. Irreversible Electroporation for Nonthermal Tumor Ablation in the Clinical Setting: A Systematic Review of Safety and Efficacy. J. Vasc. Interv. Radiol. 2014, 25, 997–1011. [Google Scholar] [CrossRef]
- Ringel-Scaia, V.M.; Beitel-White, N.; Lorenzo, M.F.; Brock, R.M.; Huie, K.E.; Coutermarsh-Ott, S.; Eden, K.; McDaniel, D.K.; Verbridge, S.S.; Rossmeisl, J.H.; et al. High-frequency irreversible electro-poration is an effective tumor ablation strategy that induces immunologic cell death and promotes systemic anti-tumor immunity. Ebiomedicine 2019, 44, 112–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, T.J.; Passeri, M.; Lorenzo, M.F.; Sulzer, J.K.; Lyman, W.B.; Swet, J.H.; Vrochides, D.; Baker, E.H.; Iannitti, D.A.; Davalos, R.V.; et al. Experimental High-Frequency Irreversible Electroporation Using a Single-Needle Delivery Approach for Nonthermal Pancreatic Ablation In Vivo. J. Vasc. Interv. Radiol. 2019, 30, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Beebe, S.J.; Lassiter, B.P.; Guo, S. Nanopulse Stimulation (NPS) Induces Tumor Ablation and Immunity in Orthotopic 4T1 Mouse Breast Cancer: A Review. Cancers 2018, 10, 97. [Google Scholar] [CrossRef] [Green Version]
- Edelblute, C.M.; Guo, S.; Hornef, J.; Yang, E.; Jiang, C.; Schoenbach, K.; Heller, R. Moderate Heat Application Enhances the Efficacy of Nanosecond Pulse Stimulation for the Treatment of Squamous Cell Carcinoma. Technol. Cancer Res. Treat. 2018, 17, 1533033818802305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, A.; Pakhomova, O.N.; Pakhomov, A.G.; Weygandt, S.; Bulysheva, A.A.; Murray, L.E.; Mollica, P.A.; Muratori, C. Mechanisms and immunogenicity of nsPEF-induced cell death in B16F10 melanoma tumors. Sci. Rep. 2019, 9, 431. [Google Scholar] [CrossRef]
- Rossi, A.; Pakhomova, O.N.; Mollica, P.A.; Casciola, M.; Mangalanathan, U.; Pakhomov, A.G.; Muratori, C. Nanosecond Pulsed Electric Fields Induce Endoplasmic Reticulum Stress Accompanied by Immunogenic Cell Death in Murine Models of Lymphoma and Colorectal Cancer. Cancers 2019, 11, 2034. [Google Scholar] [CrossRef] [Green Version]
- Munavalli, G.S.; Zelickson, B.D.; Selim, M.M.; Kilmer, S.L.; Rohrer, T.E.; Newman, J.; Jauregui, L.; Knape, W.A.; Ebbers, E.; Uecker, D.; et al. Safety and Efficacy of Nanosecond Pulsed Electric Field Treatment of Sebaceous Gland Hyperplasia. Dermatol. Surg. 2020, 46, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Zupanic, A.; Ribaric, S.; Miklavčič, D. Increasing the repetition frequency of electric pulse delivery reduces unpleasant sensations that occur in electrochemotherapy. Neoplasma 2007, 54, 246–250. [Google Scholar] [PubMed]
- Ball, C.; Thomson, K.R.; Kavnoudias, H. Irreversible Electroporation: A new chanllenge in “out of operating theater” anesthesia. Anesth. Analg. 2010, 110, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Miklavčič, D.; Pucihar, G.; Pavlovec, M.; Ribarič, S.; Mali, M.; Maček-Lebar, A.; Petkovšek, M.; Nastran, J.; Kranjc, S.; Čemažar, M.; et al. The effect of high frequency electric pulses on muscle contractions and antitumor efficiency in vivo for a potential use in clinical electrochemotherapy. Bioelectrochemistry 2005, 65, 121–128. [Google Scholar] [CrossRef]
- Teissie, J.; Golzio, M.; Rols, M.-P. Mechanisms of cell membrane electropermeabilization: A minireview of our present (lack of?) knowledge. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2005, 1724, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, B.; Teissie, J. Direct observation in the millisecond time range of fluorescent molecule asymmetrical interaction with the electropermeabilized cell membrane. Biophys. J. 1997, 73, 2630–2637. [Google Scholar] [CrossRef] [Green Version]
- Hibino, M.; Itoh, H.; Kinosita, K. Time courses of cell electroporation as revealed by submicrosecond imaging of transmembrane potential. Biophys. J. 1993, 64, 1789–1800. [Google Scholar] [CrossRef] [Green Version]
- Bier, M.; Hammer, S.M.; Canaday, D.J.; Lee, R.C. Kinetics of sealing for transient electropores in isolated mammalian skeletal muscle cells. Bioelectromagnetics 1999, 20, 194–201. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Pakhomova, O.N. The interplay of excitation and electroporation in nanosecond pulse stimulation. Bioelectrochemistry 2020, 136, 107598. [Google Scholar] [CrossRef] [PubMed]
- Azarov, J.E.; Semenov, I.; Casciola, M.; Pakhomov, A.G. Excitation of murine cardiac myocytes by nanosecond pulsed electric field. J. Cardiovasc. Electrophysiol. 2019, 30, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Semenov, I.; Casciola, M.; Xiao, S. Neuronal excitation and permeabilization by 200-ns pulsed electric field: An optical membrane potential study with FluoVolt dye. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 1273–1281. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Xiao, S.; Novickij, V.; Casciola, M.; Semenov, I.; Mangalanathan, U.; Kim, V.; Zemlin, C.; Sozer, E.; Muratori, C.; et al. Excitation and electroporation by MHz bursts of nanosecond stimuli. Biochem. Biophys. Res. Commun. 2019, 518, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Semenov, I.; Grigoryev, S.; Neuber, J.U.; Zemlin, C.W.; Pakhomova, O.N.; Casciola, M.; Pakhomov, A.G. Excitation and injury of adult ventricular cardiomyocytes by nano- to millisecond electric shocks. Sci. Rep. 2018, 8, 8233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, G.; Shires, P.K.; Plescia, D.; Beebe, S.J.; Kolb, J.F.; Schoenbach, K.H. Targeted Tissue Ablation With Nanosecond Pulses. IEEE Trans. Biomed. Eng. 2011, 58, 2161–2167. [Google Scholar] [CrossRef]
- Casciola, M.; Xiao, S.; Apollonio, F.; Paffi, A.; Liberti, M.; Muratori, C.; Pakhomov, A.G. Cancellation of nerve excitation by the reversal of nanosecond stimulus polarity and its relevance to the gating time of sodium channels. Cell. Mol. Life Sci. 2019, 76, 4539–4550. [Google Scholar] [CrossRef]
- Gianulis, E.C.; Casciola, M.; Zhou, C.; Yang, E.; Xiao, S.; Pakhomov, A.G. Selective distant electrostimulation by synchronized bipolar nanosecond pulses. Sci. Rep. 2019, 9, 13116. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Grigoryev, S.; Semenov, I.; Casciola, M.; Jiang, C.; Xiao, S. The second phase of bipolar, nanosecond-range electric pulses determines the electroporation efficiency. Bioelectrochemistry 2018, 122, 123–133. [Google Scholar] [CrossRef]
- Polajžer, T.; Dermol–Černe, J.; Reberšek, M.; O’Connor, R.; Miklavčič, D. Cancellation effect is present in high-frequency reversible and irreversible electroporation. Bioelectrochemistry 2020, 132, 107442. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Gudvangen, E.; Xiao, S.; Semenov, I. Interference targeting of bipolar nanosecond electric pulses for spatially focused electroporation, electrostimulation, and tissue ablation. Bioelectrochemistry 2021, 141, 107876. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.B.; Sano, M.B.; Rossmeisl, J.H., Jr.; Caldwell, J.L.; Garcia, P.A.; Rylander, M.N.; Davalos, R.V. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomed. Eng. Online 2011, 10, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercadal, B.; Arena, C.B.; Davalos, R.V.; Ivorra, A. Avoiding nerve stimulation in irreversible electroporation: A numerical modeling study. Phys. Med. Biol. 2017, 62, 8060–8079. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, D.C.; Rebersek, M.; Dermol, J.; Rems, L.; Miklavcic, D.; Davalos, R.V. Quantification of cell membrane permeability induced by monopolar and high-frequency bipolar bursts of electrical pulses. Biochim. Biophys. Acta 2016, 1858, 2689–2698. [Google Scholar] [CrossRef]
- Boinagrov, D.; Loudin, J.; Palanker, D. Strength–Duration Relationship for Extracellular Neural Stimulation: Numerical and Analytical Models. J. Neurophysiol. 2010, 104, 2236–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostock, H. The strength-duration relationship for excitation of myelinated nerve: Computed dependence on membrane parameters. J. Physiol. 1983, 341, 59–74. [Google Scholar] [CrossRef] [Green Version]
- Reilly, J.P. Applied Bioelectricity: From Electrical Stimulation to Electropathology; Springer: New York, NY, USA, 1998. [Google Scholar]
- Reilly, J.P.; Freeman, V.T.; Larkin, W.D. Sensory effects of transient electrical stimulation-evaluation with a neuroelectric model. IEEE Trans. Biomed. Eng. 1985, 32, 1001–1011. [Google Scholar] [CrossRef]
- Castellví, Q.; Mercadal, B.; Moll, X.; Fondevila, D.; Andaluz, A.; Ivorra, A. Avoiding neuromuscular stimulation in liver irreversible electroporation using radiofrequency electric fields. Phys. Med. Biol. 2017, 63, 035027. [Google Scholar] [CrossRef] [Green Version]
- Rogers, W.R.; Merritt, J.H.; Comeaux, J.A.; Kuhnel, C.T.; Moreland, D.F.; Teltschik, D.G.; Lucas, J.H.; Murphy, M.R. Strength-Duration Curve for an Electrically Excitable Tissue Extended Down to Near 1 Nanosecond. IEEE Trans. Plasma Sci. 2004, 32, 1587–1599. [Google Scholar] [CrossRef]
- Aycock, K.N.; Zhao, Y.; Lorenzo, M.F.; Davalos, R.V. A theoretical argument for extended interpulse delays in therapeutic high-frequency irreversible electroporation treatments. IEEE Trans. Biomed. Eng. 2021, 68, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Nesin, V.; Pakhomov, A.G. Inhibition of voltage-gated Na+ current by nanosecond pulsed electric field (nsPEF) is not mediated by Na+ influx or Ca2+ signaling. Bioelectromagnetics 2012, 33, 443–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesin, V.; Bowman, A.M.; Xiao, S.; Pakhomov, A.G. Cell permeabilization and inhibition of voltage-gated Ca2+ and Na+ channel currents by nanosecond pulsed electric field. Bioelectromagnetics 2012, 33, 394–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakhomov, A.G.; Pakhomova, O.N. Nanopores: A distinct transmembrane passageway in electroporated cells. In Advanced Electroporation Techniques in Biology in Medicine; Pakhomov, A.G., Miklavcic, D., Markov, M.S., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 178–194. [Google Scholar]
- Bowman, A.M.; Nesin, O.M.; Pakhomova, O.N.; Pakhomov, A.G. Analysis of plasma membrane integrity by fluorescent detection of Tl+ uptake. J. Membr. Biol. 2010, 236, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakhomov, A.G.; Bowman, A.M.; Ibey, B.L.; Andre, F.M.; Pakhomova, O.N.; Schoenbach, K.H. Lipid nanopores can form a stable, ion channel-like conduction pathway in cell membrane. Biochem. Biophys. Res. Commun. 2009, 385, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Pierce, S.; Chatterjee, I.; Craviso, G.L.; Leblanc, N. Paradoxical effects on voltage-gated Na+ conductance in adrenal chromaffin cells by twin vs single high intensity nanosecond electric pulses. PLoS ONE 2020, 15, e0234114. [Google Scholar] [CrossRef] [PubMed]
- Zaklit, J.; Craviso, G.L.; Leblanc, N.; Vernier, P.T.; Sözer, E.B. 2-ns Electrostimulation of Ca2+ Influx into Chromaffin Cells: Rapid Modulation by Field Reversal. Biophys. J. 2021, 120, 556–567. [Google Scholar] [CrossRef]
- Gianulis, E.C.; Lee, J.; Jiang, C.; Xiao, S.; Ibey, B.L.; Pakhomov, A.G. Electroporation of mammalian cells by nanosecond electric field oscillations and its inhibition by the electric field reversal. Sci. Rep. 2015, 5, 13818. [Google Scholar] [CrossRef]
- Sozer, E.B.; Pakhomov, A.G.; Semenov, I.; Casciola, M.; Kim, V.; Vernier, P.T.; Zemlin, C.W. Analysis of electrostimulation and electroporation by high repetition rate bursts of nanosecond stimuli. Bioelectrochemistry 2021, 140, 107811. [Google Scholar] [CrossRef] [PubMed]
- Casciola, M.; Xiao, S.; Pakhomov, A.G. Damage-free peripheral nerve stimulation by 12-ns pulsed electric field. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunel, N.; van Rossum, M.C.W. Quantitative investigations of electrical nerve excitation treated as polarization (Re-printed from J. Physiol. Pathol. Gen. 1907, 9, 620–635). Biol. Cybern. 2007, 97, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Ibey, B.L.; Xiao, S.; Schoenbach, K.H.; Murphy, M.R.; Pakhomov, A.G. Plasma membrane permeabilization by 60- and 600-ns electric pulses is determined by the absorbed dose. Bioelectromagnetics 2009, 30, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianulis, E.C.; Casciola, M.; Xiao, S.; Pakhomova, O.N.; Pakhomov, A.G. Electropermeabilization by uni- or bipolar nanosecond electric pulses: The impact of extracellular conductivity. Bioelectrochemistry 2018, 119, 10–19. [Google Scholar] [CrossRef]
- Xiao, S.; Zhou, C.; Yang, E.; Rajulapati, S.R. Nanosecond bipolar pulse generators for bioelectrics. Bioelectrochemistry 2018, 123, 77–87. [Google Scholar] [CrossRef]
- Ryan, H.A.; Hirakawa, S.; Yang, E.; Zhou, C.; Xiao, S. High-Voltage, Multiphasic, Nanosecond Pulses to Modulate Cellular Responses. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 338–350. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, V.; Gudvangen, E.; Kondratiev, O.; Redondo, L.; Xiao, S.; Pakhomov, A.G. Peculiarities of Neurostimulation by Intense Nanosecond Pulsed Electric Fields: How to Avoid Firing in Peripheral Nerve Fibers. Int. J. Mol. Sci. 2021, 22, 7051. https://doi.org/10.3390/ijms22137051
Kim V, Gudvangen E, Kondratiev O, Redondo L, Xiao S, Pakhomov AG. Peculiarities of Neurostimulation by Intense Nanosecond Pulsed Electric Fields: How to Avoid Firing in Peripheral Nerve Fibers. International Journal of Molecular Sciences. 2021; 22(13):7051. https://doi.org/10.3390/ijms22137051
Chicago/Turabian StyleKim, Vitalii, Emily Gudvangen, Oleg Kondratiev, Luis Redondo, Shu Xiao, and Andrei G. Pakhomov. 2021. "Peculiarities of Neurostimulation by Intense Nanosecond Pulsed Electric Fields: How to Avoid Firing in Peripheral Nerve Fibers" International Journal of Molecular Sciences 22, no. 13: 7051. https://doi.org/10.3390/ijms22137051
APA StyleKim, V., Gudvangen, E., Kondratiev, O., Redondo, L., Xiao, S., & Pakhomov, A. G. (2021). Peculiarities of Neurostimulation by Intense Nanosecond Pulsed Electric Fields: How to Avoid Firing in Peripheral Nerve Fibers. International Journal of Molecular Sciences, 22(13), 7051. https://doi.org/10.3390/ijms22137051







