The Synergic Role of Actomyosin Architecture and Biased Detachment in Muscle Energetics: Insights in Cross Bridge Mechanism beyond the Lever-Arm Swing
Abstract
1. Introduction
2. Results
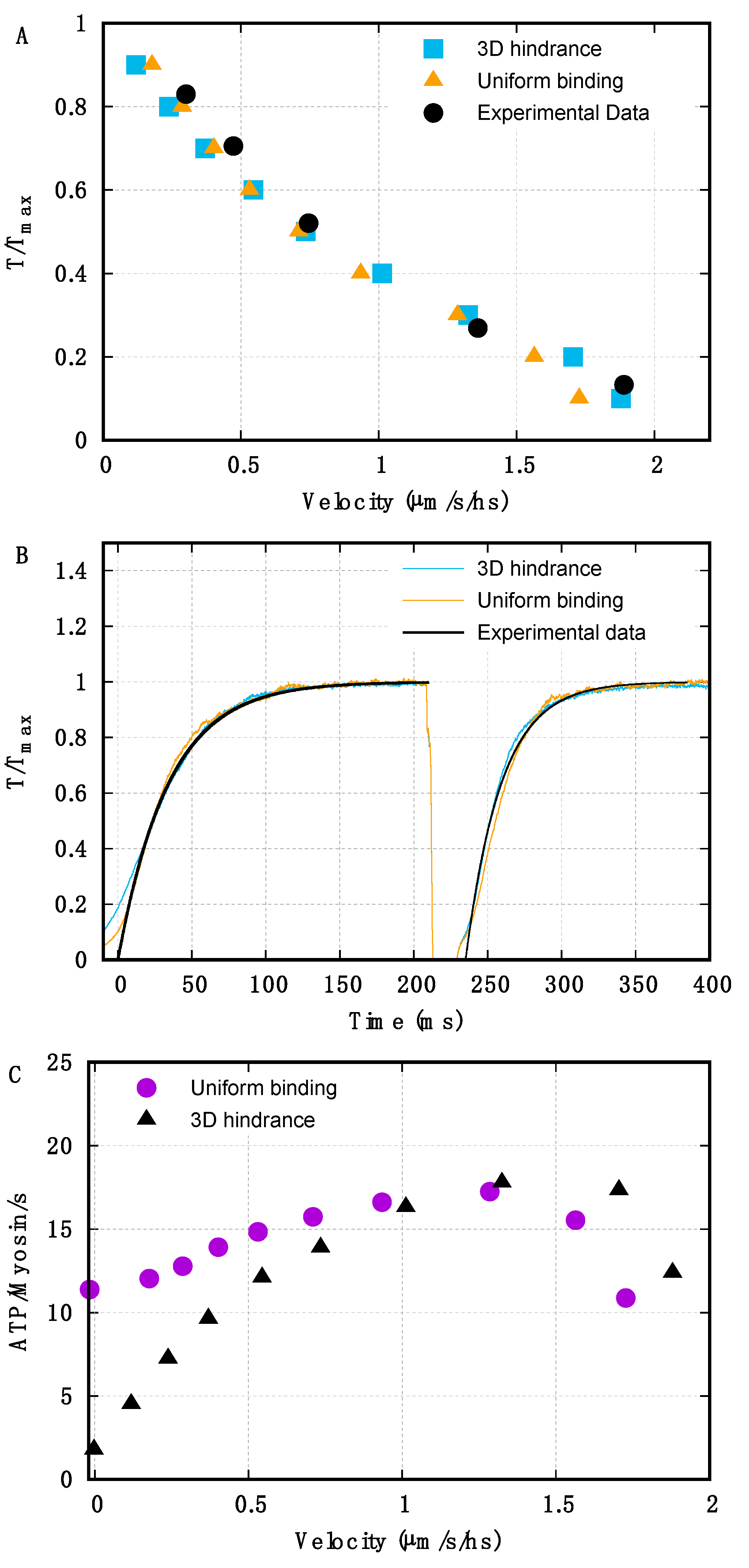
2.1. Geometrical Hindrance Limits ATP Consumption in Isometric Conditions and at Low but Not Intermediate Velocities of Shortening
2.2. Preferential Detachment Has a Synergistic Effect with Geometrical Hindrance to Increase the Dependence of ATP-ase Activity on the Velocity of Shortening
2.3. Thermal Ratchet Components Can Account for up to 15% of Maximum Velocity in Physiological Conditions
3. Discussion
4. Materials and Methods
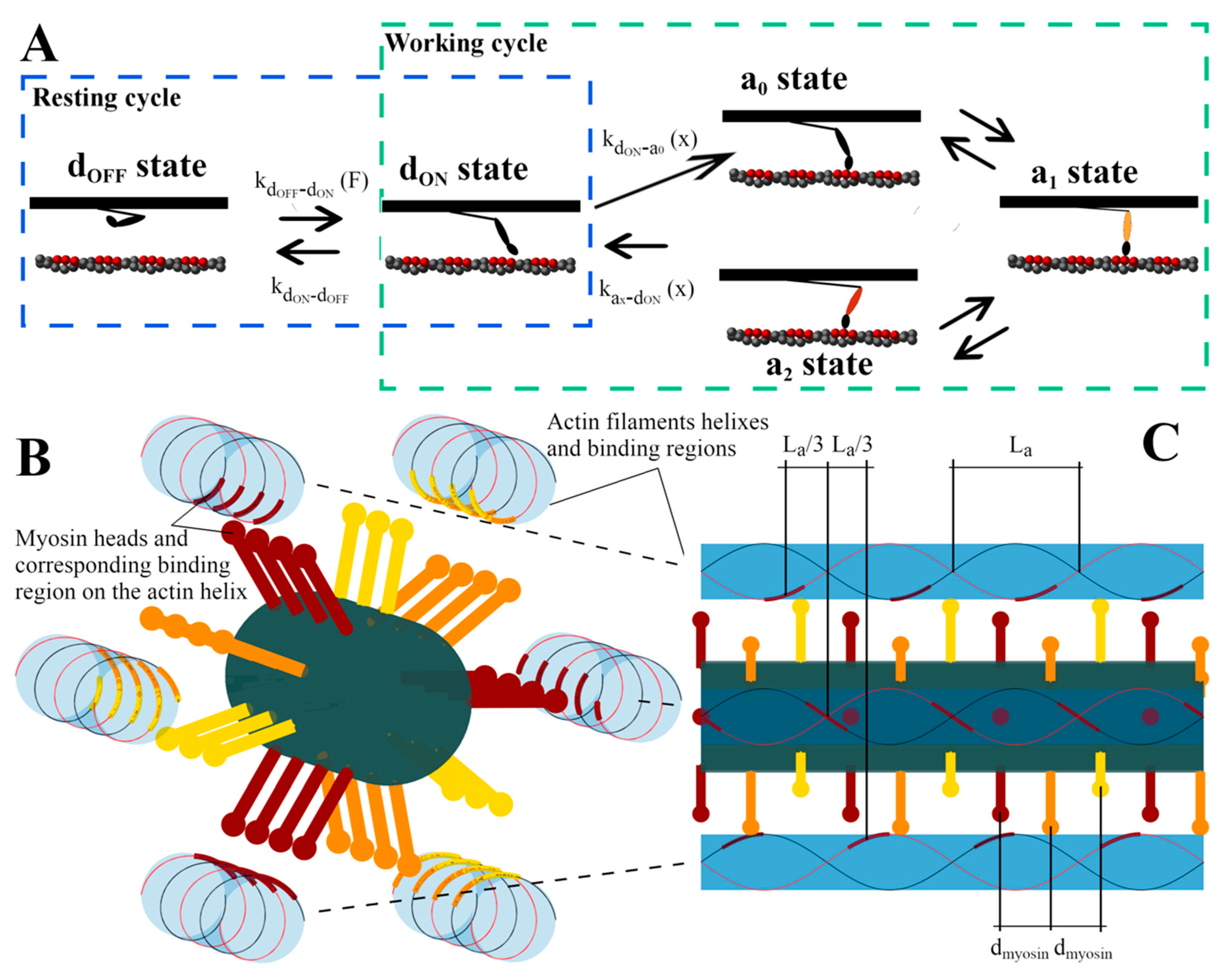
4.1. Model
4.2. Construction of DNA Origami
4.3. Myosin Construct
4.4. Protein Expression and Purification
4.5. Oligonucleotide Labeling to Myosin
4.6. Labeling of Qdot to Actin Filament
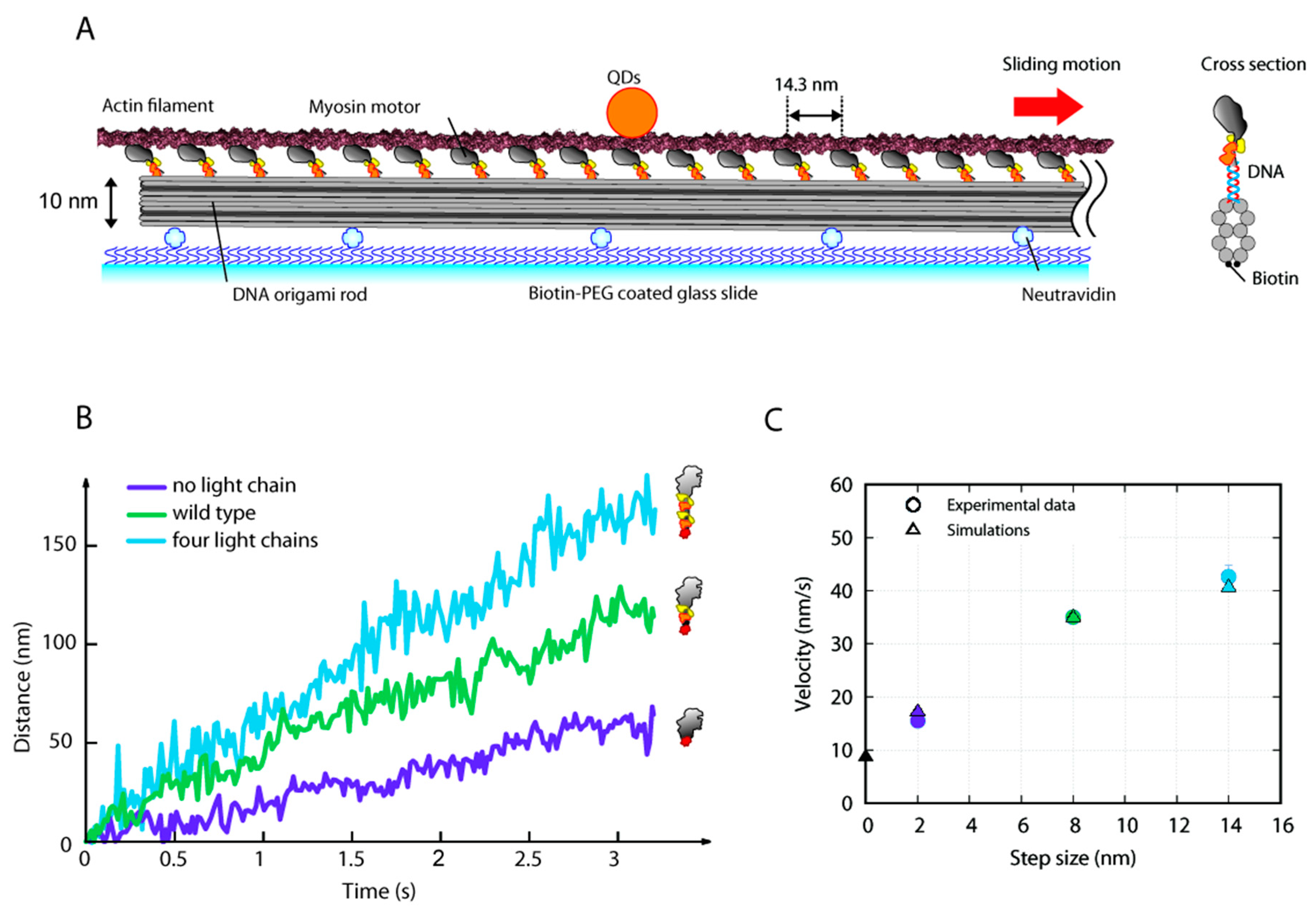
4.7. Observation of Actin Sliding along DNA-Origami Thick Filament
4.8. Sliding Filament Model
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hanson, J.; Huxley, H.E. Structural Basis of the Cross-Striations in Muscle. Nature 1953, 172, 530–532. [Google Scholar] [CrossRef]
- Huxley, H.E. The mechanism of muscular contraction. Science 1969, 164, 1356–1365. [Google Scholar] [CrossRef]
- Huxley, A.F.; Niedergerke, R. Structural Changes in Muscle During Contraction: Interference Microscopy of Living Muscle Fibres. Nature 1954, 173, 971–973. [Google Scholar] [CrossRef]
- Lymn, R.W.; Taylor, E.W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry 1971, 10, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Linari, M.; Woledge, R.C. Comparison of energy output during ramp and staircase shortening in frog muscle fibres. J. Physiol. 1995, 487, 699–710. [Google Scholar] [CrossRef]
- Huxley, A.F. Muscle Structure and Theories of Contraction. Prog. Biophys. Biophys. Chem. 1957, 7, 255–318. [Google Scholar] [CrossRef]
- Barclay, C.; Woledge, R.; Curtin, N. Inferring crossbridge properties from skeletal muscle energetics. Prog. Biophys. Mol. Biol. 2010, 102, 53–71. [Google Scholar] [CrossRef]
- Piazzesi, G.; Lombardi, V. A cross-bridge model that is able to explain mechanical and energetic properties of shortening muscle. Biophys. J. 1995, 68, 1966–1979. [Google Scholar] [CrossRef]
- Piazzesi, G.; Reconditi, M.; Linari, M.; Lucii, L.; Bianco, P.; Brunello, E.; Decostre, V.; Stewart, A.; Gore, D.B.; Irving, T.C.; et al. Skeletal Muscle Performance Determined by Modulation of Number of Myosin Motors Rather Than Motor Force or Stroke Size. Cell 2007, 131, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.V. The effect of load on the heat of shortening of muscle. Proc. R. Soc. B Biol. Sci. 1964, 159, 297–318. [Google Scholar] [CrossRef]
- Marcucci, L.; Reggiani, C. Mechanosensing in Myosin Filament Solves a 60 Years Old Conflict in Skeletal Muscle Modeling between High Power Output and Slow Rise in Tension. Front. Physiol. 2016, 7, 427. [Google Scholar] [CrossRef] [PubMed]
- Linari, M.; Brunello, E.; Reconditi, M.; Fusi, L.; Caremani, M.; Narayanan, T.; Piazzesi, G.; Lombardi, V.; Irving, M. Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature 2015, 528, 276–279. [Google Scholar] [CrossRef]
- Stewart, M.A.; Franks-Skiba, K.; Chen, S.; Cooke, R. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc. Natl. Acad. Sci. USA 2010, 107, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Fusi, L.; Brunello, E.; Yan, Z.; Irving, M. Thick filament mechano-sensing is a calcium-independent regulatory mechanism in skeletal muscle. Nat. Commun. 2016, 7, 13281. [Google Scholar] [CrossRef]
- Irving, M. Regulation of Contraction by the Thick Filaments in Skeletal Muscle. Biophys. J. 2017, 113, 2579–2594. [Google Scholar] [CrossRef]
- Caremani, M.; Pinzauti, F.; Reconditi, M.; Piazzesi, G.; Stienen, G.; Lombardi, V.; Linari, M. Size and speed of the working stroke of cardiac myosin in situ. Proc. Natl. Acad. Sci. USA 2016, 113, 3675–3680. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.L.; Trivedi, D.V.; Sarkar, S.S.; Henze, M.; Ma, W.; Gong, H.; Rogers, C.S.; Gorham, J.M.; Wong, F.L.; Morck, M.M.; et al. Deciphering the super relaxed state of human β-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc. Natl. Acad. Sci. USA 2018, 115, E8143–E8152. [Google Scholar] [CrossRef]
- Marcucci, L.; Washio, T.; Yanagida, T. Titin-mediated thick filament activation, through a mechanosensing mechanism, introduces sarcomere-length dependencies in mathematical models of rat trabecula and whole ventricle. Sci. Rep. 2017, 7, 5546. [Google Scholar] [CrossRef]
- Marcucci, L.; Washio, T.; Yanagida, T. Proposed mechanism for the length dependence of the force developed in maximally activated muscles. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rohde, J.A.; Roopnarine, O.; Thomas, D.D.; Muretta, J.M. Mavacamten stabilizes an autoinhibited state of two-headed cardiac myosin. Proc. Natl. Acad. Sci. USA 2018, 115, E7486–E7494. [Google Scholar] [CrossRef] [PubMed]
- Woody, M.S.; Greenberg, M.J.; Barua, B.; Winkelmann, D.A.; Goldman, Y.E.; Ostap, E.M. Positive cardiac inotrope omecamtiv mecarbil activates muscle despite suppressing the myosin working stroke. Nat. Commun. 2018, 9, 3838. [Google Scholar] [CrossRef]
- Greenberg, M.; Shuman, H.; Ostap, E.M. Inherent Force-Dependent Properties of β -Cardiac Myosin Contribute to the Force-Velocity Relationship of Cardiac Muscle. Biophys. J. 2014, 107, L41–L44. [Google Scholar] [CrossRef]
- Steffen, W.; Smith, D.; Simmons, R.; Sleep, J. Mapping the actin filament with myosin. Proc. Natl. Acad. Sci. USA 2001, 98, 14949–14954. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Ohmachi, M.; Ikezaki, K.; Yanagida, T.; Iwaki, M. Direct visualization of human myosin II force generation using DNA origami-based thick filaments. Commun. Biol. 2019, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.V. The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. B Biol. Sci. 1938, 126, 136–195. [Google Scholar] [CrossRef]
- Caremani, M.; Melli, L.; Dolfi, M.; Lombardi, V.; Linari, M. The working stroke of the myosin II motor in muscle is not tightly coupled to release of orthophosphate from its active site. J. Physiol. 2013, 591, 5187–5205. [Google Scholar] [CrossRef]
- Månsson, A. Actomyosin-ADP States, Interhead Cooperativity, and the Force-Velocity Relation of Skeletal Muscle. Biophys. J. 2010, 98, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Mijailovich, S.M. Toward a Unified Theory of Muscle Contraction. II: Predictions with the Mean-Field Approximation. Ann. Biomed. Eng. 2008, 36, 1353–1371. [Google Scholar] [CrossRef]
- Uyeda, T.Q.; Abramson, P.D.; Spudich, J.A. The neck region of the myosin motor domain acts as a lever arm to generate movement. Proc. Natl. Acad. Sci. USA 1996, 93, 4459–4464. [Google Scholar] [CrossRef]
- Fusi, L.; Percario, V.; Brunello, E.; Caremani, M.; Bianco, P.; Powers, J.D.; Reconditi, M.; Lombardi, V.; Piazzesi, G. Minimum number of myosin motors accounting for shortening velocity under zero load in skeletal muscle. J. Physiol. 2017, 595, 1127–1142. [Google Scholar] [CrossRef]
- Fujita, K.; Iwaki, M.; Iwane, A.H.; Marcucci, L.; Yanagida, T. Switching of myosin-V motion between the lever-arm swing and Brownian search-and-catch. Nat. Commun. 2012, 3, 956. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Magnasco, M.O. Forced thermal ratchets. Phys. Rev. Lett. 1993, 71, 1477–1481. [Google Scholar] [CrossRef]
- Prost, J.; Chauwin, J.-F.; Peliti, L.; Ajdari, A. Asymmetric pumping of particles. Phys. Rev. Lett. 1994, 72, 2652–2655. [Google Scholar] [CrossRef]
- Reimann, P. Brownian motors: Noisy transport far from equilibrium. Phys. Rep. 2002, 361, 57–265. [Google Scholar] [CrossRef]
- Douglas, S.; Dietz, H.; Liedl, T.; Högberg, B.; Graf, F.; Shih, W.M. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 2009, 459, 414–418. [Google Scholar] [CrossRef]
- Iwaki, M.; Wickham, S.F.; Ikezaki, K.; Yanagida, T.; Shih, W.M. A programmable DNA origami nanospring that reveals force-induced adjacent binding of myosin VI heads. Nat. Commun. 2016, 7, 13715. [Google Scholar] [CrossRef]
- Davis, J.S. Assembly Processes in Vertebrate Skeletal Thick Filament Formation. Annu. Rev. Biophys. Biophys. Chem. 1988, 17, 217–239. [Google Scholar] [CrossRef]
- Brown, A.E.; Hategan, A.; Safer, D.; Goldman, Y.E.; Discher, D.E. Cross-Correlated TIRF/AFM Reveals Asymmetric Distribution of Force-Generating Heads along Self-Assembled, “Synthetic” Myosin Filaments. Biophys. J. 2009, 96, 1952–1960. [Google Scholar] [CrossRef]
- Smith, N.P.; Barclay, C.J.; Loiselle, D.S. The efficiency of muscle contraction. Prog. Biophys. Mol. Biol. 2005, 88, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Flechsig, H.; Mikhailov, A.S. Simple mechanics of protein machines. J. R. Soc. Interface 2019, 16, 20190244. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Higuchi, H. Stiffness, working stroke, and force of single-myosin molecules in skeletal muscle: Elucidation of these mechanical properties via nonlinear elasticity evaluation. Cell. Mol. Life Sci. 2013, 70, 4275–4292. [Google Scholar] [CrossRef]
- Linari, M.; Piazzesi, G.; Pertici, I.; Dantzig, J.A.; Goldman, Y.E.; Lombardi, V. Straightening Out the Elasticity of Myosin Cross-Bridges. Biophys. J. 2020, 118, 994–1002. [Google Scholar] [CrossRef]
- Månsson, A.; Persson, M.; Shalabi, N.; Rassier, D.E. Nonlinear Actomyosin Elasticity in Muscle? Biophys. J. 2019, 116, 330–346. [Google Scholar] [CrossRef]
- Marcucci, L.; Canato, M.; Protasi, F.; Stienen, G.J.M.; Reggiani, C. A 3D diffusional-compartmental model of the calcium dynamics in cytosol, sarcoplasmic reticulum and mitochondria of murine skeletal muscle fibers. PLoS ONE 2018, 13, e0201050. [Google Scholar] [CrossRef]
- Brunello, E.; Fusi, L.; Ghisleni, A.; Park-Holohan, S.-J.; Ovejero, J.G.; Narayanan, T.; Irving, M. Myosin filament-based regulation of the dynamics of contraction in heart muscle. Proc. Natl. Acad. Sci. USA 2020, 117, 8177–8186. [Google Scholar] [CrossRef]
- Previs, M.J.; Prosser, B.L.; Mun, J.Y.; Previs, S.B.; Gulick, J.; Lee, K.; Robbins, J.; Craig, R.; Lederer, W.J.; Warshaw, D.M. Myosin-binding protein C corrects an intrinsic inhomogeneity in cardiac excitation-contraction coupling. Sci. Adv. 2015, 1, e1400205. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.; Spudich, J.A. Is the Lever Arm of Myosin a Molecular Elastic Element? Proc. Natl. Acad. Sci. USA 1996, 93, 4462–4464. [Google Scholar] [PubMed]
- Daniel, T.L.; Trimble, A.C.; Chase, P.B. Compliant Realignment of Binding Sites in Muscle: Transient Behavior and Mechanical Tuning. Biophys. J. 1998, 74, 1611–1621. [Google Scholar] [CrossRef]
- Chase, P.B.; MacPherson, J.M.; Daniel, T.L. A Spatially Explicit Nanomechanical Model of the Half-Sarcomere: Myofilament Compliance Affects Ca2+-Activation. Ann. Biomed. Eng. 2004, 32, 1559–1568. [Google Scholar] [CrossRef]
- Smith, D.; Geeves, M.; Sleep, J.; Mijailovich, S. Towards a Unified Theory of Muscle Contraction. I: Foundations. Ann. Biomed. Eng. 2008, 36, 1624–1640. [Google Scholar] [CrossRef]
- Mijailovich, S.M.; Kayser-Herold, O.; Stojanović, B.; Nedic, D.; Irving, T.C.; Geeves, M.A. Three-dimensional stochastic model of actin–myosin binding in the sarcomere lattice. J. Gen. Physiol. 2016, 148, 459–488. [Google Scholar] [CrossRef]
- Vilfan, A.; Duke, T. Instabilities in the Transient Response of Muscle. Biophys. J. 2003, 85, 818–827. [Google Scholar] [CrossRef]
- Månsson, A. Comparing models with one versus multiple myosin-binding sites per actin target zone: The power of simplicity. J. Gen. Physiol. 2019, 151, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, L.; Washio, T.; Yanagida, T. Including Thermal Fluctuations in Actomyosin Stable States Increases the Predicted Force per Motor and Macroscopic Efficiency in Muscle Modelling. PLoS Comput. Biol. 2016, 12, e1005083. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.W. Handbook of Stochastic Methods for Physics, Chemistry, and the Natural Sciences; Springer: Berlin, Germany, 1985; ISBN 978-0-387-15607-1. [Google Scholar]
- Marcucci, L.; Truskinovsky, L. Mechanics of the power stroke in myosin II. Phys. Rev. E 2010, 81, 051915. [Google Scholar] [CrossRef]
- Marcucci, L.; Truskinovsky, L. Muscle contraction: A mechanical perspective. Eur. Phys. J. E 2010, 32, 411–418. [Google Scholar] [CrossRef] [PubMed]
- I Bell, G. Models for the specific adhesion of cells to cells. Science 1978, 200, 618–627. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcucci, L.; Fukunaga, H.; Yanagida, T.; Iwaki, M. The Synergic Role of Actomyosin Architecture and Biased Detachment in Muscle Energetics: Insights in Cross Bridge Mechanism beyond the Lever-Arm Swing. Int. J. Mol. Sci. 2021, 22, 7037. https://doi.org/10.3390/ijms22137037
Marcucci L, Fukunaga H, Yanagida T, Iwaki M. The Synergic Role of Actomyosin Architecture and Biased Detachment in Muscle Energetics: Insights in Cross Bridge Mechanism beyond the Lever-Arm Swing. International Journal of Molecular Sciences. 2021; 22(13):7037. https://doi.org/10.3390/ijms22137037
Chicago/Turabian StyleMarcucci, Lorenzo, Hiroki Fukunaga, Toshio Yanagida, and Mitsuhiro Iwaki. 2021. "The Synergic Role of Actomyosin Architecture and Biased Detachment in Muscle Energetics: Insights in Cross Bridge Mechanism beyond the Lever-Arm Swing" International Journal of Molecular Sciences 22, no. 13: 7037. https://doi.org/10.3390/ijms22137037
APA StyleMarcucci, L., Fukunaga, H., Yanagida, T., & Iwaki, M. (2021). The Synergic Role of Actomyosin Architecture and Biased Detachment in Muscle Energetics: Insights in Cross Bridge Mechanism beyond the Lever-Arm Swing. International Journal of Molecular Sciences, 22(13), 7037. https://doi.org/10.3390/ijms22137037





