Non-Genetic Diversity in Chemosensing and Chemotactic Behavior
Abstract
1. Introduction
2. Molecular Mechanisms Underlying Phenotypic Diversity in E. coli Chemotaxis
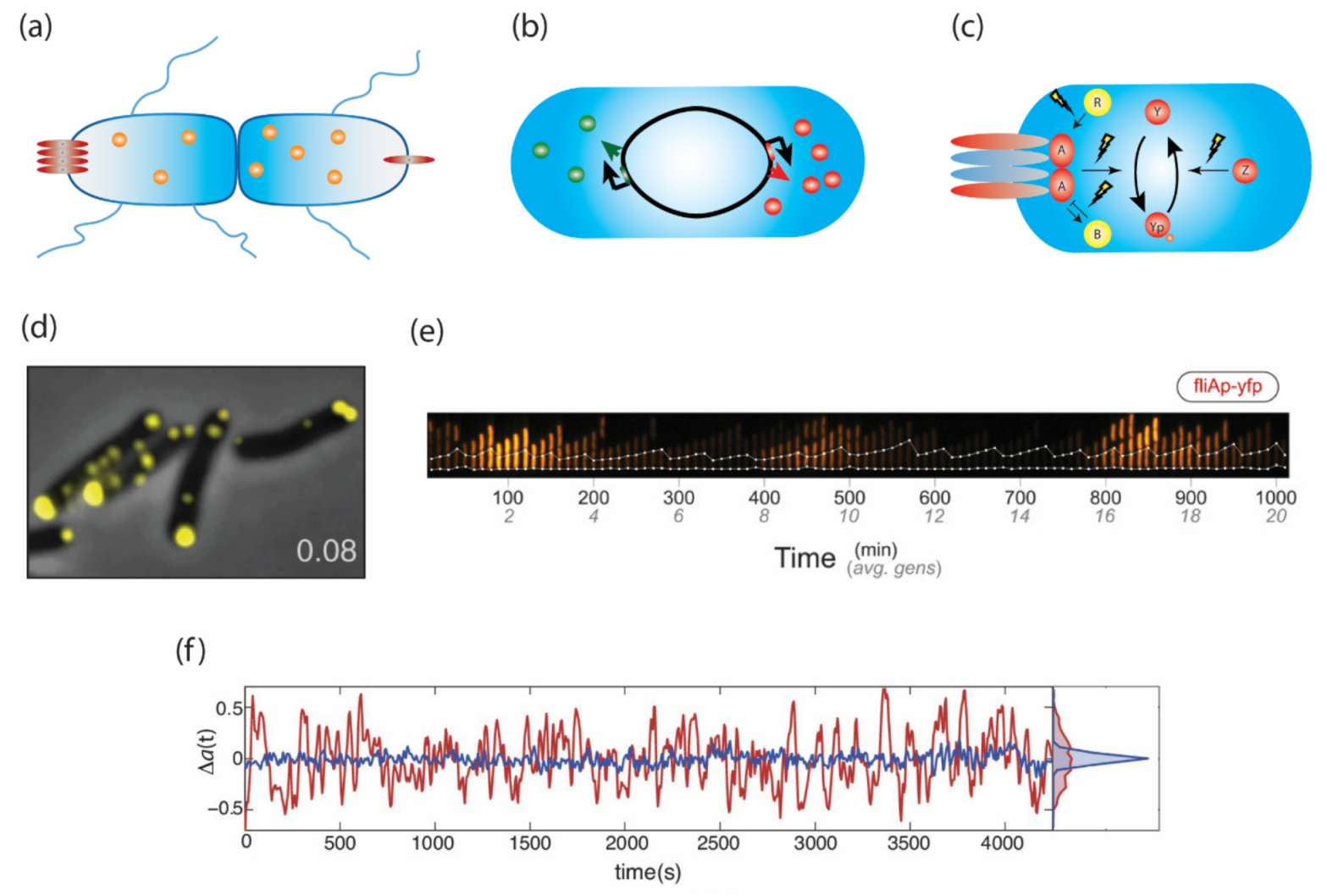
2.1. Variation Arising at Cell Division
2.2. Stochastic Pulses of Motility Gene Expression
2.3. Spontaneous Temporal Fluctuations in Pathway Activity
3. Functional Consequences of Phenotypic Diversity on Chemosensing and Chemotactic Performance
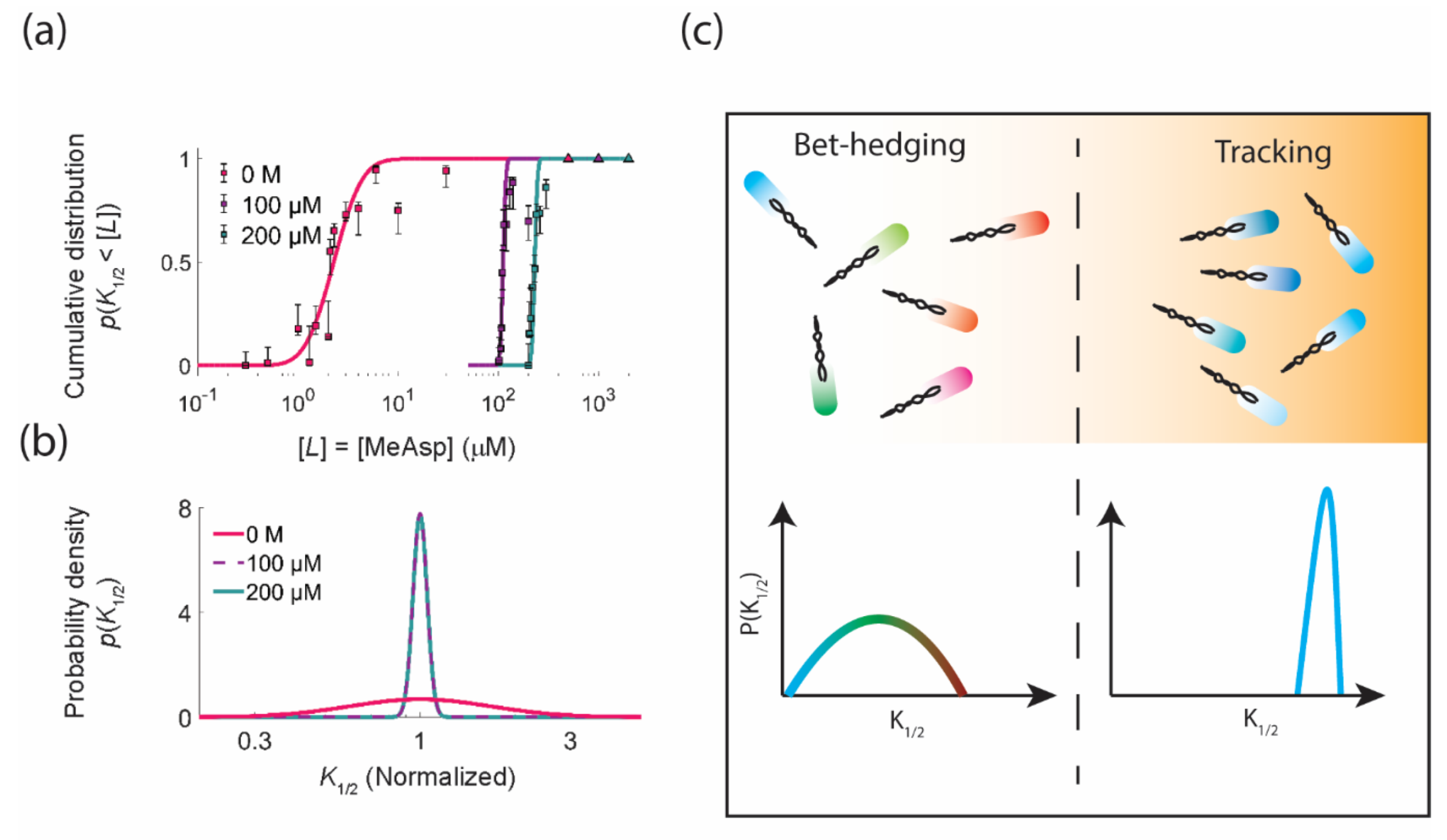
3.1. Functional Consequences of Phenotypic Diversity
3.2. Consequences of Temporal Variation in the Chemosensory Pathway
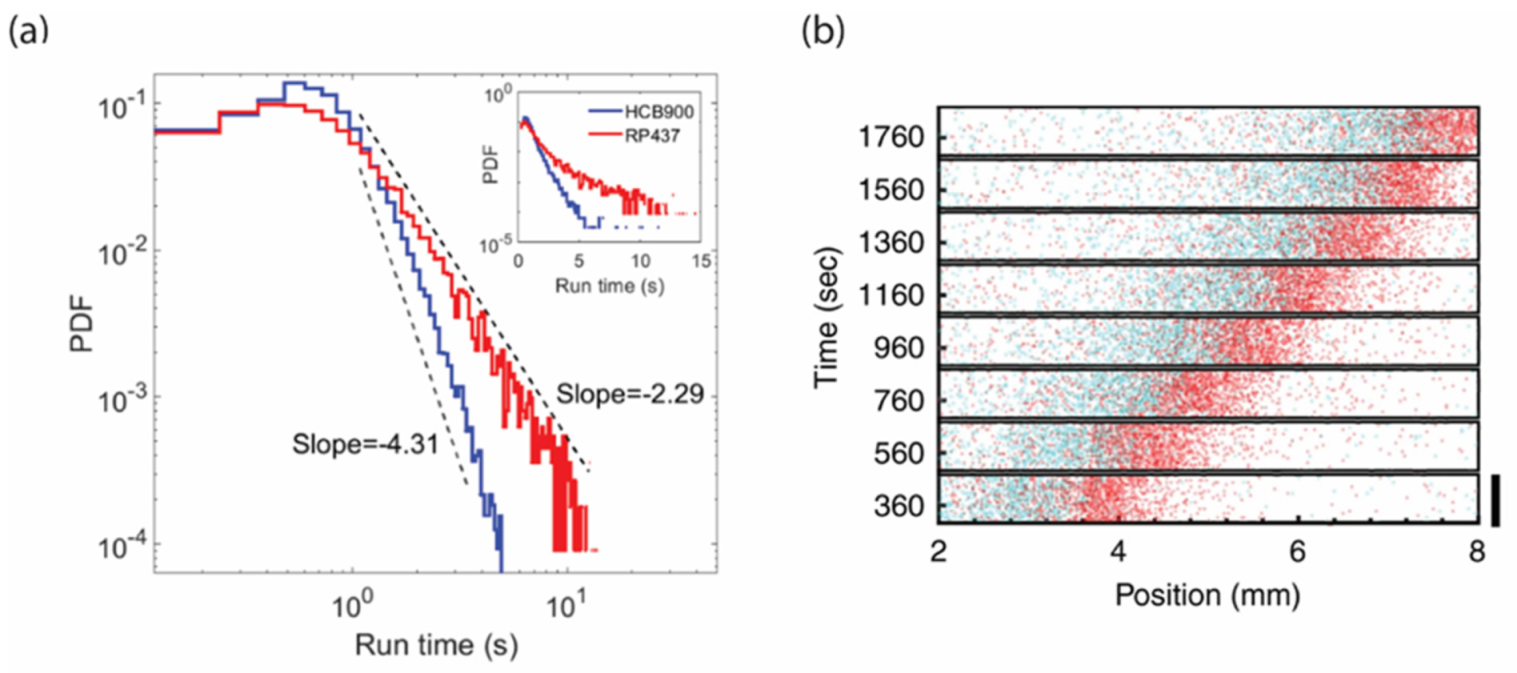
3.3. Spatial Sorting of Chemotaxis Phenotypes
4. Diversity Tuning: Modulating the Degree of Phenotypic Diversity in Response to the Environment
5. Conclusions and Outlook
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Liu, W.; Cremer, J.; Li, D.; Hwa, T.; Liu, C. An evolutionarily stable strategy to colonize spatially extended habitats. Nature 2019, 575, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Genet. 2019, 17, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M. A functional perspective on phenotypic heterogeneity in microorganisms. Nat. Rev. Genet. 2015, 13, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Martins, B.M.; Locke, J.C. Microbial individuality: How single-cell heterogeneity enables population level strategies. Curr. Opin. Microbiol. 2015, 24, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Norman, T.M.; Lord, N.D.; Paulsson, J.; Losick, R. Stochastic Switching of Cell Fate in Microbes. Annu. Rev. Microbiol. 2015, 69, 381–403. [Google Scholar] [CrossRef]
- Raj, A.; Van Oudenaarden, A. Nature, Nurture, or Chance: Stochastic Gene Expression and Its Consequences. Cell 2008, 135, 216–226. [Google Scholar] [CrossRef]
- Macnab, R.M.; Koshland, D.E., Jr. The gradient-sensing mechanism in bacterial chemotaxis. Proc. Natl. Acad. Sci. USA 1972, 69, 2509–2512. [Google Scholar] [CrossRef]
- Spudich, J.L.; Koshland, D.E. Non-genetic individuality: Chance in the single cell. Nature 1976, 262, 467–471. [Google Scholar] [CrossRef]
- Adler, J. Chemotaxis in Bacteria. Science 1966, 153, 708–716. [Google Scholar] [CrossRef]
- Berg, H.C.; Brown, D.A. Chemotaxis in Escherichia coli analysed by Three-dimensional Tracking. Nature 1972, 239, 500–504. [Google Scholar] [CrossRef]
- Cai, L.; Friedman, N.; Xie, X.S. Stochastic protein expression in individual cells at the single molecule level. Nature 2006, 440, 358–362. [Google Scholar] [CrossRef]
- Elowitz, M.B.; Levine, A.J.; Siggia, E.D.; Swain, P. Stochastic Gene Expression in a Single Cell. Science 2002, 297, 1183–1186. [Google Scholar] [CrossRef]
- Huh, D.; Paulsson, J. Non-genetic heterogeneity from stochastic partitioning at cell division. Nat. Genet. 2010, 43, 95–100. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Choi, P.J.; Li, G.-W.; Chen, H.; Babu, M.; Hearn, J.; Emili, A.; Xie, X.S. Quantifying E. coli Proteome and Transcriptome with Single-Molecule Sensitivity in Single Cells. Science 2010, 329, 533–538. [Google Scholar] [CrossRef]
- Giri, S.; Waschina, S.; Kaleta, C.; Kost, C. Defining Division of Labor in Microbial Communities. J. Mol. Biol. 2019, 431, 4712–4731. [Google Scholar] [CrossRef]
- Takhaveev, V.; Heinemann, M. Metabolic heterogeneity in clonal microbial populations. Curr. Opin. Microbiol. 2018, 45, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Sourjik, V. Stimulus sensing and signal processing in bacterial chemotaxis. Curr. Opin. Microbiol. 2018, 45, 22–29. [Google Scholar] [CrossRef]
- Colin, R.; Sourjik, V. Emergent properties of bacterial chemotaxis pathway. Curr. Opin. Microbiol. 2017, 39, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Hazelbauer, G.L.; Falke, J.J.; Parkinson, J.S. Bacterial chemoreceptors: High-performance signaling in networked arrays. Trends Biochem. Sci. 2008, 33, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, J.S.; Hazelbauer, G.L.; Falke, J.J. Signaling and sensory adaptation in Escherichia coli chemoreceptors: 2015 update. Trends Microbiol. 2015, 23, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Briegel, A. Diversity of Bacterial Chemosensory Arrays. Trends Microbiol. 2020, 28, 68–80. [Google Scholar] [CrossRef]
- Piñas, G.E.; Frank, V.; Vaknin, A.; Parkinson, J.S. The source of high signal cooperativity in bacterial chemosensory arrays. Proc. Natl. Acad. Sci. USA 2016, 113, 3335–3340. [Google Scholar] [CrossRef]
- Karmakar, R. State of the art of bacterial chemotaxis. J. Basic Microbiol. 2021, 61, 366–379. [Google Scholar] [CrossRef]
- Tu, Y. Quantitative modeling of bacterial chemotaxis: Signal amplification and accurate adaptation. Annu. Rev. Biophys. 2013, 42, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Waite, A.J.; Frankel, N.W.; Emonet, T. Behavioral Variability and Phenotypic Diversity in Bacterial Chemotaxis. Annu. Rev. Biophys. 2018, 47, 595–616. [Google Scholar] [CrossRef]
- Figueroa-Morales, N.; Soto, R.; Junot, G.; Darnige, T.; Douarche, C.; Martinez, V.A.; Lindner, A.; Clément, É. 3D Spatial Exploration by E. coli Echoes Motor Temporal Variability. Phys. Rev. X 2020, 10, 21004. [Google Scholar] [CrossRef]
- Grognot, M.; Taute, K.M. More than propellers: How flagella shape bacterial motility behaviors. Curr. Opin. Microbiol. 2021, 61, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Masson, J.B.; Voisinne, G.; Wong-Ng, J.; Celani, A.; Vergassola, M. Noninvasive inference of the molecular chemotactic response using bacterial trajectories. Proc. Natl. Acad. Sci. USA 2012, 109, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Taute, K.M.; Gude, S.; Tans, S.J.; Shimizu, T.S. High-throughput 3D tracking of bacteria on a standard phase contrast microscope. Nat. Commun. 2015, 6, 8776. [Google Scholar] [CrossRef]
- Turner, L.; Ping, L.; Neubauer, M.; Berg, H.C. Visualizing Flagella while Tracking Bacteria. Biophys. J. 2016, 111, 630–639. [Google Scholar] [CrossRef]
- Dufour, Y.S.; Gillet, S.; Frankel, N.W.; Weibel, D.B.; Emonet, T. Direct Correlation between Motile Behavior and Protein Abundance in Single Cells. PLoS Comput. Biol. 2016, 12, e1005041. [Google Scholar] [CrossRef]
- Jordan, D.; Kuehn, S.; Katifori, E.; Leibler, S. Behavioral diversity in microbes and low-dimensional phenotypic spaces. Proc. Natl. Acad. Sci. USA 2013, 110, 14018–14023. [Google Scholar] [CrossRef]
- Pleška, M.; Jordan, D.; Frentz, Z.; Xue, B.; Leibler, S. Nongenetic individuality, changeability, and inheritance in bacterial behavior. Proc. Natl. Acad. Sci. USA 2021, 118, 2023322118. [Google Scholar] [CrossRef] [PubMed]
- Koler, M.; Peretz, E.; Aditya, C.; Shimizu, T.S.; Vaknin, A. Long-term positioning and polar preference of chemoreceptor clusters in E. coli. Nat. Commun. 2018, 9, 4444. [Google Scholar] [CrossRef]
- Kim, J.M.; Garcia-Alcala, M.; Balleza, E.; Cluzel, P. Stochastic transcriptional pulses orchestrate flagellar biosynthesis in Escherichia coli. Sci. Adv. 2020, 6, eaax0947. [Google Scholar] [CrossRef] [PubMed]
- Keegstra, J.M.; Kamino, K.; Anquez, F.; Lazova, M.D.; Emonet, T.; Shimizu, T.S. Phenotypic diversity and temporal variability in a bacterial signaling network revealed by single-cell FRET. eLife 2017, 6, e27455. [Google Scholar] [CrossRef]
- Baptista, I.; Ribeiro, A.S. Stochastic models coupling gene expression and partitioning in cell division in Escherichia coli. Biosystem 2020, 193–194, 104154. [Google Scholar] [CrossRef]
- Jones, C.W.; Armitage, J.P. Positioning of bacterial chemoreceptors. Trends Microbiol. 2015, 23, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Solari, J.; Anquez, F.; Scherer, K.M.; Shimizu, T.S. Bacterial Chemoreceptor Imaging at High Spatiotemporal Resolution Using Photoconvertible Fluorescent Proteins; Springer: New York, NY, USA, 2018; pp. 203–231. [Google Scholar]
- Brameyer, S.; Hoyer, E.; Bibinger, S.; Burdack, K.; Lassak, J.; Jung, K. Molecular Design of a Signaling System Influences Noise in Protein Abundance under Acid Stress in Different Gammaproteobacteria. J. Bacteriol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Brewster, R.C.; Phillips, R. Promoter architecture dictates cell-to-cell variability in gene expression. Science 2014, 346, 1533–1536. [Google Scholar] [CrossRef]
- Kollmann, M.; Løvdok, L.; Bartholomé, K.; Timmer, J.; Sourjik, V. Design principles of a bacterial signalling network. Nature 2005, 438, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Dufour, Y.S.; Sneddon, M.W.; Emonet, T. Thermal Robustness: Lessons from Bacterial Chemotaxis. Curr. Biol. 2011, 21, R465–R468. [Google Scholar] [CrossRef][Green Version]
- Oleksiuk, O.; Jakovljevic, V.; Vladimirov, N.; Carvalho, R.; Paster, E.; Ryu, W.S.; Meir, Y.; Wingreen, N.S.; Kollmann, M.; Sourjik, V. Thermal Robustness of Signaling in Bacterial Chemotaxis. Cell 2011, 145, 312–321. [Google Scholar] [CrossRef]
- Frankel, N.W.; Pontius, W.; Dufour, Y.S.; Long, J.; Hernandez-Nunez, L.; Emonet, T. Adaptability of non-genetic diversity in bacterial chemotaxis. eLife 2014, 3, e3526. [Google Scholar] [CrossRef]
- Yoney, A.; Salman, H. Precision and Variability in Bacterial Temperature Sensing. Biophys. J. 2015, 108, 2427–2436. [Google Scholar] [CrossRef][Green Version]
- Kalinin, Y.; Neumann, S.; Sourjik, V.; Wu, M. Responses of Escherichia coli bacteria to two opposing chemoattractant gradients depend on the chemoreceptor ratio. J. Bacteriol. 2010, 192, 1796–1800. [Google Scholar] [CrossRef]
- Apel, D.; Surette, M.G. Bringing order to a complex molecular machine: The assembly of the bacterial flagella. Biochim. Biophys. Acta (BBA) Biomembr. 2008, 1778, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Sassi, A.S.; Garcia-Alcala, M.; Kim, M.J.; Cluzel, P.; Tu, Y. Filtering input fluctuations in intensity and in time underlies stochastic transcriptional pulses without feedback. Proc. Natl. Acad. Sci. USA 2020, 117, 26608–26615. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; O’Shea, E.K. Signal-dependent dynamics of transcription factor translocation controls gene expression. Nat. Struct. Mol. Biol. 2011, 19, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Locke, J.C.W.; Young, J.W.; Fontes, M.; Jimenez, M.J.H.; Elowitz, M.B. Stochastic Pulse Regulation in Bacterial Stress Response. Science 2011, 334, 366–369. [Google Scholar] [CrossRef]
- Korobkova, E.; Emonet, T.; Vilar, J.M.G.; Shimizu, T.S.; Cluzel, P. From molecular noise to behavioural variability in a single bacterium. Nature 2004, 428, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Barkai, N.; Leibler, S. Robustness in simple biochemical networks. Nature 1997, 387, 913–917. [Google Scholar] [CrossRef]
- Emonet, T.; Cluzel, P. Relationship between cellular response and behavioral variability in bacterial chemotaxis. Proc. Natl. Acad. Sci. USA 2008, 105, 3304–3309. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Pontius, W.; Guet, C.C.; Marko, J.F.; Emonet, T.; Cluzel, P. Interdependence of behavioural variability and response to small stimuli in bacteria. Nature 2010, 468, 819–823. [Google Scholar] [CrossRef]
- Shimizu, T.S.; Tu, Y.; Berg, H.C. A modular gradient-sensing network for chemotaxis in Escherichia coli revealed by responses to time-varying stimuli. Mol. Syst. Biol. 2010, 6, 382. [Google Scholar] [CrossRef]
- Li, M.; Hazelbauer, G.L. Cellular Stoichiometry of the Components of the Chemotaxis Signaling Complex. J. Bacteriol. 2004, 186, 3687–3694. [Google Scholar] [CrossRef]
- Goldbeter, A.; Koshland, D.E. An amplified sensitivity arising from covalent modification in biological systems. Proc. Natl. Acad. Sci. USA 1981, 78, 6840–6844. [Google Scholar] [CrossRef]
- Sourjik, V.; Berg, H.C. Receptor sensitivity in bacterial chemotaxis. Proc. Natl. Acad. Sci. USA 2002, 99, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Colin, R.; Rosazza, C.; Vaknin, A.; Sourjik, V. Multiple sources of slow activity fluctuations in a bacterial chemosensory network. eLife 2017. [Google Scholar] [CrossRef]
- Kamino, K.; Keegstra, J.M.; Long, J.; Emonet, T.; Shimizu, T.S. Adaptive tuning of cell sensory diversity without changes in gene expression. Sci. Adv. 2020, 6, eabc1087. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, H.H.; Kamino, K.; Machta, B.B.; Emonet, T. E. coli chemotaxis is information-limited. arXiv 2021, arXiv:2102.11732. [Google Scholar]
- Frank, V.; Piñas, G.E.; Cohen, H.; Parkinson, J.S.; Vaknin, A. Networked Chemoreceptors Benefit Bacterial Chemotaxis Performance. mBio 2016, 7, e1824. [Google Scholar] [CrossRef]
- Endres, R.G.; Wingreen, N.S. Precise adaptation in bacterial chemotaxis through “assistance neighborhoods”. Proc. Natl. Acad. Sci. USA 2006, 103, 13040–13044. [Google Scholar] [CrossRef]
- Li, M.; Hazelbauer, G.L. Adaptational assistance in clusters of bacterial chemoreceptors. Mol. Microbiol. 2005, 56, 1617–1626. [Google Scholar] [CrossRef]
- Pontius, W.; Sneddon, M.W.; Emonet, T. Adaptation Dynamics in Densely Clustered Chemoreceptors. PLoS Comput. Biol. 2013, 9, e1003230. [Google Scholar] [CrossRef] [PubMed]
- Bódi, Z.; Farkas, Z.; Nevozhay, D.; Kalapis, D.; Lázár, V.; Csörgő, B.; Nyerges, Á.; Szamecz, B.; Fekete, G.; Papp, B.; et al. Phenotypic heterogeneity promotes adaptive evolution. PLoS Biol. 2017, 15, e2000644. [Google Scholar] [CrossRef]
- Min, T.L.; Mears, P.J.; Chubiz, L.M.; Rao, C.V.; Golding, I.; Chemla, Y.R. High-resolution, long-term characterization of bacterial motility using optical tweezers. Nat. Methods 2009, 6, 831–835. [Google Scholar] [CrossRef]
- Min, T.L.; Mears, P.J.; Golding, I.; Chemla, Y.R. Chemotactic adaptation kinetics of individual Escherichia coli cells. Proc. Natl. Acad. Sci. USA 2012, 109, 9869–9874. [Google Scholar] [CrossRef]
- Waite, A.J.; Frankel, N.W.; Dufour, Y.S.; Johnston, J.F.; Long, J.; Emonet, T. Non-genetic diversity modulates population performance. Mol. Syst. Biol. 2016, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- Dufour, Y.; Fu, X.; Hernandez-Nunez, L.; Emonet, T. Limits of Feedback Control in Bacterial Chemotaxis. PLoS Comput. Biol. 2014, 10, e1003694. [Google Scholar] [CrossRef] [PubMed]
- Salek, M.M.; Carrara, F.; Fernandez, V.; Guasto, J.S.; Stocker, R. Bacterial chemotaxis in a microfluidic T-maze reveals strong phenotypic heterogeneity in chemotactic sensitivity. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Jensen, J.L.W.V. Sur les fonctions convexes et les inégalités entre les valeurs moyennes. Acta Math. 1906, 30, 175–193. [Google Scholar] [CrossRef]
- De Jong, I.G.; Haccou, P.; Kuipers, O.P. Bet hedging or not? A guide to proper classification of microbial survival strategies. BioEssays 2011, 33, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Kussell, E.; Leibler, S. Phenotypic diversity, population growth, and information in fluctuating environments. Science 2005, 309, 2075–2078. [Google Scholar] [CrossRef] [PubMed]
- Shoval, O.; Sheftel, H.; Shinar, G.; Hart, Y.; Ramote, O.; Mayo, A.; Dekel, E.; Kavanagh, K.; Alon, U. Evolutionary trade-offs, Pareto optimality, and the geometry of phenotype space. Science 2012, 336, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, T.; Datta, S.S. Bacterial hopping and trapping in porous media. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Bhattacharjee, T.; Datta, S.S. Confinement and activity regulate bacterial motion in porous media. Soft Matter 2019, 15, 9920–9930. [Google Scholar] [CrossRef]
- Tostevin, F.; Ten Wolde, P.R. Mutual Information between Input and Output Trajectories of Biochemical Networks. Phys. Rev. Lett. 2009, 102, 218101. [Google Scholar] [CrossRef]
- Sartori, P.; Tu, Y. Free Energy Cost of Reducing Noise while Maintaining a High Sensitivity. Phys. Rev. Lett. 2015, 115, 118102. [Google Scholar] [CrossRef]
- Tu, Y.; Grinstein, G. How White Noise Generates Power-Law Switching in Bacterial Flagellar Motors. Phys. Rev. Lett. 2005, 94, 208101. [Google Scholar] [CrossRef]
- Matthäus, F.; Mommer, M.S.; Curk, T.; Dobnikar, J. On the Origin and Characteristics of Noise-Induced Lévy Walks of E. Coli. PLoS ONE 2011, 6, e18623. [Google Scholar] [CrossRef]
- Flores, M.; Shimizu, T.S.; Ten Wolde, P.R.; Tostevin, F. Signaling Noise Enhances Chemotactic Drift of E. coli. Phys. Rev. Lett. 2012, 109, 148101. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, M.W.; Pontius, W.; Emonet, T. Stochastic coordination of multiple actuators reduces latency and improves chemotactic response in bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, 805–810. [Google Scholar] [CrossRef]
- Huo, H.; He, R.; Zhang, R.; Yuan, J. Swimming Escherichia coli Cells Explore the Environment by Lévy Walk. Appl. Environ. Microbiol. 2021, 87, e02429-20. [Google Scholar] [CrossRef]
- Ariel, G.; Rabani, A.; Benisty, S.; Partridge, J.D.; Harshey, R.M.; Be’er, A. Swarming bacteria migrate by Lévy Walk. Nat. Commun. 2015, 6, 8396. [Google Scholar] [CrossRef]
- Fu, X.; Kato, S.; Long, J.; Mattingly, H.H.; He, C.; Vural, D.C.; Zucker, S.W.; Emonet, T. Spatial self-organization resolves conflicts between individuality and collective migration. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Adler, J. Effect of Amino Acids and Oxygen on Chemotaxis in Escherichia coli. J. Bacteriol. 1966, 92, 121–129. [Google Scholar] [CrossRef]
- Cremer, J.; Honda, T.; Tang, Y.; Ng, J.W.; Vergassola, M.; Hwa, T. Chemotaxis as a navigation strategy to boost range expansion. Nature 2019, 575, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Dragoš, A.; Kiesewalter, H.; Martin, M.; Hsu, C.-Y.; Hartmann, R.; Wechsler, T.; Eriksen, C.; Brix, S.; Drescher, K.; Stanley-Wall, N.; et al. Division of Labor during Biofilm Matrix Production. Curr. Biol. 2018, 28, 1903–1913.e5. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, H.; Emonet, T. A rule from bacteria to balance growth and expansion. Nature 2019, 575, 602–603. [Google Scholar] [CrossRef]
- Tsoi, R.; Wu, F.; Zhang, C.; Bewick, S.; Karig, D.; You, L. Metabolic division of labor in microbial systems. Proc. Natl. Acad. Sci. USA 2018, 115, 2526–2531. [Google Scholar] [CrossRef]
- Xue, B.; Sartori, P.; Leibler, S. Environment-to-phenotype mapping and adaptation strategies in varying environments. Proc. Natl. Acad. Sci. USA 2019, 116, 13847–13855. [Google Scholar] [CrossRef]
- Lambert, G.; Kussell, E. Memory and Fitness Optimization of Bacteria under Fluctuating Environments. PLoS Genet. 2014, 10, e1004556. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, F.; Littmann, S.; Lavik, G.; Escrig, S.; Meibom, A.; Kuypers, M.M.M.; Ackermann, M. Phenotypic heterogeneity driven by nutrient limitation promotes growth in fluctuating environments. Nat. Microbiol. 2016, 1, 16055. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.; Mettert, E.L.; Roggiani, M.; Myers, K.S.; Kiley, P.J.; Goulian, M. Regulated Stochasticity in a Bacterial Signaling Network Permits Tolerance to a Rapid Environmental Change. Cell 2018, 173, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Roggiani, M.; Goulian, M. Oxygen-Dependent Cell-to-Cell Variability in the Output of the Escherichia coli Tor Phosphorelay. J. Bacteriol. 2015, 197, 1976–1987. [Google Scholar] [CrossRef]
- Keymer, J.E.; Endres, R.G.; Skoge, M.; Meir, Y.; Wingreen, N.S. Chemosensing in Escherichia coli: Two regimes of two-state receptors. Proc. Natl. Acad. Sci. USA 2006, 103, 1786–1791. [Google Scholar] [CrossRef]
- Mello, B.A.; Tu, Y. An allosteric model for heterogeneous receptor complexes: Understanding bacterial chemotaxis responses to multiple stimuli. Proc. Natl. Acad. Sci. USA 2005, 102, 17354–17359. [Google Scholar] [CrossRef]
- Karin, O.; Alon, U. Cell-Cell Variation in Chemotaxis Gain Implements a Simulated Tempering Strategy for Efficient Navigation in Complex Environments. iScience 2021. [Google Scholar] [CrossRef]
- Ni, B.; Colin, R.; Link, H.; Endres, R.G.; Sourjik, V. Growth-rate dependent resource investment in bacterial motile behavior quantitatively follows potential benefit of chemotaxis. Proc. Natl. Acad. Sci. USA 2020, 117, 595–601. [Google Scholar] [CrossRef]
- Wuichet, K.; Zhulin, I. Origins and Diversification of a Complex Signal Transduction System in Prokaryotes. Sci. Signal. 2010, 3, ra50. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, J.P.; Kamino, K.; Emonet, T. Non-Genetic Diversity in Chemosensing and Chemotactic Behavior. Int. J. Mol. Sci. 2021, 22, 6960. https://doi.org/10.3390/ijms22136960
Moore JP, Kamino K, Emonet T. Non-Genetic Diversity in Chemosensing and Chemotactic Behavior. International Journal of Molecular Sciences. 2021; 22(13):6960. https://doi.org/10.3390/ijms22136960
Chicago/Turabian StyleMoore, Jeremy Philippe, Keita Kamino, and Thierry Emonet. 2021. "Non-Genetic Diversity in Chemosensing and Chemotactic Behavior" International Journal of Molecular Sciences 22, no. 13: 6960. https://doi.org/10.3390/ijms22136960
APA StyleMoore, J. P., Kamino, K., & Emonet, T. (2021). Non-Genetic Diversity in Chemosensing and Chemotactic Behavior. International Journal of Molecular Sciences, 22(13), 6960. https://doi.org/10.3390/ijms22136960





