Chemotaxis of Beneficial Rhizobacteria to Root Exudates: The First Step towards Root–Microbe Rhizosphere Interactions
Abstract
:1. Introduction
2. Chemoeffectors in Root Exudates Sensed by Beneficial Rhizobacteria
3. Rhizobacterial MCPs Sensing the Rhizosphere Chemoeffectors
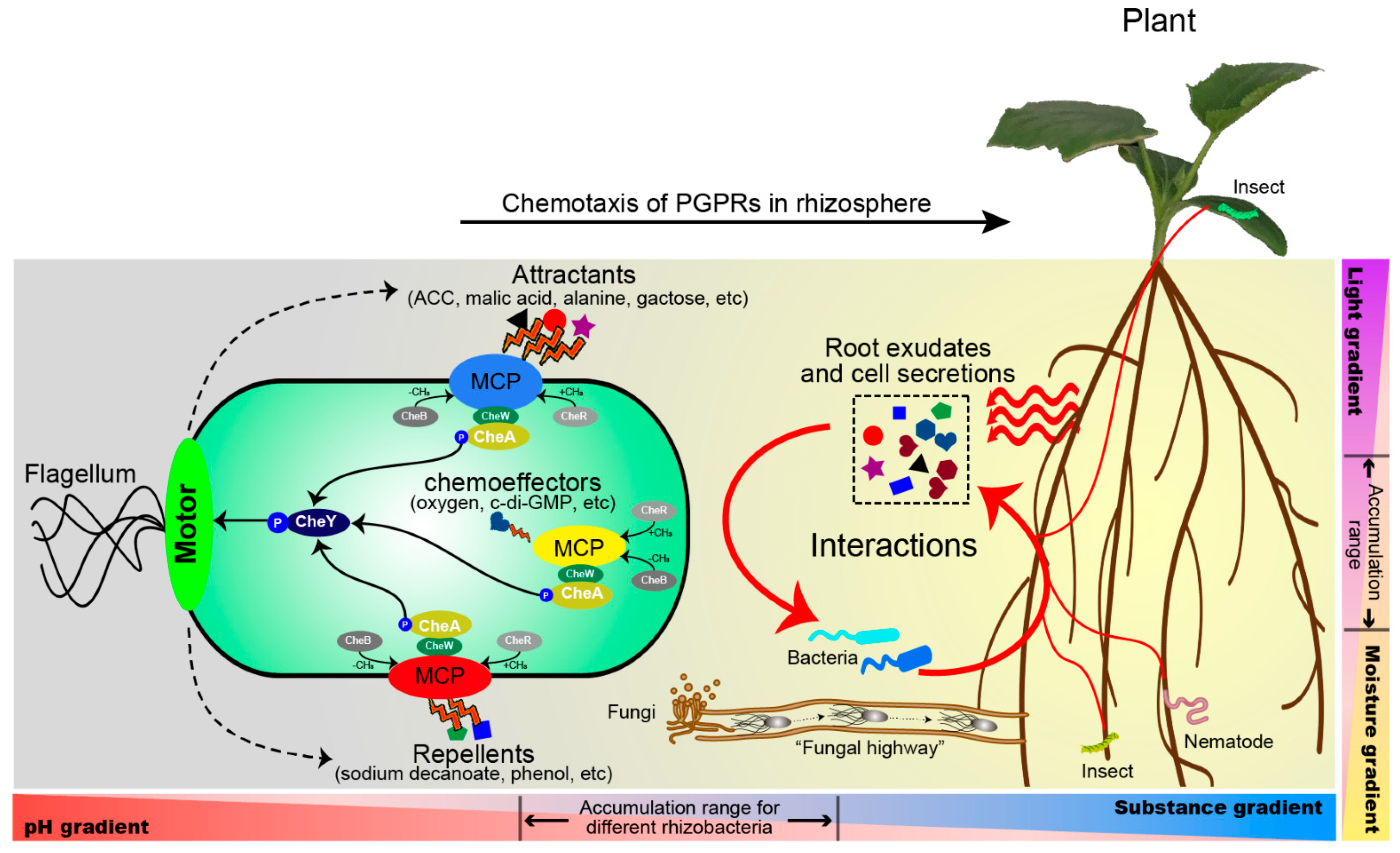
4. Role of Chemotaxis in Root–Microbe Interactions in Rhizosphere
5. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.; et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- De Vries, F.T.; Griffiths, R.I.; Knight, C.G.; Nicolitch, O.; Williams, A. Harnessing Rhizosphere Microbiomes for Drought-Resilient Crop Production. Science 2020, 368, 270–274. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Van Le, A.; Dufresne, A. The Importance of the Microbiome of the Plant Holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [Green Version]
- Compant, S.; Clément, C.; Sessitsch, A. Plant Growth-Promoting Bacteria in the Rhizo- and Endosphere of Plants: Their Role, Colonization, Mechanisms Involved and Prospects for Utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Kamilova, F.; Validov, S.; Gafurova, L.; Kucharova, Z.; Lugtenberg, B. High Incidence of Plant Growth-Stimulating Bacteria Associated with the Rhizosphere of Wheat Grown on Salinated Soil in Uzbekistan. Environ. Microbiol. 2008, 10, 1–9. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The Role of Root Exudates in Rhizosphere Interactions with Plants and Other Organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, H.G.; Song, G.C.; Sim, H.-J.; Ryu, C.-M. Achieving Similar Root Microbiota Composition in Neighbouring Plants through Airborne Signalling. ISME J. 2021, 15, 397–408. [Google Scholar] [CrossRef]
- Mönchgesang, S.; Strehmel, N.; Schmidt, S.; Westphal, L.; Taruttis, F.; Müller, E.; Herklotz, S.; Neumann, S.; Scheel, D. Natural Variation of Root Exudates in Arabidopsis thaliana-Linking Metabolomic and Genomic Data. Sci. Rep. 2016, 6, 29033. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere Microbiome Assemblage Is Affected by Plant Development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef] [Green Version]
- Carvalhais, L.C.; Dennis, P.G.; Fan, B.; Fedoseyenko, D.; Kierul, K.; Becker, A.; von Wiren, N.; Borriss, R. Linking Plant Nutritional Status to Plant–Microbe Interactions. PLoS ONE 2013, 8, e68555. [Google Scholar] [CrossRef]
- Frey, M.; Schullehner, K.; Dick, R.; Fiesselmann, A.; Gierl, A. Benzoxazinoid Biosynthesis, a Model for Evolution of Secondary Metabolic Pathways in Plants. Phytochemistry 2009, 70, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Kudjordjie, E.N.; Sapkota, R.; Steffensen, S.K.; Fomsgaard, I.S.; Nicolaisen, M. Maize Synthesized Benzoxazinoids Affect the Host Associated Microbiome. Microbiome 2019, 7, 1–17. [Google Scholar] [CrossRef]
- Chagas, F.O.; Pessotti, R.D.C.; Caraballo-Rodríguez, A.M.; Pupo, M.T. Chemical Signaling Involved in Plant–Microbe Interactions. Chem. Soc. Rev. 2018, 47, 1652–1704. [Google Scholar] [CrossRef] [PubMed]
- Bardy, S.L.; Briegel, A.; Rainville, S.; Krell, T. Recent Advances and Future Prospects in Bacterial and Archaeal Locomotion and Signal Transduction. J. Bacteriol. 2017, 199, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allard-Massicotte, R.; Tessier, L.; Lecuyer, F.; Lakshmanan, V.; Lucier, J.F.; Garneau, D.; Caudwell, L.; Vlamakis, H.; Bais, H.P.; Beauregard, P.B. Bacillus subtilis Early Colonization of Arabidopsis thaliana Roots Involves Multiple Chemotaxis Receptors. mBio 2016, 7, e01664-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharf, B.E.; Hynes, M.F.; Alexandre, G.M. Chemotaxis Signaling Systems in Model Beneficial Plant–Bacteria Associations. Plant Mol. Biol. 2016, 90, 549–559. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and Function of Root Exudates. Plant. Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Pétriacq, P.; Williams, A.; Cotton, A.; McFarlane, A.E.; Rolfe, S.A.; Ton, J. Metabolite Profiling of Non-Sterile Rhizosphere Soil. Plant J. 2017, 92, 147–162. [Google Scholar] [CrossRef]
- Pérez-García, A.; Romero, D.; de Vicente, A. Plant Protection and Growth Stimulation by Microorganisms: Biotechnological Applications of Bacilli in Agriculture. Curr. Opin. Biotechnol. 2011, 22, 187–193. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Z.; Ling, N.; Yuan, Y.; Zheng, X.; Shen, B.; Shen, Q. Bacillus subtilis SQR9 Can Control Fusarium Wilt in Cucumber by Colonizing Plant Roots. Biol. Fertil. Soils 2011, 47, 495–506. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, D.; Liu, Y.; Li, S.; Shen, Q.; Zhang, R. Effects of Different Plant Root Exudates and Their Organic Acid Components on Chemotaxis, Biofilm Formation and Colonization by Beneficial Rhizosphere-Associated Bacterial Strains. Plant Soil 2013, 374, 689–700. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, N.; Du, W.; Zhang, H.; Liu, Y.; Fu, R.; Shao, J.; Zhang, G.; Shen, Q.R.; Zhang, R. Identification of Chemotaxis Compounds in Root Exudates and Their Sensing Chemoreceptors in Plant Growth-Promoting Rhizobacteria Bacillus amyloliquefaciens SQR9. Mol. Plant Microbe Interact. 2018, 31, 995–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, Á.; Zhulin, I.B.; Krell, T. Sensory Repertoire of Bacterial Chemoreceptors. Microbiol. Mol. Biol. Rev. 2017, 81, e00033-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compton, K.K.; Hildreth, S.B.; Helm, R.F.; Scharf, B.E. Sinorhizobium meliloti Chemoreceptor McpV Senses Short-Chain Carboxylates via Direct Binding. J. Bacteriol. 2018, 200, e00519-18. [Google Scholar] [CrossRef] [Green Version]
- Hanlon, D.W.; Ordal, G.W. Cloning and Characterization of Genes Encoding Methyl-Accepting Chemotaxis Proteins in Bacillus subtilis. J. Biol. Chem. 1994, 269, 14038–14046. [Google Scholar] [CrossRef]
- Tohidifar, P.; Bodhankar, G.A.; Pei, S.; Cassidy, C.K.; Walukiewicz, H.E.; Ordal, G.W.; Stansfeld, P.J.; Rao, C.V. The Unconventional Cytoplasmic Sensing Mechanism for Ethanol Chemotaxis in Bacillus subtilis. mBio 2020, 11, e02177-20. [Google Scholar] [CrossRef]
- Glekas, G.D.; Mulhern, B.J.; Kroc, A.; Duelfer, K.A.; Lei, V.; Rao, C.V.; Ordal, G.W. The Bacillus subtilis Chemoreceptor McpC Senses Multiple Ligands Using Two Discrete Mechanisms. J. Biol. Chem. 2012, 287, 39412–39418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corral-Lugo, A.; De la Torre, J.; Matilla, M.A.; Fernández, M.; Morel, B.; Espinosa-Urgel, M.; Krell, T. Assessment of the Contribution of Chemoreceptor-Based Signalling to Biofilm Formation. Environ. Microbiol. 2016, 18, 3355–3372. [Google Scholar] [CrossRef]
- Reyes-Darias, J.A.; García, V.; Rico-Jiménez, M.; Corral-Lugo, A.; Lesouhaitier, O.; Juárez-Hernández, D.; Yang, Y.; Bi, S.; Feuilloley, M.; Muñoz-Rojas, J.; et al. Specific Gamma-Aminobutyrate Chemotaxis in Pseudomonads with Different Lifestyle. Mol. Microbiol. 2015, 97, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Morel, B.; Corral-Lugo, A.; Krell, T. Identification of a Chemoreceptor That Specifically Mediates Chemotaxis toward Metabolizable Purine Derivatives. Mol. Microbiol. 2016, 99, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.; Reyes-Darias, J.A.; Martin-Mora, D.; Morel, B.; Matilla, M.A.; Krell, T. Identification of a Chemoreceptor for C2 and C3 Carboxylic Acids. Appl. Environ. Microbiol. 2015, 81, 5449–5457. [Google Scholar] [CrossRef] [Green Version]
- Gavira, J.A.; Ortega, Á.; Martín-Mora, D.; Conejero-Muriel, M.T.; Corral-Lugo, A.; Morel, B.; Matilla, M.A.; Krell, T. Structural Basis for Polyamine Binding at the DCACHE Domain of the McpU Chemoreceptor from Pseudomonas putida. J. Mol. Biol. 2018, 430, 1950–1963. [Google Scholar] [CrossRef] [PubMed]
- Corral-Lugo, A.; Matilla, M.A.; Martín-Mora, D.; Silva Jiménez, H.; Mesa Torres, N.; Kato, J.; Hida, A.; Oku, S.; Conejero-Muriel, M.; Gavira, J.A.; et al. High-Affinity Chemotaxis to Histamine Mediated by the TlpQ Chemoreceptor of the Human Pathogen Pseudomonas aeruginosa. mBio 2018, 9, e01894-18. [Google Scholar] [CrossRef] [Green Version]
- Oku, S.; Komatsu, A.; Tajima, T.; Nakashimada, Y.; Kato, J. Identification of Chemotaxis Sensory Proteins for Amino Acids in Pseudomonas fluorescens Pf0-1 and Their Involvement in Chemotaxis to Tomato Root Exudate and Root Colonization. Microbes Environ. 2012, 27, 462–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oku, S.; Komatsu, A.; Nakashimada, Y.; Tajima, T.; Kato, J. Identification of Pseudomonas fluorescens Chemotaxis Sensory Proteins for Malate, Succinate, and Fumarate, and Their Involvement in Root Colonization. Microbes Environ. 2014, 29, 413–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, B.A.; Helm, R.F.; Scharf, B.E. Contribution of Individual Chemoreceptors to Sinorhizobium meliloti Chemotaxis Towards Amino Acids of Host and Nonhost Seed Exudates. Mol. Plant Microbe Interact. 2016, 29, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Meier, V.M.; Muschler, P.; Scharf, B.E. Functional Analysis of Nine Putative Chemoreceptor Proteins in Sinorhizobium meliloti. J. Bacteriol. 2007, 189, 1816–1826. [Google Scholar] [CrossRef] [Green Version]
- Webb, B.A.; Karl Compton, K.; Castaneda Saldana, R.; Arapov, T.D.; Keith Ray, W.; Helm, R.F.; Scharf, B.E. Sinorhizobium meliloti Chemotaxis to Quaternary Ammonium Compounds Is Mediated by the Chemoreceptor McpX. Mol. Microbiol. 2017, 103, 333–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, M.; Compton, K.K.; Mancl, J.M.; Webb, B.A.; Brown, A.M.; Scharf, B.E.; Schubot, F.D. Structure of the Sensory Domain of McpX from Sinorhizobium meliloti, the First Known Bacterial Chemotactic Sensor for Quaternary Ammonium Compounds. Biochem. J. 2018, 475, 3949–3962. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Dam, N.M.; Bouwmeester, H.J. Metabolomics in the Rhizosphere: Tapping into Belowground Chemical Communication. Trends Plant Sci. 2016, 21, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, L.; Wu, G.; Feng, H.; Zhang, G.; Shen, Q.; Zhang, R. Identification of Root-Secreted Compounds Involved in the Communication Between Cucumber, the Beneficial Bacillus amyloliquefaciens, and the Soil-Borne Pathogen Fusarium Oxysporum. Mol. Plant Microbe Interact. 2017, 30, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; Da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P. Dynamic Root Exudate Chemistry and Microbial Substrate Preferences Drive Patterns in Rhizosphere Microbial Community Assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Bais, H.P.; Walker, T.S.; Schweizer, H.P.; Vivanco, J.M. Root Specific Elicitation and Antimicrobial Activity of Rosmarinic Acid in Hairy Root Cultures of Ocimum Basilicum. Plant Physiol. Biochem. 2002, 40, 983–995. [Google Scholar] [CrossRef]
- Zhang, J.; Subramanian, S.; Stacey, G.; Yu, O. Flavones and Flavonols Play Distinct Critical Roles during Nodulation of Medicago Truncatula by Sinorhizobium meliloti. Plant J. Cell Mol. Biol. 2009, 57, 171. [Google Scholar] [CrossRef]
- Lombardi, N.; Vitale, S.; Turrà, D.; Reverberi, M.; Fanelli, C.; Vinale, F.; Marra, R.; Ruocco, M.; Pascale, A.; D’Errico, G.; et al. Root Exudates of Stressed Plants Stimulate and Attract Trichoderma Soil Fungi. Mol. Plant Microbe Interact. 2018, 31, 982–994. [Google Scholar] [CrossRef] [Green Version]
- Oota, M.; Tsai, A.Y.L.; Aoki, D.; Matsushita, Y.; Toyoda, S.; Fukushima, K.; Saeki, K.; Toda, K.; Perfus-Barbeoch, L.; Favery, B.; et al. Identification of Naturally Occurring Polyamines as Root-Knot Nematode Attractants. Mol. Plant 2020, 13, 658–665. [Google Scholar] [CrossRef]
- Gao, X.; Li, T.; Liu, W.; Zhang, Y.; Shang, D.; Gao, Y.; Qi, Y.; Qiu, L. Enhancing the 1-Aminocyclopropane-1-Carboxylate Metabolic Rate of Pseudomonas Sp. UW4 Intensifies Chemotactic Rhizocompetence. Microorganisms 2020, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Tohidifar, P.; Plutz, M.J.; Ordal, G.W.; Rao, C.V. The Mechanism of Bidirectional PH Taxis in Bacillus subtilis. J. Bacteriol. 2019, 202, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Ulrich, L.E.; Zhulin, I.B.; Alexandre, G. PAS Domain Containing Chemoreceptor Couples Dynamic Changes in Metabolism with Chemotaxis. Proc. Natl. Acad. Sci. USA 2010, 107, 2235–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandre, G. Coupling Metabolism and Chemotaxis-Dependent Behaviours by Energy Taxis Receptors. Microbiology 2010, 156, 2283–2293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, B.L.; Zhulin, I.B.; Johnson, M.S. Aerotaxis and Other Energy-Sensing Behavior in Bacteria. Annu. Rev. Microbiol. 1999, 53, 103–128. [Google Scholar] [CrossRef]
- Rebbapragada, A.; Johnson, M.S.; Harding, G.P.; Zuccarelli, A.J.; Fletcher, H.M.; Zhulin, I.B.; Taylor, B.L. The Aer Protein and the Serine Chemoreceptor Tsr Independently Sense Intracellular Energy Levels and Transduce Oxygen, Redox, and Energy Signals for Escherichia Coli Behavior. Proc. Natl. Acad. Sci. USA 1997, 94, 10541–10546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrebola, E.; Cazorla, F.M. Aer Receptors Influence the Pseudomonas chlororaphis PCL1606 Lifestyle. Front. Microbiol. 2020, 11, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, X.G.; Sun, K.; Tang, M.J.; Xu, F.J.; Zhang, M.; Dai, C.C. Mycelial Network-Mediated Rhizobial Dispersal Enhances Legume Nodulation. ISME J. 2020, 14, 1015–1029. [Google Scholar] [CrossRef]
- Muok, A.R.; Claessen, D.; Briegel, A. Microbial Hitchhiking: How Streptomyces Spores Are Transported by Motile Soil Bacteria. ISME J. 2021, 1–10. [Google Scholar] [CrossRef]
- Parkinson, J.S.; Hazelbauer, G.L.; Falke, J.J. Signaling and Sensory Adaptation in Escherichia Coli Chemoreceptors: 2015 Update. Trends Microbiol. 2015, 23, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Matilla, M.A.; Martín-Mora, D.; Krell, T. The Use of Isothermal Titration Calorimetry to Unravel Chemotactic Signalling Mechanisms. Environ. Microbiol. 2020, 22, 3005–3019. [Google Scholar] [CrossRef] [PubMed]
- Flack, C.E.; Parkinson, J.S. A Zipped-Helix Cap Potentiates HAMP Domain Control of Chemoreceptor Signaling. Proc. Natl. Acad. Sci. USA 2018, 115, E3519–E3528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sourjik, V.; Wingreen, N.S. Responding to Chemical Gradients: Bacterial Chemotaxis. Curr. Opin. Cell Biol. 2012, 24, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Lacal, J.; García-Fontana, C.; Muñoz-Martínez, F.; Ramos, J.L.; Krell, T. Sensing of Environmental Signals: Classification of Chemoreceptors According to the Size of Their Ligand Binding Regions. Environ. Microbiol. 2010, 12, 2873–2884. [Google Scholar] [CrossRef] [PubMed]
- Matilla, M.A.; Krell, T. Chemoreceptor-Based Signal Sensing. Curr. Opin. Biotechnol. 2017, 45, 8–14. [Google Scholar] [CrossRef]
- Alexandre, G.; Greer-phillips, S.; Zhulin, I.B. Ecological Role of Energy Taxis in Microorganisms. FEMS Microbiol. Rev. 2004, 28, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Krell, T.; Lacal, J.; Muñoz-Martínez, F.; Reyes-Darias, J.A.; Cadirci, B.H.; García-Fontana, C.; Ramos, J.L. Diversity at Its Best: Bacterial Taxis. Environ. Microbiol. 2011, 13, 1115–1124. [Google Scholar] [CrossRef]
- Fernandez, M.; Corral-Lugo, A.; Krell, T. The Plant Compound Rosmarinic Acid Induces a Broad Quorum Sensing Response in Pseudomonas aeruginosa PAO1. Environ. Microbiol. 2018, 20, 4230–4244. [Google Scholar] [CrossRef] [PubMed]
- Raina, J.-B.; Fernandez, V.; Lambert, B.; Stocker, R.; Seymour, J.R. The Role of Microbial Motility and Chemotaxis in Symbiosis. Nat. Rev. Microbiol. 2019, 17, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, I.; Parales, R.E.; Krell, T.; Hill, J.E. Pseudomonas Chemotaxis. FEMS Microbiol. Rev. 2015, 39, 17–46. [Google Scholar]
- Kato, J.; Kim, H.E.; Takiguchi, N.; Kuroda, A.; Ohtake, H. Pseudomonas aeruginosa as a Model Microorganism for Investigation of Chemotactic Behaviors in Ecosystem. J. Biosci. Bioeng. 2008, 106, 1–7. [Google Scholar] [CrossRef]
- Fernández, M.; Matilla, M.A.; Ortega, Á.; Krell, T. Metabolic Value Chemoattractants Are Preferentially Recognized at Broad Ligand Range Chemoreceptor of Pseudomonas putida KT2440. Front. Microbiol. 2017, 8, 990. [Google Scholar] [CrossRef] [Green Version]
- Glekas, G.D.; Foster, R.M.; Cates, J.R.; Estrella, J.A.; Wawrzyniak, M.J.; Rao, C.V.; Ordal, G.W. A PAS Domain Binds Asparagine in the Chemotaxis Receptor McpB in Bacillus subtilis. J. Biol. Chem. 2010, 285, 1870–1878. [Google Scholar] [CrossRef] [Green Version]
- Zatakia, H.M.; Arapov, T.D.; Meier, V.M.; Scharf, B.E. Cellular Stoichiometry of Methyl-Accepting Chemotaxis Proteins in Sinorhizobium meliloti. J. Bacteriol. 2018, 200, e00614-17. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.; Liu, W.; Li, Y.; Wu, H.; Zhang, Z.; Alexandre, G.; Elmerich, C.; Xie, Z. A Chemotaxis Receptor Modulates Nodulation during the Azorhizobium Caulinodans-Sesbania Rostrata Symbiosis. Appl. Environ. Microbiol. 2016, 82, 3174–3184. [Google Scholar] [CrossRef] [Green Version]
- López-Farfán, D.; Reyes-Darias, J.A.; Matilla, M.A.; Krell, T. Concentration Dependent Effect of Plant Root Exudates on the Chemosensory Systems of Pseudomonas putida KT2440. Front. Microbiol. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zhang, N.; Fu, R.; Liu, Y.; Krell, T.; Du, W.; Shao, J.; Shen, Q.; Zhang, R. Recognition of Dominant Attractants by Key Chemoreceptors Mediates Recruitment of Plant Growth-Promoting Rhizobacteria. Environ. Microbiol. 2019, 21, 402–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Li, S.; Liu, X.; Wang, Z.; Jiang, M.; Wang, R.; Xie, L.; Liu, Q.; Xie, X.; Shang, D.; et al. Sensing of Autoinducer-2 by Functionally Distinct Receptors in Prokaryotes. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rico-Jiménez, M.; Muñoz-Martínez, F.; García-Fontana, C.; Fernandez, M.; Morel, B.; Ortega, Á.; Ramos, J.L.; Krell, T. Paralogous Chemoreceptors Mediate Chemotaxis towards Protein Amino Acids and the Non-Protein Amino Acid Gamma-Aminobutyrate (GABA). Mol. Microbiol. 2013, 88, 1230–1243. [Google Scholar] [CrossRef]
- Matilla, M.A.; Ortega, Á.; Krell, T. The Role of Solute Binding Proteins in Signal Transduction. Comput. Struct. Biotechnol. J. 2021, 19, 1786–1805. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, H.; Fu, R.; Zhang, N.; Du, W.; Shen, Q.; Zhang, R. Induced Root-Secreted D -Galactose Functions as a Chemoattractant and Enhances the Biofilm Formation of Bacillus velezensis SQR9 in an McpA-Dependent Manner. Appl. Microbiol. Biotechnol. 2020, 104, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Machuca, M.A.; Beckham, S.A.; Gunzburg, M.J.; Roujeinikova, A. Structural Basis for Amino-Acid Recognition and Transmembrane Signalling by Tandem Per-Arnt-Sim (Tandem PAS) Chemoreceptor Sensory Domains. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, S.I.; Takahashi, Y.; Yamamoto, K.; Suzuki, D.; Itoh, Y.; Sumita, K.; Uchida, Y.; Homma, M.; Imada, K.; Kawagishi, I. Identification of a Vibrio Cholerae Chemoreceptor That Senses Taurine and Amino Acids as Attractants. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Machuca, M.A.; Johnson, K.S.; Liu, Y.C.; Steer, D.L.; Ottemann, K.M.; Roujeinikova, A. Helicobacter Pylori Chemoreceptor TlpC Mediates Chemotaxis to Lactate. Sci. Rep. 2017, 7, 14089. [Google Scholar] [CrossRef]
- Gavira, J.A.; Gumerov, V.M.; Rico-Jiménez, M.; Petukh, M.; Upadhyay, A.A.; Ortega, A.; Matilla, M.A.; Zhulin, I.B.; Krell, T. How Bacterial Chemoreceptors Evolve Novel Ligand Specificities. mBio 2020, 11, e03066-19. [Google Scholar] [CrossRef] [Green Version]
- Olson, M.S.; Ford, R.M.; Smith, J.A.; Fernandez, E.J. Quantification of Bacterial Chemotaxis in Porous Media Using Magnetic Resonance Imaging. Environ. Sci. Technol. 2004, 38, 3864–3870. [Google Scholar] [CrossRef]
- Armitage, J.P.; Gallagher, A.; Johnston, A.W.B. Comparison of the Chemotactic Behaviour of Rhizobium Leguminosarum with and without the Nodulation Plasmid. Mol. Microbiol. 1988, 2, 743–748. [Google Scholar] [CrossRef]
- Ni, B.; Huang, Z.; Fan, Z.; Jiang, C.Y.; Liu, S.J. Comamonas Testosteroni Uses a Chemoreceptor for Tricarboxylic Acid Cycle Intermediates to Trigger Chemotactic Responses towards Aromatic Compounds. Mol. Microbiol. 2013, 90, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Hida, A.; Oku, S.; Kawasaki, T.; Nakashimada, Y.; Tajima, T.; Kato, J. Identification of the McpA and McpM Genes, Encoding Methyl-Accepting Proteins Involved in Amino Acid and l-Malate Chemotaxis, and Involvement of McpM-Mediated Chemotaxis in Plant Infection by Ralstonia pseudosolanacearum (Formerly Ralstonia solanacearum Phylotypes I and III). Appl. Environ. Microbiol. 2015, 81, 7420–7430. [Google Scholar] [PubMed] [Green Version]
- Yost, C.K.; Rochepeau, P.; Hynes, M.F. Rhizobium Leguminosarum Contains a Group of Genes That Appear to Code for Methyl-Accepting Chemotaxis Proteins. Microbiology 1998, 144, 1945–1956. [Google Scholar] [CrossRef] [Green Version]
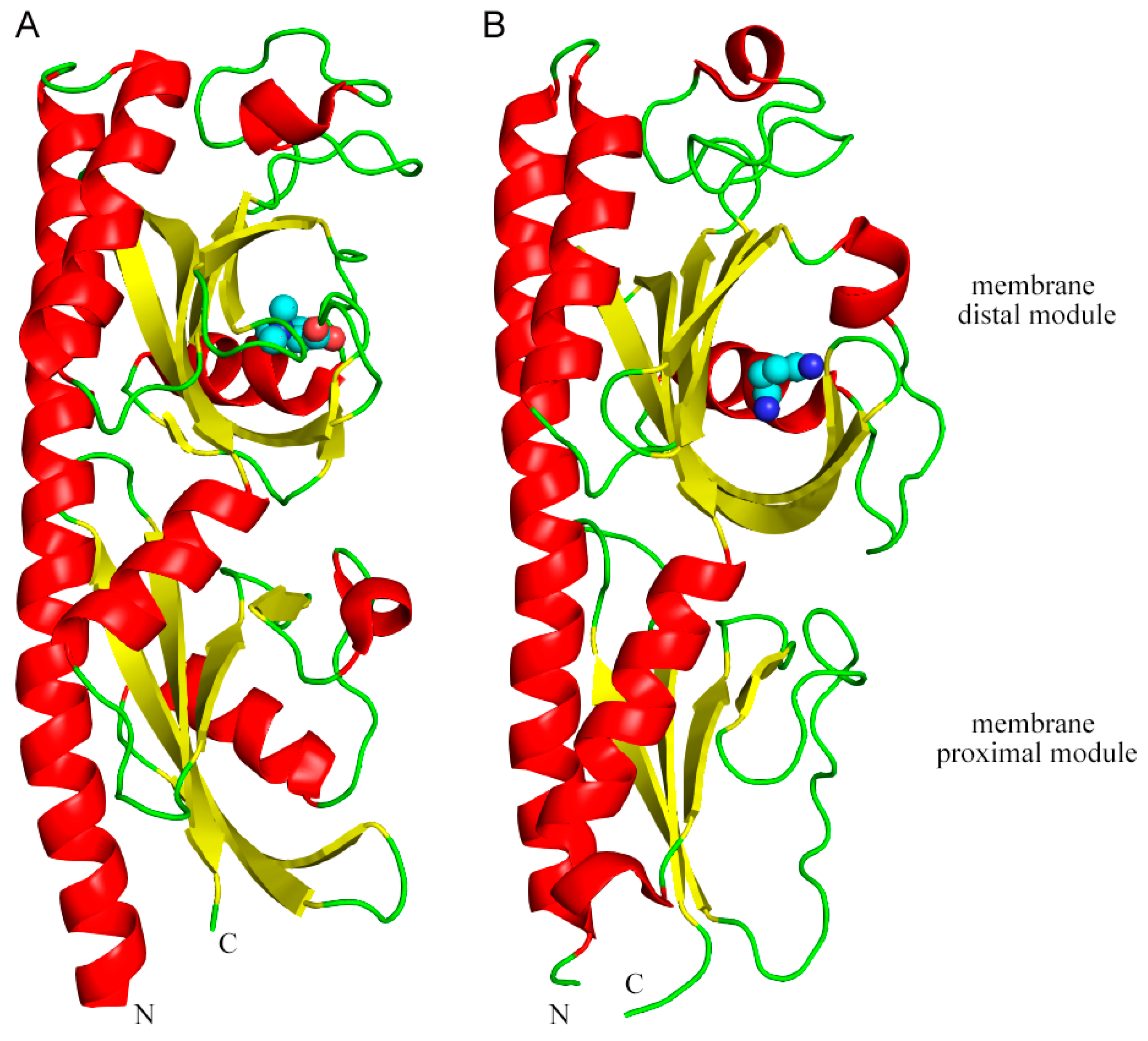
| Strains (Total Number of MCPs) | MCP | Chemoeffector | Binding Mode | References |
|---|---|---|---|---|
| Bacillus subtilis OI1085 (10) | McpA | Glucose, alpha-D-ethylglucoside | unknown | [27] |
| McpB | Asparagine, aspartic acid, glutamine, histidine; methanol | direct | [27,28] | |
| McpC | Proline, threonine, glycine, serine, valine, alanine, tyrosine, isoleucine, tryptophan, phenylalanine, leucine | direct | [29] | |
| Asparagine, lysine, glutamine, methionine | indirect * | |||
| Bacillus amyloliquefaciens SQR9 (8) | McpA | Citric acid, aspartic acid | direct | [24] |
| Glutamic acid, isoleucine, lysine, tyrosine, serine; phthalic acid, oxalic acid, malic acid, succinic acid, fumaric acid, adipic acid, dehydroascorbic acid, glyceric acid, 3-hydroxypropionic acid, gluconic acid, sodium decanoate; ribitol, mannose, ribose, fucose, hydroxycarbamate (r), fructose, galactose | unknown | |||
| McpB | Glycine, tryptophan, asparagine, glutamine, serine, cysteine, methionine; salicylic acid (r), sodium decanoate (r), adipic acid, ribose, glyceric acid, 3-hydroxypropionic acid, gluconic acid, fructose | unknown | ||
| McpC | Valine, alanine, proline, leucine, histidine, serine, threonine, cysteine, methionine; gluconic acid, succinic acid, maltose | unknown | ||
| TlpA | DL-dithiothreitol (r), hydroxycarbamate (r), sodium decanoate (r), gluconic acid, maltose | unknown | ||
| TlpB | Phenylalanine, threonine; malic acid, succinic acid, fumaric acid, salicylic acid (r), sodium decanoate (r), gluconic acid, pentadecanoic acid (r); ribose, fucose, maltose, fructose; dulcitol, inosine | unknown | ||
| Pseudomonas putida KT2440 (27) | McpA | Glycine, alanine, cysteine, serine, asparagine, glutamine, phenylalanine, tyrosine, valine, isoleucine, methionine, arginine | direct | [30] |
| McpG | γ-aminobutyric acid (GABA) | direct | [31] | |
| McpH | Adenine, guanine, hypoxanthine, purine, xanthine, uric acid | direct | [32] | |
| McpP (s) | Acetate, pyruvate, propionate, L-lactate | direct | [33] | |
| McpU | Agmatine, cadaverine, ethylenediamine, histamine, putrescine, spermidine | direct | [30,34,35] | |
| Pseudomonas fluorescens Pf0-1 (37) | CtaA | Alanine, arginine, asparagine, cystine, glycine, histidine, isoleucine, lysine, methionine, phenylalanine, serine, threonine, tyrosine, valine, leucine, proline | unknown | [36] |
| CtaB | Alanine, arginine, asparagine, cystine, glycine, histidine, isoleucine, lysine, methionine, phenylalanine, serine, threonine, tyrosine, valine, glutamine, glutamic acid | unknown | ||
| CtaC | Arginine, cystine, glycine, methionine, threonine | unknown | ||
| McpT (s) | L-malate, succinate | unknown | [37] | |
| Sinorhizobium meliloti MV II-1 (8) | McpU | Proline | direct | [38,39] |
| McpV (s) | Propionate, acetate, glycolate, pyruvate, acetoacetate, formate | direct | [26,39] | |
| Butyrate, L-lactate, glyoxylate, methyl pyruvate, α-hydroxybutyrate, α-ketobutyrate; | unknown | |||
| McpX | Choline, glycine betaine, stachydrine, trigonelline, choline, betonicine, proline betaine | direct | [39,40,41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Fu, R.; Hou, X.; Lv, Y.; Zhang, N.; Liu, Y.; Xu, Z.; Miao, Y.; Krell, T.; Shen, Q.; et al. Chemotaxis of Beneficial Rhizobacteria to Root Exudates: The First Step towards Root–Microbe Rhizosphere Interactions. Int. J. Mol. Sci. 2021, 22, 6655. https://doi.org/10.3390/ijms22136655
Feng H, Fu R, Hou X, Lv Y, Zhang N, Liu Y, Xu Z, Miao Y, Krell T, Shen Q, et al. Chemotaxis of Beneficial Rhizobacteria to Root Exudates: The First Step towards Root–Microbe Rhizosphere Interactions. International Journal of Molecular Sciences. 2021; 22(13):6655. https://doi.org/10.3390/ijms22136655
Chicago/Turabian StyleFeng, Haichao, Ruixin Fu, Xueqin Hou, Yu Lv, Nan Zhang, Yunpeng Liu, Zhihui Xu, Youzhi Miao, Tino Krell, Qirong Shen, and et al. 2021. "Chemotaxis of Beneficial Rhizobacteria to Root Exudates: The First Step towards Root–Microbe Rhizosphere Interactions" International Journal of Molecular Sciences 22, no. 13: 6655. https://doi.org/10.3390/ijms22136655
APA StyleFeng, H., Fu, R., Hou, X., Lv, Y., Zhang, N., Liu, Y., Xu, Z., Miao, Y., Krell, T., Shen, Q., & Zhang, R. (2021). Chemotaxis of Beneficial Rhizobacteria to Root Exudates: The First Step towards Root–Microbe Rhizosphere Interactions. International Journal of Molecular Sciences, 22(13), 6655. https://doi.org/10.3390/ijms22136655








