Level of Murine DDX3 RNA Helicase Determines Phenotype Changes of Hepatocytes In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Murine Cell Cultures
2.2. siRNA Design and LNP Formulation
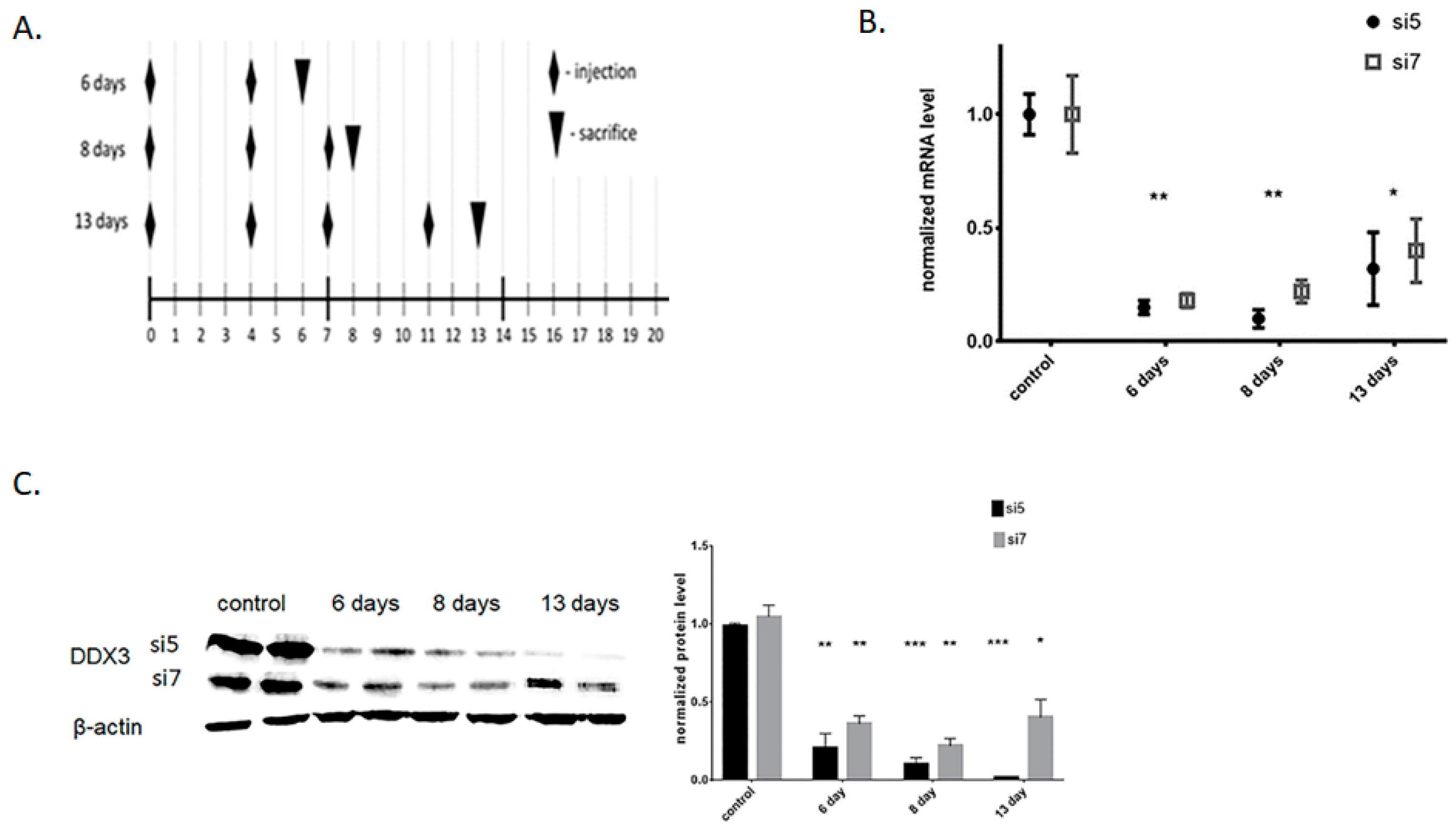
2.3. Animal Care and Treatments
2.4. Histological Analysis
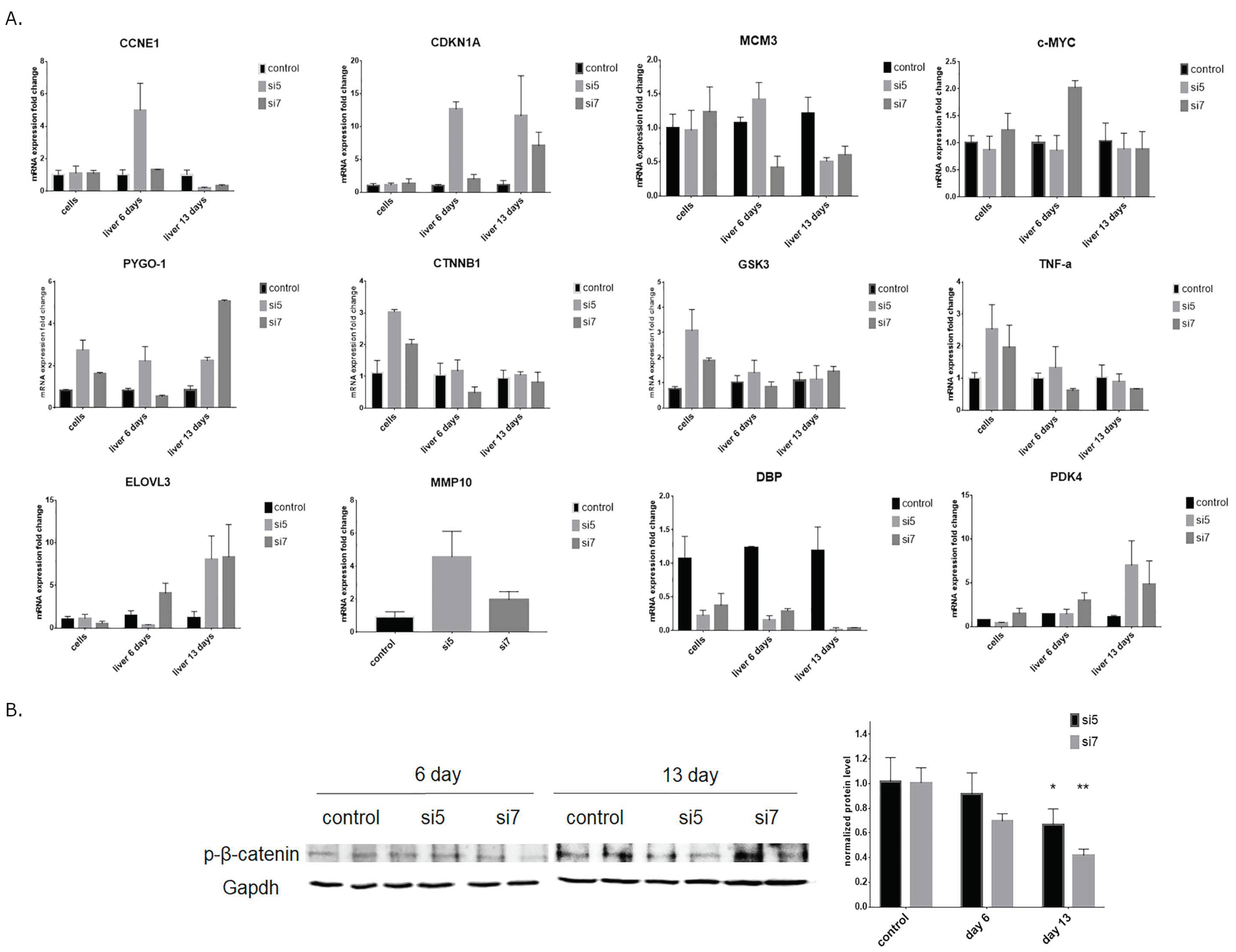
2.5. RNA Isolation and RT-qPCR
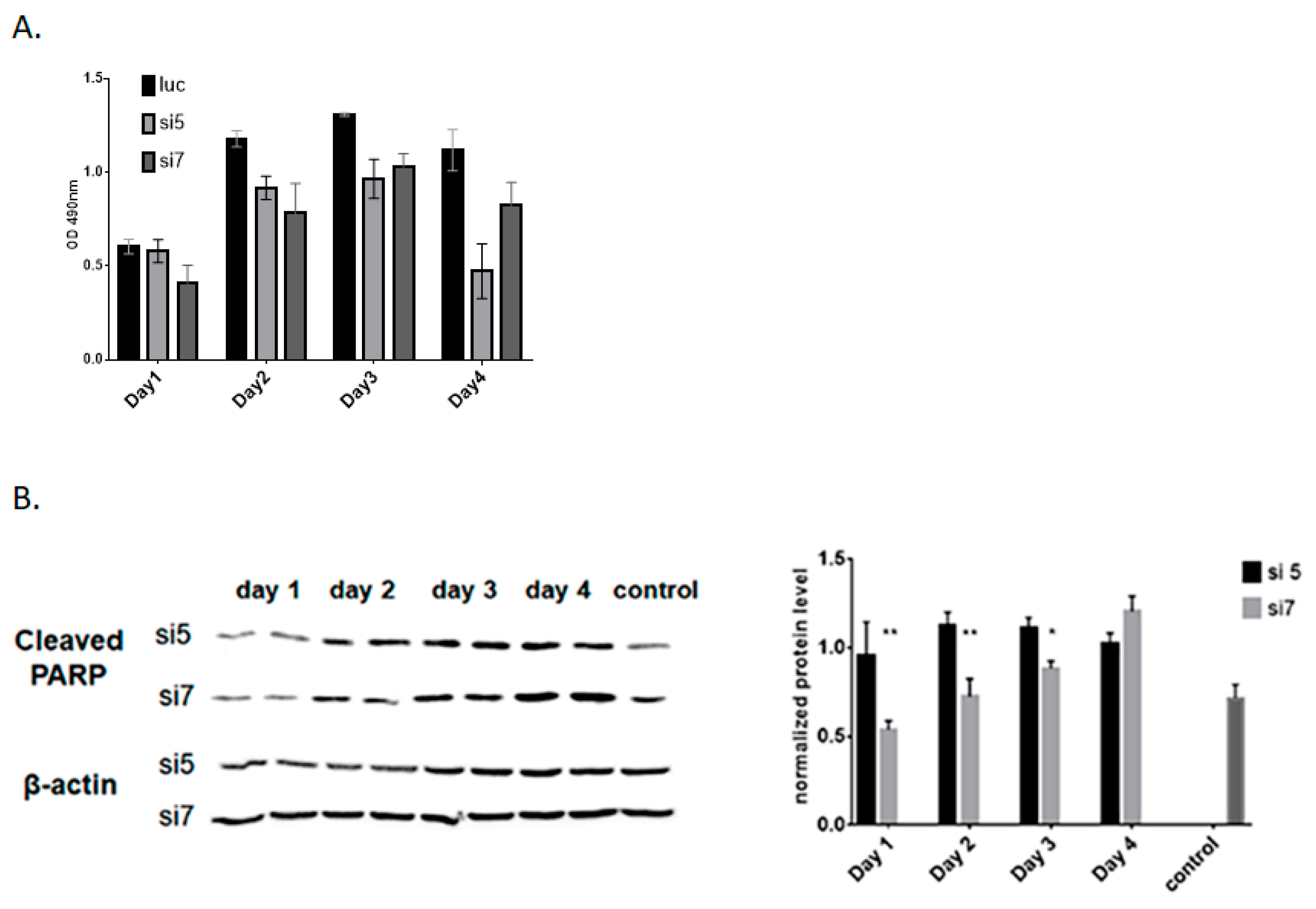
2.6. Western Blotting
2.7. Cell Viability Assay
2.8. mRNA-seq Data Processing and Analysis
2.9. Statistical Analysis of the Experimental Data
3. Results
3.1. Knockdown of DDX3 RNA Helicase in vitro
3.2. Knockdown of DDX3 RNA Helicase in vivo
3.3. Efficient Knockdown of DDX3 in the Murine Liver and Hepa1-6 Cells Induces Apoptosis of Hepatocytes
3.4. Analysis of Gene Expression after Knockdown of DDX3 in vitro and in vivo
3.5. GO Pathway Enrichment Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Honek, J. Preclinical research in drug development. MEW 2017, 26, 5–8. [Google Scholar]
- Polson, A.G.; Fuji, R.N. The successes and limitations of preclinical studies in predicting the pharmacodynamics and safety of cell-surface-targeted biological agents in patients. Br. J. Pharmacol. 2012, 166, 1600–1602. [Google Scholar] [CrossRef]
- Pyle, A.M. Translocation and unwinding mechanisms of RNA and DNA helicases. Annu. Rev. Biophys. 2008, 37, 317–336. [Google Scholar] [CrossRef]
- Heaton, S.M.; Atkinson, S.C.; Sweeney, M.N.; Yang, S.N.Y.; Jans, D.A.; Borg, N.A. Exportin-1-dependent nuclear export of DEAD-box helicase DDX3X is central to its role in antiviral immunity. Cells 2019, 8, 1181. [Google Scholar] [CrossRef]
- Gaete-Argel, A.; Marques, C.; Barriga, G.; Soto-Rifo, R.; Valiente-Echeverria, F. Strategies for Success. Viral infections and membraneless organelles. Front. Cell Infect. Microbiol. 2019, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, C.F.; Mortreux, F.; Auboeuf, D. The multiple functions of RNA helicases as drivers and regulators of gene expression. Nat. Rev. Mol. Cell. Biol. 2016, 17, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Khadivjam, B.; Stegen, C.; Hogue-Racine, M.-A.; el Bilali, N.; Döhner, K.; Sodeik, B.; Lippé, R. The ATP-dependent RNA Helicase DDX3X modulates herpes simplex virus 1 gene expression. J. Virol. 2017, 91, e02411-16. [Google Scholar] [CrossRef]
- Fuller-Pace, F.; Nikol, S. DEAD-box RNA helicases as transcription cofactors. Methods Enzymol. 2012, 511, 347–367. [Google Scholar] [CrossRef] [PubMed]
- Geissler, R.; Golbik, R.; Behrens, S. DEAD-box helicase DDX3 supports the assembly of functional 80S ribosomes. Nucl. Acids Res. 2012, 40, 4998–5011. [Google Scholar] [CrossRef] [PubMed]
- Soto-Rifo, R.; Rubilar, P.; Limousin, T.; de Breyne, S.; Décimo, D.; Ohlmann, T. DEAD-box protein DDX3 associates with eIF4F to promote translation of selected mRNAs. EMBO J. 2012, 31, 3745–3756. [Google Scholar] [CrossRef]
- Tsai, T.-Y.; Wang, W.-T.; Li, H.-K.; Chen, W.-J.; Tsai, Y.-H.; Chao, C.-H.; Lee, Y.-H.W. RNA helicase DDX3 maintains lipid homeostasis through upregulation of the microsomal triglyceride transfer protein by interacting with HNF4 and SHP. Sci. Rep. 2017, 7, 41452. [Google Scholar] [CrossRef]
- Adjibade, P.; St-Sauveur, V.; Bergeman, J.; Huot, M.-E.; Khandjian, E.; Mazroui, R. DDX3 regulates endoplasmic reticulum stress-induced ATF4 expression. Sci. Rep. 2017, 7, 13832. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-J.; Wang, W.-T.; Tsai, T.-Y.; Li, H.-K.; Lee, Y.-H.W. DDX3 localizes to the centrosome and prevents multipolar mitosis by epigenetically and translationally modulating p53 expression. Sci. Rep. 2017, 7, 9411. [Google Scholar] [CrossRef]
- Taschuk, F.; Cherry, S. DEAD-box helicases: Sensors, regulators, and effectors for antiviral defense. Viruses 2020, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Lenarcic, E.M.; Ziehr, B.J.; Moorman, N.J. An unbiased proteomics approach to identify human cytomegalovirus RNA-associated proteins. Virology 2015, 481, 13–23. [Google Scholar] [CrossRef]
- Yedavalli, V.S.R.K.; Neuveut, C.; Chi, Y.-H.; Kleiman, L.; Jeang, K.-T. Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell 2004, 119, 381–392. [Google Scholar] [CrossRef]
- Owsianka, A.M.; Patel, A.H. Hepatitis C virus core protein interacts with a human DEAD box protein DDX3. Virology 1999, 257, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ge, L.; Li, P.; Wang, Y.; Dai, J.; Sun, M.; Huang, L.; Shen, Z.; Hu, X.; Ishag, H.; et al. Cellular DDX3 regulates Japanese encephalitis virus replication by interacting with viral un-translated regions. Virology 2014, 449, 70–81. [Google Scholar] [CrossRef]
- Noble, C.G.; Chen, Y.-L.; Dong, H.; Gu, F.; Lim, S.P.; Schul, W.; Wang, Q.-Y.; Shi, P.-Y. Strategies for development of Dengue virus inhibitors. Antiviral Res. 2010, 85, 450–462. [Google Scholar] [CrossRef]
- Brai, A.; Fazi, R.; Tintori, C.; Zamperini, C.; Bugli, F.; Sanguinetti, M.; Stigliano, E.; Esté, J.; Badia, R.; Franco, S.; et al. Human DDX3 protein is a valuable target to develop broad spectrum antiviral agents. Proc. Natl. Acad. Sci. USA 2016, 113, 5388–5393. [Google Scholar] [CrossRef]
- Chahar, H.S.; Chen, S.; Manjunath, N. P-body components LSM1, GW182, DDX3, DDX6 and XRN1 are recruited to WNV replication sites and positively regulate viral replication. Virology 2013, 436, 1–7. [Google Scholar] [CrossRef]
- Vashist, S.; Urena, L.; Chaudhry, Y.; Goodfellow, I. Identification of RNA-protein interaction networks involved in the Norovirus life cycle. J. Virol. 2012, 86, 11977–11990. [Google Scholar] [CrossRef] [PubMed]
- Stunnenberg, M.; Geijtenbeek, T.; Gringhuis, S. DDX3 in HIV-1 infection and sensing: A paradox. Cytokine Growth Factor Rev. 2018, 40, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Yedavalli, V.S.R.K.; Zhang, N.; Cai, H.; Zhang, P.; Starost, M.F.; Hosmane, R.S.; Jeang, K.-T. Ring expanded nucleoside analogues inhibit RNA helicase and intracellular human immunodeficiency virus type 1 replication. J. Med. Chem. 2008, 51, 5043–5051. [Google Scholar] [CrossRef] [PubMed]
- Shadrick, W.R.; Ndjomou, J.; Kolli, R.; Mukherjee, S.; Hanson, A.M.; Frick, D.N. Discovering new medicines targeting helicases: Challenges and recent Progress. J. Biomol. Screen. 2013, 18, 761–781. [Google Scholar] [CrossRef] [PubMed]
- Radi, M.; Falchi, F.; Garbelli, A.; Samuele, A.; Bernardo, V.; Paolucci, S.; Baldanti, F.; Schenone, S.; Manetti, F.; Maga, G.; et al. Discovery of the first small molecule inhibitor of human DDX3 specifically designed to target the RNA binding site: Towards the next generation HIV-1 inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 2094–2098. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.N.Y.; Atkinson, S.C.; Audsley, M.D.; Heaton, S.M.; Jans, D.A.; Borg, N.A. RK-33 is a broad-spectrum antiviral agent that targets DEAD-Box RNA helicase DDX3X. Cells 2020, 9, 170. [Google Scholar] [CrossRef]
- Bol, G.M.; Vesuna, F.; Xie, M.; Zeng, J.; Aziz, K.; Gandhi, N.; Levine, A.; Irving, A.; Korz, D.; Tantravedi, S.; et al. Targeting DDX3 with a small molecule inhibitor for lung cancer therapy. EMBO Mol. Med. 2015, 7, 648–669. [Google Scholar] [CrossRef]
- Xie, M.; Vesuna, F.; Tantravedi, S.; Bol, G.M.; van Voss, M.R.H.; Nugent, K.; Malek, R.; Gabrielson, K.; van Diest, P.J.; Tran, P.T.; et al. RK-33 radiosensitizes prostate cancer cells by blocking the RNA Helicase DDX3. Cancer Res. 2016, 76, 6340–6350. [Google Scholar] [CrossRef]
- Sergeeva, O.; Zatsepin, T. RNA Helicases as shadow modulators of cell cycle progression. Int. J. Mol. Sci. 2021, 22, 2984. [Google Scholar] [CrossRef]
- Gopal, L.; Sapkota, P. Functions and regulation of the serine/threonine protein kinase CK1 family: Moving beyond promiscuity. Biochem. J. 2020, 447, 4603–4621. [Google Scholar] [CrossRef]
- Fullam, A.; Gu, L.; Höhn, Y.; Schröder, M. DDX3 directly facilitates IKKα activation and regulates downstream signalling pathways. Biochem. J. 2018, 475, 3595–3607. [Google Scholar] [CrossRef]
- Wang, X.; Wang, R.; Luo, M.; Li, C.; Wang, H.-X.; Huan, C.-C.; Qu, Y.-R.; Liao, Y.; Mao, X. (DEAD)-box RNA helicase 3 modulates NF-κB signal pathway by controlling the phosphorylation of PP2A-C subunit. Oncotarget 2017, 8, 33197–33213. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhou, T.; Jonasch, E.; Jope, R.S. DDX3 regulates DNA damage-induced apoptosis and p53 stabilization. Biochim. Biophys. Acta 2013, 1833, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.M.; Birmingham, A.; Baskerville, S.; Reynolds, A.; Maksimova, E.; Leake, D.; Fedorov, Y.; Karpilow, J.; Khvorova, A. Experimental validation of the importance of seed complement frequency to siRNA specificity. RNA 2008, 14, 853–861. [Google Scholar] [CrossRef]
- Dong, Y.; Siegwart, D.; Anderson, D. Strategies, design and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 2019, 114, 133–147. [Google Scholar] [CrossRef]
- Reynolds, A.; Leake, D.; Boese, Q.; Scaringe, S.; Marshall, W.S.; Khvorova, A. Rational siRNA design for RNA interference. Nat. Biotechnol. 2004, 22, 326–330. [Google Scholar] [CrossRef]
- Pei, Y.; Tuschl, T. On the art of identifying effective and specific siRNAs. Nat. Methods 2006, 3, 670–676. [Google Scholar] [CrossRef]
- Jackson, A.L.; Burchard, J.; Leake, D.; Reynolds, A.; Schelter, J.; Guo, J.; Johnson, J.M.; Lim, L.; Karpilow, J.; Nichols, K.; et al. Position-specific chemical modification of siRNAs reduces “off-target” transcript silencing. RNA 2006, 12, 1197–1205. [Google Scholar] [CrossRef]
- Jayaraman, M.; Ansell, S.M.; Mui, B.L.; Tam, Y.K.; Chen, J.; Du, X.; Butler, D.; Eltepu, L.; Matsuda, S.; Narayanannair, J.K.; et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int. Ed. Engl. 2012, 51, 8529–8533. [Google Scholar] [CrossRef]
- Walsh, C.; Ou, K.; Belliveau, N.M.; Leaver, T.J.; Wild, A.W.; Huft, J.; Lin, P.J.; Chen, S.; Leung, A.K.; Lee, J.B.; et al. Microfluidic-based manufacture of siRNA-lipid nanoparticles for therapeutic applications. Methods Mol. Biol. 2014, 1141, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Roces, C.; Lou, G.; Jain, N.; Abraham, S.; Thomas, A.; Halbert, G.; Perrie, Y. Manufacturing considerations for the development of lipid nanoparticles using microfluidics. Pharmaceutics 2020, 12, 1095. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Love, M.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Leek, J.T. svaseq: Removing batch effects and other unwanted noise from sequencing data. Nucl. Acids Res. 2014, 42, e161. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Shah, A.; Rashid, F.; Awan, H.M.; Hu, S.; Wang, X.; Chen, L.; Shan, G. The DEAD-Box RNA helicase DDX3 interacts with m6A RNA demethylase ALKBH5. Stem Cells Int. 2017, 2017, e8596135. [Google Scholar] [CrossRef]
- Kasim, V.; Wu, S.; Taira, K.; Miyagishi, M. Determination of the Role of DDX3 a factor involved in mammalian RNAi pathway using an shRNA-expression library. PLoS ONE 2013, 8, e59445. [Google Scholar] [CrossRef]
- Su, C.-Y.; Lin, T.-C.; Lin, Y.-F.; Chen, M.-H.; Lee, C.-H.; Wang, H.-Y.; Lee, Y.-C.; Liu, Y.-P.; Chen, C.-L.; Hsiao, M. DDX3 as a strongest prognosis marker and its downregulation promotes metastasis in colorectal cancer. Oncotarget 2015, 6, 18602–18612. [Google Scholar] [CrossRef]
- Love, K.T.; Mahon, K.P.; Levins, C.G.; Whitehead, K.A.; Querbes, W.; Dorkin, J.R.; Qin, J.; Cantley, W.; Qin, L.L.; Racie, T.; et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl. Acad. Sci. USA 2010, 107, 1864–1869. [Google Scholar] [CrossRef]
- Bogorad, R.L.; Yin, H.; Zeigerer, A.; Nonaka, H.; Ruda, V.M.; Zerial, M.; Anderson, D.G.; Koteliansky, V. Nanoparticle-formulated siRNA targeting integrins inhibits hepatocellular carcinoma progression in mice. Nat. Commun. 2014, 5, 3869. [Google Scholar] [CrossRef]
- Valiente-Echeverría, F.; Hermoso, M.; Soto-Rifo, R. RNA helicase DDX3: At the crossroad of viral replication and antiviral immunity. Rev. Med. Virol. 2015, 25, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Garbelli, A.; Radi, M.; Falchi, F.; Beermann, S.; Zanoli, S.; Manetti, F.; Dietrich, U.B.M.; Maga, G. Targeting the human DEAD-Box polypeptide 3 (DDX3) RNA helicase as a novel strategy to inhibit viral replication. Curr. Med. Chem. 2011, 18, 3015–3027. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.; Baran, M.; Bowie, A.G. Viral targeting of DEAD box protein 3 reveals its role in TBK1/IKKepsilon-mediated IRF activation. EMBO J. 2008, 27, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-H.; Chen, C.-M.; Cheng, P.-L.; Shih, J.-W.; Tsou, A.-P.; Lee, Y.-H.W. DDX3, a DEAD box RNA helicase with tumor growth-suppressive property and transcriptional regulation activity of the p21waf1/cip1 promoter, is a candidate tumor suppressor. Cancer Res. 2006, 66, 6579–6588. [Google Scholar] [CrossRef]
- Botlagunta, M.; Vesuna, F.; Mironchik, Y.; Raman, A.; Lisok, A.; Winnard, P.; Mukadam, S.; van Diest, P.; Chen, J.H.; Farabaugh, P.; et al. Oncogenic role of DDX3 in breast cancer biogenesis. Oncogene 2008, 27, 3912–3922. [Google Scholar] [CrossRef]
- Fuller-Pace, F.V. DEAD box RNA helicase functions in cancer. RNA Biol. 2013, 10, 121–132. [Google Scholar] [CrossRef]
- Shih, J.-W.; Tsai, T.-Y.; Chao, C.-H.; Lee, Y.-H.W. Candidate tumor suppressor DDX3 RNA helicase specifically represses cap-dependent translation by acting as an eIF4E inhibitory protein. Oncogene 2008, 27, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Ariumi, Y. Multiple functions of DDX3 RNA helicase in gene regulation, tumorigenesis, and viral infection. Front. Genet. 2014, 5, 423. [Google Scholar] [CrossRef]
- Hilliker, A.; Gao, Z.; Jankowsky, E.; Parker, R. The DEAD-box protein Ded1 modulates translation by the formation and resolution of an eIF4F-mRNA complex. Mol. Cell. 2011, 43, 962–972. [Google Scholar] [CrossRef]
- van Voss, M.R.H.; Kammers, K.; Vesuna, F.; Brilliant, J.; Bergman, Y.; Tantravedi, S.; Wu, X.; Cole, R.N.; Holland, A.; van Diest, P.J.; et al. Global effects of DDX3 inhibition on cell cycle regulation identified by a combined phosphoproteomics and single cell tracking approach. Transl. Oncol. 2018, 11, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Song, L.; Li, Y.; Zhou, T.; Jope, R. Identification of an antiapoptotic protein complex at death receptors. Cell Death Differ. 2008, 15, 1887–1900. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.; Ueda, M.; Kristen, A.; Tournev, I.; Schmidt, H.; Coelho, T.; Berk, J.; et al. Patisiran an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-H.; Chen, C.-M.; Lee, Y.-H.W.; You, L.-R. DNA damage, liver injury, and tumorigenesis: Consequences of DDX3X loss. Mol. Cancer Res. 2019, 17, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mao, Y.; Zhou, J.; Zhao, Y.; Cao, Y.; Chen, X. Multifunctional DDX3: Dual roles in various cancer development and its related signaling pathways. Am. J. Cancer Res. 2016, 6, 387–402. [Google Scholar]
- Chen, H.-H.; Yu, H.-I.; Cho, W.-C.; Tarn, W.-Y. DDX3 modulates cell adhesion and motility and cancer cell metastasis via Rac1-mediated signaling pathway. Oncogene 2015, 34, 2790–2800. [Google Scholar] [CrossRef]
- Cruciat, C.-M.; Dolde, C.; de Groot, R.E.A.; Ohkawara, B.; Reinhard, C.; Korswagen, H.C.; Niehrs, C. RNA helicase DDX3 is a regulatory subunit of casein kinase 1 in Wnt-β-catenin signaling. Science 2013, 339, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- van Voss, M.R.H.; Vesuna, F.; Trumpi, K.; Brilliant, J.; Berlinicke, C.; de Leng, W.; Kranenburg, O.; Offerhaus, J.G.; Bürger, H.; van der Wall, E.; et al. Identification of the DEAD box RNA helicase DDX3 as a therapeutic target in colorectal cancer. Oncotarget 2015, 6, 28312–28326. [Google Scholar] [CrossRef] [PubMed]
- Tarn, W.-Y.; Chang, T.-H. The current understanding of Ded1p/DDX3 homologs from yeast to human. RNA Biol. 2009, 6, 17–20. [Google Scholar] [CrossRef]
- He, Y.; Zhang, D.; Yang, Y.; Wang, X.; Zhao, X.; Zhang, P.; Zhu, H.; Xu, N.; Liang, S. A double-edged function of DDX3, as an oncogene or tumor suppressor, in cancer progression. Oncol. Rep. 2018, 39, 883–892. [Google Scholar] [CrossRef]
- Kulthong, K.; Hooiveld, G.J.E.J.; Duivenvoorde, L.; Estruch, I.M.; Marin, V.; van der Zande, M.; Bouwmeester, H. Transcriptome comparisons of in vitro intestinal epithelia grown under static and microfluidic gut-on-chip conditions with in vivo human epithelia. Sci. Rep. 2021, 11, 3234. [Google Scholar] [CrossRef]
- Ord, J.J.; Streeter, E.H.; Roberts, I.S.D.; Cranston, D.; Harris, A.L. Comparison of hypoxia transcriptome in vitro with in vivo gene expression in human bladder cancer. Br. J. Cancer 2005, 93, 346–354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pang, G.; Xie, J.; Chen, Q.; Hu, Z. Energy intake, metabolic homeostasis, and human health. FSHW 2014, 3, 89–103. [Google Scholar] [CrossRef]
- Putzbach, W.; Gao, Q.; Patel1, M.; Haluck-Kangas, A.; Murmann, A.; Peter, M. DISE: A seed dependent RNAi off-target effect that kills cancer cells. Trends Cancer 2018, 4, 10–19. [Google Scholar] [CrossRef]
- Bartoszewski, R.; Sikorski, A. Editorial focus: Understanding off-target effects as the key to successful RNAi therapy. Cell Mol. Biol. Lett. 2019, 24, 69. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.; Linsley, P. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat. Rev. Drug Discov. 2010, 9, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Janas, M.; Schlegel, M.; Harbison, C.; Yilmaz, V.; Jiang, Y.; Parmar, R.; Zlatev, I.; Castoreno, A.; Xu, H.; Shulga-Morskaya, S.; et al. Selection of GalNAc-conjugated siRNAs with limited off-target-driven rat hepatotoxicity. Nat. Commun. 2018, 9, 723. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergeeva, O.; Abakumova, T.; Kurochkin, I.; Ialchina, R.; Kosyreva, A.; Prikazchikova, T.; Varlamova, V.; Shcherbinina, E.; Zatsepin, T. Level of Murine DDX3 RNA Helicase Determines Phenotype Changes of Hepatocytes In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 6958. https://doi.org/10.3390/ijms22136958
Sergeeva O, Abakumova T, Kurochkin I, Ialchina R, Kosyreva A, Prikazchikova T, Varlamova V, Shcherbinina E, Zatsepin T. Level of Murine DDX3 RNA Helicase Determines Phenotype Changes of Hepatocytes In Vitro and In Vivo. International Journal of Molecular Sciences. 2021; 22(13):6958. https://doi.org/10.3390/ijms22136958
Chicago/Turabian StyleSergeeva, Olga, Tatiana Abakumova, Ilia Kurochkin, Renata Ialchina, Anna Kosyreva, Tatiana Prikazchikova, Varvara Varlamova, Evgeniya Shcherbinina, and Timofei Zatsepin. 2021. "Level of Murine DDX3 RNA Helicase Determines Phenotype Changes of Hepatocytes In Vitro and In Vivo" International Journal of Molecular Sciences 22, no. 13: 6958. https://doi.org/10.3390/ijms22136958
APA StyleSergeeva, O., Abakumova, T., Kurochkin, I., Ialchina, R., Kosyreva, A., Prikazchikova, T., Varlamova, V., Shcherbinina, E., & Zatsepin, T. (2021). Level of Murine DDX3 RNA Helicase Determines Phenotype Changes of Hepatocytes In Vitro and In Vivo. International Journal of Molecular Sciences, 22(13), 6958. https://doi.org/10.3390/ijms22136958






