1. Introduction
Infertility is a disease of the reproductive system. According to statistics, more than 186 million people suffer from infertility, accounting for 8–12% of couples of reproductive age worldwide [
1]. The infertility of unexplained origin account for around 10–20% of all fertility problems.
When looking for the causes of fertility problems and treatments, which are used during infertility treatment cycle, much attention is paid to one of the reproductive tissues—the endometrium. The endometrium is a hormone-dependent, cyclically changing, regenerating tissue whose stem cells play a particularly important role in a woman’s reproductive life. Studies show that endometrial dysfunction can lead to failed embryo implantation and early loss of pregnancy, making endometrial stem cell changes an attractive target for infertility studies with unexplained origin [
2]. However, endometrial stem cells can only be taken invasively by endometrial scratching, so alternatives to this type of stem cell have been sought.
Studies have shown that menstrual blood could also be a source of stem cells. Experimental studies in animal models have shown successful treatment of stroke, colitis, limb ischemia, coronary heart disease and Duchenne muscle atrophy using menstrual blood stem cells [
3]. Menstrual blood is a new and interesting source of MenSCs for researchers because these cells have advantages over stem cells obtained from other sources [
4]. Back in 2007, it was shown that MenSCs can even proliferate twice as fast as other mesenchymal stem cells. Proper rate of proliferation is essential for clinical application of cells because cell therapy depends on the number of cells, preferably when many stem cells can be obtained (or propagated) from the same source and are as early in the passage as possible, the process is non-invasive and does not face ethical issues [
5]. Because MenSCs are of endometrial origin, attempts are being made to treat conditions associated with female infertility. Nikoo and the group (2014) found that MenSCs are very important for endometrial function by comparing morphology, CD marker expression, cell proliferation, adhesion and some immunomodulatory molecules among women with endometriosis and those without endometriosis [
6,
7]. Zheng et al. (2018) were the first to show that MenSCs could be a desirable medical tool for reproductive systems cells tissue repair and regeneration [
8]. In this study, MenSCs differentiated into endometrial cells in vitro and rebuilt endometrial tissue in NOD-SCID mice after administering estrogen and progesterone in vivo. Additionally, due to results that cloning efficiency and OCT-4 expression of MenSCs from patients with intrauterine adhesions were lower compared with those from healthy women, this study revealed new possibilities to use MenSCs for the treatment of intrauterine adhesions, which remains one of the most challenging fertility problems until now [
8]. Moreover, MenSCs are shown to exert a vascular remodeling role and an immunoregulatory role by inhibiting the expression of proinflammatory factors and promoting so-called T helper type 2 (Th2) cytokines such as interleukin-4 (IL-4), which could play a critical role in the embryo implantation process and successful pregnancy development [
3,
9,
10,
11].
In our study, we analyzed the characteristics and the potential for decidualization of menstrual blood stem cells isolated from healthy volunteers and women diagnosed with infertility. We have shown that the most analyzed gene expression did not differ in MenSCs of healthy volunteers and females with diagnosed infertility except some epigenetic and stemness gene expression was lower in patients with diagnosed infertility. Therefore, we suggest that MenSCs could be a promising therapeutic source as an auxiliary tool for the females with diagnosed unexplained infertility treatment.
3. Discussion
Mesenchymal stem cells hypothetically can be obtained from almost any tissue within the human body, for example bone marrow, adipose tissue, dental pulp, exfoliated deciduous teeth, perinatal derivatives such as amniotic fluid and placenta, peripheral blood, synovium and synovial fluid, endometrium, skin, muscle, etc. Still, there are practical limitations concerning the difficulty and invasiveness of the procurement process and various donor characteristics [
12,
13,
14,
15]. To study the properties of MenSCs, we analyzed the growth rate, cell surface marker expression and proteome profile of MenSCs characteristic for healthy volunteers and patients with unexplained infertility. Additionally, the potential of MenSCs for decidualization and changes in gene expression, intracellular and secreted proteins during decidualization were revealed.
In 2007, Meng and coauthors identified a new source of stem cells, menstrual blood, and named the stem cells found in it endometrial regenerative cells, now called menstrual stem cells. Despite the similar phenotypes and characteristics of EnSCs and MenSCs, the therapeutic effects and mechanisms of these cells are unique [
11]. In this study, we found that the cell culture doubling time is about 3.2–3.4 days. Analysis of the proliferation of MenSCs in healthy volunteers and patients with unexplained infertility suggests that the rate of proliferation is not related to pathology but is determined by the individual biological characteristics of each patient.
The results of our study demonstrated that MenSCs from both groups, healthy volunteers, as well as patients with unexplained infertility, could be characterized by the high expression of mesenchymal stem cell markers (CD9, CD44, CD73, CD90 and CD166). Our data also coincide with the work of Sheikholeslami et al. (2021) who showed that MenSCs from healthy and infertile women (having endometriosis or polycystic ovary syndrome) do possess similar CD marker patterns [
16]. However, our study revealed that MenSCs obtained from patients with unexplained infertility are different from MenSCs from healthy volunteers in more pronounced expression of mesenchymal cell surface markers CD56, SUSD2. These observations illustrate subtle, though significant, variations in characteristics of MenSCs, obtained from different sources.
Decidualization, or differentiation in the direction of the epithelium, is the restructuring of endometrial tissue necessary to create favorable conditions for the implantation of a fertilized oocyte. In order to use MenSCs for the treatment of infertility associated with endometrial disorders, it is necessary to elucidate the potential of decidualization of these cells [
17]. Elevated progesterone and cellular cAMP levels after ovulation are known to activate the transcription factor Foxo1 in EnSCs, which arrests the cell cycle, and cells differentiate into decidualized cells that control embryo implantation [
18]. In this study, we showed that in vitro treatment of MenSCs with medium enriched with 8-bromo-cAMP and MPA successfully induced the decidualization process. We also studied the changes in MenSCs that occur during decidualization.
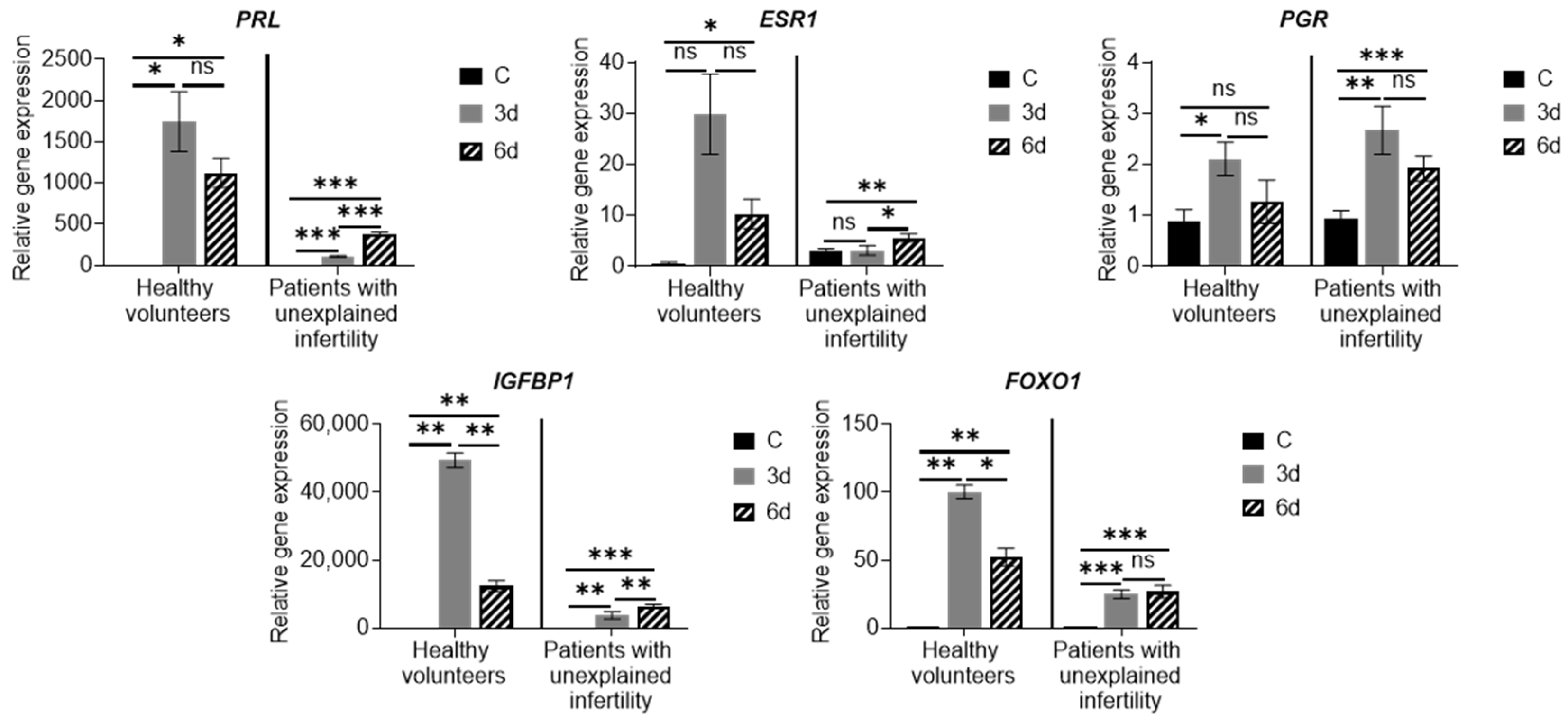
Various scientific groups investigated and identified molecular mechanisms for regulating endometrial decidualization in vitro using cultured EnSCs with functional progesterone and estrogen receptors. Morphological signs of differentiation and expression of decidualization markers, such as prolactin (
PRL) and insulin-like growth factor binding protein 1 (
IGFBP-1), have been observed after 12 days of progesterone treatment of EnSCs [
19,
20,
21]. An increase in cyclic adenosine monophosphate (cAMP) levels in cells was also found to occur. Ovarian hormones as well as relaxin, a corticotropin-releasing factor, and prostaglandin E2 promote the accumulation of cellular cAMP. In EnSCs cultures, exposure to cAMP alone increases
PRL expression, which is further enhanced if cells are exposed to cAMP and progesterone. A critical pathway for the regulation of decidualization consists of progesterone and proteins regulated by progesterone and/or cAMP, such as HOXA10, FOXO1, signal transducers and transcriptional activators, and Hand2, which plays an important role in endometrial receptivity [
22]. Thus, the increase in the expression of the main markers of decidualization confirms the results of morphological observation of cell culture in the differentiation medium and shows that MenSCs can differentiate into epithelial cells.
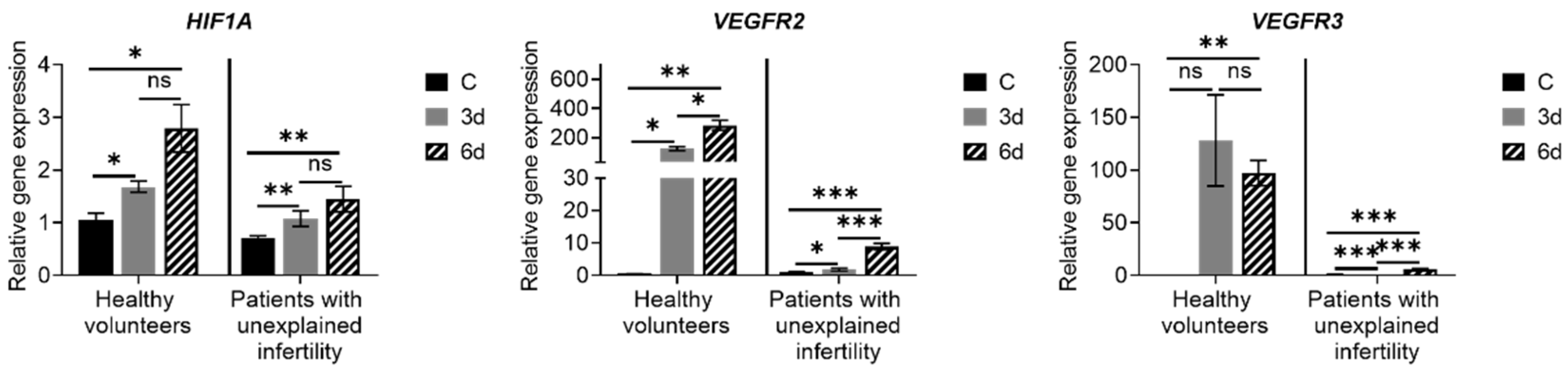
Examining the expression of MenSC genes during decidualization, we found that the expression of the main decidualization markers PRL, PCR, ESR, IGFBP and FOXO1 is upregulated, the expression of NOTCH1, NANOG, WNT4, KLF4, OCT4, SOX2 and LIN28A increased, and expression of angiogenesis-related genes HIF1A, VEGFR-2 and VEGFR-3 is upregulated as well. Many genes and proteins are responsible for endometrial development and regeneration, and the best known are Wnt, c-kit (CD117), Oct-4, CD34/Klf4 and Musashi-1. The WNT gene activates a signaling cascade, binds to cell surface markers in the Frizzled family and determines cell fate.
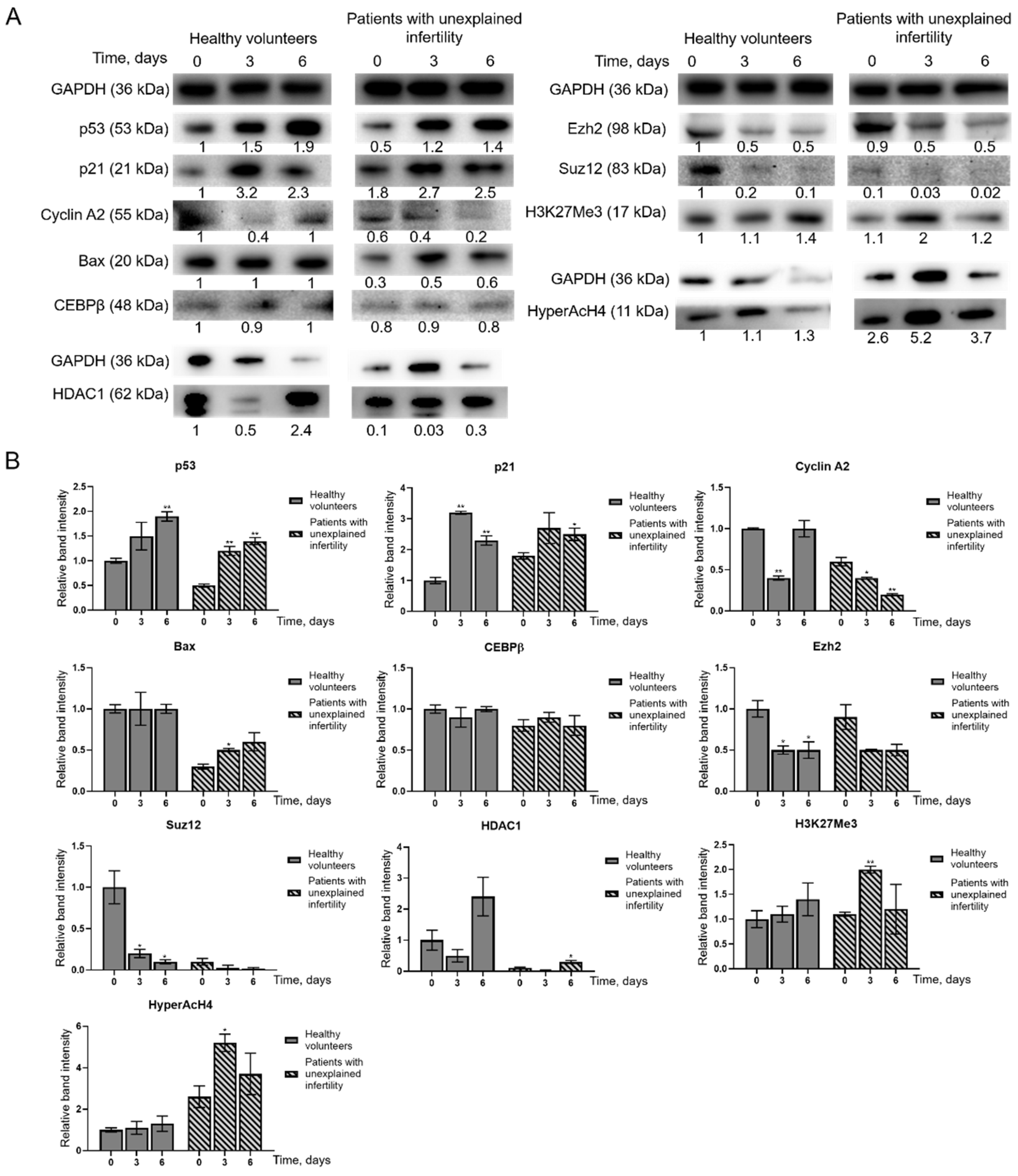
In a study of changes in protein levels during MenSCs decidualization, we found that p53 and p21 protein levels increased in MenSCs. Bax and Cyclin A2 levels in MenSCs from healthy volunteers remain constant, HDAC1 levels increase, and in MenSCs from patients with infertility change: Bax levels decrease and Cyclin A2 levels increase. The levels of epigenetic regulatory proteins Ezh2, Suz12 and HDAC1 decreased during MenSCs decidualization, while the levels of modified histones H3K27me3 and HyperAcH4 increased. Grimaldi and colleagues in 2011 showed that the amount of histone methyltransferase enhancer Ezh2 decreases during decidualization, resulting in a decrease in the level of H3K27me3 in the proximal promoters of the major decidualization marker genes
PRL and
IGFBP1. Demethylation of H3K27me3 has been associated with acetylation of the same lysine groups, indicating active conversion from transcriptionally inactive to transcriptionally active chromatin [
23]. In 2012, Estella and the group analyzed the effect of histone acetylation on the expression of tissue remodeling enzymes and EnSC activity related to the control of trophoblast invasion. Treatment of EnSCs with the HDAC inhibitor trichostatin A (TSA) increased the expression of Timp-1 and Timp-3 and decreased the expression of metalloproteinases. Acetylation of histones has been shown to disturb the balance of modulators of the extracellular filler and to limit the invasion of trophoblasts [
24]. Sakai and colleagues in 2003 showed that the HDAC inhibitor TSA increases the levels of the decidualization markers IGFBP-1 and prolactin in a dose-dependent manner via 17β-estradiol (E2) and progesterone (P4). Morphological changes similar to decidual transformation also manifest more strongly in the decidualization medium with the addition of TSA [
25]. In a 1996 study of apoptosis in rat decidualized tissues, Akcali and colleagues found that control of progesterone and estrogen for endometrial differentiation and possible apoptosis includes control of
BCL-2 gene family expression. Exposure of rat ovaries to 3.5 mg medroxyprogesterone acetate and 200 ng estradiol decreased
BCL-2 expression. In situ analysis revealed a cell type-specific increase in Bax expression after hormonal treatment and decidualization. Data suggest that the balance between Bax and Bcl-2 expression is altered during stromal cell differentiation. Increased expression of Bax is characteristic of apoptosis, and apoptosis plays a significant role in placental development [
26].
It has been found by various groups of researchers that even before the stage of direct embryo attachment to the uterus, there is a precisely regulated exchange of cellular signaling molecules between the embryo and the maternal endometrium. Decidualized EnSCs secrete many different factors, including immunologically active ones, that ensure this maternal connection. However, the cell secretion profile is quite complex and depends strictly on which stage of the menstrual cycle the cells are being examined. EnSC transition into the G0/G1 phase begins with increased secretion of various inflammatory chemokines, cytokines, C-reactive protein and other inflammatory mediators (e.g., IL-2, IL-12 and Interferon-γ). These pro-inflammatory factors are thought to promote endometrial receptivity and early stages of implantation by auto/paracrine activity, as they increase the expression of major genes required for embryo implantation, including growth factors (e.g., HB-EGF), cytokines (e.g., LIF, IL1β, PROKL and IL-11) and various morphogens (e.g., IHH, WNT4, BMP2). The phase of such pro-inflammatory secretion is tightly controlled and usually lasts for 2 to 4 days. The time-limiting factor in this case is a change in secretion profile. It is the cells that begin to secrete anti-inflammatory soluble factors and receptors and the potent anti-inflammatory hormone cortisol. Such a transition from the anti-inflammatory stage of the process is characterized by inhibition of many anti-inflammatory genes below basal levels and induction of various anti-inflammatory cytokines, including IL-4, IL-5 and IL-10 [
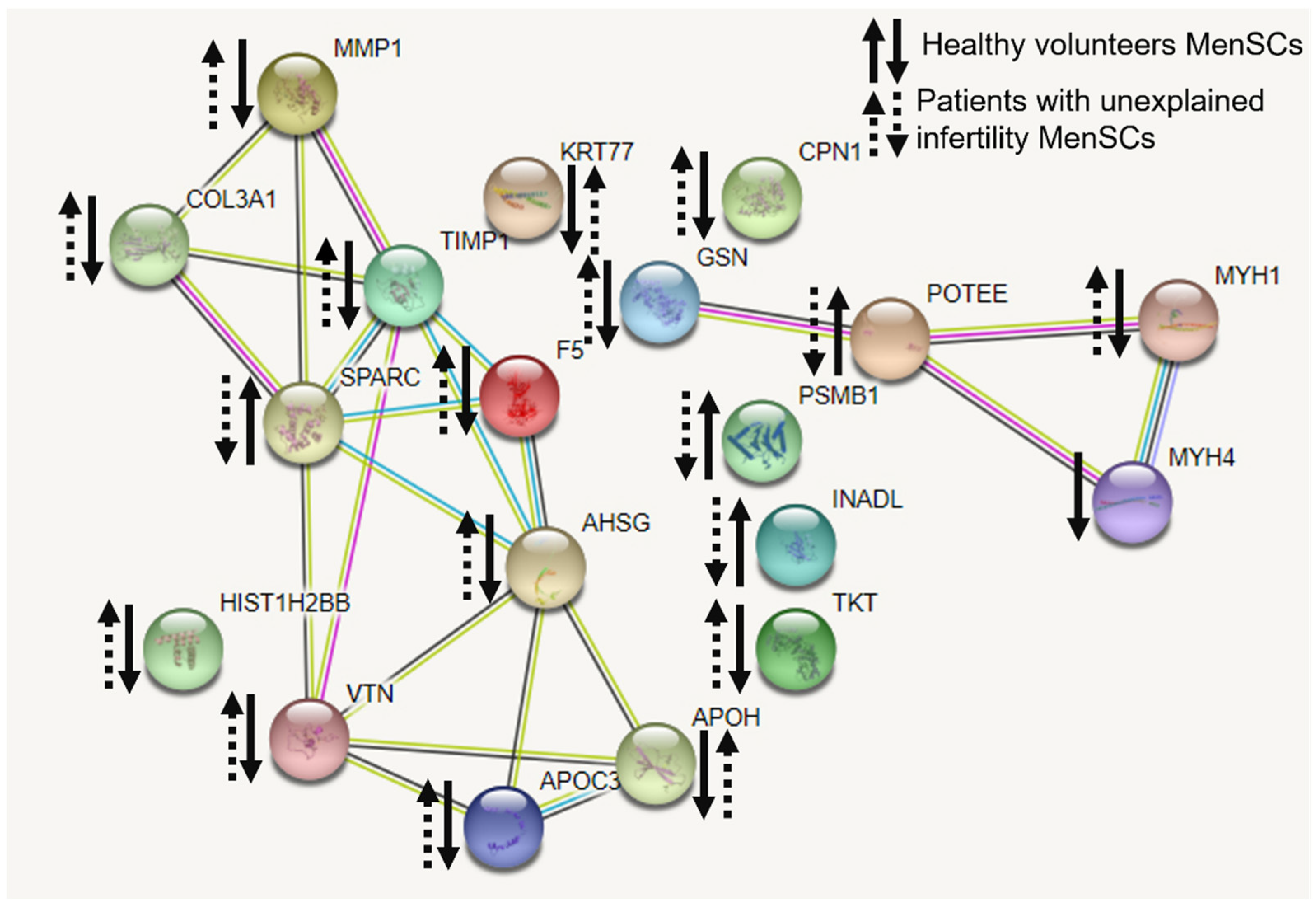
27]. In our study, detected secreted proteins were identified to be involved in the regulation of the actin cytoskeleton, the regulation of estrogen and relaxin signaling pathways, the provision of cell–cell connections and the management of inflammatory processes. Based on the literature, these identified secretory proteins confirm the successful decidualization of MenSCs. Several other study groups analyzed secreted proteins during pregnancy. It is known that an appropriate transcriptional profile in the placenta and perinatal membranes is required for successful implantation and pregnancy [
28]. Our study showed that secretion of proteins COL3A1, TIMP1 and MMP1, and PPAR taking part in relaxin signaling pathway decreased in healthy volunteers’ MenSCs while increased in patients with unexplained infertility. Matrix metalloproteinase (MMP)-mediated extracellular matrix degradation plays a key role in normal growth and remodeling of fetal membranes throughout gestation and are involved in the weakening and subsequent rupture of the fetal membranes at the time of labor [
28]. Sundrani et al., 2012, discovered that placental MMP-1 levels are higher in women delivering preterm as compared to those delivering at term [
29]. So far, the regulation mechanisms involved in matrix metalloproteinase expression and the motility of human endometrial and decidual stromal cells during decidualization remain unclear [
30]. Nevertheless, the estrogen signaling pathway in which the matrix metalloproteinase is involved should be considered for the treatment of infertility of unknown origin. For the decidualization and successful implantation, relaxin action with an accompanied increase in estrogen-associated factors is important [
31]. Accordingly, the main identified proteins demonstrate that at least relaxin and estrogen signaling pathways and the proteins involved in them are important for successful conception. Data suggest that these identified secretory proteins could be one of underlying causes for unexplained infertility.
4. Materials and Methods
4.1. Patient Recruitment
We conducted an interventional prospective cohort study. Patients were recruited from 2017 to 2020 at the Vilnius University Hospital Santaros Klinikos Obstetrics and Gynecology Center Santaros Fertility Center. Females with unexplained infertility or poor ovarian reserve were enrolled in this study. Protocols approved by the Ethics Committee of Biomedical Research of Vilnius District, No 158200-18/7-1049-550. The inclusion criteria were as follows: (a) age of woman at the time of enrollment is 30–35 years (average—35 years old), (b) the average duration of infertility—4.2 years (range 1.5–6.5 years), (c) couples with unexplained infertility diagnosis are confirmed after laboratory and instrumental investigation, (d) woman confirms the participation in the study. The exclusion criteria were as follows: (a) woman is pregnant or breastfeeding, (b) oncological disease was confirmed for woman during the last three years, (c) other infertility causes, except unexplained infertility, are confirmed, (d) woman is addicted to alcohol or other substances, (e) pregnancy is contraindicated, (f) uncontrolled endocrine or other medical conditions, such as prolactinemia or thyroid diseases.
Healthy volunteers were enrolled in the study in age of 18–45 years at the time of enrollment. The female volunteers had regular menstrual cycle (range 24–35) with normal hormonal status and no history of any other surgeries, diseases, smoking, medications, alcohol and other substance abuse.
4.2. Menstrual Blood Sample Collection and MenSCs Isolation and Cultivation
Menstrual blood samples were collected with a DivaCup during the second and third days of menstruation. Once received in the laboratory, menstrual blood samples were transferred into a 50 mL tube and treated with 50 U/mL collagenase II (Sigma-Aldrich, St. Louis, MO, USA) for 20 min at 37 °C in a humidified 5% CO2. MenSC isolation was immediately performed using the “Ficoll-Paque™ premium” (Sigma-Aldrich, St. Louis, MO, USA) according to manufacturer’s protocol. After isolation MenSC washed in phosphate-buffered saline (PBS) (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) twice and suspended in growth medium DMEM/F12 medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin (100 U/mL)—streptomycin (100 µg/mL) solution (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and, then, seeded into a 75 cm2 plastic cell culture flasks and cultivated at 37 °C in a humidified 5% CO2 atmosphere. After 3–5 days of culture, non-adherent cells were washed away with PBS (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) leaving behind adherent fibroblastic cells growing in clusters. The growth medium was replaced every 3 days.
4.3. Proliferation, Doubling Time and Viability Assay
MenSCs proliferation and viability were evaluated by trypan blue exclusion test. Cells were mixed with 0.2% of trypan blue dye (final concentration) (Pharmacia LKB, Uppsala, Sweden). Viable and dead (blue colored) cell numbers were determined by counting the cells in a hemocytometer under the light microscope. Cell doubling time was estimated by monitoring cell proliferation and calculated using the online tool
https://doubling-time.com, retrieved November 15, 2020. Doubling time is expressed in days and shows how long it takes to increase the number of cells by 2 times.
4.4. Flow Cytometry Analysis
For phenotypical characterization of MenSCs, 0.05 × 10
6 cells for one assay were collected by centrifugation at 500×
g for 5 min. Pelleted cells were washed twice in PBS supplemented with 1% bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO, USA). Then, cells were suspended in 50 μL PBS with 1% BSA and incubated with the antibodies (dilution ratio 1:25) against cell surface markers in the dark at 4 °C for 30 min. Antibodies are presented in
Table S1. After incubation samples were washed twice with PBS with 1% BSA. Finally, cells were suspended and fixated in 200 μL PBS with 1% paraformaldehyde (PFA) (Sigma-Aldrich, St. Louis, MO, USA) and analyzed using Partec flow cytometer (Sysmex Corporation, Cobe, Hioko, Japan) with Flowing Software 2 software.
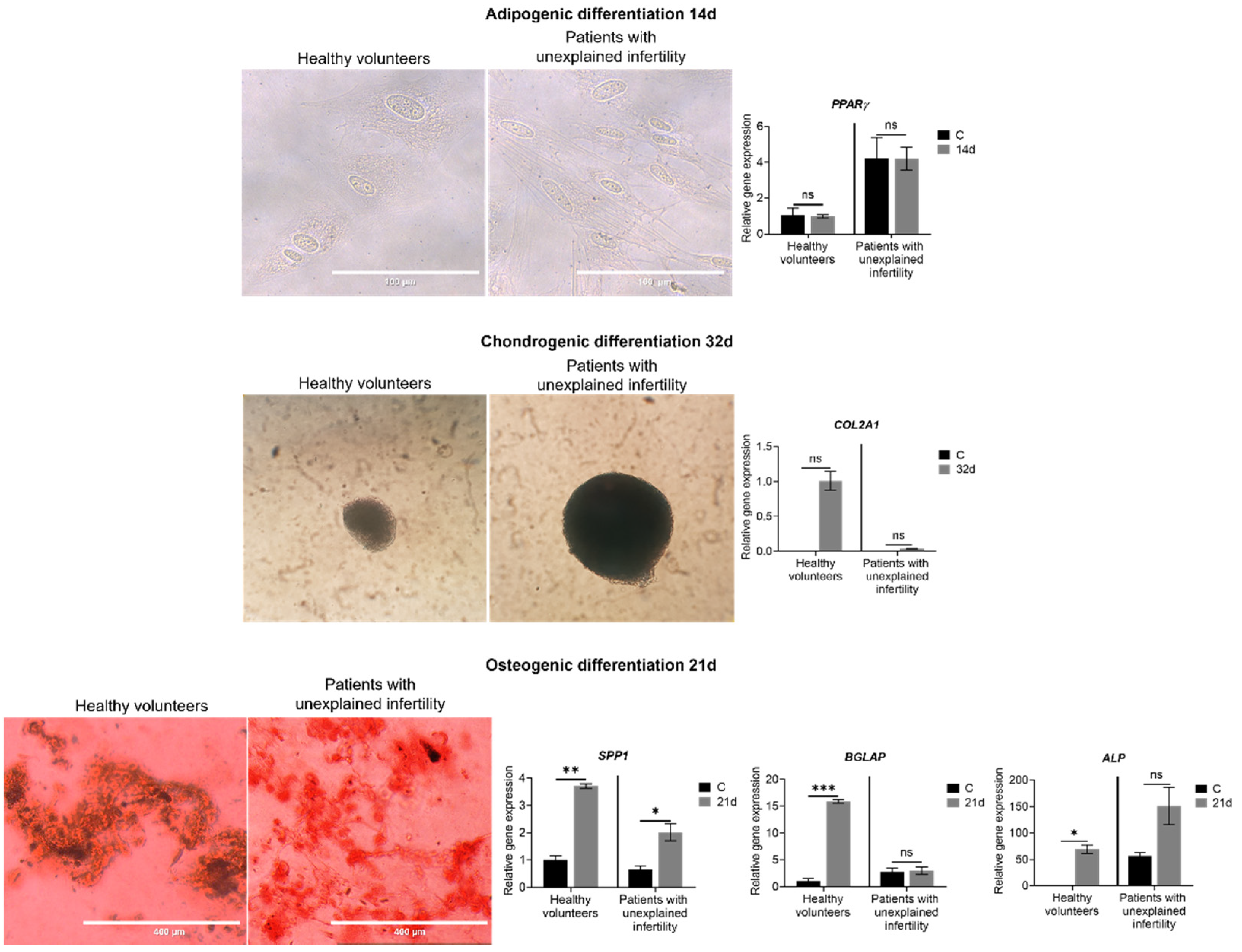
4.5. Adipogenic, Chondrogenic and Osteogenic Differentiation
MenSCs were cultured at 80–90% confluence and subsequently differentiated with differentiation medium at 37 °C in 5% CO2. Briefly, for differentiation assays, after cultivation, the growth medium was changed to either adipogenic differentiation medium: 4.5 g/L glucose DMEM (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) +1% penicillin–streptomycin (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) +1 μM dexamethasone (Cayman Chemical Company, Ann Arbor, MI, USA) +60 µM indomethacin (Sigma-Aldrich, St. Louis, MO, USA) +0.5 mM IBMX (Sigma-Aldrich, St. Louis, MO, USA), for 14 days; osteogenic differentiation medium: 1 g/L glucose DMEM (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) +1% penicillin– streptomycin (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) +10% FBS (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) +0.1 μM dexamethasone (Cayman Chemical Company, Ann Arbor, MI, USA) +50 μg/mL ascorbic acid (Cayman Chemical Company, Ann Arbor, MI, USA) +10 mM β-glycerophosphate (Cayman Chemical Company, Ann Arbor, MI, USA), for 21 days; chondrogenic differentiation medium: 4.5 g/L glucose DMEM (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) +1% penicillin–streptomycin (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) +0.1 μM dexamethasone (Cayman Chemical Company, Ann Arbor, MI, USA) +50 μg/mL ascorbic acid (Cayman Chemical Company, Ann Arbor, MI, USA) +1X ITS (PeproTech, London, UK) +1 mM sodium pyruvate (Genaxxon bioscience, Ulm, Germany) +0.35 mM proline (Genaxxon bioscience, Ulm, Germany), for 32 days, the induction medium was replaced every 3 days. Control cells were cultured in growth medium. At the end of the induction periods, the cells were washed and fixed. For cell staining, MenSCs were seeded into a 4-well (3.85 cm2) plate (Nunc, Thermo Fisher Scientific, Roskilde, Denmark) at a 2 × 104 cells/cm2 density. Adipogenic differentiation was confirmed by Oil Red O (Sigma-Aldrich, St. Louis, MO, USA) staining and osteogenic differentiation was confirmed by Alizarin Red (Sigma-Aldrich, St. Louis, MO, USA) staining and differentiation-specific genetic marker expression.
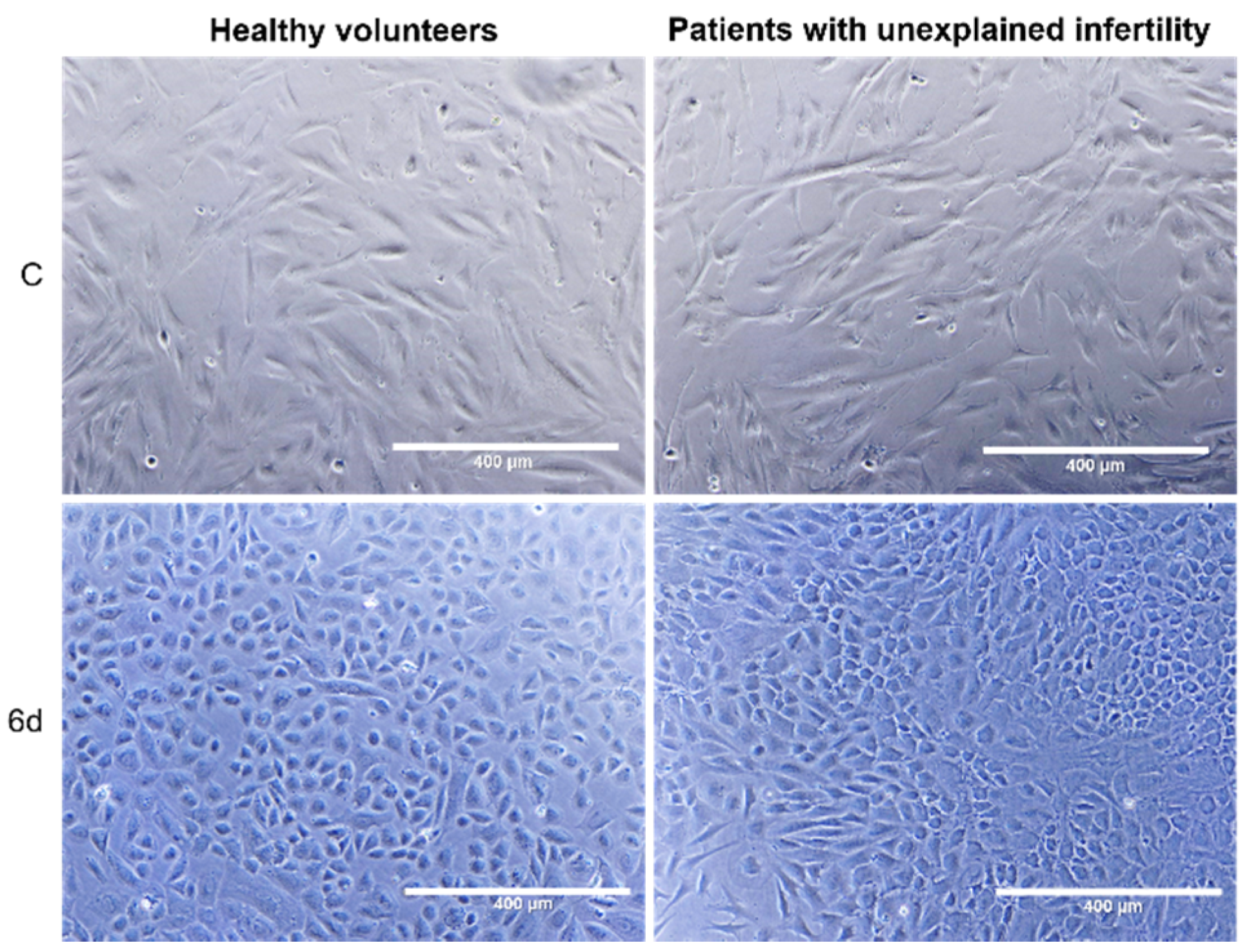
4.6. Decidualization Induction
MenSCs at 1 × 104 cells/cm2 density were cultured to 80–90% confluence and differentiated with decidualization induction medium: phenol red free RPMI 1640 (Hyclone Laboratories, Logan, UT, USA) +2% charcoal stripped FBS (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) +1% penicillin–streptomycin (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) +0.5 mM 8-brom-cAMP (Cayman Chemical Company, Ann Arbor, MI, USA) +1 µM medroxyprogesterone acetate (MPA) (Cayman Chemical Company, Ann Arbor, MI, USA) at 37 °C in 5% CO2 for 6 days. Differentiation was confirmed by observation of cell morphology changes with light microscope “EVOS™” (Thermo Fisher Scientific, Waltham, MA, USA).
4.7. Gene Expression Analysis by RT-qPCR
Total RNA was purified using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), cDNA was synthesized using SensiFAST™ cDNA Synthesis Kit (Bioline, Memphis, TN, USA), and qPCR was performed using SensiFAST™ SYBR
® No-ROX Kit (Bioline Memphis, TN, USA) on the RotorGene 6000 system (Corbett Life Science, QIAGEN, Hilden, Germany). Primer sequences (Metabion international AG, Planegg/Steinkirchen, Germany) are presented in
Table S2. mRNA levels were normalized to
GAPDH expression. The reference gene was selected using the NormFinder tool (Aarhus University Hospital, Aarhus, Denmark). Three candidate genes were tested with stability values of 0.143 for
GAPDH, 0.411 for
HPRT1 and 1.224 for
TUBB. Relative gene expression was calculated using the ΔΔCt method.
4.8. Protein Isolation and Immunoanalysis
MenSCs (1–2 × 106) were harvested, washed twice in ice-cold PBS, and 0.1 volume of benzonase (Merck, Darmstadt, Germany) was added to the tubes with patient cells and incubated for 30 min on ice, then 1 volume of 2× SDS lysis buffer (125 mM Tris, pH 6.8; 4% SDS, 600 mM DTT, 5% glycerol) and up to 20 volumes of 1× SDS lysis buffer were added. The mixture in the tubes was fragmented with an insulin syringe until non-viscous. After lysis, the tubes were incubated at 95 °C for 5 min. The ready-to-use samples were immediately subjected to electrophoresis or stored in a freezer at −20 °C.
Proteins were fractionated on a 7–15% polyacrylamide gradient SDS/PAGE gel using Tris-glycine buffer. After protein transfer to a PVDF membrane (Immobilon P; Millipore, Billerica, MA, USA), the membrane was blocked with “AdvanBlock-Chemi Antibody-antigen enhancing and blocking solution” (Advansta, San Jose, CA, USA), washed in PBS–Tween-20 and probed with the primary antibody (
Table S3) in accordance with the manufacturer’s instructions. The membrane was subsequently washed four times with PBS–Tween-20 and then incubated with horseradish peroxidase (HRP)-linked secondary antibody for 1 h at room temperature. GAPDH was used as loading control. “Clarity Western ECL Substrate” (BIORAD, Hercules, CA, USA) was used for chemiluminescent detection. Signal detection was carried out on ChemiDoc XRS+ System (BIORAD, Hercules, CA, USA). Quantitative evaluation was performed using ImageJ software.
4.9. Secretome Analysis by Mass Spectrometry
MenSCs’ culture medium is aspirated and collected in 5 mL tubes and centrifuged for 10 min at 2000×
g to remove cells and cell debris. After centrifugation, the supernatant is collected and filtered through a 0.22 μm filter. After filtration, the samples are cleaned and concentrated using ProteoMiner
TM Sequential Elution Large-Capacity kit (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions. The secretome protein concentration was measured using the Pierce Detergent Compatible Bradford Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The secretome proteins were trypsinized according FASP protocol, as described by Wisniewski et al. [
32]. Briefly, proteins were diluted in 8 M urea; following two washes with urea, proteins were alkylated with 50 mM iodoacetamide (GE Healthcare Life Sciences, MA, USA). Protein concentrators were washed twice with urea and twice with 50 mM NH
4HCO
3. Proteins were digested overnight with TPCK Trypsin 20233 (Thermo Scientific, Vilnius, Lithuania). After overnight digestion, peptides were collected from the concentrators by centrifugation at 14,000×
g for 10 min and additionally eluted using 20% CH
3CN. The eluates were combined, acidified with 10% CF
3COOH and lyophilized in vacuum centrifuge. The lyophilized peptides were redissolved in 0.1% formic acid.
4.10. LC-MS-Based Protein Identification
Liquid chromatographic (LC) analysis was performed in a Waters Acquity ultra performance LC system (Waters Corporation, Wilmslow, UK). Peptide separation was performed on an ACQUITY UPLC HSS T3 250 mm analytical column. Data were acquired using Synapt G2 mass spectrometer (MS) and Masslynx 4.1 software (Waters Corporation) in positive ion mode using data-independent acquisition (
UDMSE). Raw data were lock mass-corrected using the doubly charged ion of [Glu1]-fibrinopeptide B (
m/
z 785.8426; [M+2H]2+). Raw data files were processed and searched using ProteinLynx Global SERVER (PLGS) version 3.0.1 (Waters Corporation, UK). Data were analyzed using trypsin as the cleavage protease; one missed cleavage was allowed, and fixed modification was set to carbamidomethylation of cysteines; variable modification was set to oxidation of methionine. Minimum identification criteria included 1 fragment ions per peptide, 3 fragment ions and one peptide per protein. The following parameters were used to generate peak lists: (i) low energy threshold was set to 150 counts, (ii) elevated energy threshold was set to 50 counts, (iii) intensity threshold was set to 750 counts. UniprotKB/SwissProt human databases (2020-09-24) were used. Protein quantification was calculated using the ISOQuant software. The obtained results of mass spectrometry analysis were also analyzed with the online tool STRING (String consortium,
https://string-db.org, retrieved 20 January 2021).
4.11. Statistical Analysis
Data are expressed as mean ± standard deviation (S.D.). T-test (Holm–Sidak method) was used to calculate the significance of difference between samples; significance was set at p ≤ 0.05 (*); p ≤ 0.01 (**); p ≤ 0.001 (***).