Placental Complement Activation in Fetal and Neonatal Alloimmune Thrombocytopenia: An Observational Study
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics
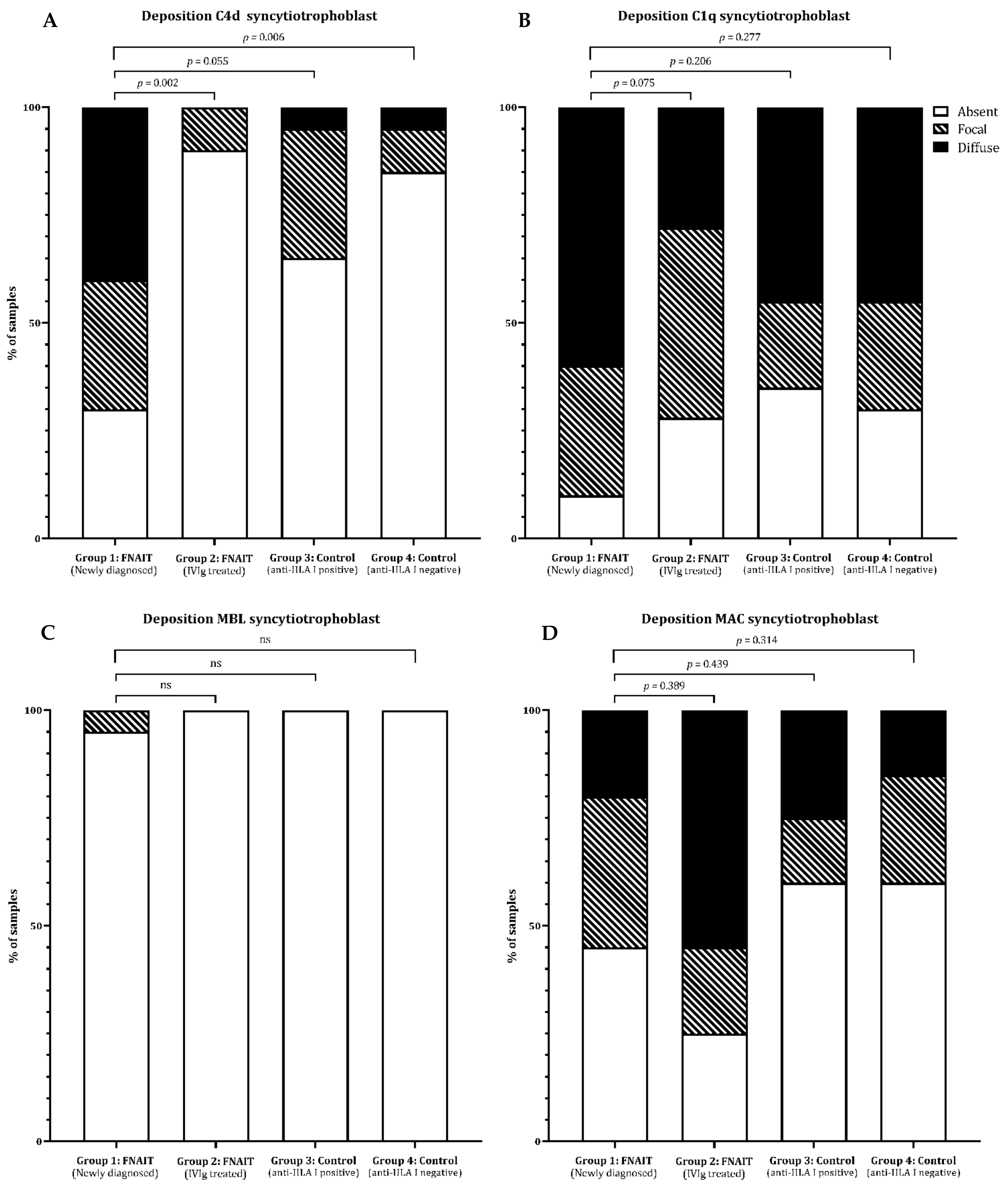
2.2. C4d Deposition on Syncytiotrophoblast Is Increased in Newly Detected FNAIT Cases
2.3. Placenta Maturation Was Delayed in Newly Diagnosed FNAIT Cases
3. Discussion
3.1. Main Findings
3.2. Strengths and Limitations
3.3. Interpretation
3.4. Future Perspectives
4. Materials and Methods
4.1. Study Cohort and Placenta Collection
4.2. Clinical Data Collection and Definitions
4.3. Ethics
4.4. HPA Antibody Detection
4.5. HLA Antibody Detection and HLA Typing
4.6. Histopathology
4.7. Immunohistochemistry
4.8. Quantification of Immunohistochemical Staining
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dreyfus, M.; Kaplan, C.; Verdy, E.; Schlegel, N.; Durand-Zaleski, I.; Tchernia, G. Frequency of immune thrombocytopenia in newborns: A prospective study. Immune Thrombocytopenia Working Group. Blood 1997, 89, 4402–4406. [Google Scholar] [CrossRef]
- Winkelhorst, D.; Kamphuis, M.M.; Steggerda, S.J.; Rijken, M.; Oepkes, D.; Lopriore, E.; van Klink, J.M.M. Perinatal Outcome and Long-Term Neurodevelopment after Intracranial Haemorrhage due to Fetal and Neonatal Alloimmune Thrombocytopenia. Fetal Diagn. Ther. 2019, 45, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Winkelhorst, D.; Murphy, M.F.; Greinacher, A.; Shehata, N.; Bakchoul, T.; Massey, E.; Baker, J.; Lieberman, L.; Tanael, S.; Hume, H.; et al. Antenatal management in fetal and neonatal alloimmune thrombocytopenia: A systematic review. Blood 2017, 129, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Van Gils, J.M.; Stutterheim, J.; van Duijn, T.J.; Zwaginga, J.J.; Porcelijn, L.; de Haas, M.; Hordijk, P.L. HPA-1a alloantibodies reduce endothelial cell spreading and monolayer integrity. Mol. Immunol. 2009, 46, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Refsum, E.; Meinke, S.; Gryfelt, G.; Wikman, A.; Höglund, P. Adding to the complexity of fetal and neonatal alloimmune thrombocytopenia: Reduced fibrinogen binding in the presence of anti-HPA-1a antibody and hypo-responsive neonatal platelets. Thromb. Res. 2018, 162, 69–76. [Google Scholar] [CrossRef]
- Santoso, S.; Wihadmadyatami, H.; Bakchoul, T.; Werth, S.; Al-Fakhri, N.; Bein, G.; Kiefel, V.; Zhu, J.; Newman, P.J.; Bayat, B.; et al. Antiendothelial αvβ3 Antibodies Are a Major Cause of Intracranial Bleeding in Fetal/Neonatal Alloimmune Thrombocytopenia. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1517–1524. [Google Scholar] [CrossRef]
- Yougbare, I.; Zdravic, D.; Ni, H. Angiogenesis and bleeding disorders in FNAIT. Oncotarget 2015, 6, 15724–15725. [Google Scholar] [CrossRef]
- Tiller, H.; Killie, M.K.; Husebekk, A.; Skogen, B.; Ni, H.; Kjeldsen-Kragh, J.; Øian, P. Platelet antibodies and fetal growth: Maternal antibodies against fetal platelet antigen 1a are strongly associated with reduced birthweight in boys. Acta Obstet. Gynecol. Scand. 2012, 91, 79–86. [Google Scholar] [CrossRef]
- Winkelhorst, D.; Oostweegel, M.; Porcelijn, L.; Middelburg, R.A.; Zwaginga, J.J.; Oepkes, D.; van der Bom, J.G.; de Haas, M.; Lopriore, E. Treatment and outcomes of fetal/neonatal alloimmune thrombocytopenia: A nationwide cohort study in newly detected cases. Br. J. Haematol. 2019, 184, 1026–1029. [Google Scholar] [CrossRef]
- Tiller, H.; Kamphuis, M.M.; Flodmark, O.; Papadogiannakis, N.; David, A.L.; Sainio, S.; Koskinen, S.; Javela, K.; Wikman, A.T.; Kekomaki, R.; et al. Fetal intracranial haemorrhages caused by fetal and neonatal alloimmune thrombocytopenia: An observational cohort study of 43 cases from an international multicentre registry. BMJ Open 2013, 3. [Google Scholar] [CrossRef]
- Tiller, H.; Killie, M.K.; Chen, P.; Eksteen, M.; Husebekk, A.; Skogen, B.; Kjeldsen-Kragh, J.; Ni, H. Toward a prophylaxis against fetal and neonatal alloimmune thrombocytopenia: Induction of antibody-mediated immune suppression and prevention of severe clinical complications in a murine model. Transfusion 2012, 52, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Yougbaré, I.; Tai, W.S.; Zdravic, D.; Oswald, B.E.; Lang, S.; Zhu, G.; Leong-Poi, H.; Qu, D.; Yu, L.; Dunk, C.; et al. Activated NK cells cause placental dysfunction and miscarriages in fetal alloimmune thrombocytopenia. Nat. Commun. 2017, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Colvin, R.B.; Daha, M.R.; Drachenberg, C.B.; Haas, M.; Nickeleit, V.; Salmon, J.E.; Sis, B.; Zhao, M.H.; Bruijn, J.A.; et al. Pros and cons for C4d as a biomarker. Kidney Int. 2012, 81, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Buyon, J.P.; Guerra, M.M.; Rana, S.; Zhang, D.; Laskin, C.A.; Petri, M.; Lockshin, M.D.; Sammaritano, L.R.; Branch, D.W.; et al. Angiogenic factor imbalance early in pregnancy predicts adverse outcomes in patients with lupus and antiphospholipid antibodies: Results of the PROMISSE study. Am. J. Obstet. Gynecol. 2016, 214, 108.e1–108.e14. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Buurma, A.; Goemaere, N.N.; Girardi, G.; le Cessie, S.; Scherjon, S.; Bloemenkamp, K.W.; de Heer, E.; Bruijn, J.A.; Bajema, I.M. Classical complement activation as a footprint for murine and human antiphospholipid antibody-induced fetal loss. J. Pathol. 2011, 225, 502–511. [Google Scholar] [CrossRef]
- Buurma, A.; Cohen, D.; Veraar, K.; Schonkeren, D.; Claas, F.H.; Bruijn, J.A.; Bloemenkamp, K.W.; Baelde, H.J. Preeclampsia is characterized by placental complement dysregulation. Hypertension 2012, 60, 1332–1337. [Google Scholar] [CrossRef]
- Agostinis, C.; Bulla, R.; Tripodo, C.; Gismondi, A.; Stabile, H.; Bossi, F.; Guarnotta, C.; Garlanda, C.; De Seta, F.; Spessotto, P.; et al. An alternative role of C1q in cell migration and tissue remodeling: Contribution to trophoblast invasion and placental development. J. Immunol. 2010, 185, 4420–4429. [Google Scholar] [CrossRef]
- Girardi, G. Complement activation, a threat to pregnancy. Semin. Immunopathol. 2018, 40, 103–111. [Google Scholar] [CrossRef]
- Wabnitz, H.; Khan, R.; Lazarus, A.H. The use of IVIg in fetal and neonatal alloimmune thrombocytopenia- Principles and mechanisms. Transfus. Apher. Sci. 2020, 59, 102710. [Google Scholar] [CrossRef]
- Pierik, E.; Prins, J.R.; van Goor, H.; Dekker, G.A.; Daha, M.R.; Seelen, M.A.J.; Scherjon, S.A. Dysregulation of Complement Activation and Placental Dysfunction: A Potential Target to Treat Preeclampsia? Front. Immunol. 2019, 10, 3098. [Google Scholar] [CrossRef]
- Jaiman, S.; Romero, R.; Pacora, P.; Jung, E.J.; Kacerovsky, M.; Bhatti, G.; Yeo, L.; Hsu, C.D. Placental delayed villous maturation is associated with evidence of chronic fetal hypoxia. J. Perinat. Med. 2020, 48, 516–518. [Google Scholar] [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Kim, J.S. Chronic inflammation of the placenta: Definition, classification, pathogenesis, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213, S53–S69. [Google Scholar] [CrossRef]
- Althaus, J.; Weir, E.G.; Askin, F.; Kickler, T.S.; Blakemore, K. Chronic villitis in untreated neonatal alloimmune thrombocytopenia: An etiology for severe early intrauterine growth restriction and the effect of intravenous immunoglobulin therapy. Am. J. Obstet. Gynecol. 2005, 193, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Dubruc, E.; Lebreton, F.; Giannoli, C.; Rabilloud, M.; Huissoud, C.; Devouassoux-Shisheboran, M.; Allias, F. Placental histological lesions in fetal and neonatal alloimmune thrombocytopenia: A retrospective cohort study of 21 cases. Placenta 2016, 48, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Kumpel, B.M.; Sibley, K.; Jackson, D.J.; White, G.; Soothill, P.W. Ultrastructural localization of glycoprotein IIIa (GPIIIa, beta 3 integrin) on placental syncytiotrophoblast microvilli: Implications for platelet alloimmunization during pregnancy. Transfusion 2008, 48, 2077–2086. [Google Scholar] [CrossRef]
- Eksteen, M.; Heide, G.; Tiller, H.; Zhou, Y.; Nedberg, N.H.; Martinez-Zubiaurre, I.; Husebekk, A.; Skogen, B.R.; Stuge, T.B.; Kjær, M. Anti-human platelet antigen (HPA)-1a antibodies may affect trophoblast functions crucial for placental development: A laboratory study using an in vitro model. Reprod. Biol. Endocrinol. RBE 2017, 15, 28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paridaans, N.P.; Kamphuis, M.M.; Taune Wikman, A.; Tiblad, E.; Van den Akker, E.S.; Lopriore, E.; Challis, D.; Westgren, M.; Oepkes, D. Low-Dose versus Standard-Dose Intravenous Immunoglobulin to Prevent Fetal Intracranial Hemorrhage in Fetal and Neonatal Alloimmune Thrombocytopenia: A Randomized Trial. Fetal Diagn. Ther. 2015, 38, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.; Skogen, B.; Kjaer, M.; Husebekk, A.; Kjeldsen-Kragh, J.; Tiller, H. A combined effect of anti-HPA-1a and anti-HLA Class I in pregnancy? Transfusion 2020. [Google Scholar] [CrossRef] [PubMed]
- Pinar, H.; Sung, C.J.; Oyer, C.E.; Singer, D.B. Reference values for singleton and twin placental weights. Pediatr. Pathol. Lab. Med. 1996, 16, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Hoftiezer, L.; Hukkelhoven, C.W.; Hogeveen, M.; Straatman, H.M.; van Lingen, R.A. Defining small-for-gestational-age: Prescriptive versus descriptive birthweight standards. Eur. J. Pediatr. 2016, 175, 1047–1057. [Google Scholar] [CrossRef]
- Porcelijn, L.; Huiskes, E.; de Haas, M. Progress and development of platelet antibody detection. Transfus. Apher. Sci. 2020, 59, 102705. [Google Scholar] [CrossRef] [PubMed]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.; Boyd, T.K.; Brundler, M.A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef] [PubMed]
| Group 1 FNAIT Cases Newly Diagnosed n = 9 | Group 2 FNAIT Cases IVIg-Treated n = 14 | Group 3 Controls Anti-HLA I Positive n = 10 | Group 4 Controls Anti-HLA I Negative n = 10 | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Maternal age (years)—median (IQR) | 31 (29–34) | 33 (31–36) | 33 (31–35) | 33 (30–39) |
| Gravidity—median (IQR) | 2 (1–2) | 3 (2–4) | 3 (2–3) | 2 (1–4) |
| Parity—median (IQR) | 0 (0–1) | 1 (1–2) | 1 (0–1) | 1 (0–1) |
| Multiparous women—n (%) | 6 (67%) | 14 (100%) | 9 (90%) | 7 (70%) |
| Pre-eclampsia—n (%) | 0 | 0 | 0 | 0 |
| Delivery | ||||
| Spontaneous vaginal delivery—n (%) | 1 (11%) | 8 (57%) | 4 (40%) | 4 (40%) |
| CS fetal distress—n (%) | 3 (33%) | 1 (7%) | 0 | 0 |
| GA at delivery (weeks+days) **—median (IQR) | 37+1 (33+5–40+5) | 38+3 (37+2–38+6) | 39+4 (39+1–40+4) | 37+1 (38+4–40+2) |
| Neonatal data | ||||
| Sex (male)—n (%) | 5 (56%) | 7 (50%) | 5 (50%) | 5 (50%) |
| Birthweight (grams) **—median (IQR) | 2405 (2099–3535) | 3133 (2674–3493) | 3700 (3319–3805) | 3323 (3214–3814) |
| Small for gestational age *—n (%) | 4 (44%) | 1 (7%) | 0 | 0 |
| Skin bleeding only *—n (%) | 4 (44%) | 0 | 0 | 0 |
| Intracranial hemorrhage *—n (%) | 2 (22%) | 1 (7%) Ф | 0 | |
| Perinatal asphyxia—n (%) | 1 (11%) | 0 | 0 | 0 |
| Platelet count nadir (×109/l) **—median (IQR) | 17 (9–43) | 64 (21–170) | NT | 0 |
| HPA alloantibodies Mother HPA-1a-negative—n (%) Fetus HPA-1a-positive—n (%) Antibodies directed against; HPA-1a—n (%) HPA-1a and 3a—n (%) HPA-1a and 5b—n (%) | 9 (100%) 9 (100%) 6 (67%) 1 (11%) 2 (22%) | 14 (100%) 14 (100%) 14 (100%) | 0 NT NT | 0 NT NT |
| Anti-HLA class I present fetus-specific †—n (%) | 4 (57%), 2 missing | 4 (44%), 5 missing | 10 (100%) | 0 |
| Placenta characteristics ¶ | n = 9 | n = 9 | n = 7 | n = 8 |
| Placenta weight (grams)—median (IQR) | 460 (268–636) | 500 (440–577) | 572 (420–790) | 610 (533–730) |
| Placenta weight < p10 *—n (%) | 4 (44%) | 1 (7%) | 0 | 0 |
| Pathology | Group 1 FNAIT Cases Newly Diagnosed n = 9 | Group 2 FNAIT Cases IVIg-Treated n = 14 | Group 3 Controls Anti-HLA I-Positive n = 10 | Group 4 Controls Anti-HLA I-Negative n = 10 |
|---|---|---|---|---|
| Maturation | ||||
| Delayed | 4 (44%) | 5 (36%) | 1 (10%) | 0 |
| Corresponding with GA | 3 (33%) | 9 (64%) | 9 (90%) | 10 (100%) |
| Accelerated | 2 (22%) | 0 | 0 | 0 |
| Maternal vascular malperfusion | 3 (33%) | 3 (21%) | 0 | 0 |
| Retroplacental hematoma | 1 | 0 | 0 | 0 |
| Infarction | 3 | 0 | 0 | 0 |
| Ischemia | 0 | 2 | 0 | 0 |
| Distal villous hypoplasia | 1 | 3 | 0 | 0 |
| Fetal vascular malperfusion | 1 (11%) | 0 | 0 | 3 (30%) |
| Avascular villi | 1 | 0 | 0 | 3 |
| Ascending intrauterine infection | 2 (22%) | 1 (7%) | 1 (10%) | 2 (20%) |
| Stage 1 | 2 | 1 | 0 | 2 |
| Fetal response | 0 | 0 | 1 | 0 |
| Villitis of unknown etiology | 3 (33%) | 3 (21%) | 2 (20%) | 1 (10%) |
| Low-grade focal | 3 | 3 | 1 | 1 |
| Low-grade multifocal | 0 | 0 | 1 | 0 |
| Massive perivillous fibrin depositions | 0 | 1 (7%) | 0 | 0 |
| Signs of fetal hypoxia | 2 (22%) | 1 (7%) | 0 | 0 |
| Mild hypoxia | 2 | 1 | 0 | 0 |
| Chorangiosis | 0 | 0 | 2 (20%) | 0 |
| Meconium | 1 (11%) | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Vos, T.W.; Winkelhorst, D.; Baelde, H.J.; Dijkstra, K.L.; van Bergen, R.D.M.; van der Meeren, L.E.; Nikkels, P.G.J.; Porcelijn, L.; van der Schoot, C.E.; Vidarsson, G.; et al. Placental Complement Activation in Fetal and Neonatal Alloimmune Thrombocytopenia: An Observational Study. Int. J. Mol. Sci. 2021, 22, 6763. https://doi.org/10.3390/ijms22136763
de Vos TW, Winkelhorst D, Baelde HJ, Dijkstra KL, van Bergen RDM, van der Meeren LE, Nikkels PGJ, Porcelijn L, van der Schoot CE, Vidarsson G, et al. Placental Complement Activation in Fetal and Neonatal Alloimmune Thrombocytopenia: An Observational Study. International Journal of Molecular Sciences. 2021; 22(13):6763. https://doi.org/10.3390/ijms22136763
Chicago/Turabian Stylede Vos, Thijs W., Dian Winkelhorst, Hans J. Baelde, Kyra L. Dijkstra, Rianne D. M. van Bergen, Lotte E. van der Meeren, Peter G. J. Nikkels, Leendert Porcelijn, C. Ellen van der Schoot, Gestur Vidarsson, and et al. 2021. "Placental Complement Activation in Fetal and Neonatal Alloimmune Thrombocytopenia: An Observational Study" International Journal of Molecular Sciences 22, no. 13: 6763. https://doi.org/10.3390/ijms22136763
APA Stylede Vos, T. W., Winkelhorst, D., Baelde, H. J., Dijkstra, K. L., van Bergen, R. D. M., van der Meeren, L. E., Nikkels, P. G. J., Porcelijn, L., van der Schoot, C. E., Vidarsson, G., Eikmans, M., Kapur, R., van der Keur, C., Trouw, L. A., Oepkes, D., Lopriore, E., van der Hoorn, M.-L. P., Bos, M., & de Haas, M. (2021). Placental Complement Activation in Fetal and Neonatal Alloimmune Thrombocytopenia: An Observational Study. International Journal of Molecular Sciences, 22(13), 6763. https://doi.org/10.3390/ijms22136763








